Abstract
Objectives:
Little is known about platelet transfusions in pediatric critical illness. We sought to describe the epidemiology, indications, and outcomes of platelet transfusions among critically ill children.
Design:
Prospective cohort study
Setting:
Multicenter (82 pediatric intensive care units), international (16 countries) from September 2016 to April 2017
Patients:
Children ages 3 days to 16 years prescribed a platelet transfusion in the ICU during screening days
Interventions:
None
Measurements and Main Results:
Over six weeks, 16,934 patients were eligible and 559 received at least one platelet transfusion (prevalence 3.3%). The indications for transfusion included prophylaxis (67%), minor bleeding (21%) and major bleeding (12%). Thirty-four percent of prophylactic platelet transfusions were prescribed when the platelet count was ≥ 50×109 cells/L. The median (IQR) change in platelet count post transfusion was 48×109 cells/L (17–82) for major bleeding, 42×109 cells/L (16–80) for prophylactic transfusions to meet a defined threshold, 38×109 cells/L (17–72) for minor bleeding, and 25×109 cells/L (10–47) for prophylaxis in patients at risk of bleeding from a device. Overall ICU mortality was 25%, but varied from 18–35% based on indication for transfusion. Upon adjusted analysis, total administered platelet dose was independently associated with increased ICU mortality (odds ratio for each additional 1 mL/kg platelets transfused 1.002; 95% CI 1.001–1.003; p=0.005).
Conclusions:
The majority of platelet transfusions are given as prophylaxis to non-bleeding children and significant variation in platelet thresholds exists. Studies are needed to clarify appropriate indications, with focus on prophylactic transfusions.
MESH Terms: pediatrics, platelet transfusions, thrombocytopenia, critical care
INTRODUCTION
Platelet transfusions are commonly prescribed in critical illness but uncertainty remains regarding their efficacy and safety for all indications. In 2015, an American Association of Blood Banks (AABB) survey reported that 48,000 children in the United States received 165,000 apheresis platelet units.1 Unfortunately, there is no information regarding the epidemiology, indications, and outcomes for critically ill children receiving platelet transfusions.
Evidence-based guidelines for pediatric platelet transfusions are generally lacking and based primarily on expert opinion. Recommendations published by the AABB state that pediatric platelet transfusions are indicated for: 1) total platelet count (TPC) < 10×109 cells/L due to hypoproliferative thrombocytopenia; 2) active bleeding in association with qualitative platelet defects; 3) unexplained excessive bleeding when undergoing cardiopulmonary bypass; or 4) patients undergoing extracorporeal membrane oxygenation (ECMO) with TPC < 100×109 cells/L.2
Little is known about the practice of platelet transfusion in pediatric critical illness. In order to evaluate platelet transfusion strategies in critically ill children and guide platelet transfusion practices, it is important to understand the epidemiology. The primary objective of the study was to describe patterns of platelet transfusions among critically ill children, including transfusion thresholds, indications, post transfusion platelet count increment, and outcomes. The international nature supports its generalizability.
METHODS
Study Population
This point prevalence study was an exploratory, international, prospective, cross-sectional design. Pediatric intensive care units were recruited through the Pediatric Critical Care Blood Research Network (BloodNet), Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) network, several international critical care societies, and sites who previously participated in an epidemiologic study of plasma transfusions (PlasmaTV).3 Sites were encouraged to recruit across all pediatric critical care areas, including specialized pediatric cardiac critical care units, but were not required to do so. Each site was assigned six random weeks (between September 2016 to April 2017) and screened all seven days of each week. A child was considered eligible if admitted to the pediatric intensive care unit (PICU) during one of the screening days and between 3 days and 16 years old. A potential subject was enrolled if he/she received a platelet transfusion prescribed by the intensive care team during one of the screening days. Patients were excluded if life expectancy was less than 24 hours, gestational age was less than 37 weeks, or the patient was previously enrolled. The study was approved by the Institutional Review Board at each site, except for the UK where it was approved by the Health Research Authority of the National Health Service. Waiver of consent was granted at all sites except one in Italy that required written consent. Sites in Switzerland required a description of the study to be posted with an opt-out possibility for families.
Data Collection
For each enrolled subject, the site chose from the following indications for the transfusion (more than one could be selected): (1) TPC < 10×109 cells/L with failure of platelet production; (2) TPC < 30×109 cells/L in neonate with failure of platelet production; (3) major bleeding: as defined by (a) bleeding requiring massive transfusion; (b) intracranial, intraocular, retroperitoneal, intra-spinal, or non-traumatic intra-articular bleeding; or (c) bleeding requiring surgical intervention (i.e. hemothorax requiring drainage); (4) minor bleeding (surgical or non-surgical): as defined by any bleeding not meeting criteria for major bleeding; (5) preparation for surgery; (6) preparation for invasive procedure; (7) known qualitative platelet defect with risk of bleeding; or (8) at risk of bleeding from device. Indications 1, 2, 5, 6, 7 and 8 were all categorized as “prophylactic” transfusions. When sites were unsure of indication, the record was reviewed by the study PI and an assignment was made after discussion with the local PI.
