Introduction
Stem cell tourism has become popular for the treatment of neurological disorders, but the safety of a wide variety of the associated stem cell therapies remains a concern. Few reports illustrate the potential complications of intrathecal a neural stem cell injection, including cauda equina syndrome due to glioproliferative lesions of the spinal cord.1, 2, 3, 4 In the current paper, we report a case of growth of glioneuronal tissue on lumbosacral nerve roots causing polyradiculopathies following serial intrathecal stem cell injections from an international stem cell clinic with the intention to “cure” Parkinson's disease. This case underscores the serious concern regarding potential complications of stem cell transplantation at these unregulated international stem cell clinics.
Case Report
A 63‐year‐old woman, who developed left upper extremity tremors, bradykinesia, and shuffling gait at 56 years of age, was diagnosed with Parkinson's disease and started on Sinemet.
Eight years later, the patient traveled to Moscow, Russia and received intrathecal stem cell transplantation as an experimental treatment modality to “cure” her Parkinson's disease. She received three doses beginning in January 2014, with repeated doses January 2016 and January 2017. The stem cells were reported to be fetal progenitor cells from a 12‐week old embryo, and were injected into the intrathecal space at the level of L3‐L4. The premise of stem cells injection was that these cells would secrete cytokines, growth factors, and hormones essential for neuron repair.5 The patient tolerated the first two doses and reported a subjective improvement; however, at two to three months following the third intrathecal injection, she developed a moderate intensity low backache, bilateral lower extremities radicular pain, progressive weakness in proximal lower extremities, and urinary incontinence.
An MRI of the Lumbosacral spine showed prominent clumping of the cauda equina and diffused abnormal thickening with enhancement of lumbosacral nerve roots (Fig. 1 A‐D). Gadolinium‐enhanced MRI of the brain and the cervical and thoracic spine were unremarkable. Electromyography and nerve conduction studies showed a mild subacute L5 and S1 radiculopathy. Cerebrospinal fluid (CSF) studies revealed a lymphocytic pleocytosis, Glucose 20 (45–80 mg/dL), protein 127 (12–45 mg/dL), and negative cytology and flow cytometry. CSF infectious and paraneoplastic panel was negative. The patient was immunocompetent and not on any immunosuppressive agents. Subsequently, the patient had L2‐L3 laminectomy and nerve sheath biopsy performed for symptomatic relief and diagnostic purpose. Intraoperatively, abnormal tubular shaped tissue encasing nerve rootlets were identified and biopsied (Fig. 2A‐B). Circumferential dissection of the tissue was performed, sparing the normal appearing nerve rootlets. Neuropathological evaluation of biopsied material was performed at Allegheny General Hospital Pittsburgh, PA and Brigham and Women's Hospital Boston, MA. This revealed mature glioneuronal tissue with focal chronic inflammation (Fig. 2C‐D). Few glial cells showed nuclear atypia, with the majority within the spectrum of reactive changes. Immunohistochemistry was positive for glial and neuronal cell markers (OLIG2, GFAP, NeuN, and NF). Glial cells were MIB‐1/P53, H3(K27 M), and IDH1(R132H) negative, arguing against a neoplastic growth. The patient is being treated conservatively, and has been advised against receiving any further stem cell injections. Her backache, lower extremity radicular symptoms, and gait imbalance improved gradually. Two subsequent MRIs of the Lumbosacral spine, performed at six monthly intervals, did not show any progression of her lesions. We plan to continue to monitor the lesions with follow‐up MRI and PET scans if needed.
Figure 1.
