Abstract
We have previously shown that a higher 24-h respiratory quotient (24-h RQ) predicts greater ad-libitum food intake and that nighttime eaters (NE) ingested more calories during an in-patient food intake study and gained more weight over time. We investigated whether 24-h RQ was higher in individuals who exhibited nighttime eating behavior. Healthy nondiabetic Pima Indians (PI; n = 97, 54 male/43 female) and whites (W; n = 32, 22 male/10 female) were admitted to our Clinical Research Unit. After 3 days of a weight maintaining diet, 24-h energy expenditure (24-h EE), 24-h RQ, rates of carbohydrate (CHOX) and lipid oxidation (LIPOX), and spontaneous physical activity (SPA) were measured in a metabolic chamber whereas volunteers were in energy balance and unable to consume excess calories. Individuals subsequently ate ad libitum from a computerized vending machine for 3 days with amount and timing of food intake recorded. Fifty-five individuals (36%; 39 PI, 16 W) were NE, who ate between 11 am and 5 am on at least one of the 3 days on the vending machines. There were no differences in BMI or percentage body fat between NE and non-NE. After adjusting for age, sex, race, fat-free mass, fat mass, and energy balance, NE had a higher 24-h RQ (P = 0.01), higher CHOX (P = 0.009), and lower LIPOX (P = 0.03) and higher 24-h SPA (P = 0.04) compared to non-NE. There were no differences in adjusted 24-h EE or sleep RQ between the groups. Individuals with nighttime eating behavior have higher 24-h RQ, higher CHOX and lower LIPOX, a phenotype associated with increased food intake and weight gain.
INTRODUCTION
It is well established that sleep plays an integral role in energy balance (1). In rodents, total sleep deprivation leads to marked hyperphagia (2) and recent studies also indicate a relationship between sleep restriction and weight gain in humans (3). Although the connection between sleep-related problems and obesity is well documented (4), the mechanisms underlying this relationship are unclear. A number of studies have observed abnormal hormone levels during sleep deprivation, including decreased leptin (1), and elevated ghrelin (4) and orexin (5) levels, which could be related to increased appetite (3). Decreased energy expenditure (EE) might also be a pathway that mediates the effect of altered sleep on weight gain, but this has not been directly tested in humans (3).
Numerous behavioral, social, and environmental factors have been linked to weight gain including, the “toxic” environment (6), stress (7), dieting (8), and binge eating (9). There is evidence of a relationship between the night eating syndrome and obesity (10–12) and night eating behaviors often precede the onset of obesity (13,14). More recently, two studies have demon strated that eating at night predicts weight gain (15,16).
Surprisingly, no differences have been observed between individuals with night eating syndrome and control subjects in sleep onset and offset, as measured by actigraphy among outpatients (17) and by polysomnography among in-patients (18). Despite their comparable circadian sleep rhythms, night eaters did, however, have significantly greater sleep disturbances than control subjects, including more frequent awakenings and lower sleep efficiency (18).
While there does not appear to be an altered circadian rhythm in terms of sleep there does seem to be a delayed circadian rhythm related to food intake. Indeed, the core features of night eating syndrome are evening hyperphagia (25% or more of total daily food intake after the evening meal) and/or nocturnal awakening and ingestion of food (19). Compared to control subjects, circadian caloric intake rhythms of night eaters were delayed by about 1.5 h (20), accompanied by a similar delay in the circadian rhythm of the food regulatory hormones, insulin, and leptin, as well as melatonin, cortisol, prolactin, and thyroid-stimulating hormone.
Although the relationship between altered sleep, altered food intake pattern, and weight gain has been established, the mechanism of weight gain in nighttime eaters (NE) has not been explored. We recently reported an association between nighttime eating behavior during a controlled in-patient study and subsequent weight gain over a 3-year follow-up period (15). As measurements of substrate oxidation (in particular respiratory quotient (RQ) and carbohydrate oxidation) have been associated with food intake (21), we retrospectively examined whether the cohort identified in our previous study as having nighttime eating behaviors differed by these measures compared to controls.
