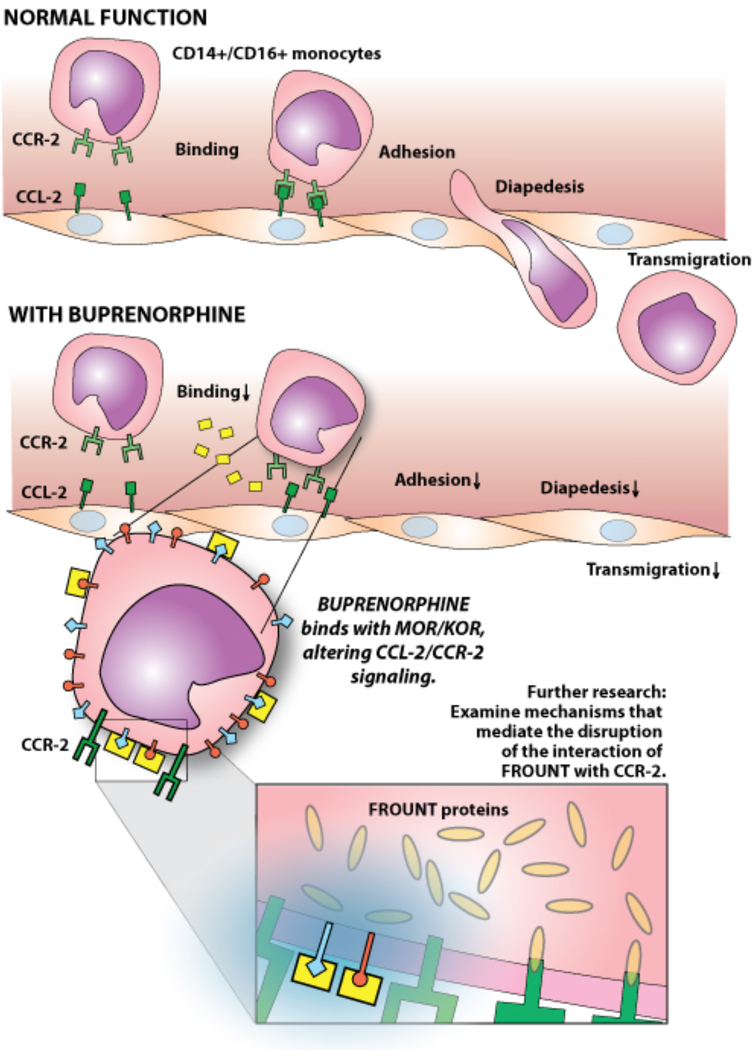
Opioid drug abuse is an important and increasing problem, and appears to contribute significantly to HIV pathogenesis, including HIV associated CNS disorders. In the paper appearing in this issue from Dr. Joan Berman’s laboratory, “Buprenorphine decreases CCL2-mediated migration of CD14+CD16+ monocytes,” the authors present novel findings demonstrating the ability of Buprenorphine, a drug used therapeutically to treat addiction, to prevent steps involved in transmigration of CD14+/CD16+ monocytes. Such monocytes, although normally relatively infrequent and having a more mature phenotype, have been implicated in the pathogenesis of HIV associated neurological disorders, as well as several other inflammatory conditions including atherosclerosis/cardiovascular disease, rheumatoid arthritis, Crohn’s disease, metabolic syndrome. Here the authors demonstrate the ability of buprenorphine to reduce binding, transmigration, and chemotaxis of CD16+ monocytes in response to CCL-2, via a process that appears to reduce binding of monocytes to endothelial cell ICAM-I, as well as reduced association of the CCL-2 receptor, CCR2, with the protein FROUNT. As pointed out by the authors, these results support the notion that buprenorphine may have an additional benefit beyond addiction control, namely the treatment of disorders where monocyte/macrophage invasion and inflammation may be involved in diseases pathogenesis. This paper also opens additional avenues for investigation regarding the mechanism whereby buprenorphine disrupts or prevents the interaction of FROUNT with CCR2. This could result from decreased recycling of CCR2 receptors, previously noted as a consequence of buprenorphine treatment, although the rapidity of the effects in vitro would seem to argue this mechanism.1 Alternatively, buprenorphine may induce destabilization of FROUNT, and/or CCR2, and thereby decrease FROUNT:CCR2 interactions. These interactions normally result in CCR2/FROUNTclustering at the leading cell surface and likely represent an important step in the migration process. The mechanism whereby buprenorphine negatively impacts the association of CCL2 and FROUNT is unknown and may further affect the process of oligomerization and clustering between FROUNT:CCL2 complexes.2 Since decreased CCR2/FROUNT interaction has been observed in response to buprenorphine treatment, it is likely that downstream PI(3)K activity, rapidly induced by CCR2/FROUNT interaction, and contributing to transmigration, would be predicted to be reduced secondarily.2
Interestingly, in view of buprenorphine’s unique dual activities as a kappa opioid receptor (KOR) antagonist and Mu opioid receptor (MOR) partial antagonist activities, studies have been performed investigating its potential as an anti depressant3,4. Animal models and successful clinical trials have elucidated that low doses of buprenorphine may be a novel medication for the treatment of major depressive disorder (MDD). The use of buprenorphine as an antidepressant is promising, however, the mechanism responsible for such an effect remains elusive. Recent studies have identified that HIV positive individuals show a higher prevalence of MDD diagnosis as compared to uninfected individuals. Psychiatric comorbidities such as MDD are also highly prevalent in opioid users. Thus, HIV positive opioid users are highly susceptible to developing MDD, when compared to uninfected individuals and buprenorphine may have benefit.