Data collected included patient demographics, reason for admission, any prior platelet transfusions, attributes of the platelet product (dose, source and processing), any adverse reactions to the transfusion, and laboratory assays assessed before and after transfusion. All lab data was collected at the discretion of the medical team and recorded by the research team up to 24 hours prior to and 36 hours following the transfusion of interest. Major clinical outcome data, including length of stay, length of mechanical ventilation, mortality, and degree of organ dysfunction, as measured by PELOD-2 score4 were collected. Study data were managed using REDCap electronic data capture tools hosted at Weill Cornell Medicine.
Statistical Approach
Demographic and clinical characteristics were described as N (%) or mean +/− standard deviation (SD) or median and interquartile range (IQR), as appropriate. Categorical data were compared by Fisher’s Exact/Chi-square tests. Continuous variables were compared by ANOVA/Kruskal-Wallis tests or t-test/Wilcoxon Rank Sum for parametric/non-parametric data, respectively. All p-values were two-sided with statistical significance evaluated at the 0.05 alpha level. All analyses for descriptive statistics were performed in R version 3.4.0 (R Foundation for Statistical Computing, Vienna, Austria).
To assess the independent association between total administered platelet dose and mortality, a multivariable logistic regression model was developed. Candidate variables with a p-value of less than 0.1 on unadjusted analyses were included. These were: full days since PICU admission, ECMO, PELOD-2 score on day of transfusion, bleeding as indication for transfusion, post-operative status on admission to PICU, and lowest platelet count on day of admission. Multicollinearity between variables was assessed and determined not to be an issue. The regression was performed using a backward, stepwise model. All analyses for outcomes data were conducted using SPSS version 25 (IBM Corp, Armonk, NY).
RESULTS
Prevalence
Eighty two sites from sixteen countries participated. The majority of sites were urban (97%), academic (98%), trauma centers (85%) and ECMO centers (80%). Of the participating sites, 48/82 (58%) were located in North America, 23/82 (28%) in Europe, 4/82 (5%) in Oceania, 4/82 (5%) in the Middle East and 3/82 (4%) in Asia.
During the six weeks of screening, 16,934 patients were eligible. Of those, 559 received platelet transfusions and were enrolled yielding a platelet transfusion prevalence of 3.3% per patient. There were 9/82 sites (11%) that did not transfuse platelets during the entire study period. The sites that did not transfuse any eligible subjects were limited to North America and Europe.
Subjects
Subject demographics are summarized in Table 1 and categorized according to indication for platelet transfusion. The median (IQR) age was 4.1 years (0.5–10.8) and 55% were male. The three most common reasons for admission were respiratory insufficiency/failure (39.2%), septic shock (22.4%), and cardiac surgery involving bypass (12.1%). Nearly half the subjects (43.5%) had an underlying oncologic diagnosis. The majority were mechanically ventilated at time of transfusion (64.4%) and admitted to the PICU for a median (IQR) length of 2 days (2–7) before enrollment. The median (IQR) PELOD-2 score at enrollment was 7 (5–10). The frequency of subjects receiving medications that may affect platelet function was: milrinone (17.2%), aspirin (2.6%) and non-steroidal anti-inflammatories (2.2%). Subjects were supported by the following devices at time of platelet transfusion: ECMO (16.8%), continuous renal replacement therapy (10.6%), intermittent hemodialysis (1.3%), and molecular adsorbent recirculation system (0.9%). Approximately one-third (36%) of the children had received at least one other platelet transfusion in the PICU during the admission prior to enrollment.
Table 1.
