Abstract
JAK2 mutations are rare in de novo acute myeloid leukemia (AML), and JAK2-mutated acute myeloid leukemia (AML) patients usually have a previous history of myeloproliferative neoplasms (MPNs). Current advances in laboratory techniques, such as single nucleotide polymorphism array (SNPa) and next-generation sequencing (NGS), have facilitated new insight into the molecular basis of hematologic diseases. Herein, we present two cases of JAK2-mutated AML in which both SNPa and NGS methods added valuable information. Both cases had leukemogenic collaboration, namely, copy-neutral loss of heterozygosity (CN-LOH), detected on chromosome 9. One of the cases exhibited both JAK2 and IDH2 mutations, most likely having originated as an MPN with leukemic transformation, while the other case was classified as a de novo AML with JAK2, CEBPA, and FLT3 mutations.
Keywords: Cytogenetics; Leukemia, Myeloid, Acute; Polymorphism, Single Nucleotide; Sequence Analysis, DNA
INTRODUCTION
Myeloproliferative neoplasms (MPNs) are clonal disorders of hematopoietic stem cells with increased proliferation of myeloid cells and effective maturation, leading to peripheral blood leukocytosis and excess erythrocytes or platelets.1 According to the World Health Organization (WHO) 2016 revision of the classification of myeloid neoplasms and acute leukemia, MPNs are subdivided into (i) chronic myeloid leukemia (BCR-ABL1–positive); (ii) chronic neutrophilic leukemia; (iii) polycythemia vera; (iv) primary myelofibrosis; (v) essential thrombocythemia; (vi) chronic eosinophilic leukemia; and (vii) unclassifiable myeloproliferative neoplasm.2 The final evolution of MPNs involves progression to fibrosis or leukemic transformation. Acute myeloid leukemia (AML) following MPNs is characterized by 20% or more blasts being present in the bone marrow (BM) or peripheral blood (PB).3 MPN patients who develop AML have a poor prognosis, with a median survival of fewer than 6 months.4 Leukemic transformation occurs in 1%, 4% and 20% of patients with essential thrombocythemia (ET), polycythemia vera (PV) and primary myelofibrosis (PM), respectively, over a 10-year period.5
Mutations in JAK2 have been identified in the majority of patients with PV, ET, and PM, underscoring the importance of constitutive activation of JAK2 signaling caused by mutations.6 Genetic studies of paired samples before and after AML transformation suggest that there are at least two distinct routes for leukemic transformation. Patients who exhibit the JAK2 mutation in MPNs progress to AML, suggesting that this mutation might be associated with the acquisition of additional genetic alterations. In contrast, patients who harbor the JAK2 mutation in their MPNs have no evidence of the mutation in leukemic blasts post-MPN AML.6,7 It is important to highlight that, despite the cooccurrence of JAK2 mutations with other genetic alterations, JAK2 mutations have rarely been reported in de novo AML (<5%).8,9
Cytogenetic and molecular cytogenetic techniques combined may provide a comprehensive analysis of chromosomal aberrations. Advances in laboratory techniques, such as single nucleotide polymorphism array (SNPa), a molecular cytogenetic method, and high-throughput sequencing, have facilitated new insight into the molecular basis of hematologic diseases. SNPa is a sensitive technique used to perform high-resolution genome-wide DNA copy number analysis and to detect copy-neutral loss of heterozygosity (CN-LOH). Next-generation sequencing (NGS) is capable of detecting single nucleotide variants (SNVs) or small insertions and deletions that have recently been shown as important molecular phenomena in AML.10 To substantiate this idea, we describe two JAK2-mutated AML cases for which both SNPa and NGS added valuable information.
METHODS
Patients
The patients described in this report were seen for follow up at the Hospital São Paulo of the Federal University of São Paulo, São Paulo, Brazil. The Ethics Committee of the institution approved the study, and written informed consent was obtained from all patients (CAAE 00547512.5.0000.5505).
Conventional Cytogenetics
Karyotypes were made from BM samples using standard methods, and aberrations were described according to the International System for Human Cytogenetic Nomenclature (ISCN) 2016.11
Single Nucleotide Polymorphism Array
SNPa was performed on total BM (cases 1 and 2) and buccal cells (only case 1) samples. Two hundred and fifty nanograms of DNA were extracted and digested, amplified, purified, fragmented, labeled and hybridized using the Affymetrix CytoScan® HD Array. CEL files were created using the GeneChip® System 3000 7G according to the manufacturer’s instructions. CEL files were analyzed using Chromosome Analysis Suite v3.0 (ChAS) software. Regions of copy number variations (CNVs) larger than 1 Mb and CN-LOH larger than 10 Mb identified by the ChAS Software or detected by visual inspection, regardless of gene content, are considered true aberrations, with the exception of those known to be normal genomic variants (present in the Genomic Variants Database [http://projects.tcag.ca/variation]) or identified in constitutional (buccal cells) SNPa analysis.10 Aberrations identified by SNPa were described according to the ISCN 2016.