Methods and Procedures
Subjects
Ninety-seven Pima Indians (PI; 54 male/43 female) and 32 whites (W; 22 male/10 female) were recruited between December 1999 and November 2005. Before participation, all volunteers were fully informed of the nature and purpose of the study and written informed consent was obtained. The experimental protocol was approved by the Institutional Review Board of the National Institute of Diabetes and Digestive and Kidney Diseases.
All subjects were found to be free of disease according to physical examination, medical history, and laboratory testing. Upon admission to the metabolic ward, subjects were fed a standard weight maintaining diet (20, 30, and 50% of daily calories provided as protein, fat, and carbohydrate, respectively) for 3 days before testing. Weight maintenance energy needs (WMEN) on the metabolic ward were calculated for each subject based on weight and gender (men: WMEN = 9.5 × weight (kg) + 1,973; women: WMEN = 9.5 × weight (kg) + 1,745) (22). Body composition was measured by dual-energy X-ray absorptiometry (Lunar, Madison, WI). Percentage body fat, fat mass, and fat-free mass were calculated as previously described (23,24). Glucose tolerance was assessed by a 75-g oral glucose tolerance test according to the criteria of the World Health Organization. Only nondiabetic subjects participated in this study.
Respiratory chamber
The method for measuring EE and substrate oxidation in the respiratory chamber was previously described (25). In brief, volunteers entered the chamber at 0745 after having fasted overnight and remained therein for 23 h. Meals were provided at 0800, 1130, 1700, and 2000 (evening snack). Because of the confinement within the chamber, only 80% of WMEN on the metabolic ward were provided in the respiratory chamber, as previously described (25), and were therefore unable to consume excess calories. The rate of EE was measured continuously, calculated for each 15-min interval, and then extrapolated for the 24-h interval (24-h EE). Spontaneous physical activity (SPA) was detected by radar sensors and expressed as the percentage of time over the 24-h period in which activity was detected (SPA). Sleeping metabolic rate was defined as the average EE of all 15-min periods between 2330 and 0500 during which SPA was <1.5%. Carbon dioxide production (V˙ CO2) and oxygen consumption (V˙ O2) were calculated for every 15-min interval and were extrapolated for the 24-h interval. The 24-h RQ was calculated as the ratio of 24-h V˙ CO2 and 24-h V˙ O2. Sleep RQ was calculated in the same manner during the same period as the sleeping metabolic rate (above). The substrate balances were calculated from the 24-h energy intake, 24-h EE, and 24-h RQ. Carbohydrate and fat oxidation rates were calculated from the 24-h RQ, accounting for protein oxidation (calculated from the measurement of 24-h urinary nitrogen excretion). Night SPA and night RQ (separate from sleep as defined above) was computed using all data from 11 pm to 5 am. EE and substrate oxidation were measured in the chamber after ≥3 days on the weight maintenance diet and ≥1 day before the 3-day unrestricted access to the vending machines. Body weights before and after the chamber stay were recorded (change in body weight (mean ± s.d.: −0.82 ± 0.86 kg).
Assessment of food preferences
After admission to the metabolic ward, subjects completed an 80-item Food Preferences Questionnaire (FPQ) containing typical breakfast, lunch, dinner, and snack items as previously described (26). Briefly, foods were categorized as being high (>45% kcal) or low (<20% kcal) in fat and within each of these categories, high in simple sugar (>30% kcal), complex carbohydrate (>30% kcal), or protein (>13% kcal) (27). Individuals were asked to assign each food a hedonic rating using a 9-point Likert scale with the following anchors: 1 = dislike extremely; 5 = neutral; 9 = like extremely; an option to indicate that the food item had never been tasted was also included. Ratings from the FPQ were used to determine the foods made available to the volunteers during the ad libitum food intake portion of the study, described below.
Ad libitum food intake using a computerized vending machine system
After completion of their stay in the respiratory chamber and during the final 3 days on the metabolic ward, subjects were asked to self-select all their food using a computer-operated vending machine system, which has been previously described, validated, and tested for reproducibility (intraclass correlation for energy intake = 0.90, P < 0.0001 (26,28–30)). Briefly, an automated food-selection system is made up of a refrigerated vending machine (model 3007; U-Select-It, Des Moines, IA) that contains 40 trays.