Demographics of Subjects
| Variable | Major Bleeding (n=64) | Minor Bleeding (n=115) | Prophylactic Transfusions in Patients on Devices (n=79) | Prophylactic Transfusions to Meet Threshold (n=278) | p-Values |
|---|---|---|---|---|---|
| Age (yr), median (IQR) | 3 (0–11) | 4 (0–10) | 0 (0–3) | 5 (1–11) | < 0.001 |
| Sex (male), n (%) | 33 (52%) | 66 (57%) | 44 (56%) | 152 (55%) | 0.897 |
| Weight (kg), median (IQR) | 13.7 (5.9–36.5) | 16.0 (8.5–28.5) | 6.4 (3.5–18.8) | 19.1 (8.6–38.1) | < 0.001 |
| Days Since Admission, median (IQR) | 1 (0–3) | 2 (0–8.8) | 5 (2–13) | 2 (0–6) | < 0.001 |
| Mechanical Ventilation, n (%) | 48 (75%) | 82 (71%) | 74 (94%) | 141 (51%) | < 0.001 |
| Underlying Oncologic Diagnosis, n (%) | 21 (34%) | 35 (31%) | 9 (12%) | 155 (59%) | < 0.001 |
| Reason for PICU Admission, n(%) | |||||
| Respiratory | 15 (23%) | 51 (44%) | 48 (61%) | 96 (35%) | < 0.001 |
| Septic Shock | 4 (6%) | 22 (19%) | 9 (11%) | 85 (31%) | < 0.001 |
| Hemorrhagic Shock | 16 (25%) | 5 (4%) | 2 (3%) | 6 (2%) | < 0.001 |
| Other Shock | 4 (6%) | 5 (4%) | 4 (5%) | 11 (4%) | 0.810 |
| Trauma | 4 (6%) | 7 (6%) | 1 (1%) | 2 (1%) | 0.002 |
| Traumatic Brain Injury | 3 (5%) | 2 (2%) | 0 (0%) | 3 (1%) | 0.114 |
| Burn | 0 (0%) | 1 (1%) | 0 (0%) | 1 (0%) | 0.731 |
| Cardiac surgery-bypass | 14 (22%) | 17 (15%) | 13 (16%) | 21 (8%) | 0.004 |
| Cardiac surgery-no bypass | 1 (2%) | 4 (3%) | 1 (1%) | 3 (1%) | 0.366 |
| Cardiac - non-surgical | 3 (5%) | 10 (9%) | 12 (15%) | 16 (6%) | 0.047 |
| Emergency surgery | 4 (6%) | 1 (1%) | 3 (4%) | 6 (2%) | 0.131 |
| Elective surgery | 3 (5%) | 7 (6%) | 5 (6%) | 8 (3%) | 0.312 |
| Seizure | 1 (2%) | 6 (5%) | 0 (0%) | 8 (3%) | 0.181 |
| Encephalopathy | 6 (9%) | 15 (13%) | 4 (5%) | 16 (6%) | 0.078 |
| Meningitis | 0 (0%) | 1 (1%) | 2 (3%) | 2 (1%) | 0.390 |
| Renal failure | 3 (5%) | 15 (13%) | 8 (10%) | 29 (10%) | 0.371 |
| Hepatic failure | 3 (5%) | 8 (7%) | 2 (3%) | 13 (5%) | 0.576 |
| Post-op liver transplant | 2 (3%) | 3 (3%) | 1 (1%) | 7 (3%) | 0.879 |
| Other | 23 (36%) | 27 (23%) | 15 (19%) | 92 (33%) | 0.026 |
| PELOD-2 Score prior to transfusion, median (IQR) | 7 (5.5–10.5) | 7 (6–11) | 8 (7–11) | 7 (4–9) | 0.001 |
Of the 559 patients transfused and enrolled, 536 had reported complete demographic data..
Indications
The indications for each platelet transfusion are summarized in Figure 1. The majority (67%) of transfusions were prophylactic; major or minor bleeding was the indication in only one-third of transfusions (33%). Of the 79 patients who received prophylactic transfusions while on mechanical circulatory devices, sixty-seven (85%) were supported by ECMO, and twelve (15%) were supported with CRRT.
Figure 1.
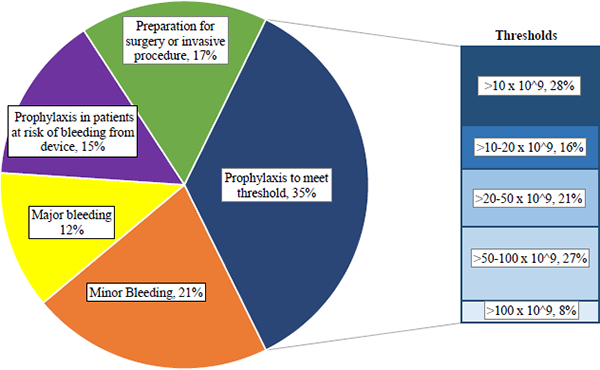
Indications for Platelet Transfusions
Blood Product/Transfusion Event
The majority of platelets transfused were collected by apheresis (87.1%) or whole blood-derived (12.9%). The transfusions were commonly leukoreduced (93.4%), and irradiated (79.9%). They were infrequently volume-reduced (8.3%), pathogen-inactivated (4.5%), or HLA-matched (0.8%). The median (IQR) administered platelet dose per transfusion event was 9.4 mL/kg (5.5–13.1). Subjects received a median (IQR) 4 (2–11) platelet transfusion events during their PICU course for a total median (IQR) administered dose of 32.4 mL/kg (14.0–91.4). The total number of transfusions that the 559 enrolled subjects received over their PICU admissions was 6090.
Reported adverse reactions were uncommon. In the 515 transfusions in which this data was entered, there were a total of 32 (6.2%) reactions. A new fever or increase in temperature by 1°C, if already febrile, occurred most frequently (3.0%), followed by hypotension (2.6%), urticaria (0.6%), and bronchospasm (0.2%). There were no hemolytic reactions or confirmed septic reactions.
