Abstract
Intracranial EEG (iEEG), also known as electrocorticography (ECoG) using subdural grid electrodes or stereotactic EEG (sEEG) using depth electrodes, is blossoming in various fields of human neuroscience. In this article, we highlight the potentials of iEEG in exploring functions of the human brain while also considering its limitations. The iEEG signal provides anatomically precise information about the selective engagement of neuronal populations at the millimeter scale and about the temporal dynamics of their engagement at the millisecond scale. If several nodes of a given network are monitored simultaneously with implanted electrodes, the iEEG signals can also reveal information about functional interactions within and across networks during different stages of neural computation. As such, human iEEG can complement other methods of neuroscience beyond simply replicating what is already known, or can be known, from non-invasive lines of research in humans or from invasive recordings in non-human mammalian brains.
INTRODUCTION
Inspired by the reports of electrical signals recorded directly from the brains of rabbits, cats, dogs and monkeys1,2, Hans Berger (1873–1941) performed the first recordings of human electrical brain activity with electrodes attached to the scalp surface in patients with skull bone removed or healthy individuals without much hair (e.g., bald men) and called his method electroencephalography3, nowadays referred to as scalp EEG. When the EEG recordings are obtained with intracranial electrodes, we refer to it as intracranial EEG (iEEG) either in the form of electrocorticography (ECoG) using strips or grids of electrodes implanted in the subdural space), or stereotaxic-EEG (sEEG) using wires of electrodes penetrating the brain and targeting pre-defined deeper sites (e.g., hippocampus) without open craniotomy (Figure 1).
Figure 1: Two Methods of Intracranial EEG: Electrocorticography (ECoG) and Stereo-EEG (sEEG).
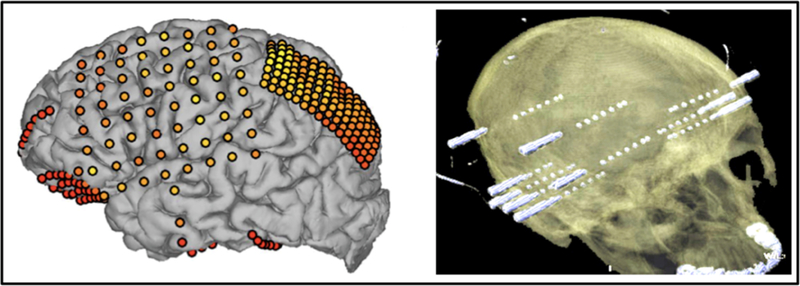
While grids and strips of subdural electrodes (left) provide a large coverage over the bare surface of the cerebral cortex, they are often implanted in one hemisphere and do not reach deeper brain structures (e.g., hippocampus or insula). By comparison, depth electrodes (right) can enable bilateral monitoring of superficial and deep cortical structures but only the most superficial and deep contacts will be within the cortical gray matter while the rest of the contacts are placed in the white matter. ECoG electrodes have a circular plate shape while depth electrode contacts have a cylinder shape. The diameter of subdural plate electrodes is often 1.2 to 3mm while the diameter of depth electrodes is 0.86–1.1mm with 2.29 or 2.41mm height. The distance between the centers of two adjacent electrodes (subdural or depth) is often in the range of 4 to 10mm. The total area of the brain covered with electrodes can be in the range of 1mm2 to 15mm2. Lastly, it should be noted that the number of electrodes and the coverage areas are defined according to the patient’s clinical needs. Because majority of patients have seizures originating from medial temporal and frontal lobes, it is exceedingly rare to find coverage outside these regions of the brain. This often explains the relatively small number of subjects in iEEG publications reporting data from non-temporal and non-frontal sites.
Today, combined with neuroimaging and computational tools, human iEEG has become increasingly more amenable for scientific explorations and its popularity trend among neuroscientists is on a steady rise (Figure 2). As such, human iEEG provides the opportunity to confirm and extend cognitive neuroscience research from other modalities. This review is intended to provide an overview of the promise and limitation of human iEEG for complementing other methods of scientific inquiry in cognitive neuroscience.
Figure 2: Recent Surge in the Number of iEEG Publications.
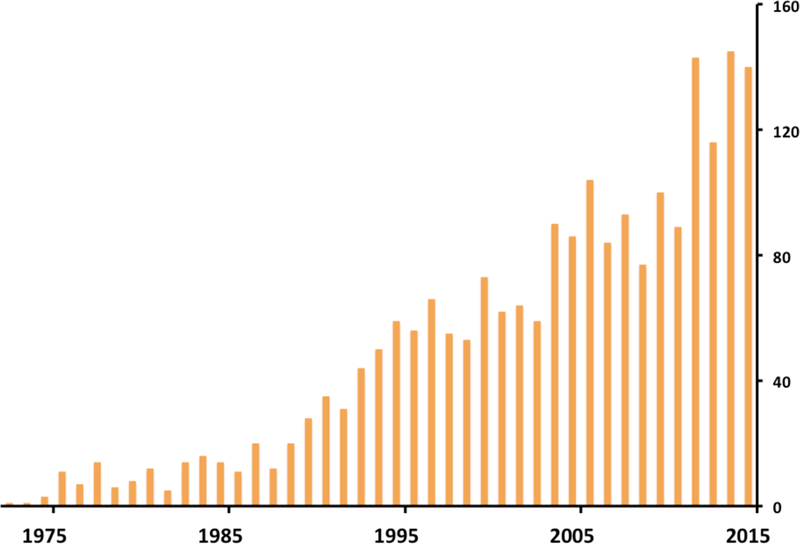
Number of publications in PubMed using the search terms “sEEG”, “depth electrodes”, “iEEG”, “iEEG”, “ECoG”, or electrocorticography.
CLINICAL RATIONALE FOR INVASIVE RECORDINGS WITH SUBDURAL OR DEPTH ELECTRODES
Most human iEEG studies are conducted in patients with epilepsy. About 1% of the world’s population suffers from epilepsy and approximately 1/3 of these patients suffer from medication-resistant epilepsy4. These patients have breakthrough seizures while on medications. Those with focal epilepsy can gain seizure freedom if the source of their seizures can be identified and surgically removed5. Prior to that, iEEG monitoring is often needed to identify the precise origin of seizures. Before the patient is implanted for invasive monitoring, clinicians use non-invasive diagnostic tools to form a hypothesis about the approximate origin of patient’s seizures. Scalp-EEG recordings are used to determine (if possible) the laterality and approximate lobar origin of seizures; structural brain MRI to detect anatomical abnormalities that are often associated with seizures; brain FDG-PET to determine focal hypometabolic tissue; and neuropsychological evaluations to detect lateralizing cognitive deficits (e.g., verbal memory deficits associated with seizures involving the left medial temporal lobe). Invasive implantation of electrodes is planned if clinicians have a high confidence that the patient suffers from focal epilepsy – though the exact focus if often not known.
If the laterality of seizures is unknown, or if the seizure onset zone is hypothesized to be in the deeper structures of the brain (such as the hippocampus or the insula) the sEEG approach is preferred. In these cases, each patient is often implanted with 5–15 depth electrodes, unilaterally or bilaterally (each consisting of 10–14 recording contacts). These electrodes often target the limbic structures (medial temporal lobes, cingulate, orbitofrontal and insular regions), but since they penetrate the brain from its lateral surface, they also offer recordings from the lateral sites as well (Figure 1).
If the preoperative evidence is strong enough to suggest laterality and lobar origin of seizures, but the extent of the epileptic tissue is unknown, the ECoG method with subdural electrodes is preferred. In these cases, grids and strips of electrodes are placed over the suspected lobe to confirm the precise extent of the pathologic brain tissue and to delineate the safe boundaries of cortical resection.
Because clinical needs dictate the pattern and type of implantation in each patient, and given that the majority of patients with epilepsy suffer from limbic or frontal lobe seizures, most of the implanted electrodes will cover these regions of the brain. Recordings from occipital and parietal lobes by comparison quite rare and unique. In a study of 2200 patients with epilepsy, clinical non-invasive data suggested focal origin in approximately 62%. In this subgroup of patients, about 66% were suspected to have temporal lobe epilepsy while 24% frontal lobe, 3% occipital, and 2% parietal, and 3% multilobar epilepsies6. The high percentage of cases with suspected temporal lobe epilepsy explains why the majority of human iEEG reports are from the temporal or frontal lobes.