The 40-food items made available to the subjects on each of the 3 days consisted of those foods to which the subject had assigned an intermediate high (between 4 and 8) hedonic rating on the food-preference questionnaire. In addition, a core group of condiments, beverages, breads, and spreads were provided to each subject each day. The same selection was offered each day and accommodated the appropriateness of certain foods for breakfast, lunch, dinner, and evening snacks. Subjects had free access to the vending machines ad libitum for 23½ h/day. The refrigerated machines were housed in a separate eating area and subjects were instructed to eat only in the vending room, to eat whatever they wished whenever they desired. They were instructed to return all uneaten portions and wrappers. Television viewing during food consumption was prohibited.
The time of day and foods eaten were recorded during each visit to the vending machine. Daily energy, protein, fat, and carbohydrate intakes were calculated from the actual weights of food and condiments consumed using the CBORD Professional Diet Analyzer Program (CBORD, Ithaca, NY) with the database modified to reflect the nutrient content of specific food items as indicated by the food manufacturer. Results for daily energy intake are presented as the mean ± s.d. of the 3 days; percent WMEN was calculated as (mean kcal over vend/WMEN) × 100.
Nighttime eating status
The Clinical Research Unit requires that all subjects be in their rooms with the lights out at 11 pm, and subjects are routinely awakened at 5 am to obtain morning weights. Therefore, subjects who had energy intakes recorded between 11 pm and 5 am on any of the 3 days during which they used the vending machines were considered NE and those who never consumed any food after 11 pm were categorized as non-NE.
Statistical analyses
Statistical analyses were performed by using the procedures of the SAS statistical package (version 9.0; SAS Institute, Cary, NC). Unless otherwise specified, all data are expressed as means ± s.d. Race and sex differences in the general, anthropometric, metabolic, and energy intake characteristics were evaluated by Student’s t-test and χ2 analyses for continuous and categorical variables, respectively. Spearman correlation was used to assess the association between variables. Linear regression models adjusted for covariates were used to calculate least square means and 95% confidence intervals for differences in anthropometric and metabolic characteristics, as well as energy intake and weight change by night eating status. The 24-h EE and sleeping metabolic rate were adjusted for age, sex, fat-free mass, fat mass, and race (25,31). 24-h RQ, sleep RQ, and night RQ and 24-h carbohydrate and fat oxidation rates (24-h CHOX and 24-h LIPOX, respectively) were adjusted for the same covariates plus energy balance (32). Mixed models were also used to account for repeated measures in examining SPA and RQ.
RESULTS
Forty-seven volunteers (36%; 13 W, 34 PI) were categorized as NE and 82 (19 W, 63 PI) were categorized as non-NE. Frequency of nighttime eating was similar between W and PI (χ2 = 0.32, P = 0.57), and between men and women (χ2 = 0.74, P = 0.39). The physical, metabolic, and food intake characteristics of the study participants are shown in Table 1. These variables did not differ by race (data not shown) but females had a higher BMI (kg/m2) and percent body fat than males (P = 0.002 and P < 0.0001, respectively). There were no differences between NE and non-NE in age or body weight, percent body fat, fat mass, fat-free mass, or BMI adjusted for age, sex, and race.
Table 1.