There is an association between inflammation including elevated levels of certain cytokines and chemokines with MDD.5,6 Examination of postmortem prefrontal cortex of individuals with MDD demonstrated an increase in IL-6, TNFα, and IL-1β. Along with elevated proinflammatory cytokines, CCL2 is also increased in patients suffering MDD.7 Increased tryptophan catabolism as determined by plasma kynurenine: tryptophan ratio has been found associated with MDD.8 Elevated proinflammatory cytokines, particularly TNFα and IFNγ, increase tryptophan catabolism resulting in increased plasma kynurenine and decreased tryptophan concentrations in plasma. It has been postulated that a decrease in the availability of tryptophan due to excessive breakdown, could be responsible for the development of MDD via the consequent decrease in serotonin production from tryptophan via the methoxy-indole pathway. The mechanism of action whereby low dose buprenorphine may relieve MDD is unknown, however CCL2 levels are elevated during MDD and could contribute to depressive disorders. Dr. Berman’s group has previously described that buprenorphine decreases CCL2 expression. Buprenorphine has also demonstrated to reduce proinflammatory cytokines such as IFNγ, and TNFα, which have been previously linked to the development of MDD. Thus, buprenorphine may function via CCL2, and exert its antidepressant action via its effect in modulating tryptophan catabolism.
Opioids have immune modulatory effects, and immunosuppressive effects have been established. In evaluating the potential of buprenorphine as a treatment for HIV associated neurocognitive disorders in the absence of addiction, the potential effects on immunity should be considered. In support of buprenorphine use, there do not appear to be substantial effects on macrophage function, however, differential effects on M1 and M2 macrophages have been noted.9 As a partial MOR antagonist, buprenorphine may have reduced immune modulatory activity relative to morphine. It is unclear if the effects of buprenorphine occur via MOR or KOR, and more selective MOR or KOR antagonists might provided even more valuable therapeutics for the treatment of diseases involving monocyte/macrophage transmigration. Caution should be taken in the extrapolation of these findings to the potential therapeutic use of buprenorphine, beyond its use an opioid substitute, particularly in non-opioid users. Buprenorphine has been demonstrated to induce both tolerance and hyperalgesia that may be an important concern during longer-term administration at higher doses.10 The studies from Dr. Berman’s laboratory presented in this issue provide major advances in establishing the potential of buprenorphine in modulating monocyte transmigration. Further studies, potentially with analogues, could promote additional advances toward more effective and selective therapeutic approaches for neurocognitive impairment in HIV infection as well as for other disorders involving monocyte activation and trans-endothelial migration.
References
- 1.Carvallo L, Lopez L, Che FY, et al. Buprenorphine decreases the CCL2-mediated chemotactic response of monocytes. J Immunol. 2015;194(7):3246–3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Terashima Y, Onai N, Murai M, et al. Pivotal function for cytoplasmic protein FROUNT in CCR2-mediated monocyte chemotaxis. Nat Immunol. 2005;6(8):827–835. [DOI] [PubMed] [Google Scholar]
- 3.Emrich HM, Vogt P, Herz A. Possible antidepressive effects of opioids: action of buprenorphine. Ann N Y Acad Sci. 1982;398:108–112. [DOI] [PubMed] [Google Scholar]
- 4.Vignau J Preliminary assessment of a 10-day rapid detoxification programme using high dosage buprenorphine. Eur Addict Res. 1998;4 Suppl 1:29–31. [DOI] [PubMed] [Google Scholar]
- 5.Mechawar N, Savitz J. Neuropathology of mood disorders: do we see the stigmata of inflammation? Transl Psychiatry. 2016;6(11):e946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Setiawan E, Wilson AA, Mizrahi R, et al. Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry. 2015;72(3):268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahajan GJ, Vallender EJ, Garrett MR, et al. Altered neuro-inflammatory gene expression in hippocampus in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2018;82:177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Umehara H, Numata S, Watanabe SY, et al. Altered KYN/TRP, Gln/Glu, and Met/methionine sulfoxide ratios in the blood plasma of medication-free patients with major depressive disorder. Sci Rep. 2017;7(1):4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun J, Guo W, Du X. Buprenorphine differentially affects M1- and M2-polarized macrophages from human umbilical cord blood. Eur Cytokine Netw. 2017;28(2):85–92. [DOI] [PubMed] [Google Scholar]
- 10.Athanasos P, Ling W, Bochner F, White JM, Somogyi AA. Buprenorphine Maintenance Subjects Are Hyperalgesic and Have No Antinociceptive Response to a Very High Morphine Dose. Pain Med. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]