Laboratory Assays
The TPC was known prior to transfusion in the vast majority of cases (99.1%). The median (IQR) TPC prior to transfusion was 40×109 cells/L (20–66). Thirty-four percent of transfusions were prescribed when the TPC was ≥ 50×109 cells/L. Of the 278 prophylactic transfusions prescribed to meet a threshold, forty-six (17%) were given to surgical patients. The median (IQR) TPC prior to transfusion in patients with surgical indications for PICU admission was 48×109 cells/L (30–81). This differed significantly (p < 0.001) from medical patients whose median (IQR) TPC prior to transfusion was 26×109 cells/L (15–42) (see Supplemental Figure 1).
Viscoelastic testing, such as thromboelastography (TEG) and rotational thromboelastometry (ROTEM) were uncommonly analyzed prior to transfusion (4.5% and 2.2% respectively).
Figure 2 depicts the change in TPC following transfusion at various times and for various indications corrected for transfusion dose. The median change in TPC varied across groups based on indication (p=0.03). For every 10 mL/kg of platelets transfused, the median (IQR) change in TPC was 48×109 cells/L (17–82) for patients with major bleeding, 42×109 cells/L (16–80) for prophylactic transfusions to meet a threshold, 38×109 cells/L (17–72) for patients with minor bleeding, and 25×109 cells/L (10–47) for prophylactic transfusions in patients at risk of bleeding from a device. The incremental change in TPC did not vary between those who had an underlying oncologic diagnosis and those who did not (p=0.57).
Figure 2.

Change in Platelet Count Following Transfusion by Indication
Outcomes
Outcome data was available for 532 of the 559 enrolled patients. The median (IQR) length of stay in the PICU was 13 days (6–29). The median (IQR) number of ventilator free days was 4 (1–9). The mortality rate for all children was 25%. Outcomes based on indication are summarized in Table 2. Variables associated with ICU mortality by univariate regression analysis are described in Supplemental Table 1. In the unadjusted model, each additional 1 mL/kg of platelets transfused was associated with an increase in mortality of 0.3% (odds ratio 1.003; 95% CI 1.002–1.004; p< 0.001). Multivariable logistic regression showed a 2% increase in mortality for every additional standard dose (10mL/kg) of platelets administered after adjustment for confounding variables (odds ratio for each additional 1 mL/kg transfused of 1.002; 95% CI 1.001–1.003; p = 0.005), shown in Table 3.
Table 2.
Outcomes Based on Indication for Platelet Transfusion
| Variable | Major Bleeding (n=64) | Minor Bleeding (n=113) | Prophylactic Transfusions in Patients on Devices (n=77) | Prophylactic Transfusions to Meet Threshold (n=278) | p-Values |
|---|---|---|---|---|---|
| PICU Length of Stay (days), median (IQR) | 10 (4–27) | 14 (7–27) | 25 (12–50) | 11.5 (5–26) | < 0.001 |
| Mechanical Ventilator Free Days, median (IQR) | 2 (0–8) | 3 (0–9) | 2 (0–7) | 5 (1–10) | < 0.001 |
| Total platelet dose (mL/kg), median (IQR) | 30.9 (13.5–67.3) | 27.3 (10.4–62.1) | 97.7 (40.0–243.0) | 28.6 (12.3–79.8) | < 0.001 |
| Mortality, n (%) | 18 (28.1%) | 40 (35.4%) | 27 (35.1%) | 48 (17.3%) | < 0.001 |
Of the 559 patients transfused and enrolled, 532 had reported outcome data.
Table 3.
Multiple Logistic Regression Model of Independent Variables and ICU Mortality
| Candidate Independent Variables | OR (95% CI) | p-value |
|---|---|---|
| Full days since PICU admission | 1.021 (1.006–1.036) | 0.007 |
| Post-operative at admission | 1.676 (0.940–2.990) | 0.080 |
| Extracorporeal Life Support (ECLS) | 3.256 (1.782–5.951) | <0.001 |
| Lowest platelet count on admission | 0.995 (0.990–1.001) | 0.089 |
| PELOD Score on day of transfusion | 1.311 (1.220–1.408) | <0.001 |
| Indication for transfusion = major or minor bleeding | 2.391 (1.463–3.906) | 0.001 |
| Total administered dose of platelets | 1.002 (1.001–1.003) | 0.005 |
CRRT and PELOD score at admission removed from model based on likelihood ratio test in backward stepwise model.
DISCUSSION
This international point prevalence study is the first published analysis of the epidemiology, indications, and outcomes of critically ill children transfused platelets. Approximately two–thirds of platelet transfusions were prescribed in a prophylactic manner to non-bleeding children. Thirty-four percent of prophylactic transfusions were prescribed when the TPC was ≥ 50×109 cells/L. While the observed rise in TPC following transfusion varied based on indication, those at risk of bleeding from a circulatory device had the smallest increase. Mortality in patients who receive platelet transfusions was high, ranging from 17–35% according to indication. There was an independent association between administered platelet dose and mortality.