Metabolic characteristics and intake data for non-nighttime eaters (non-NE) and nighttime eaters (NE)
| Non-NE (n = 82) (46 male, 36 female) | NE (n = 47) (30 male, 17 female) | P value | |
|---|---|---|---|
| Agea | 34 ± 8 | 32 ± 8 | 0.15 |
| Weight (kg)b | 94 (88, 100) | 95 (88, 103) | 0.74 |
| Body fat (%)b | 39 (37, 41) | 40 (38, 42) | 0.61 |
| BMI (kg/m2)b | 33 (31, 35) | 33 (31, 36) | 0.99 |
| Fat mass (kg)b | 23 (21, 25) | 24 (21, 26) | 0.54 |
| Fat-free mass (kg)b | 35 (32, 38) | 34 (31, 38) | 0.74 |
| 24-h EE (kcal/day)c | 2,363 (2,292, 2,433) | 2,336 (2,248, 2,424) | 0.62 |
| 24-h Radar (%) | 7 (5, 9) | 10 (7, 13) | 0.04 |
| Sleep RQc | 0.82 (0.81, 0.83) | 0.83 (0.82, 0.85) | 0.28 |
| 24-h RQd | 0.84 (0.83, 0.85) | 0.85 (0.84, 0.86) | 0.01 |
| CHOX (kcal/day)d | 973 (912,1,032) | 1,093 (1,019, 1,169) | 0.009 |
| LIPOX (kcal/day)d | 1,045 (973, 1,117) | 921 (832, 1,011) | 0.03 |
| DEI (kcal/day)b | 4,130 (3,861, 4,398) | 4,567 (4,232, 4,901) | 0.03 |
| Energy intake (%WMEN)b | 148 (138, 157) | 164 (152, 176) | 0.03 |
CHOX, carbohydrate oxidation; DEI, daily energy intake (mean of the 3 days); EE, energy expenditure; FFM, fat-free mass; FM, fat mass; RQ, respiratory quotient; LIPOX, lipid oxidation; WMEN, weight maintenance energy needs.
Student’s t-test; expressed as mean ± s.d.
General linear regression models, adjusted for sex, age, and race; expressed as adjusted means and 95% confidence intervals.
24-h EE and sleeping metabolic rate were adjusted for race, age, sex, FFM, and FM.
24-h RQ, 24-h carbohydrate, and 24-h fat oxidation rates were adjusted for race, age, sex, FFM, FM, and energy balance.
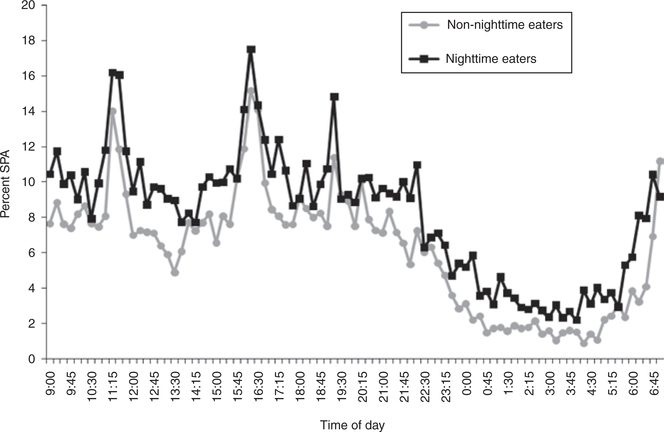
Consistent with our previous results (15), the nighttime eating group had a greater daily energy intake compared to the non-NE group before and after adjustment for age, sex, and race (4,715 mean ± 1,217 s.d. kcal vs. 4,161 ± 1,220; P = 0.01, see Table 1 for adjusted results). NE had a higher adjusted 24-h RQ, adjusted 24-h CHOX, and lower adjusted 24-h LIPOX (see Table 1). There were no differences in NE compared to non-NEs in adjusted sleep RQ or adjusted 24-h EE. After controlling for age, sex, and race, the non-NE had a significantly higher CHO balance compared to NE (104 vs. −10 kcal; P = 0.01). There were no differences in overall energy balance, fat or protein balance between the groups (data not shown). Twenty-four hour SPA was higher in NE vs. non-NE (10 vs. 7%, P = 0.04); night SPA was also consistently but not significantly higher (P = 0.11) (Figure 1). Night RQ adjusted for the same covariates was not different between the groups. Results of nighttime SPA between the groups did not differ in repeated measures analyses (data not shown).
Figure 1.
Percent of spontaneous physical activity (SPA) during the 24-h stay in the respiratory chamber was higher in NE vs. non-NE (P = 0.04). Values are presented from 9 am through 7 am (volunteers are being taken out or put in the chamber between 7 and 9 am, data not shown).
Because the definition of night eating syndrome has also included evening hyperphagia (defined as eating 25% of the day’s calories after the evening meal) we also performed the analysis defining groups by this definition in three different ways (using 5 pm, 6 pm, or 7 pm as the cut-off for their evening meal) and there were no differences in weight gain or substrate oxidation between those with and without evening hyperphagia.