Next-Generation Sequencing
Total BM was used for DNA extraction. A 19-gene panel was screened using the Ion AmpliSeq AML Research Panel (Thermo Fisher). Reads were mapped to the HG19 reference human genome, and alignment results were analyzed using CLC Genomics Workbench software.10 FLT3-ITD was tested in addition to the gene panel by PCR and capillary electrophoresis using the following primers: forward 5’−6FAM- tgcctattcctaactgactcatc-3’ and reverse 5’- tctttgttgctgtccttccac-3’.
CASE REPORTS
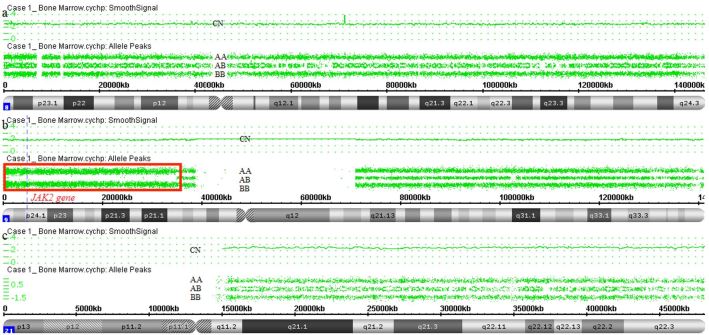
Case 1: A 66-year-old female patient was referred to the Hematology service for investigation of polyglobulia (hemoglobin 17.0 g/dL and hematocrit 53.4%) associated with weight loss (18 kg over the last six months). However, admission lab tests showed hemoglobin of 9.4 g/dL, hematocrit of 33.4%, WBC count of 47.8 × 109/L (with 22% blasts) and platelet counts of 119.0 × 109/L. Bone marrow aspirate smear revealed 20% of blasts, and multiparametric flow cytometry immunophenotyping was compatible with AML (Positive: CD45 moderate, CD34, HLA-DR, CD117, CD33 heterogeneous, CD13 heterogeneous, CD11b partial, CD64 partial heterogeneous, CD36 partial, CD7 partial. Negative: CD14, CD65, CD41, CD2, CD4, CD56, CD19, CD10). The bone marrow karyotype is as follows: 48,XX,+8,+21[11]/46,XX[1]. SNPa results are as follows: arr[hg19] (8)x2-3; arr[hg19] (21)x2-3; arr[hg19] 9p24.3p13.3(192128_35098008) hmz (Figure 1). NGS analysis revealed IDH2 R140Q and JAK2 V617F mutations. The patient was on chronic osteomyelitis treatment prior to initiating chemotherapy and died one month after admission due to infection.
Figure 1. Genomic alterations detected by SNPa (Affymetrix CytoScan® HD array) in case 1. a – Mosaic gain of one entire chromosome 8 (CN: 2.57). b – Red box showing CN-LOH in 9p24.3p13.3. The JAK2 gene is located in this region. c – Mosaic gain of chromosome 21 (CN: 2.54).
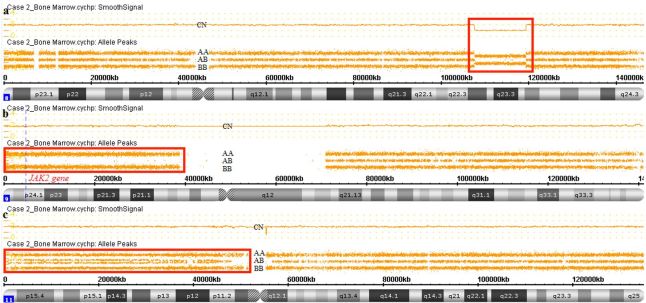
Case 2: A 77-year-old female patient was admitted to hematology service with complaints of fatigue and easy bruising. Lab tests on admission showed hemoglobin of 10.3 g/dL, WBC count of 99.9 × 109/L (with 45% blasts) and platelet counts of 23.0 × 109/L. Bone marrow aspirate, showing 40% blasts and multiparametric flow cytometry immunophenotyping, was compatible with AML (Positive: CD34, CD117, CD13, CD45 moderate, CD33, HLA-DR, CD64, CD7. Negative: CD11b, CD10, CD19, CD15, CD36, CD14, CD65, CD2, CD56). The bone marrow karyotype did not present metaphases. SNPa results are as follows: arr[hg19] 8q23.1q24.12(107621735_119398978)×1; arr[hg19] 9p24.3p13.1(192128_40087758) hmz; arr[hg19] 11p15.5p11.2(198509_47404314) hmz (mosaic) (Figure 2). NGS results revealed mutations in FLT3-ITD, CEBPA E309_T310insE and JAK2 V617F. The patient was on conventional chemotherapy treatment and died three months after admission due to infection.