Platelet transfusion practices observed in this study resemble those published in adults. In one large observational study of critically ill adults in the UK, wide variation in platelet use was reported.5 The prevalence of platelet transfusion was 9%. More than half of platelet units were given as “prophylactic transfusions” in patients with no documented bleeding. One-third of transfusions were given when the TPC > 50×109 cells/L. Similar findings were seen in a retrospective study from 3 adult ICUs in Canada; the median TPC reported prior to platelet transfusion was 87×109 cells/L.6 In these same two studies, the median (IQR) increment in TPC following transfusion was modest, 15×109 cells/L (2–36) and 23×109 cells/L (7–44).5,6
Pediatric data from a single center prospective study reported the prevalence of critically ill children receiving at least one platelet transfusion to be 7.2%.7 The average TPC was 49 ± 34×109 cells/L prior to transfusion, similar to thresholds reported here. They reported a wide range of incremental changes in TPC following transfusion (from 30 to nearly 100×109 cells/L), based on indication for transfusion. The variation in TPC noted in our study is likely also due to differences in the indication for transfusion but also due to differences in patient characteristics or etiology of thrombocytopenia. For example, change in TPC is expected to be different between patients transfused prophylactically in preparation for neurosurgery compared to a bleeding patient with an oncologic disorder. Of note, the incremental change in TPC we report did not vary with the presence or absence of an underlying oncologic diagnosis. The incremental change in TPC in critically ill children following platelet transfusion may be higher than that observed in critically ill adults because of better ABO compatibility, which was implicated in improved post-transfusion platelet increment in adults with hematologic malignancies.8 The incremental change in TPC we report also varied by indication for transfusion. The smallest incremental change was seen in those at risk of bleeding from a device which may be theoretically explained by alloimmunization and consumption related to the device itself. Counter-intuitively, children with major bleeding had a greater median rise in TPC than those with minor or no bleeding. Variations in platelet products, including storage solution, age and temperature, may have impacted the median change in TPC post transfusion. Differences in storage solution affect post transfusion platelet count at 1 and 24 hours and may have played a role.9, 10 In addition, differences in age of the platelets transfused has been related to clinical complications in trauma patients.11 Platelets stored at room temperature may be relatively inert and not consumed at a high enough rate to show reduced increments compared to non-bleeding patients. “Cold” platelets (stored at 4°C) have been shown to have increased hemostatic efficacy and increased safety relative to bacterial contamination.12, 13, 14
Our study confirms that clinicians rely on few assays, other than TPC alone, to prescribe platelet transfusions. Whiting et al15 suggest that viscoelastic testing is more effective than standard lab testing to guide transfusion therapy in adults undergoing cardiac surgery or following trauma. A recent Cochrane review analyzing the benefit of viscoelastic testing to monitor hemostatic treatment versus usual care in adults or children with bleeding reported that application of TEG- or ROTEM-guided transfusion strategies may reduce the need for blood products and improve morbidity.16 The PLADO trial, comparing different platelet doses in adults and children with hematological malignancies, reported a poor relationship between degree of severity of thrombocytopenia and bleeding risk.17 More work needs to be done to define bleeding risk, other than isolated platelet counts, in critically ill children.
Given there are few evidence-based guidelines for platelet transfusion in critically ill children, it is not surprising that significant variation in prescribing practices exists. Randomized trials represent the goal for evidence-based practitioners but we must recognize considerable challenges undertaking trials of platelet transfusions in critically ill children. Alternative designs, such as comparative effectiveness methods, need to be considered. Since nearly half the children in this cohort had an underlying oncologic diagnosis, this specific population should be investigated. Future studies should also focus on children supported by circulatory devices since they receive the highest exposure to platelet transfusions with high mortality.
This study represents the largest prospective study of platelet transfusions in critically ill children reported to date. Since the data was predominantly collected with waiver of consent, it is without selection bias. Given the number of international sites, results should be externally valid for PICUs with platelet transfusions readily available in their blood banks. The results, though primarily descriptive in nature, provide important preliminary data that identifies at risk patient populations and will facilitate the design of future trials.
Some limitations exist. Unfortunately, there is no validated bleeding assessment tool in critically ill children, so it was not included as an outcome. Data was not collected on transfusion of other hemostatic products, such as plasma, cryoprecipitate, or anti-fibrinolytics. Since we did not collect data on the 16,375 non-transfused patients, we cannot directly compare patients transfused and not transfused. The number of sites that did not transfuse any subjects were limited to North America and Europe and may represent different regional approaches to platelet transfusions. Data was not collected on platelet transfusions ordered by anesthesiologists or surgeons, so only reflects practices of pediatric intensivists. Data on adverse events related to the transfusions was collected passively. The population of children transfused had increased acuity compared to the general PICU population, in both median PELOD-2 scores (7 versus 4) and mortality (25% versus 3%).18 Finally, the association between platelet dose and mortality must be interpreted with caution as patients who are sicker are often more heavily transfused and this confounding cannot be removed entirely.