Once intracranial electrodes are implanted in the operating room and under general anesthesia, the patient is transferred to a hospital room to be connected with wires to a recording rig for continuous streaming of raw electrophysiological data from the implanted electrodes. The patient’s antiepileptic medications are gradually discontinued and if needed analgesic medications are administered. Given the large number of wires connecting the electrodes to the recording rig, the patient is literally tethered to bed for several days. To determine the source of seizures, one often needs to record for several days to capture several seizures. It is during these days of monitoring and in this clinical setting that neuroscience experiments are conducted.
Characteristics of Human iEEG:
Limited Accessibility and Clinical Setting:
One of the main limitations of the iEEG method is that it is only doable in clinical settings at few hospitals and by specially trained teams of clinicians and investigators. This introduces a significant limitation of accessibility. Furthermore, the experimental subjects suffer from a pathological condition (See Box 1). Clinical and hospital constraints do not permit experimental setups for sophisticated psychophysics measurements, and the experiments often suffer from low number of trials and simplicity of design. Moreover, the location of electrodes is decided clinically and, once implanted in the operating room, cannot be changed - unlike animal recordings in which the investigator penetrate the cortex many times until the responsive neurons are found.
BOX 1: PATHOLOGICAL BRAINS?
Findings from patients with epilepsy have contributed significantly to our understanding of the brain throughout the history of neuroscience. For instance, experiments in patients with epilepsy led to the finding of somatotopic organization in the postcentral gyrus85, significance of medial temporal lobe in memory consolidation (patient HM)86, and lateralization of functions across the hemispheres (split brains)87.
A major concern regarding iEEG is that recordings are made from patients with longstanding epilepsy. A pertinent question that needs to be addressed is the extent to which epilepsy brains provide a suitable model for studying regular neural mechanisms underlying various aspects of human cognition and behavior. Are epilepsy brains too pathological to begin with? Will intracranial recordings in epileptic brains reflect the normal neural substrates of human brain function? These are valid questions and have to be carefully considered. However, the answer to these questions depends on two important factors: the patient population recruited for the iEEG study, and the relative health of the recorded brain areas.
Epilepsy is a heterogeneous disease with diverse severity, clinical appearance, and pathogenic mechanisms. At one end of the spectrum, it includes severe epilepsy syndromes, with onset in early childhood, that are associated with bilateral and multifocal epileptiform discharges, diffuse slowing of baseline brain activity, mental deterioration, and behavioral regression. At the other end of the spectrum, it includes focal epilepsies in high functioning adult patients, who have normal intelligence and few localized deficits depending on the focal brain network that is involved 88–91. In addition, the cognitive and behavioral burden of the disease is directly related to factors such as early onset, duration of the disease and seizure control. It is therefore imperative that the iEEG reports are interpreted in light of the severity of the disease and the details of administered medications in the studied patient population. Unfortunately, current iEEG reports vary significantly in the clinical details that they provide (See Opportunities for Growth for suggestions).
The main goal of invasive recordings is to find a single seizure focus and offer focal resection and thereby seizure freedom. IEEG monitoring is only chosen if the preoperative work up suggests focality of disease and a higher chance for finding the seizure focus – even though most of the time the exact focus is not known prior to implantation and even though many times multifocal disease is confirmed only after the implantation. Invasive monitoring is mostly avoided in patients with presurgical evidence for known multifocal or diffuse disease. In these patients, intracranial recordings will reveal widespread and bilateral pathological activity.
During invasive monitoring, often a large number of electrodes (100–200 per patient) are implanted across lobes or hemispheres to ensure that the source of seizures is not missed. As a result, intracranial electrodes often cover a large extent of the brain. In a patient with focal disease, and with a relatively large number of implanted electrodes, many recording sites will be void of epileptic activity while a few will capture the source of seizures.
The ratio of epileptic to non-epileptic electrodes will naturally depend on the total number of implanted electrodes and the location of electrodes across the brain. For instance, a patient who reports flickering stars in the right visual field every time he has a seizure, and scalp EEG evidence of epileptic discharges in the left posterior quadrant of the head, one may choose to implant strips and grids of ECoG electrodes covering a large extent of the occipital and ventral temporal cortices in the left hemisphere. In this patient, one will find that a small piece of cortex in the peri-calcarine area is epileptic while the rest of implanted sites are void of pathological epileptic discharges. In another patient with limbic seizures (e.g., rising nausea) and scalp EEG evidence of bilateral discharges and no MRI or PET abnormality, one will implant the hippocampi, amygdalae, anterior and posterior cingulate, as well as orbitofrontal and insular cortices bilaterally with the sEEG method. In this patient, few electrodes in the medial temporal lobe regions may show epileptic activity while others will be void of such activity. An electrode needle with 14 recording contacts will target the insula traversing through the frontal lobe. While the first couple of electrodes capture insular activity, the other contacts along the electrode needle will provide unique recordings from structures such as the claustrum.
As a safety measure, one often concentrates the signal analysis on the channels that are free from pathological activity (i.e., without pathological slowing of background activity and without epileptiform discharges). This is especially important when the analysis is focused on the power of HFB because transient and paroxysmal pathological high frequency oscillations (HFOs) are often present in the epileptic tissue (see main text), and it is important to ensure that these HFOs are not mixed with induced time-locked physiological HFBs.
An issue that is often discussed in the clinical epilepsy literature, but has yet to be settled is to what extent the focal epileptic activity in one region of the brain affects the normal activity of the rest the brain. It has been known that isolated epileptic discharges (that correspond to large amplitude intracellular depolarization with evoked action potentials in a group of neurons) and subclinical subtle seizure activity in the same region cause transitory cognitive impairment (TCI) with the type of deficit dependent on where in the cortex the epileptic activity arises 92–94. It has been hypothesized that focal epileptic discharges (i.e., seizures or isolated interictal discharges) cause material and site-specific deficits in cognitive functions that are mediated by the focal network of the brain in the hemisphere in which the discharges occur94. As such, material specific deficits in a patient can indirectly be used to highlight the brain network that is affected by seizures. In fact, for many decades, neuropsychological preoperative evaluation of patients who are candidates for epilepsy surgery is successfully used in clinical practice to highlight dysfunction in specific domains of cognition and thereby provide functional information about the possible lobe and hemisphere of origin for the patient’s seizures95. For instance, patients with left temporal lobe seizures are expected to score lower on verbal memory tests and those with frontal lobe seizures do poorly in executive function tests95. Cognitive dysfunction in these patients is directly correlated with the frequency of interictal epileptiform discharges and are greater with generalized than with focal discharges, but more specific with the latter 94,96. The subsequent treatment of a discharging focus leads to modest improvement in patient’s cognition97.
Given the current state of our knowledge about focal epilepsy and the effect of epileptic activity on the rest of the brain, we believe epilepsy brains can only be used as a proxy for normal human brains if the confounding effects of epilepsy on the acquired intracranial electrophysiological data are minimized by rigorous measures. Such measures include: 1) acquiring data from non-epileptic tissue; 2) obtaining data several hours outside the window of seizures; 3) excluding trials in which epileptic discharges were occurring; and most importantly 4) documenting that the observed findings in a patient are anatomically and functionally consistent across other patients with other types of epilepsies and seizure foci. It would be beneficial to show that findings in epilepsy subjects are akin to findings reported in non-invasive studies of healthy subjects. This will naturally not be possible if the findings are novel and unreported in literature. As we will discuss in the following text, such reliable results from iEEG will have the potential to yield unique information about human cognition and subjective experience.
Sparse Sampling:
Since clinical needs dictate the pattern and type of implantation in each patient, parietal, occipital, and inter-hemispheric areas are much less frequently implanted with iEEG electrodes. Moreover, those brain areas that are covered with electrodes are often probed with electrodes that are 5–10 mm apart from each other. Thus, unlike functional MRI, an excellent global coverage across the whole brain is not possible.
Corticocentric Bias:
Except studies of amygdala and hippocampus with depth electrodes in epilepsy patients or the subthalamic nucleus in patients with Parkinson’s disease7,8, subcortical structures such as basal ganglia, thalamus, brainstem and cerebellar regions are not implanted for pre-surgical EEG monitoring in epilepsy patients because of lack of clinical motivation. As a consequence, there is little study of these subcortical regions using iEEG. Thereby, the field of iEEG fuels the current “corticocentric myopia” where contributions of subcortical structures to cognition and behavior are often not considered9. Direct recordings in non-human primates and other animal models are needed to fill this gap (e.g.10,11).
Neuronal population activity at millimeter resolution:
Electrodes used in sEEG method are cylinder shaped with contact length of ~2mm, diameter of ~1mm, and total surface area of ~10mm2 penetrating the cortical layers. By comparison, grid or strip electrodes used in ECoG are circular plates with a diameter of ~2mm and surface area of ~4mm2 placed over the bare cortex. Thus, iEEG electrodes capture signals from a relatively large and diverse population of cells. Given the diameter of human iEEG electrodes and the known estimates of the number of neurons per cortical area, one may assume that there are ~500,000 cells underneath or surrounding these electrodes12.