Figure 2. Genomic alterations detected by SNPa (Affymetrix CytoScan® HD array) in case 2. a – Red box showing deletion in 8q23.1q24.12 (CN: 1.00). b – Red box showing CN-LOH in 9p24.3p13.1. The JAK2 gene is located in this region. c – Red box showing mosaic CN-LOH in 11p15.5p11.2.
DISCUSSION
The two cases presented herein were classified as AML, one of which had a previous history of polyglobulia and weight loss (case 1), while the other had become recently symptomatic (case 2). Based on the clinical history, one may interpret the first case as leukemic transformation of a myeloproliferative neoplasm and the second as de novo AML.
Which tests could confirm the above-mentioned hypotheses: Conventional cytogenetics (karyotype), molecular cytogenetics (SNPa) or molecular tests (NGS)?
Cytogenetic abnormalities identified by karyotype allow the detection of clonal abnormalities, classifying cases into favorable, intermediate or unfavorable prognosis. Karyotype remains one of the most powerful predictors of outcome in AML.12 However, approximately 50% of AML cases present a normal karyotype or present noninformative results, requiring other tests to better characterize the disease.10
Our first case presented a hyperdiploid karyotype, (trisomies 8 and 21), an aberration observed in 0.7% of AMLs,13 allowing no additional clarification to the posed question. However, it allowed the classification of this case as intermediate risk by the European Leukemia Net genetic classification,14 although this is a conceptually a high-risk disease as it fits into the “AML with myelodysplasia-related changes” (post-MPN AML). For case 2, the karyotype was noninformative, and in this condition, risk stratification could not be correctly interpreted.
SNPa is a complementary tool to karyotype, and may be applied for the evaluation of chromosomal losses, gains and CN-LOH. Combining SNPa with karyotype increases the detection rate of abnormalities in 28%.15 Advances in high-throughput sequencing techniques have facilitated new insights into the molecular basis of AML.12,16 Indeed, each test has distinct performances characteristics, with variability in the detection rate being defined by the type of genomic alteration (Table 1).
Table 1. Technique comparison for identifying genetic abnormalities.
| Technique | Characteristics | ||||
|---|---|---|---|---|---|
| Resolution | Gain and Loss | Translocation | CN-LOH | SNVs/INDELs | |
| Karyotype | 5Mb | Yes | Yes | No | No |
| SNPa | <1Mb | Yes | No | Yes | No |
| NGS | 1Bp | Yes* | No | No | Yes |
SNPa: Affymetrix CytoScan™ HD. NGS: Ion AmpliSeq™ AML Research Panel.
SNVs (Single Nucleotide Variants) / INDELs (Insertions and Deletions <50 base pairs).