In conclusion, the majority of platelet transfusions prescribed in the PICU are given prophylactically to non-bleeding children and significant variation in platelet thresholds exists. Studies are needed to clarify appropriate indications for platelet transfusion and subsequent responses in critically ill children according to their illness, with particular focus on prophylactic transfusions. Further work should investigate the association between administered platelet dose and mortality.
Supplementary Material
Supplemental Figure 1. Comparison of Medical and Surgical Patients Receiving Prophylactic Transfusions to Meet a Defined Threshold
Supplemental Table 1. Unadjusted Logistic Regression Models of Independent Variables and ICU Mortality
ACKNOWLEDGEMENTS
We would like to thank all of the P3T investigators for their contribution, as well as the Clinical Translational Science Center (CTSC) at Weill Cornell Medical College.
P3T investigators: Australia: Warwick Butt, Carmel Delzoppo (Royal Children’s Hospital, Melbourne); Simon Erickson, Elizabeth Croston, Samantha Barr (Princess Margaret Hospital, Perth); Elena Cavazzoni (Children’s Hospital at Westmead, Sydney). Belgium: Annick de Jaeger (Princess Elisabeth Children’s University Hospital, Ghent). Canada: Marisa Tucci, Mary-Ellen French, Marion Ropars, Lucy Clayton (CHU Sainte-Justine, Montreal QC); Srinivas Murthy, Gordon Krahn (British Columbia Children’s Hospital, Vancouver, BC). China: Dong Qu, Yi Hui (Children’s Hospital Capital Institute of Pediatrics, Beijing). Denmark: Mathias Johansen, Anne-Mette Baek Jensen, Inge-Lise Jarnvig, Ditte Strange (Rigshospitalet, University of Copenhagen, Copenhagen). India: Muralidharan Jayashree, Mounika Reddy (Postgraduate Institute of Medical Education and Research, Chandigarh); Jhuma Sankar, U Vijay Kumar, Rakesh Lodha (All India Institute of Medical Sciences, New Delhi). Israel: Reut Kassif Lerner, Gideon Paret (The Edmond and Lily Safra Children’s Hospital, Sheba Medical Center, Ramat Gan); Ofen Schiller, Eran Shostak, Ovadia Dagan (Schneider Children’s Medical Center, Petah Tikva); Yuval Cavari (Soroka University Medical Center, Beersheva). Italy: Fabrizio Chiusolo, Annagrazia Cillis (Bambino Gesu Children’s Hospital, Rome); Anna Camporesi (Children’s Hospital Vittore Buzzi, Milano). Netherlands: Martin Kneyber (Beatrix Children’s Hospital, Groningen); Suzan Cochius-den Otter, Ellen Van Hemeldonck (Erasmus MC-Sophia Children’s Hospital, Rotterdam). New Zealand: John Beca, Claire Sherring, Miriam Rea (Starship Child Health, Auckland). Portugal: Clara Abadesso, Marta Moniz (Hospital Prof. Dr. Fernando Fonseca, Amadora). Saudi Arabia: Saleh Alshehri (King Saud Medical City, Riyadh). Spain: Jesus Lopez-Herce, Irene Ortiz, Miriam Garcia (Hospital General Universitario Gregorio Maranon, Madrid); Iolanda Jordan (Institut de Recerca Hospital Sant Joan de Deu, Barcelona); J Carlos Flores Gonzalez (Hospital Universitario Puerta del Mar, Cadiz); Antonio Perez-Ferrer, Ana Pascual-Albitre (La Paz University Hospital, Madrid). Switzerland: Serge Grazioli (University Hospital of Geneva, Geneva); Carsten Doell (University Children’s Hospital Zurich, Zurich). United Kingdom: Peter J. Davis (Bristol Royal Hospital for Children, Bristol); Ilaria Curio, Andrew Jones, Mark J. Peters (Great Ormond St Hospital NHS Foundation Trust, London); Jon Lillie (Evelina London Children’s Hospital, London); Angela Aramburo, Medhat Shabana, Priya Ramachandran, Helena Sampaio (Royal Brompton Hospital, London); Kalaimaran Sadasivam (Royal London Hospital, Barts Health NHS Trust, London); Nicholas J Prince (St George’s Hospital, London); Hari Krishnan Kanthimathinathan (Birmingham Children’s Hospital, Birmingham); Ricardo Garcia Branco (Cambridge University Hospitals NHS Trust, Cambridge); Kim L. Sykes, Christie Mellish (University Hospital Southampton, Southampton); Avishay Sarfatti, James Weitz (Oxford University Hospitals NHS Foundation Trust, Oxford). United States: Ron C. Sanders Jr, Glenda Hefley (Arkansas Children’s Hospital, Little Rock, AR); Rica Sharon