DISCUSSION
We found that individuals with nighttime eating behavior, a group previously found to consume more calories during an in-patient study and gain more weight over a 3-year follow-up period than non-NE (15), have higher adjusted 24-h RQ and carbohydrate oxidation rates and lower lipid oxidation rates. In addition, we found that SPA was higher in NEs both during the day and extending overnight. These measurements were made in a whole room calorimeter on a weight maintaining diet, and preceded the period of ad libitum food intake. These differences in substrate oxidation and SPA indicate that the night eating behavior phenotype may have physiologic underpinnings.
Individuals with relatively higher RQ are known to be at risk for weight gain. Individuals in the highest decile of RQ have a 2.5-fold increased risk of at least a 5 kg weight gain compared to those in the lowest decile (32). In our study of food intake, we previously found that nighttime eating lead to an increase in calorie consumption and was a risk factor for weight gain (15). We have also demonstrated that higher adjusted RQ and in particular higher adjusted carbohydrate oxidation rates are associated with increased food intake (21). Our findings of higher 24-h RQ and 24-h CHOX in NEs are consistent with a phenotype associated with increased food intake and weight gain.
NE also had higher levels of 24-h SPA compared to the non-NE. Although the SPA at night was not significantly different when analyzed as a separate block of time (from 11 pm to 5 am), the increased activity in the NE was also clearly present in the nighttime hours (see Figure 1). This increased activity indicates more restless sleep and could be indicative of nighttime arousals. Although the difference in SPA is small in absolute terms, the increased activity may indicate the presence of disordered sleep and hence nighttime arousals which could lead to nighttime energy intake.
In linking nighttime eating behavior with differences in substrate metabolism and SPA it is unclear whether previous eating at night might lead to or be a consequence of such differences ultimately resulting in long-term weight gain. In the present study, we did not assess whether the nighttime eating behavior had been occurring before admission. Thus, we do not have information on the duration of such behavior if it had previously existed. However, all subjects were on a weight maintenance diet for 3 days before their chamber study and during the metabolic chamber measurements no food was available to them during the night. Thus, the differences observed do not appear to be an immediate consequence of simply overeating or recent nighttime eating, but may be involved in the primary drive to consume excess calories at night. At this point, it is not possible to suggest clinical interventions which would alter substrate oxidation and thus influence nighttime eating behavior. In the case of SPA, this may be a marker of subtle sleep disturbance, so future clinical interventions might focus on establishing bedtime routines to improve sleeping habits or performing sleep studies to look for clinical causes of mildly disordered sleep.
Flatt previously noted that lower carbohydrate balance in mice on 1 day predicted greater food intake on the following day indicating that lower glycogen stores stimulated food intake (33). In NEs, CHO balance was lower (and 24-h CHOX higher). These findings could reflect more rapid depletion of glycogen stores with resultant increased calorie consumption, although it is not clear why this behavior should necessarily occur at night. Another possible explanation for the increase in food intake could involve neurohormonal differences defined by the elevated RQ. RQ is negatively associated with sympathetic nervous system activity (34). Glucocorticoids inhibit sympathetic nervous system activity and are associated with increased RQ. In healthy males, glucocorticoid administration increased food intake whereas simultaneously lowering urinary norepinephrine (35). Thus, the elevated RQ may reflect peripheral nervous system or hormonal differences between these groups. Unfortunately, neither sympathetic nervous system activity or urinary cortisol was measured in this study. As noted above, it must be acknowledged that neither differences in the ability to maintain glycogen stores, sympathetic nervous system activity, nor cortisol explain why the increased food intake would occur at night. The differences in SPA, which persist during the overnight period indicate an altered circadian rhythm. In support of this, mice whose circadian rhythm has been genetically disrupted are more active and overeat on a high fat diet during their usual sleep period (36).
In summary, we found that SPA, adjusted 24-h RQ, 24-h CHOX were higher and adjusted 24-h LIPOX was lower in individuals with nighttime eating, a behavior identified as leading to weight gain (15,16). These measurements preceded the period of ad libitum food intake which characterized these individuals, thus linking physiologic differences with this behavior. In particular, the increased SPA which extends overnight provides preliminary evidence of an altered circadian rhythm in these individuals and provide direction for future research.