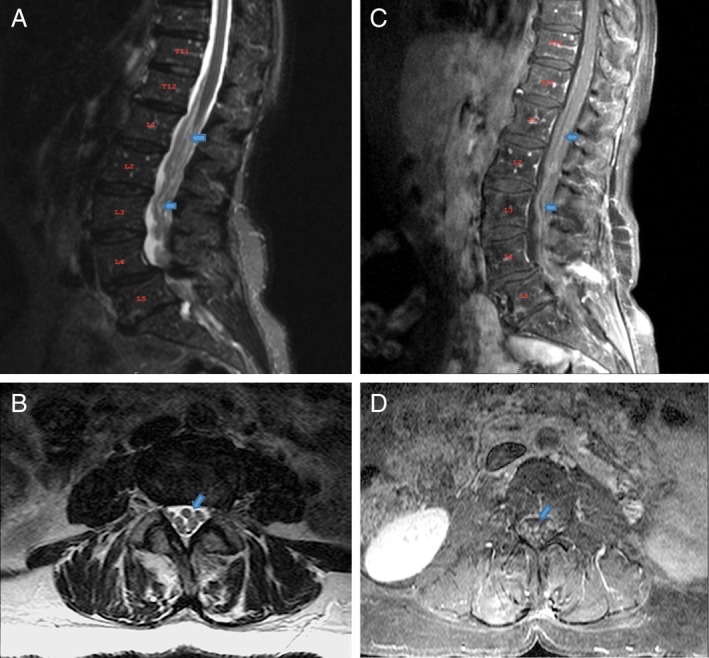
MRI of the Lumbosacral spine. A, B T2 sagittal and axial view with diffuse abnormal thickening of nerve roots; C, D T1 post‐contrast sagittal and axial view with enhancement of nerve roots.
Figure 2.
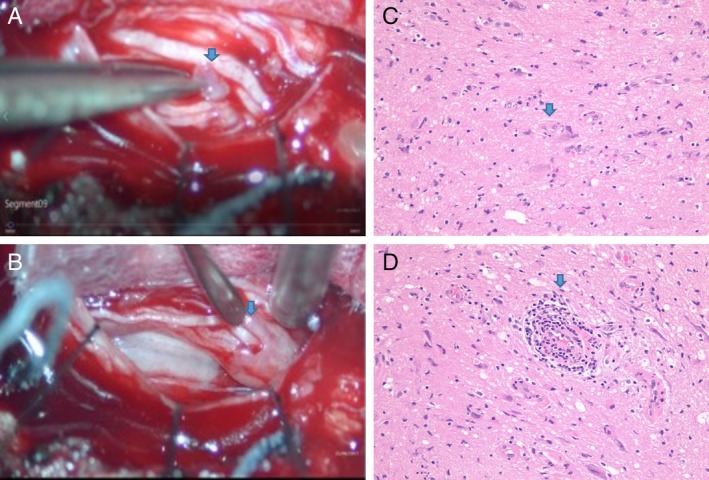
A Abnormal tubular shaped tissue encasing nerve rootlets; B tissue dissected open revealing nerve rootlets; C, D mature glioneuronal tissue and perivascular chronic inflammation.
Discussion
Many for‐profit stem cell clinics outside of the United States are beyond the FDA purview, and it is unclear if the treating physicians at these clinics are counseling patients about the potential risks.6 Given the variety of stem cell products, guidelines are made to ensure the integrity, function, and safety of cells destined for use in patients. Even minimal manipulation could lead to altered cell function or malignancy. ISSCR (International Society for Stem Cell Research) condemns the administration of unproven stem cell‐based interventions, and as a matter of professional ethics, warns scientists and clinicians not to participate in such activities.7 Our case study illustrates a serious complication of intrathecal fetal stem cell therapy, whereby these cells resulted in infiltrative growth of glioneuronal tissue causing lumbosacral polyradiculopathies.