P. Morzov, Barry Markovitz (Children’s Hospital Los Angeles, Los Angeles, CA); Anna Ratiu, Anil Sapru (Mattel’s Children’s Hospital, Los Angeles, CA); Allison S. Cowl (Connecticut Children’s Medical Center, Hartford, CT); E. Vincent S Faustino (Yale School of Medicine, New Haven, CT); Shruthi Mahadevaiah (University of Florida Shands Children’s Hospital, Gainesville, FL); Fernando Beltramo (Nicklaus Children’s Hospital, Miami, FL); Asumthia S Jeyapalan, Mary K Cousins (University of Miami/Holtz Children’s Hospital, Miami, FL); Cheryl Stone, James Fortenberry (Children’s Hospital of Atlanta, Atlanta, GA); Neethi P. Pinto, Chiara Rodgers, Allison Kniola (The University of Chicago, Chicago, IL); Melissa Porter, Erin Owen, Kristen Lee, Laura J. Thomas (University of Louisville, Kosair Charities Pediatric Clinical Research Unit, and Norton Children’s Hospital, Louisville, KY); Melania M Bembea, Ronke Awojoodu (Johns Hopkins University, Baltimore, MD); Daniel Kelly, Kyle Hughes (Boston Children’s Hospital, Boston, MA); Zenab Mansoor, Carol Pineda (Tufts Floating Hospital for Children, Boston, MA); Phoebe H Yager, Maureen Clark (Massachusetts General Hospital, Boston, MA); Scot T. Bateman (UMass Memorial Children’s Medical Center, Worcester, MA); Kevin W. Kuo, Erin F. Carlton (C.S. Mott Children’s Hospital, University of Michigan, Ann Arbor, MI); Brian Boville, Mara Leimanis (Helen DeVos Children’s Hospital, Grand Rapids, MI); Marie E Steiner, Dan Nerheim (University of Minnesota Masonic Children’s Hospital, Minneapolis, MN); Kenneth E. Remy, Lauren Langford, Melissa Schicker (Washington University in St. Louis, St. Louis, MO); Marcy N Singleton, J Dean Jarvis, Sholeen T Nett (Dartmouth-Hitchcock Medical Center, Lebanon, NH); Shira Getz (Hackensack University Medical Center, Hackensack, NJ); Ruchika Goel (New York Presbyterian Hospital – Weill Cornell Medicine, New York, NY); James S. Killinger, Meghan Sturhahn (Memorial Sloan Kettering Cancer Center, New York, NY); Margaret M. Parker, Ilana Harwayne-Gidansky (Stony Brook Children’s Hospital, Stony Brook, NY); Laura A. Watkins (Cohen Children’s Medical Center, Northwell Health, Queens, NY); Gina Cassel, Adi Aran, Shibhi Kaushik (The Children’s Hospital at Montefiore, Bronx, NY); Andy Y. Wen (NYU Langone Medical Center, NYU School of Medicine, New York, NY); Amanda B. Hassinger (Women and Children’s Hospital of Buffalo, Buffalo, NY); Caroline P. Ozment, Candice M. Ray (Duke Children’s Hospital and Health Center, Durham, NC); Michael C. McCrory, Andora L Bass (Wake Forest Brenner Children’s Hospital, Winston-Salem, NC); Michael T Bigham, Heather Anthony (Akron Children’s Hospital, Akron, OH); Jennifer A. Muszynski, Jill Popelka (Nationwide Children’s Hospital, Columbus, OH); Julie C. Fitzgerald, Susan Doney Leonard (Children’s Hospital of Philadelphia, Philadelphia, PA); Neal J. Thomas, Debbie Spear (Penn State Hershey Children’s Hospital, Hershey, PA); Whitney E. Marvin (Medical University of South Carolina, Charleston, SC); Arun Saini; Alina Nico West (University of Tennessee Health Science Center and Le BonHeur Children’s Hospital, Memphis, TN); Jennifer McArthur, Angela Norris, Saad Ghafoor, Ashlea Anderson (St. Jude Children’s Research Hospital, Memphis, TN); Tracey Monjure, Kris Bysani (Medical City Children’s Hospital, Dallas, TX); LeeAnn M. Christie (Dell Children’s Medical Center, Austin, TX); Laura L Loftis (Baylor College of Medicine, Texas Children’s Hospital, Houston, TX); Andrew D. Meyer, Robin Tragus, Holly Dibrell, David Rupert (University of Texas Health Science Center at San Antonio, San Antonio, TX); Claudia Delgado-Corcoran, Stephanie Bodily (University of Utah, Primary Children’s Hospital, Salt Lake City, UT); Douglas Willson (Children’s Hospital of Richmond at VCU, Richmond, VA); Leslie A. Dervan (Seattle Children’s, University of Washington, Seattle, WA); Sheila J. Hanson (Medical College of Wisconsin/ Children’s Hospital of Wisconsin, Milwaukee, WI); Scott A. Hagen, Awni M. Al-Subu (University of Wisconsin School of Medicine and Public Health, Madison, WI).