IEEG signals recorded from such a large and diverse population of cells is understandably complex and carry information in different bands of oscillatory activity (e.g., delta (1–3Hz), theta (4–7Hz), alpha (8–12Hz), beta (13–20Hz), and gamma (21 to ~40 or 50Hz)) as well as higher frequency activity known as high-gamma activity or high frequency broadband (HFB) (above 50Hz). The HFB signal is currently interpreted as a reflection of a non-oscillatory broadband signal13,14. Importantly, HFB is different than pathological High Frequency Oscillations (HFOs) that are seen in epileptic recording sites. For instance, HFOs are associated or coupled with interictal epileptiform discharges and are randomly interspersed with pathological background activity and can be present across several adjacent electrodes15,16 whereas HFBs are time locked to the presentation of specific stimuli or cognitive or behavioral conditions (see Box 1).
HFB activity is a reliable electrophysiological correlate of underlying averaged spiking activity generated by thousands of neurons adjacent to the recording electrode 17–21. The extent of brain tissue from which the HFB signals are recorded remains largely unknown. However, based on studies in non-human brains using research grade micro-electrodes estimates fall around several hundred micrometers 20,22,23. Moreover, the number of neurons contributing to the high frequency signal may be as scarce of ~16% of neurons sampled by a given electrode24.
HFB signals also correlate with hemodynamic signals detected with fMRI 21,25–28. Thus, an increase in the HFB power in a recording site represents the local engagement of the cortical tissue underneath or around the electrode. There is strong evidence that, unlike slow oscillatory activity, the HFB signal has a remarkably localized anatomical precision and originates from the cortical tissue immediately around or underneath the recording electrode (for human iEEG evidence see for example29–35 and for direct measures from non-human primate studies see for example18–20,22,27).
While HFB activity appears to reflect responses of local populations of cells around or underneath a recording electrode, slow frequency oscillations in the theta, alpha, and beta frequency bands may serve as carrier frequencies that are used by distant nodes within large-scale networks to communicate36. Importantly, slow frequency oscillations may control the excitability of local neuronal populations, as evidenced by the coupling between the higher frequency activity and slow oscillations during cognitive tasks36. Here we remind the reader that locking to phases of higher frequencies is technically difficult because a small amount of jitter in precision abolishes locking. The measure of coupling between the phase of slow oscillations and the power of higher frequencies (particularly HFB) or the rate of neuronal spikes (cross-frequency, or spike phase coupling), as well as the measure of coupling of phases of two oscillatory rhythms (phase-phase locking) can inform about important aspects of the functional dynamics of brain activity such as the directionality of information flow across a network (for recent reviews see 37,38). Cross-frequency interactions may also be important for folding nodes into a common network or for integration of interactions across different cognitive networks39. As relevant examples, in a recent study, it was found that theta phase (4–8 Hz) and HFB coupling in the human prefrontal cortex were predictive of trial-by-trial response times40. In another study, HFB power modulated by the phase of an ongoing 2–5 Hz oscillations remained elevated throughout the period of attentional allocation in the prefrontal, parietal, and visual cortices, and the strength of this phase-amplitude coupling predicted reaction times to detected targets on a trial-by-trial basis41.
Millisecond Temporal Resolution:
Given that the sampling rate of human iEEG data is typically in the range of 1000–3000Hz, the intracranial signal contains temporal information with millisecond resolution – though down sampling and temporal smoothing can hamper the resolution of the signal (See Box 2). Conventional scalp EEG and magnetoencephalography have similar temporal resolution as iEEG; however, the iEEG signal is highly localized, and the source of the signal is spatially better defined. Observation of fast dynamics of activity between precisely localizable populations of neurons across distinct brain regions can inform neuromechanistic accounts of perceptual and cognitive functions. Moreover, knowing the exact onset of activations or deactivations in a region of the brain can inform us about the details of possible neural computations taking place in the targeted brain area. For instance, finding 10s of milliseconds of lag time between activations in region A compared to region B suggests that neural computations in A precede computations in B (and activity in A may even be causing activity in B). Precise temporal information can also help us understand the pattern with which different regions of the brain interact with each other. In a recent study42, it was found that within the first 300ms of object presentation, several different subregions within the human inferior temporal and lateral parietal cortex come online (i.e., become activated) together but at different time windows, clearly depicting a detailed account of recurrent information flow between the inferior temporal and lateral parietal regions. A different study of object recognition targeted at neuronal populations in ventral visual cortex illustrates how iEEG’s millisecond resolution can inform mechanistic accounts of perceptual processes. When subjects viewed incomplete images of the objects, neural responses in the human inferior temporal cortex required ~100ms of additional processing time as compared to those of whole objects. This pattern of time information clearly suggested that recognition of partially presented objects depends on recurrent signals from not only feed forward but more likely from feedback connections, as also proposed by attractor networks or Bayesian inference models43.
BOX 2: HANDLING iEEG SIGNALS WITH CARE.
For the reader of iEEG literature, it is important to be aware of potential problems that may degrade the value of the findings:
-
1)
Electrode localization: An important advantage of the iEEG method is that the anatomical coordinates of the recording site can be identified with great precision. However, one must be cautious about the reported anatomical coordinates of the electrodes. Especially in ECoG, when the electrode hardware is implanted after a relatively large craniotomy, the brain of each patient will necessarily be shifted in space from its pre-operative coordinates. Therefore, reconstructing the location of electrodes on the subject’s pre-operative brain MRIs (as is often done) may be problematic. One way of avoiding this issue is construct the 3D location of the electrodes using post-operative MRIs. Another way is to use methods that account for the shift and take great deal of care to ensure correct alignment between the two series of images98,99.
-
2)
Data Processing: Multiple steps are involved in handling complex iEEG signals and each step can cause data distortion. Processing signals obtained from patients with healthy and pathological signals mixed across recording sites can also be problematic if the data processing has not been performed correctly. While the precise steps taken in the processing of data may vary across labs and research aims, several key steps are commonly applied to the raw iEEG data before the actual statistical analyses are performed: concatenating several data files (e.g. across blocks or conditions across days); down sampling of the data, most commonly from 1000–10,000 to 250–1000 samples per second and thus changing the temporal resolution of the reported data; identifying and removing artifacts and noise or pathological signals (notch filtering, excluding signals from electrodes with abundant pathological activity and common-averaging or re-referencing to remove the common signal).
-
3)
Defining baseline: Control condition in electrophysiology is the “baseline” condition which is commonly defined as the average pre-stimulus signal (usually ~100–200ms prior to onset of the trial). Normalizing the raw signal to the baseline activity is important given that the electrodes are not homogenously covering all pixels of the brain and they may vary in shape, size, or distance from each other. By correcting for baseline, the signal of interest is not conflated with average noise. Thus, differences between conditions during stimulus presentation can be accurately compared and contrasted. Depending on the hypotheses that are being tested, it is exceedingly important to ensure that the right baseline is chosen. For instance, shorter baseline will be problematic for slow oscillations (too few waveforms per unit of time), or the task design may not allow using pre-stimulus phase as the baseline.
-
4)
Multiple Comparisons: One of the greatest attributes of intracranial EEG is the ability to measure signals within a specific frequency range such as delta, theta, alpha, beta and gamma oscillations as well as the broadband signal. One can either process the amount of activity in each bandwidth (i.e., the amplitude of the filtered signal) or look at the interaction and coupling of activity across frequencies. One may study the association of multiple frequencies across different bands of activity or across neural systems. One can study the sustained versus transient burst of activity or coupling within or across certain frequencies or neural systems. The richness of the iEEG data is a true blessing but it can also be a curse and lead to spurious findings. In a large amount of data with different frequencies and multiple sites and different measures of power and phase, almost any pattern can be interpreted as a “result”, and multiple comparisons correction becomes close to infinite. Having an a priori hypothesis with a mechanistic approach linking the work to previous literature and existing theories, showing consistency of the same findings across subjects, and selectivity and specificity of the finding to a given experimental condition, region, or a network are must haves before a result can be taken as a serious finding. Combining passive recordings with active electrical stimulations and prove causality will be the greatest ultimate means by which one can beat the curse of pseudo-findings out of iEEG literature.