Stem cell therapies are primarily advertised online, targeting a wide variety of neurological diseases. Patients may seek out stem cell tourism for many reasons, including desperation in the face of significant disability, lack of known cures despite best medical treatment, and dissatisfaction of the current medical options. In the setting of a debilitating and non‐curable illness, patients are especially vulnerable to “success” stories presented on the web.8 Physicians should address therapeutic hope and distress associated with progressive, incurable neurologic disease by engaging patients about hopes and goals for the future and providing alternatives outside of stem cell therapy.9
The premature commercialization or inaccurate marketing of unproven stem cell therapy not only puts patients at risk, but also represents a serious threat to the stem cell research community. Regulation of stem cell therapy and enforcement of pre‐existing legislation varies from country to country and has allowed stem cell tourism to outpace legislation. Government authorities and professional societies are strongly encouraged to establish and strictly enforce regulations governing the use of such therapies commercially. It has been proposed that, to counter the attraction of these unregulated stem cell clinics, policy changes to current FDA regulation should be considered, allowing patients easier access to investigative interventions.7, 10 Unfortunately, access to high‐quality trials of stem cell therapies will likely remain limited in the near future.
Author Roles
1. Research Project: A. Conception, B. Organization, C. Execution; 2. Statistical Analysis: A. Design, B. Execution, C. Review and Critique; 3. Manuscript Preparation: A. Writing the First Draft, B. Review and Critique.
R.R.: 1A, 1B, 1C, 3A, 3B
S.R.: 1B, 1C, 3B
P.P.: 1B, 3A
M.A.: 1B
M.F.: 3A
A.S.: 3B
S.B.: 1A, 3B
T.S.: 1B, 1C, 3A, 3B
Disclosures
Ethical Compliance Statement: Full consent was obtained from the patient for the case report publication. The authors have read the journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflict of Interest: No specific funding was received for this work. We have no conflicts of interest relevant to this work.
Financial disclosure for the previous 12 months: The authors declare that there are no additional disclosures to report.
Acknowledgments
We thank Dr. Cunfeng Pu and Dr. Kymberly Gyure (Department of Pathology, Allegheny General Hospital, Pittsburgh PA) for assistance with neuropathological interpretations.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Berkowitz AL, Miller MB, Mir SA, et al. Glioproliferative Lesion of the Spinal Cord as a Complication of "Stem‐Cell Tourism." N Engl J Med 2016;75(2):196–198. [DOI] [PubMed] [Google Scholar]
- 2. Hurst RW, Bosch EP, Morris JM, Dyck PJ, Reeves RK. Inflammatory hypertrophic cauda equina following intrathecal neural stem cell injection. Muscle Nerve 2013;48(5):831–835. [DOI] [PubMed] [Google Scholar]
- 3. Lee BS, Achey RL, Yeaney GA, et al. Stem cell injection‐induced glioneuronal lesion of the cauda equina. Neurology 2018;90(13):613–615. [DOI] [PubMed] [Google Scholar]
- 4. Bauer G, Elsallab M, Abou‐El‐Enein M. Concise Review: A comprehensive analysis of reported adverse events in patients receiving unproven stem cell‐based interventions. Stem Cells Transl Med 2018. Sep;7(9):676–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Longevity Medicine LLC. Neurological Conditions. http://www.stemcells4life.net/what‐conditions‐we‐treat/neurological‐conditions/. Accessed October 10, 2018.
- 6. Connolly R, O'Brien T, Flaherty G. Stem cell tourism–a web‐based analysis of clinical services available to international travellers. Travel Med Infect Dis 2014;12(6 Pt B):695–701. [DOI] [PubMed] [Google Scholar]
- 7. International Society for Stem Cell Research . Guidelines for stem cell research and clinical translation http://www.isscr.org/membership/policy/2016‐guidelines. Accessed January 28, 2019.
- 8. Bowman RL, Busque L, Levine RL. Clonal hematopoiesis and evolution to hematopoietic malignancies. Cell Stem Cell 2018; 22(2):157–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hyun I. Bioethics and the Future of Stem Cell Research. Cambridge: Cambridge University press; 2013. [Google Scholar]
- 10. Matthews KR, Iltis AS. Unproven stem cell‐based interventions and achieving a compromise policy among the multiple stakeholders. BMC Med Ethics 2015;16(1):75. [DOI] [PMC free article] [PubMed] [Google Scholar]