SNPa confirmed the karyotype results in case 1 and added new information in both cases: CN-LOH on chromosome 9 (cases 1 and 2), CN-LOH on chromosome 11 (case 2) and partial deletion of chromosome 8 (case 2). CN-LOH indicates that an individual possesses two identical copies of one chromosome or chromosome region, i.e., this abnormality represents an important mechanism by which point mutations and other micro lesions can be established in a homozygous state.17 JAK2 and FLT3 mutations share a common mechanism that confers augmented reactive oxygen species, which induce DNA damage, resulting in homologous recombination events that initiate CN-LOH.18
NGS identified mutations in both cases, two in case 1 and three in case 2. The JAK2 V617F mutation was identified in both cases. Case 1 presented 72.5% allelic frequency, and case 2 exhibited 94.3% allelic frequency. These values are compatible with a CN-LOH on chromosome 9 detected by SNPa, where the JAK2 is gene located. In MPNs, the high JAK2 V617F allele burden in PV represents a risk factor for progression to myelofibrosis. In contrast, high JAK2 V617F allele burden is not significantly related to leukemic transformation.19 The JAK2 mutation constitutively activates the JAK/STAT signaling pathway, but the prognostic implications of this alteration remain unclear.20
Regarding the other mutations, IDH2 (case 1) is involved in DNA methylation, and the relationship of this mutation with prognosis is still uncertain.21 However, some groups have identified the IDH2 mutation in a subset of patients with MPN who underwent leukemic transformation.22,23 In addition, combined expression of JAK2 V617F and IDH2 R140Q induces MPN progression, alters stem/progenitor cell function and impairs differentiation in mice.24
The CEBPA mutation is involved in the development of myeloid progenitors into differentiated neutrophils. Previous studies suggest that monoallelic mutations in CEBPA are prognostically neutral, whereas biallelic mutations in trans are associated with a favorable prognosis in cytogenetically normal AML.25 FLT3-ITD constitutively activate tyrosine kinase by interfering with the autoinhibitory function of the juxtamembrane domain, leading to enhanced RAS, MAPK, and STAT5 signaling. Mutations in this gene are associated with worse prognosis.25
JAK2 mutations are rare in de novo AML, and AML patients with JAK2 mutations usually have a history of an antecedent MPN. The most common mutations observed in de novo AML, including NPM1, DNMT3A, and FLT3, are largely absent from AMLs that occur following an MPN.6 Despite the infrequent incidence of JAK2 mutation in AML, mutated JAK2 could be therapeutically targeted in some cases with JAK2 mutations.8
SNPa and NGS added valuable information to these analyses, as both cases exhibited leukemogenic collaboration of the detected mutations: one had JAK2 and IDH2 mutations, being most likely an MPN with leukemic transformation, while the other could be a de novo AML with JAK2, CEBPA, and FLT3 mutations.
In summary, the present work supports the use of both SNPa and NGS as important tools for patients with AML, offering insights into the molecular pathogenesis and indicating that different mutation combinations and cytogenetic results could further stratify prognosis.
The authors retain an informed consent signed by the patients, and this manuscript is in accordance with the Institutional Ethics Committee.
Footnotes
How to cite: Noronha TR, Mitne-Neto M, Chauffaille ML. JAK2-mutated acute myeloid leukemia: comparison of next-generation sequencing (NGS) and single nucleotide polymorphism array (SNPa) findings between two cases. Autops Case Rep [Internet]. 2019;9(2):e2018084. https://doi.org/10.4322/acr.2018.084
Financial support: NGS and SNPa procedures were supported by Fleury Group
REFERENCES
- 1.Chauffaille MLLF. Myeloproliferative neoplasms: a review of diagnostic criteria and clinical aspects. Rev Bras Hematol Hemoter. 2010;32(4):308-16. 10.1590/S1516-84842010005000091. [DOI] [Google Scholar]
- 2.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classi fi cation of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391-405. 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]
- 3.Mesa RA, Verstovsek S, Cervantes F, et al. Primary myelofibrosis (PMF), post polycythemia vera myelofibrosis (post-PV MF), post essential thrombocythemia myelofibrosis (post-ET MF), blast phase PMF (PMF-BP): Consensus on terminology by the international working group for myelofibrosis research and treatment (IWG-MRT). Leuk Res. 2007;31(6):737-40. 10.1016/j.leukres.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Mesa RA, Li CY, Ketterling RP, Schroeder GS, Knudson RA, Tefferi A. Leukemic transformation in myelofibrosis with myeloid metaplasia: A single-institution experience with 91 cases. Blood. 2005;105(3):973-7. 10.1182/blood-2004-07-2864. [DOI] [PubMed] [Google Scholar]