High Signal to Noise Ratio:
A clear advantage of working with the iEEG signal is the access to data with exceedingly high signal-to-noise ratio (SNR). Compared to imaging studies, the SNR of iEEG is indisputably higher. For instance, local energy consumption increase owing to a typical task-related response in fMRI is as little as 1%44. By contrast task-related increases in the local field potentials can be as high as ~300%45. Sources of SNR in imaging studies of the brain include field of view, scan parameters, magnetic field strength, slice thickness, and noise stemming from the subject (i.e. cardiac and respiratory pulsations, head motion) which vary in time. These are largely absent in iEEG recordings. Compared to scalp EEG, SNR of iEEG data can be as high as 100 times higher46. This is in part because of ~10x higher amplitude of iEEG signal compared to scalp EEG, and significantly reduced problem of electro-magnetic noise from the recording room, physiological noise from cardiac signal or muscle contractions, or skin potentials (e.g., skin cells on the scalp or ionic potential of sweat glands) with intracranial recordings. Of critical importance for the field of brain machine interface, the SNR of iEEG recordings remains stable and strong over many months without negative correlation between decoding performance and the time between model generation and model testing47 (see Box 2 for important steps in signal processing to increase the SNR).
Selectivity of iEEG Signals:
Since the iEEG signal is conveyed by the “forest” of neurons and their neuropil, one might question whether the forest signal be specific and selective enough and if so at what level and to what extent. Many recent studies from iEEG laboratories have convincingly shown selective rise of HFP power across a multitude of experiments. In this context, it is important to know that the reported iEEG findings are based on a comparison between the induced changes of the electrophysiologcal activity after the onset of a stimulus and the baseline pre-stimulus activity in the same recording site (usually ~200ms prior to onset of the trial). This is in stark contrast to some functional imaging data where the reported results are based on subtraction of two signals during two different cognitive conditions (e.g., increased hemodynamic responses to numbers minus responses to colorful images (see Box 2). In this regard, selectivity of the iEEG responses could be considered more meaningful. As examples, intracranial recordings in the human lateral temporal lobe (in areas commonly known as being part of the language system) have revealed heterogeneous patterns of neuronal population responses clearly revealing that language processing in this part of cortex is not spatially homogenous over a centimeter of cortex29. In a different study using high-density grid of electrodes in human subjects listening to natural and continuous speech showed that the acoustic properties of the phonemes were mediated by population responses distributed across millimeters of the brain30. In another study, recording from a grid of electrodes in the human temporal cortex, speech representations and identification of individual words were decoded directly from the iEEG signals during single trial sound presentations48. Thus, the selectivity profile of HFB responses across millimeters of the brain enables us to get closer to a neuromechanistic view of cortical processing that otherwise could not be studied with methods that lack the anatomical precision of the recorded signals or their temporal resolution.
It goes without saying that acquiring single unit data from the vicinity of implanted iEEG electrodes7,8 can provide more granular information about the computations performed in a given brain region. For instance, the timing at which spiking activity occurs often clusters relative to certain phases of oscillatory activity, as extracted from the local field potential. Such spike- field coherence can provide another useful tool for decoding neural computations and interregional connectivity. For instance, it has been shown that successful memory formation in humans is better predicted by a coordination of spike timing with the local theta oscillation rather than the phase of these oscillations or the average firing rate of neurons per se49. Moreover, the strength of spike-field coupling as a function of task set suggests that temporal codes play an important role in sculpting and orchestrating perceptual and cognitive functions across large-scale networks39. Examples for such temporal coding have been found in many brain regions, with spiking activity typically coupling to phases of interregional slow oscillatory activity50,51.
Simultaneous sampling of a large number of sites:
Implantations in human subjects often include a large number of electrodes and a broad coverage for simultaneous recordings across a wide range of regions. The number of ECoG electrodes (e.g., 8×8cm grid of electrodes) or the number of sEEG electrodes (e.g., 10–15 cannulas containing 10–14 electrodes) can total to 150–200 different recording sites. Such a broad spatial coverage over large-scale networks has two advantages: First it allows one to examine the involvement of a larger mantle of the cerebral cortex and identify distinct patterns of responses across multiple cortical locations at various time points during a cognitive experiment, or mapping large-scale gradients (e.g. in the visual hierarchy) within a given region of the brain and in the same individual brains. Second, it allows one to identify functional relationships between nodes of the same functional network that are incidentally covered by clinical electrodes. As such, processes in large-scale networks can be linked to more local processes, and the local processes can be understood in the context of their large-scale role in the network. For instance, in a recent iEEG study (Figure 3), simultaneous recordings from ventral temporal cortex and dorsal parietal regions explored the timing and profile of responses in discrete neuronal populations during simple arithmetic processing (e.g., 2 + 2 = 4)42. Electrodes in anatomically consistent inferior temporal and intraparietal sulcus regions showed similar profiles of time-locked HFB responses during the addition of numerals. Interestingly, the HFB responses were relatively weak to the presentation of the first digit in the equation and increased significantly after the following operator and second digit were presented. Such a precise timing and differential selectivity profile of responses across the temporal and parietal sites can provide important information about the flow of information between the two sites during an experimental condition42.
Figure 3: Simultaneous recording with a broad coverage for tracking the spatiotemporal profiles of activity of populations of neurons during a particular cognitive task.
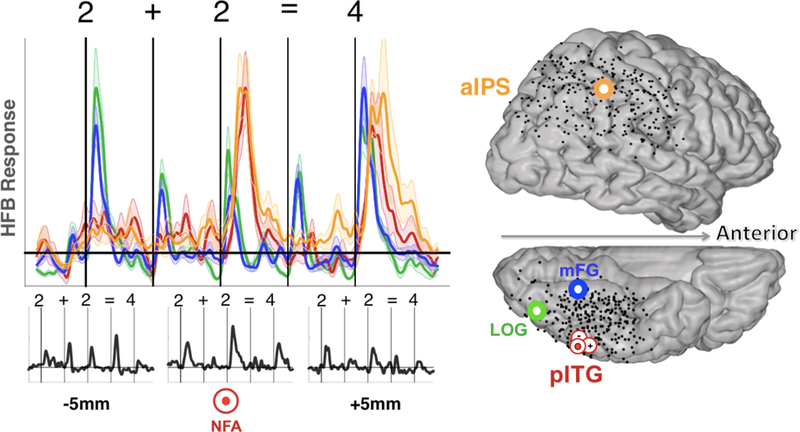
In a group of subjects simultaneous recordings in the lateral parietal and inferior temporal regions tracked HFB responses in each electrode site while the subjects were making true/false judgments on an arithmetic task in which operands (numerals 1–9) and operators (+. =) were visually presented one symbol at a time (e.g. “2”, “+”, “2”, “=”, “4”). Non-selective HFB responses in lateral occipital gyrus (LOG) and medial fusiform gyrus (mFG) contrasted the selective HFB responses to numerals in the posterior inferior temporal gyrus (pITG, red) and anterior intraparietal sulcus region (aIPS, upper left panel). Note the stronger responses in the pITG and aIPS after the presentation of the second numeral following the “+” sign and the opposite profile of HFB responses in the LOG and mFG sites. Also, of interest was the finding of HFB response selectivity across the three adjacent pITG sites (left lower panel). Neuronal populations that are ~5mm apart show clearly different profiles of responses. Note the most selective responses to numbers in the number form area (NFA) that has previously been reported in a different set of subjects100. Adapted from Daitch et al 2016.
Understanding the temporal dynamics of neuronal responses in different nodes of a specific brain network is of great importance for generating a neuromechanistic account of human brain function at the systems level. For example, neuroimaging studies have shown that the posterior cingulate cortex (PCC) and angular gyrus (AG) are engaged in autobiographical memory functions and also connected at rest52. However, based on these data, it was almost assumed that a large area of PCC should be connected to a large area of AG. In a recent ECoG study, simultaneous recordings from PCC and AG in human subjects showed, on a trial-by-trial basis, that responses in both PCC and AG during memory retrieval are coupled with zero time-lag (i.e., both areas are engaged around the same time and one does not drive the other), and more importantly, not the entire AG was functionally coupled with the entire PCC at rest. Instead, using the same metrics validated in prior studies 53,54, it was found that the coupling at rest occurred between those discrete populations of neurons in the PCC and AG that were co-activated during the experimental task 34. In summary, simultaneous iEEG recordings from distinct nodes of functional brain networks provide data with high temporal resolution and reliable anatomical precision of signal sources. Such data are presently not possible to obtain with non-invasive methods in humans.