ACKNOWLEDGMENTS
The authors thank Mr John Graves and the dietary staff and Ms Carol Massengill and the nursing staff of the NIH Clinical Unit and the staff of the Diabetes Epidemiology and Clinical Research Section, NIDDK. This study was supported by the Intramural Research Program of the NIDDK. Most of all, we thank the volunteers for their participation in the study.
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med 2004;141:846–850. [DOI] [PubMed] [Google Scholar]
- 2.Everson CA, Bergmann BM, Rechtschaffen A. Sleep deprivation in the rat: III. Total sleep deprivation. Sleep 1989;12:13–21. [DOI] [PubMed] [Google Scholar]
- 3.Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev 2007;11: 163–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Cauter E, Spiegel K, Tasali E, Leproult R. Metabolic consequences of sleep and sleep loss. Sleep Med 2008;9 Suppl 1:S23–S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sakurai T Orexin: a link between energy homeostasis and adaptive behaviour. Curr Opin Clin Nutr Metab Care 2003;6:353–360. [DOI] [PubMed] [Google Scholar]
- 6.Devlin MJ, Yanovski SZ, Wilson GT. Obesity: what mental health professionals need to know. Am J Psychiatry 2000;157:854–866. [DOI] [PubMed] [Google Scholar]
- 7.Roberts C, Troop N, Connan F, Treasure J, Campbell IC. The effects of stress on body weight: biological and psychological predictors of change in BMI. Obesity (Silver Spring) 2007;15:3045–3055. [DOI] [PubMed] [Google Scholar]
- 8.Stice E, Cameron RP, Killen JD, Hayward C, Taylor CB. Naturalistic weight-reduction efforts prospectively predict growth in relative weight and onset of obesity among female adolescents. J Consult Clin Psychol 1999;67:967–974. [DOI] [PubMed] [Google Scholar]
- 9.Fairburn CG, Cooper Z, Doll HA, Norman P, O’Connor M. The natural course of bulimia nervosa and binge eating disorder in young women. Arch Gen Psychiatry 2000;57:659–665. [DOI] [PubMed] [Google Scholar]
- 10.Aronoff NJ, Geliebter A, Zammit G. Gender and body mass index as related to the night-eating syndrome in obese outpatients. J Am Diet Assoc 2001;101:102–104. [DOI] [PubMed] [Google Scholar]
- 11.Lundgren JD, Allison KC, Crow S et al. Prevalence of the night eating syndrome in a psychiatric population. Am J Psychiatry 2006;163: 156–158. [DOI] [PubMed] [Google Scholar]
- 12.Colles SL, Dixon JB, O’Brien PE. Night eating syndrome and nocturnal snacking: association with obesity, binge eating and psychological distress. Int J Obes (Lond) 2007;31:1722–1730. [DOI] [PubMed] [Google Scholar]
- 13.Marshall HM, Allison KC, O’Reardon JP, Birketvedt G, Stunkard AJ. Night eating syndrome among nonobese persons. Int J Eat Disord 2004;35:217–222. [DOI] [PubMed] [Google Scholar]
- 14.Spaggiari MC, Granella F, Parrino L et al. Nocturnal eating syndrome in adults. Sleep 1994;17:339–344. [DOI] [PubMed] [Google Scholar]
- 15.Gluck ME, Venti CA, Salbe AD, Krakoff J. Nighttime eating: commonly observed and related to weight gain in an inpatient food intake study. Am J Clin Nutr 2008;88:900–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andersen GS, Stunkard AJ, Sørensen TI, Petersen L, Heitmann BL. Night eating and weight change in middle-aged men and women. Int J Obes Relat Metab Disord 2004;28:1338–1343. [DOI] [PubMed] [Google Scholar]
- 17.O’Reardon JP, Ringel BL, Dinges DF et al. Circadian eating and sleeping patterns in the night eating syndrome. Obes Res 2004;12:1789–1796. [DOI] [PubMed] [Google Scholar]
- 18.Rogers NL, Dinges DF, Allison KC et al. Assessment of sleep in women with night eating syndrome. Sleep 2006;29:814–819. [DOI] [PubMed] [Google Scholar]