Footnotes
Conflicts of Interest and Source of Funding: There are no conflicts of interest to disclose. This project was supported in part by funds from the Clinical Translational Science Center (CTSC), National Center for Advancing Translational Sciences (NCATS) grant #UL1-TR000457.
REFERENCES
- 1.Sapiano MRP, Savinkina AA, Ellingson KD, et al. Supplemental findings from the National Blood Collection and Utilization Surveys, 2013 and 2015. Transfusion. 2017; 57(52): 1599–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Josephson C, Meyer E. Neonatal and pediatric transfusion practice In: AABB Technical Manual. Fung MK (ed). 18th edition Bethesda, MD, AABB, 2014, pp 571–597. [Google Scholar]
- 3.Karam O, Demaret P, Shefler A, et al. Indications and Effects of Plasma Transfusions in Critically Ill Children. Am J Respir Crit Care Med. 2015. June 15; 191(12):1395–402. [DOI] [PubMed] [Google Scholar]
- 4.Leteutre S, Duhamel A, Salleron J, et al. PELOD-2: an update of the Pediatric Logistic Organ Dysfunction score. Crit Care Med 2013; 41:1761–1773. [DOI] [PubMed] [Google Scholar]
- 5.Stanworth SJ, Walsh TS, Prescott RJ, et al. Thrombocytopenia and platelet transfusion in UK critical care: a multicenter observational study. Transfusion 2013; 53:1050–1058. [DOI] [PubMed] [Google Scholar]
- 6.Ning S, Barty R, Liu Y, et al. Platelet Transfusion Practices in the ICU: Data from a Large Transfusion Registry. Chest. 2016. September; 150(3):516–23. [DOI] [PubMed] [Google Scholar]
- 7.Du Pont-Thibodeau G, Tucci M, Robitaille N, et al. Platelet Transfusions in Pediatric Intensive Care. Pediatr Crit Care Med. 2016. September; 17(9):e420–9. [DOI] [PubMed] [Google Scholar]
- 8.Inverardi D, Bocchio C, Rossi L, et al. Clinical, immunologic, and technical factors affecting recovery of platelet count after platelet transfusion. Haematologica. 2002; 87(8):893–4. [PubMed] [Google Scholar]
- 9.Dijkstra-Tiekstra MJ, van de Watering LM, Rondeel JM, Slomp J, de Wildt-Eggen J. Implementaion of a new platelet pooling system for platelet concentrates led to a higher corrected count increment after transfusion: a comparative observational study of platelet concentrates before and after implementation. Transfus Med 2014. April; 24(2): 99–104. [DOI] [PubMed] [Google Scholar]
- 10.Kerkhoofs JL, Eikenboom JC, Schipperus MS, et al. A multicenter randomized study of the efficacy of transfusions with platelets stored in platelet additive solution II versus plasma. Blood 2006. November; 108(9): 3210–5. [DOI] [PubMed] [Google Scholar]
- 11.Inaba K, Branco BC, Rhee P, et al. Impact of the duration of platelet storage in critically ill trauma patients. J Trauma 2001; 71(6):1766–73. [DOI] [PubMed] [Google Scholar]
- 12.Nair PM, Pidcoke HF, Cap AP, et al. Effect of cold storage on shear-induced platelet aggregation and clot strength. J Trauma Acute Care Surg. 2014; 77(3 Suppl 2):S88–93. [DOI] [PubMed] [Google Scholar]
- 13.Nair PM, Pandya SG, Dallo SF, et al. Platelets stored at 4°C contribute to superior clot properties compared to current standard-of-care through fibrin-crosslinking. Br J Haematol. 2017; 178(1):119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pidcoke HF, Spinella PC, Ramasubramanian AK, et al. Refrigerated platelets for the treatment of acute bleeding: a review of the literature and reexamination of current standards. Shock. 2014;41 Suppl 1:51–3 [DOI] [PubMed] [Google Scholar]
- 15.Whiting P, Al M, Westwood M, et al. Viscoelastic point-of-care testing to assist with the diagnosis, management and monitoring of haemostasis: a systematic review and cost-effectiveness analysis. Health Technol Assess. 2015. July; 19(58):1–228, v–vi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wikkelsø A, Wetterslev J, Møller AM, et al. Thromboelastography (TEG) or thromboelastometry (ROTEM) to monitor haemostatic treatment versus usual care in adults or children with bleeding. Cochrane Database Syst Rev. 2016. 22; (8):CD007871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uhl L, Assmann SF, Hamza TH, et al. Laboratory predictors of bleeding and the effect of platelet and RBC transfusions on bleeding outcomes in the PLADO trial. Blood 2017; 130(10):1247–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paediatric Intensive Care Audit Network Annual Report 2017. (published November 2017): Universities of Leeds and Leicester. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Comparison of Medical and Surgical Patients Receiving Prophylactic Transfusions to Meet a Defined Threshold
Supplemental Table 1. Unadjusted Logistic Regression Models of Independent Variables and ICU Mortality