Exploring Causality:
Another characteristic of iEEG data is that it can provide information about the causal importance of a given cortical site (and its connected network) in a given cognitive condition or behavior. Implanted electrodes can not only record signals from a specific population of neurons but also deliver electrical pulses to that population. Direct electrical stimulation of the human brain confers the ability to record and stimulate the human brain at specific sites and hence, test the causal importance of a given population of neurons (and their interconnections) for a particular function while subjective report of the human participant is instantly available. The fact that humans can explain their subjective experience during electrical stimulation of their brain makes the intracranial experiments in humans unique. For instance, in patients undergoing awake brain surgery, stimulating the right inferior parietal regions triggered a strong intention and desire to move the contralateral hand, arm, or foot, whereas stimulating the left inferior parietal region provoked the intention to move the lips and to talk55. When stimulation intensity was increased in parietal areas, participants believed they had really performed these movements, although no electromyographic activity was detected55. Other findings include that stimulation of the anterior cingulate cortex induced a complex physical (chest or neck vibrations) and autonomic state (increased heart rate) coupled with emotional (feeling of anticipated anxiety and foreboding) along with a strong motivational state (e.g., “I want to push harder and harder”, “ I want to fight against it”)56. Further, stimulation of the brain in a patient with implanted electrodes in the dorsal frontal cortex caused feeling of mirth57 or stimulation of the angular gyrus has led to illusory transformations of the patient’s own body experience58. In a study of the human fusiform face form area (FFA), stimulation of the FFA caused distortions of perceived faces, and these changes of perception were modality specific and thus applied only to faces45. These effects occurred only when the right, but not when the left FFA was stimulated59. These are just a few examples among many other cortical stimulation studies60 that go far beyond correlative approaches and can offer evidence about the link between the brain and a human’s subjective experience.
Direct electrical stimulation of the brain has a great therapeutic potential specially when coupled with real-time recordings from the surface of the brain or from a subcortical structure in a closed-loop circuits. This is evident in the current practice of Responsive Neurostimulation61 to control pathologically-driven activity such as seizures (NeuroPace) or abnormal beta-band activity with movement in PD62 or recent investigations to the possibility of modulating cognitively-driven activity to treat neuropsychiatric disorders63, or simply devising cognitive prosthetic devices64.
The Human Brain Model:
From our discussion thus far, it could be argued that iEEG is currently the method with suitable combination of anatomical precision, temporal resolution, and simultaneous coverage of multiple nodes of interest to study the human brain. The method yields the promise of providing additional insights into human brain function beyond what we have learned, or can learn, from non-invasive studies of the human brain. One advantage of human iEEG studies over those conducted in laboratory animals such as monkeys or rats is that humans can perform tasks based on verbal instructions and they do so with minimal training, and in the absence of ongoing reward or task-cueing. Such an approach allows for more ecologically valid and ethologically relevant experiments than are possible in most animal species, also avoiding the potential confound of over-training. Moreover, to develop animal models for specific cognitive functions requires a perfect phenotype that matches the human counterpart, but these phenotypes are often absent for uniquely human faculties such as language. As an example, a recent human iEEG study revised the old model of language processing and differentiated the neuromechanistic accounts of speech from language processing65. It showed that our current models of lateralized speech processing may not be entirely accurate after all. The traditional model of language processing proposes that speech production as well as language processing occurs in the language dominant (mostly left) hemisphere, and that the coupling from language perceptual sensory (lateral temporal) sites to production (inferior frontal) sites occurs primarily in the language-dominant hemisphere. Instead, the iEEG recordings revealed a clearly bilateral rather than unilateral speech sensory-motor coupling. As another example, an iEEG study in human subjects revealed that conscious and subjective perception of visual phosphenes (induced by electrical stimulation of the primary visual cortex) occurs only when stimulations in the primary visual cortex are accompanied by HFB responses in the temporoparietal junction area (TPJ)66 – suggesting that the outbound distribution of signals from the primary visual cortex to the TPJ may be necessary for human conscious visual perception. This study does not only illustrate the knowledge that can be gained from subjects who are able to report whether or not they are consciously aware of their percepts, but also the advantages of a data driven approach employing a broad coverage of the brain with hundreds of electrodes.
Lastly, invasive recordings in pathological regions within the human brain provides important human specific information about the pathophysiological mechanisms at play. For example, none of the available animal models of Parkinson’s disease (PD) accurately reproduces all of the symptoms of the human PD and animal models of PD or epilepsy do not reproduce the gradual pathological changes that occur in the brain over the course of decades7. Thus, testing pathophysiological hypotheses directly in the human brain will provide complimentary information to studies in animal models.
OPPORTUNITIES FOR GROWTH
Partnership between electrophysiologists working on human and primate brain models:
The macaque monkey is the prime animal model for human brain function. However, comparisons of brain function in humans and non-human primates have been thus far mainly indirect due to differences in methodological approaches. There are only few direct comparative studies across primate brains, and they have used a combination of invasive recordings and fMRI (e.g.67,68). Thus, comparative electrophysiology in monkey and human subjects will be a fruitful approach to establish not only the validity of the non-human primate brain to serve as the prime model for human brain function, but also to study evolutionary aspects of cognition. Such an approach will require recordings in tasks that result in common behavior across primate species and from sites that are functionally similar. As noted, the recorded iEEG signal is the sum of local field potentials generated from large populations of cells (“the forest”) that are localized under or around the recording electrode. This signal is too crude for understanding a distributed code among many cells within a small region. While recent studies with multi-contact microelectrodes that are chronically implanted in epilepsy patients provide great promise for deciphering the laminar source of signals that we record with ECoG and sEEG recordings69,70, a much more granular research with microelectrodes in non-human models (e.g., 67,71) will provide a great opportunity to uncover the neuronal mechanisms in greater detail. In non-human primates, laminar recordings across the layers of cortex can provide further details into local circuitry including detailed consideration of signals arising from feedforward and feedback pathways (e.g.,72), and simultaneous recordings of interconnected nodes of a large scale network can inform about mechanisms of interareal communication (e.g., 55 10). Importantly, sophisticated and identical behavior can be established in non-human primates in tasks motivated from human cognitive psychology, thereby taking our mechanistic models from the coarse sampling of human iEEG all the way to the microcircuitry of laminar recordings in non-human primates (e.g.,10).
Common Platforms and More Data Sharing:
Many innovative ideas can be fostered by establishing common platforms for acquiring data across labs so that a larger pool of subjects can be recruited to perform the same task. Moreover, sharing the acquired data with the rest of the world will make the iEEG data more accessible to a larger pool of researchers. Recent efforts by NIH and NSF make it easier for sharing research data with the public using their platforms.
Better Reporting:
It is important that scientific journals require iEEG findings to be accompanied by detailed reports of the patient demographics, especially the source of seizures, duration of epilepsy, frequency of seizures, type of seizures, educational level, antiepileptic medications onboard, as well as details of their neuropsychological evaluation and IQ. Such detailed documentation will allow the reader to evaluate the cohort, in which the data was collected and provide context to position the results and their interpretation.
Technical & Analytical Improvements:
Several areas of iEEG practice have unfortunately not been modernized for decades, partly because any innovation has to go through the regulatory hurdles of FDA. As it was recently highlighted73, new technologies are needed to create substantial improvements in both spatial and temporal resolution. With the growing role of engineering (including materials, computing, electronics, and hardware)74, there is great hope that we will reach major milestones in the years ahead.
One major milestone will be the development of wireless intracranial recordings and stimulations in human subjects. Currently, intracranial electrodes need to be wired to a recording apparatus. This tethers the patient to bed and increases morbidity due to prolonged bed rest. Each wire tail is also an inlet for infectious agents. Wires are also susceptible to movement artifacts.
Another milestone will be the development of new computational systems that will increase the power of current clinical recording systems that cannot process more than several hundred channels simultaneously. Large channel counts will have major implications for every downstream component, including connectors, routing, amplification, signal processing, and storage. Multiplexing the signals will be required to get all the signals in a single or few wires. Increasing the number of recording electrodes and utilizing finer grained recording sensors73 and combining conventional iEEG electrodes with microelectrodes will lead to analysis of data across multiple spatial and temporal scales, and will improve our understanding of the relationships embedded within the complex network of the human brain during normal and pathological (e.g., epilepsy) conditions. For instance, NeuroGrid75 is a step towards this aim. The largest human probe is currently ~2cmx2cm and has 512 channels, which require several parallelized chips with bulky back-end connections. In order to record 5 minutes of a study with 20μm spatial resolution and 20kHz sampling rate the system will require about 12–15Tb of memory and 320Gb/s for real-time processes. To increase the size of coverage to several centimeters, the computational requirements, in today’s standards, will be out of range of currently available tools. Signal acquisition of such large-scale data is currently limited by lack of high-channel-count electrophysiological interfacing electronics. However, in concert with advances in high-speed electronics and data processing capacity, the number of samples recorded per unit of time could be substantially increased in the future.