- 19.Allison KC, Lundgren JD, O’Reardon JP et al. Proposed diagnostic criteria for night eating syndrome. Int J Eat Disord 2010;43:241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goel N, Stunkard AJ, Rogers NL et al. Circadian rhythm profiles in women with night eating syndrome. J Biol Rhythms 2009;24:85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pannacciulli N, Salbe AD, Ortega E et al. The 24-h carbohydrate oxidation rate in a human respiratory chamber predicts ad libitum food intake. Am J Clin Nutr 2007;86:625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferraro R, Boyce VL, Swinburn B, De Gregorio M, Ravussin E. Energy cost of physical activity on a metabolic ward in relationship to obesity. Am J Clin Nutr 1991;53:1368–1371. [DOI] [PubMed] [Google Scholar]
- 23.Mazess RB, Barden HS, Bisek JP, Hanson J. Dual-energy x-ray absorptiometry for total-body and regional bone-mineral and soft-tissue composition. Am J Clin Nutr 1990;51:1106–1112. [DOI] [PubMed] [Google Scholar]
- 24.Tataranni PA, Ravussin E. Use of dual-energy X-ray absorptiometry in obese individuals. Am J Clin Nutr 1995;62:730–734. [DOI] [PubMed] [Google Scholar]
- 25.Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. J Clin Invest 1986;78:1568–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salbe AD, Tschöp MH, DelParigi A, Venti CA, Tataranni PA. Negative relationship between fasting plasma ghrelin concentrations and ad libitum food intake. J Clin Endocrinol Metab 2004;89:2951–2956. [DOI] [PubMed] [Google Scholar]
- 27.Geiselman PJ, Anderson AM, Dowdy ML et al. Reliability and validity of a macronutrient self-selection paradigm and a food preference questionnaire. Physiol Behav 1998;63:919–928. [DOI] [PubMed] [Google Scholar]
- 28.Rising R, Alger S, Boyce V et al. Food intake measured by an automated food-selection system: relationship to energy expenditure. Am J Clin Nutr 1992;55:343–349. [DOI] [PubMed] [Google Scholar]
- 29.Salbe AD, Venti CA, Franks P, Krakoff J, Tataranni, PA. Reproducibility of ad libitum energy intake in humans. Obes Res 2006;13:A174–A175. [Google Scholar]
- 30.Venti CA, Votruba SB, Franks PW, Krakoff J, Salbe AD. Reproducibility of ad libitum energy intake with the use of a computerized vending machine system. Am J Clin Nutr 2010;91:343–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weyer C, Snitker S, Rising R, Bogardus C, Ravussin E. Determinants of energy expenditure and fuel utilization in man: effects of body composition, age, sex, ethnicity and glucose tolerance in 916 subjects. Int J Obes Relat Metab Disord 1999;23:715–722. [DOI] [PubMed] [Google Scholar]
- 32.Zurlo F, Lillioja S, Esposito-Del Puente A et al. Low ratio of fat to carbohydrate oxidation as predictor of weight gain: study of 24-h RQ. Am J Physiol 1990;259:E650–E657. [DOI] [PubMed] [Google Scholar]
- 33.Flatt JP. The difference in the storage capacities for carbohydrate and for fat, and its implications in the regulation of body weight. Ann N Y Acad Sci 1987;499:104–123. [DOI] [PubMed] [Google Scholar]
- 34.Snitker S, Tataranni PA, Ravussin E. Respiratory quotient is inversely associated with muscle sympathetic nerve activity. J Clin Endocrinol Metab 1998;83:3977–3979. [DOI] [PubMed] [Google Scholar]
- 35.Tataranni PA, Larson DE, Snitker S et al. Effects of glucocorticoids on energy metabolism and food intake in humans. Am J Physiol 1996;271:E317–E325. [DOI] [PubMed] [Google Scholar]
- 36.Yang S, Liu A, Weidenhammer A et al. The role of mPer2 clock gene in glucocorticoid and feeding rhythms. Endocrinology 2009;150:2153–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]