- 5.Abdulkarim K, Girodon F, Johansson P, et al. AML transformation in 56 patients with Ph–MPD in two well defined populations. Eur J Haematol. 2009;82(2):106-11. 10.1111/j.1600-0609.2008.01163.x. [DOI] [PubMed] [Google Scholar]
- 6.Rampal R, Ahn J, Abdel-Wahab O, et al. Genomic and functional analysis of leukemic transformation of myeloproliferative neoplasms. Proc Natl Acad Sci USA. 2014;111(50):E5401-10. 10.1073/pnas.1407792111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heaney ML, Soriano G. Acute myeloid leukemia following a myeloproliferative neoplasm: Clinical characteristics, genetic features and effects of therapy. Curr Hematol Malig Rep. 2013;8(2):116-22. 10.1007/s11899-013-0154-5. [DOI] [PubMed] [Google Scholar]
- 8.Lee JW, Kim YG, Soung YH, et al. The JAK2 V617F mutation in de novo acute myelogenous leukemias. Oncogene. 2006;25(9):1434-6. 10.1038/sj.onc.1209163. [DOI] [PubMed] [Google Scholar]
- 9.Hidalgo-López JE, Kanagal-Shamanna R, Medeiros LJ, et al. Morphologic and molecular characteristics of de novo AML with JAK2 V617F mutation. J Natl Compr Canc Netw. 2017;15(6):790-6. 10.6004/jnccn.2017.0106. [DOI] [PubMed] [Google Scholar]
- 10.Noronha T, Mitne-Neto M, Chauffaille M. Mutational profiling of acute myeloid leukemia with normal cytogenetics in Brazilian patients: The value of next-generation sequencing for genomic classification. J Investig Med. 2017;65(8):1155-8. 10.1136/jim-2017-000566. [DOI] [PubMed] [Google Scholar]
- 11.Pinheiro RF, Chauffaille M. Comparison of I-FISH and G-banding for the detection of chromosomal abnormalities during the evolution of myelodysplastic syndrome. Braz J Med Biol Res. 2009;42(11):42-5. 10.1590/S0100-879X2009001100018. [DOI] [PubMed] [Google Scholar]
- 12.Papaemmanuil E, Gerstung M, Bullinger L, et al. Genomic Classification and Prognosis in Acute Myeloid Leukemia. N Engl J Med. 2016;374(23):2209-21. 10.1056/NEJMoa1516192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cortes JE, Kantarjian H, O’Brien S, et al. Clinical and prognostic significance of trisomy 21 in adult patients with acute myelogenous leukemia and myelodysplastic syndromes. Leukemia. 1995;9(1):115-7. [PubMed] [Google Scholar]
- 14.Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424-47. 10.1182/blood-2016-08-733196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noronha TR, Rohr S, Chauffaille M. Identifying the similarities and differences between single nucleotide polymorphism array (SNPa) analysis and karyotyping in acute myeloid leukemia and myelodysplastic syndromes. Rev Bras Hematol Hemoter. 2015;37(1):48-54. 10.1016/j.bjhh.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ley TJ, Miller C, Ding L, et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368(22):2059-74. 10.1056/NEJMoa1301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noronha T, Chauffaille M. Multiple long runs of homozygosity detected by SNP array: offspring of consanguineous parents and his siblings. Adv Cytol Pathol. 2018;3(3):56-9. [Google Scholar]
- 18.Gaymes TJ, Mohamedali A, Eiliazadeh AL, Darling D, Mufti GJ. FLT3 and JAK2 mutations in acute myeloid leukemia promote interchromosomal homologous recombination and the potential for copy neutral loss of heterozygosity. Cancer Res. 2017;77(7):1697-708. 10.1158/0008-5472.CAN-16-1678. [DOI] [PubMed] [Google Scholar]
- 19.Passamonti F, Rumi E, Pietra D, et al. A prospective study of 338 patients with polycythemia vera: The impact of JAK2 (V617F) allele burden and leukocytosis on fibrotic or leukemic disease transformation and vascular complications. Leukemia. 2010;24(9):1574-9. 10.1038/leu.2010.148. [DOI] [PubMed] [Google Scholar]
- 20.Lee HJ, Daver N, Kantarjian HM, Verstovsek S, Ravandi F. The role of JAK pathway dysregulation in the pathogenesis and treatment of acute myeloid leukemia. Clin Cancer Res. 2013;19(2):327-35. 10.1158/1078-0432.CCR-12-2087. [DOI] [PubMed] [Google Scholar]
- 21.Im AP, Sehgal AR, Carroll MP, et al. DNMT3A and IDH mutations in acute myeloid leukemia and other myeloid malignancies: associations with prognosis and potential treatment strategies. Leukemia. 2014;28(9):1774-83. 10.1038/leu.2014.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tefferi A, Jimma T, Sulai NH, et al. IDH mutations in primary myelofibrosis predict leukemic transformation and shortened survival: clinical evidence for leukemogenic collaboration with JAK2V617F. Leukemia. 2012;26(3):475-80. 10.1038/leu.2011.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Green A, Beer P. Somatic Mutations of IDH1 and IDH2 in the Leukemic Transformation of Myeloproliferative Neoplasms. N Engl J Med. 2010;362(4):369-70. 10.1056/NEJMc0910063. [DOI] [PubMed] [Google Scholar]
- 24.McKenney AS, Lau AN, Somasundara AVH, et al. JAK2/IDH-mutant–driven myeloproliferative neoplasm is sensitive to combined targeted inhibition. J Clin Invest. 2018;128(2):789-804. 10.1172/JCI94516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyer SC, Levine RL. Translational implications of somatic genomics in acute myeloid leukaemia. Lancet Oncol. 2014;15(9):e382-94. 10.1016/S1470-2045(14)70008-7. [DOI] [PubMed] [Google Scholar]