Technical advancements will also lead to better electrical stimulators. In current clinical practice, constant-current stimulators are used to deliver rectangular change-balanced, biphasic waveforms with minimal risk of tissue damage76. More recently, non-rectangular waveforms have been proposed which may potentially usher in novel stimulation methods77.
Technological advances in the field of iEEG will hopefully lead to a newer field of therapeutics (i.e., electroceuticals78) and newer implantable devices79. It will also lead to a more fruitful path in the field of brain-computer interface research80. This is specially promising since brain computer interfaces using LFPs in non-human models can even outperform those using spikes and may have extended lifetime81. This will offer a great opportunity for cognitive or motor prosthetics used by disabled patients to convey thoughts or actions81.
Lastly the field of iEEG can benefit from incorporating sophisticated network analytical tools from functional imaging methods (e.g., dynamic connectivity, clustering, and graph theory) that have resulted in findings about the functional architecture of the human brain that would have otherwise gone unnoticed82–84.
CONCLUSION
This piece was meant to provide an overview of human iEEG method and its limitations as well as promises. The field of human iEEG has a unique place in the study of averaged responses of populations of neurons with high temporal resolution and probe their causal importance for human subjective experience and behavior and their interaction with, and relative time of their engagement compared to, other nodes of the same or different functional networks. As such, iEEG can provide novel and unique temporal information that can be complemented with other means of scientific research to construct neuromechanistic accounts of human cognition and behavior. Human iEEG can bring novel information to the field of neuroscience beyond simply replicating what is already known, or can be known, from non-invasive lines of research in humans or from invasive recordings in non-human mammalian brains.
Figure 4: Ratio of electrodes in epileptic and non- epileptic tissue.
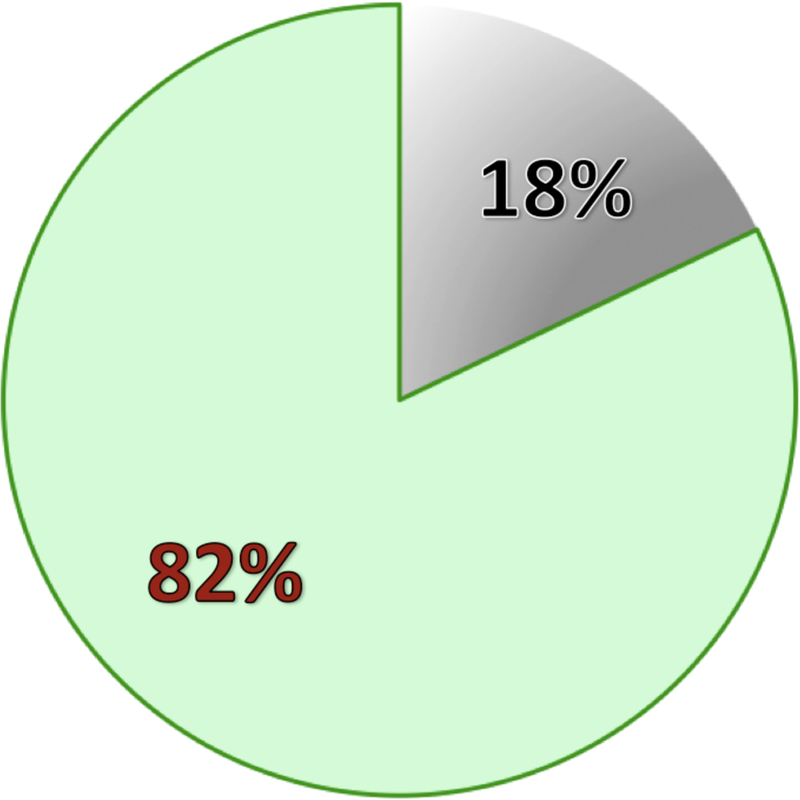
In 100 patients implanted with ECoG or sEEG electrodes at Stanford Medical Center, we reviewed the iEEGs in each patient and labeled pathological electrodes that contained epileptic activity (i.e., recorded seizures or epileptiform spikes). We used the total number of electrodes implanted in these patients to calculate the ratio of pathological (gray) to non-pathological (green) sites. In patients with focal epilepsy, sites with pathological activity are clustered to few electrode contacts. The extent of non-pathological electrodes will depend on the density, form, and size of implanted intracranial electrodes. In a patient with wide coverage and focal epileptic zone, non-pathological sites will be covered across a large region of the brain.
ACKNOWLEDGMENTS
We gratefully acknowledge funding support from the US National Institute of Health (1R01MH109954–01 to JP; 2RO1MH064043–12, 5RO1EY017699–09, Silvio O. Conte Center 21560–685 to SK), the US National Science Foundation (BCS1358907 to JP; BCS-1328270 to SK), and the James S. McDonnell Foundation to SK.
Footnotes
COMPETING FINANCIAL INTERESTS STATEMENT
None
REFERENCES
- 1.Caton R The electric currents of the brain. . Br. Med. J. 2 (1875). [Google Scholar]
- 2.Beck A Determination of Localization in the Brain and Spinal Cord by Means of Electrical Phenomena. Polsk Akad Um, 186–232 (1891). [Google Scholar]
- 3.Berger H öber das Elektroenkephalogramm des Menschen. Archiv fÅr psychiatrie und Nervenkrankheiten 87, 527–570 (1929). [Google Scholar]
- 4.Kwan P, Schachter SC & Brodie MJ Drug-resistant epilepsy. The New England journal of medicine 365, 919–926, doi: 10.1056/NEJMra1004418 (2011). [DOI] [PubMed] [Google Scholar]
- 5.Engel J Jr. Surgery for seizures. N. Engl. J. Med. 334, 647–652 (1996). [DOI] [PubMed] [Google Scholar]
- 6.Semah F et al. Is the underlying cause of epilepsy a major prognostic factor for recurrence? Neurology 51, 1256–1262 (1998). [DOI] [PubMed] [Google Scholar]
- 7.Engel AK, Moll CK, Fried I & Ojemann GA Invasive recordings from the human brain: clinical insights and beyond. Nat Rev Neurosci 6, 35–47, doi: 10.1038/nrn1585 (2005). [DOI] [PubMed] [Google Scholar]
- 8.Mukamel R & Fried I Human intracranial recordings and cognitive neuroscience. Annu. Rev. Psychol. 63, 511–537, doi: 10.1146/annurev-psych-120709-145401 (2012). [DOI] [PubMed] [Google Scholar]
- 9.Parvizi J Corticocentric myopia: old bias in new cognitive sciences. Trends Cogn Sci. 13, 354–359. (2009). [DOI] [PubMed] [Google Scholar]
- 10.Saalmann YB, Pinsk MA, Wang L, Li X & Kastner S The pulvinar regulates information transmission between cortical areas based on attention demands. Science 337, 753–756, doi: 10.1126/science.1223082 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wimmer RD et al. Thalamic control of sensory selection in divided attention. Nature 526, 705–709, doi: 10.1038/nature15398 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller KJ, Sorensen LB, Ojemann JG & den Nijs M Power-law scaling in the brain surface electric potential. PLoS Comput Biol 5, e1000609, doi: 10.1371/journal.pcbi.1000609 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller KJ Broadband spectral change: evidence for a macroscale correlate of population firing rate? The Journal of neuroscience : the official journal of the Society for Neuroscience 30, 6477–6479, doi: 10.1523/JNEUROSCI.6401-09.2010 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winawer J et al. Asynchronous Broadband Signals Are the Principal Source of the BOLD Response in Human Visual Cortex. Current biology : CB 23, 1145–1153, doi: 10.1016/j.cub.2013.05.001 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiruska P et al. Update on the mechanisms and roles of high-frequency oscillations in seizures and epileptic disorders. Epilepsia 58, 1330–1339, doi: 10.1111/epi.13830 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schevon CA et al. Spatial characterization of interictal high frequency oscillations in epileptic neocortex. Brain 132, 3047–3059, doi: 10.1093/brain/awp222 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ray S, Crone NE, Niebur E, Franaszczuk PJ & Hsiao SS Neural Correlates of High-Gamma Oscillations (60–200 Hz) in Macaque Local Field Potentials and Their Potential Implications in Electrocorticography. J. Neurosci. 28, 11526–11536, doi: 10.1523/jneurosci.2848-08.2008 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ray S & Maunsell JH Different origins of gamma rhythm and high-gamma activity in macaque visual cortex. PLoS Biol 9, e1000610, doi: 10.1371/journal.pbio.1000610 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kreiman G et al. Object selectivity of local field potentials and spikes in the macaque inferior temporal cortex. Neuron 49, 433–445, doi: 10.1016/j.neuron.2005.12.019 (2006). [DOI] [PubMed] [Google Scholar]
- 20.Liu J & Newsome WT Local field potential in cortical area MT: stimulus tuning and behavioral correlations. J. Neurosci. 26, 7779–7790, doi: 10.1523/JNEUROSCI.5052-05.2006 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mukamel R Coupling Between Neuronal Firing, Field Potentials, and fMRI in Human Auditory Cortex. Science 309, 951–954, doi: 10.1126/science.1110913 (2005). [DOI] [PubMed] [Google Scholar]
- 22.Katzner S et al. Local origin of field potentials in visual cortex. Neuron 61, 35–41, doi: 10.1016/j.neuron.2008.11.016 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Engel AK, Konig P, Gray CM & Singer W Stimulus-Dependent Neuronal Oscillations in Cat Visual Cortex: Inter-Columnar Interaction as Determined by Cross-Correlation Analysis. Eur. J. Neurosci. 2, 588–606 (1990). [DOI] [PubMed] [Google Scholar]
- 24.Park SH et al. Functional Subpopulations of Neurons in a Macaque Face Patch Revealed by Single-Unit fMRI Mapping. Neuron 95, 971–981 e975, doi: 10.1016/j.neuron.2017.07.014 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Logothetis NK, Pauls J, Augath M, Trinath T & Oeltermann A Neurophysiological investigation of the basis of the fMRI signal. Nature 412, 150–157 (2001). [DOI] [PubMed] [Google Scholar]
- 26.Nir Y et al. Coupling between neuronal firing rate, gamma LFP, and BOLD fMRI is related to interneuronal correlations. Curr. Biol. 17, 1275–1285 (2007). [DOI] [PubMed] [Google Scholar]
- 27.Manning JR, Jacobs J, Fried I & Kahana MJ Broadband shifts in local field potential power spectra are correlated with single-neuron spiking in humans. J Neurosci. 29, 13613–13620. (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niessing J et al. Hemodynamic signals correlate tightly with synchronized gamma oscillations. Science. 309, 948–951. (2005). [DOI] [PubMed] [Google Scholar]
- 29.Flinker A, Chang EF, Barbaro NM, Berger MS & Knight RT Sub-centimeter language organization in the human temporal lobe. Brain Lang. 117, 103–109, doi: 10.1016/j.bandl.2010.09.009 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mesgarani N, Cheung C, Johnson K & Chang EF Phonetic feature encoding in human superior temporal gyrus. Science 343, 1006–1010, doi: 10.1126/science.1245994 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hermes D et al. Neurophysiologic correlates of fMRI in human motor cortex. Hum. Brain Mapp, doi: 10.1002/hbm.21314 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller KJ et al. Spectral changes in cortical surface potentials during motor movement. The Journal of neuroscience : the official journal of the Society for Neuroscience 27, 2424–2432, doi: 10.1523/JNEUROSCI.3886-06.2007 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Canolty RT et al. High gamma power is phase-locked to theta oscillations in human neocortex. Science 313, 1626–1628 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Foster BL, Rangarajan V, Shirer WR & Parvizi J Intrinsic and task-dependent coupling of neuronal population activity in human parietal cortex. Neuron 86, 578–590, doi: 10.1016/j.neuron.2015.03.018 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Foster BL, Dastjerdi M & Parvizi J Neural populations in human posteromedial cortex display opposing responses during memory and numerical processing. Proc. Natl. Acad. Sci. U. S. A. 109, 15514–15519, doi: 10.1073/pnas.1206580109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buzsaki G Rhythms of the Brain. (Oxford University Press, 2006). [Google Scholar]
- 37.VanRullen R Perceptual Cycles. Trends Cogn Sci 20, 723–735, doi: 10.1016/j.tics.2016.07.006 (2016). [DOI] [PubMed] [Google Scholar]
- 38.Canolty RT & Knight RT The functional role of cross-frequency coupling. Trends in Cognitive Sciences 14, 506–515 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schroeder CE & Lakatos P Low-frequency neuronal oscillations as instruments of sensory selection. Trends Neurosci. 32, 9–18, doi: 10.1016/j.tins.2008.09.012 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Voytek B et al. Oscillatory dynamics coordinating human frontal networks in support of goal maintenance. Nat. Neurosci. 18, 1318–1324, doi: 10.1038/nn.4071 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Szczepanski SM et al. Dynamic changes in phase-amplitude coupling facilitate spatial attention control in fronto-parietal cortex. PLoS Biol 12, e1001936, doi: 10.1371/journal.pbio.1001936 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Daitch AL et al. Mapping human temporal and parietal neuronal population activity and functional coupling during mathematical cognition. Proc. Natl. Acad. Sci. U. S. A. 113, E7277–E7286, doi: 10.1073/pnas.1608434113 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang H et al. Spatiotemporal dynamics underlying object completion in human ventral visual cortex. Neuron 83, 736–748, doi: 10.1016/j.neuron.2014.06.017 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raichle ME Neuroscience. The brain’s dark energy. Science 314, 1249–1250 (2006). [PubMed] [Google Scholar]
- 45.Parvizi J et al. Electrical stimulation of human fusiform face-selective regions distorts face perception. The Journal of neuroscience : the official journal of the Society for Neuroscience 32, 14915–14920, doi: 10.1523/JNEUROSCI.2609-12.2012 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ball T, Kern M, Mutschler I, Aertsen A & Schulze-Bonhage A Signal quality of simultaneously recorded invasive and non-invasive EEG. Neuroimage 46, 708–716, doi: 10.1016/j.neuroimage.2009.02.028 (2009). [DOI] [PubMed] [Google Scholar]
- 47.Chao ZC, Nagasaka Y & Fujii N Long-term asynchronous decoding of arm motion using electrocorticographic signals in monkeys. Front Neuroeng 3, 3, doi: 10.3389/fneng.2010.00003 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pasley BN et al. Reconstructing speech from human auditory cortex. PLoS Biol 10 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rutishauser U, Ross IB, Mamelak AN & Schuman EM Human memory strength is predicted by theta-frequency phase-locking of single neurons. Nature 464, 903–907 (2010). [DOI] [PubMed] [Google Scholar]
- 50.Jacobs J, Kahana MJ, Ekstrom AD & Fried I Brain oscillations control timing of single-neuron activity in humans. J. Neurosci. 27, 3839–3844, doi: 10.1523/JNEUROSCI.4636-06.2007 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zanos S, Zanos TP, Marmarelis VZ, Ojemann GA & Fetz EE Relationships between spike-free local field potentials and spike timing in human temporal cortex. J. Neurophysiol. 107, 1808–1821, doi: 10.1152/jn.00663.2011 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Svoboda E, McKinnon M & Levine B The functional neuroanatomy of autobiographical memory: A meta-analysis. Neuropsychologia 44, 2189–2208, doi: 10.1016/j.neuropsychologia.2006.05.023 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nir Y, Dinstein I, Malach R & Heeger DJ BOLD and spiking activity. Nat. Neurosci. 11, 523–524; author reply 524, doi: 10.1038/nn0508-523 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Keller CJ et al. Neurophysiological investigation of spontaneous correlated and anticorrelated fluctuations of the BOLD signal. The Journal of neuroscience : the official journal of the Society for Neuroscience 33, 6333–6342, doi: 10.1523/JNEUROSCI.4837-12.2013 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Desmurget M et al. Movement intention after parietal cortex stimulation in humans. Science 324, 811–813, doi:324/5928/81110.1126/science.1169896 (2009). [DOI] [PubMed] [Google Scholar]
- 56.Parvizi J, Rangarajan V, Shirer WR, Desai N & Greicius MD The will to persevere induced by electrical stimulation of the human cingulate gyrus. Neuron 80, 1359–1367, doi: 10.1016/j.neuron.2013.10.057 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fried I, Wilson CL, MacDonald KA & Behnke EJ Electric current stimulates laughter [letter]. Nature 391, 650 (1998). [DOI] [PubMed] [Google Scholar]
- 58.Blanke O, Ortigue S, Landis T & Seeck M Stimulating illusory own-body perceptions. Nature 419, 269–270 (2002). [DOI] [PubMed] [Google Scholar]
- 59.Rangarajan V et al. Electrical stimulation of the left and right human fusiform gyrus causes different effects in conscious face perception. J. Neurosci. 34, 12828–12836, doi: 10.1523/JNEUROSCI.0527-14.2014 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Selimbeyoglu A & Parvizi J Electrical stimulation of the human brain: perceptual and behavioral phenomena reported in the old and new literature. Frontiers in human neuroscience 4, 46, doi: 10.3389/fnhum.2010.00046 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morrell MJ Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology 77, 1295–1304, doi: 10.1212/WNL.0b013e3182302056 (2011). [DOI] [PubMed] [Google Scholar]
- 62.Rosin B et al. Closed-loop deep brain stimulation is superior in ameliorating parkinsonism. Neuron 72, 370–384, doi: 10.1016/j.neuron.2011.08.023 (2011). [DOI] [PubMed] [Google Scholar]
- 63.Widge AS et al. Treating refractory mental illness with closed-loop brain stimulation: Progress towards a patient-specific transdiagnostic approach. Exp. Neurol. 287, 461–472, doi: 10.1016/j.expneurol.2016.07.021 (2017). [DOI] [PubMed] [Google Scholar]
- 64.Pesaran B, Musallam S & Andersen RA Cognitive neural prosthetics. Current biology : CB 16, R77–80, doi: 10.1016/j.cub.2006.01.043 (2006). [DOI] [PubMed] [Google Scholar]
- 65.Cogan GB et al. Sensory-motor transformations for speech occur bilaterally. Nature 507, 94–98, doi: 10.1038/nature12935 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Beauchamp MS, Sun P, Baum SH, Tolias AS & Yoshor D Electrocorticography links human temporoparietal junction to visual perception. Nat. Neurosci. 15, 957–959, doi: 10.1038/nn.3131 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tsao DY, Moeller S & Freiwald WA Comparing face patch systems in macaques and humans. Proc. Natl. Acad. Sci. U. S. A. 105, 19514–19519, doi: 10.1073/pnas.0809662105 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pinsk MA et al. Neural representations of faces and body parts in macaque and human cortex: a comparative FMRI study. J. Neurophysiol. 101, 2581–2600, doi: 10.1152/jn.91198.2008 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Halgren E et al. Laminar profile of spontaneous and evoked theta: Rhythmic modulation of cortical processing during word integration. Neuropsychologia 76, 108–124, doi: 10.1016/j.neuropsychologia.2015.03.021 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cash SS et al. The human K-complex represents an isolated cortical down-state. Science 324, 1084–1087, doi: 10.1126/science.1169626 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kiani R et al. Natural grouping of neural responses reveals spatially segregated clusters in prearcuate cortex. Neuron 85, 1359–1373, doi: 10.1016/j.neuron.2015.02.014 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lakatos P, Karmos G, Mehta AD, Ulbert I & Schroeder CE Entrainment of neuronal oscillations as a mechanism of attentional selection. Science 320, 110–113 (2008). [DOI] [PubMed] [Google Scholar]
- 73.Chang EF Towards large-scale, human-based, mesoscopic neurotechnologies. Neuron 86, 68–78, doi: 10.1016/j.neuron.2015.03.037 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Litt B Engineering the next generation of brain scientists. Neuron 86, 16–20, doi: 10.1016/j.neuron.2015.03.029 (2015). [DOI] [PubMed] [Google Scholar]
- 75.Khodagholy D et al. NeuroGrid: recording action potentials from the surface of the brain. Nat. Neurosci. 18, 310–315, doi: 10.1038/nn.3905 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lilly JC, Hughes JR, Alvord EC Jr. & Galkin TW Brief, noninjurious electric waveform for stimulation of the brain. Science 121, 468–469 (1955). [DOI] [PubMed] [Google Scholar]
- 77.Foutz TJ & McIntyre CC Evaluation of novel stimulus waveforms for deep brain stimulation. J Neural Eng 7, 066008, doi: 10.1088/1741-2560/7/6/066008 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Famm K, Litt B, Tracey KJ, Boyden ES & Slaoui M Drug discovery: a jump-start for electroceuticals. Nature 496, 159–161, doi: 10.1038/496159a (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Litt B Evaluating devices for treating epilepsy. Epilepsia 44 Suppl 7, 30–37 (2003). [DOI] [PubMed] [Google Scholar]
- 80.Schalk G & Leuthardt EC Brain-computer interfaces using electrocorticographic signals. IEEE Rev Biomed Eng 4, 140–154 (2011). [DOI] [PubMed] [Google Scholar]
- 81.Andersen RA, Burdick JW, Musallam S, Pesaran B & Cham JG Cognitive neural prosthetics. Trends in Cognitive Sciences 8, 486–493, doi: 10.1016/j.tics.2004.09.009 (2004). [DOI] [PubMed] [Google Scholar]
- 82.Bassett DS & Sporns O Network neuroscience. Nat. Neurosci. 20, 353–364, doi: 10.1038/nn.4502 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Power JD et al. Functional network organization of the human brain. Neuron 72, 665–678, doi: 10.1016/j.neuron.2011.09.006 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kriegeskorte N et al. Matching categorical object representations in inferior temporal cortex of man and monkey. Neuron 60, 1126–1141, doi: 10.1016/j.neuron.2008.10.043 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Penfield W & Boldrey E Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain 60, 389–443 (1937). [Google Scholar]
- 86.Milner B, Squire LR & Kandel ER Cognitive neuroscience and the study of memory. [Review] [173 refs]. Neuron 20, 445–468 (1998). [DOI] [PubMed] [Google Scholar]
- 87.Gazzaniga MS Review of the split brain. J. Neurol. 209, 75–79 (1975). [DOI] [PubMed] [Google Scholar]
- 88.Elger CE, Helmstaedter C & Kurthen M Chronic epilepsy and cognition. Lancet Neurol 3, 663–672, doi: 10.1016/S1474-4422(04)00906-8 (2004). [DOI] [PubMed] [Google Scholar]
- 89.Motamedi G & Meador K Epilepsy and cognition. Epilepsy Behav 4 Suppl 2, S25–38 (2003). [DOI] [PubMed] [Google Scholar]
- 90.Meador KJ Networks, cognition, and epilepsy. Neurology 77, 930–931, doi: 10.1212/WNL.0b013e31822cfcd6 (2011). [DOI] [PubMed] [Google Scholar]
- 91.Holmes MD & Tucker DM Identifying the epileptic network. Front Neurol 4, 84, doi: 10.3389/fneur.2013.00084 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Holmes GL & Lenck-Santini PP Role of interictal epileptiform abnormalities in cognitive impairment. Epilepsy Behav 8, 504–515, doi: 10.1016/j.yebeh.2005.11.014 (2006). [DOI] [PubMed] [Google Scholar]
- 93.Aldenkamp AP & Arends J Effects of epileptiform EEG discharges on cognitive function: is the concept of “transient cognitive impairment” still valid? Epilepsy Behav 5 Suppl 1, S25–34 (2004). [DOI] [PubMed] [Google Scholar]
- 94.Binnie CD Cognitive impairment during epileptiform discharges: is it ever justifiable to treat the EEG? Lancet Neurol 2, 725–730 (2003). [DOI] [PubMed] [Google Scholar]
- 95.Jones-Gotman M Localization of lesions by neuropsychological testing. Epilepsia 32 Suppl 5, S41–52 (1991). [PubMed] [Google Scholar]
- 96.Glennon JM et al. Interictal epileptiform discharges have an independent association with cognitive impairment in children with lesional epilepsy. Epilepsia 57, 1436–1442, doi: 10.1111/epi.13479 (2016). [DOI] [PubMed] [Google Scholar]
- 97.Loring DW, Kapur R, Meador KJ & Morrell MJ Differential neuropsychological outcomes following targeted responsive neurostimulation for partial-onset epilepsy. Epilepsia 56, 1836–1844, doi: 10.1111/epi.13191 (2015). [DOI] [PubMed] [Google Scholar]
- 98.Groppe DM et al. iELVis: An open source MATLAB toolbox for localizing and visualizing human intracranial electrode data. J. Neurosci. Methods 281, 40–48, doi: 10.1016/j.jneumeth.2017.01.022 (2017). [DOI] [PubMed] [Google Scholar]
- 99.Hermes D, Miller KJ, Noordmans HJ, Vansteensel MJ & Ramsey NF Automated electrocorticographic electrode localization on individually rendered brain surfaces. J. Neurosci. Methods 185, 293–298, doi: 10.1016/j.jneumeth.2009.10.005 (2010). [DOI] [PubMed] [Google Scholar]
- 100.Shum J et al. A brain area for visual numerals. The Journal of neuroscience : the official journal of the Society for Neuroscience 33, 6709–6715, doi: 10.1523/JNEUROSCI.4558-12.2013 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]


