Abstract
Background
Parkinson's disease (PD) management comprises of drug treatments, surgery, and physical activity/occupational therapies to relieve PD's symptoms. The aim of this study is twofold; first, to appraise recent economic evaluation studies on PD management in order to update the existing knowledge; and second, to facilitate decision making on PD management by assessing the cost‐effectiveness of all types of PD interventions.
Methods
A systematic search for studies published between 2010 and 2018 was conducted. The inclusion and exclusion of the articles were based on criteria relevant to population, intervention, comparison, outcomes, and study design (PICO). The reporting quality of the articles was assessed according to Consolidated Health Economic Evaluation Reporting Standards.
Results
Twenty‐eight articles were included, 10 of which were evaluations of drug treatments, 10 deep brain stimulation (DBS), and eight physical/occupational therapies. Among early‐stage treatments, Ti Ji dominated all physical activity interventions; however, its cost‐effectiveness should be further explored in relation to its duration, intensity, and frequency. Multidisciplinary interventions of joint medical and nonmedical therapies provided slightly better health outcomes for the same costs. In advanced PD patients, adjunct drug treatments could become more cost‐effective if introduced during early PD and, although DBS was more cost‐effective than adjunct drug therapies, the results were time‐bound.
Conclusions
Conditionally, certain PD interventions are cost‐effective. However, PD progression differs in each patient; thus, the cost‐effectiveness of individually tailored combinations of interventions that could provide more time in less severe disease states and improve patients’ and caregivers’ quality of life, should be further explored.
Keywords: cost‐effectiveness, economic evaluations, Parkinson's disease, Parkinson's disease management
Background
Parkinson's disease (PD) is the second most common neurodegenerative disease, after Alzheimer's, which causes severe morbidity and mortality globally1 and leads to motor fluctuations and psychological and behavioral disorders. PD prevalence is estimated to affect nearly one to two per 1000 people and, due to intense demographic transition towards aging societies and extended life expectancy, is expected to double in the next 20 years.2 The number of people with PD in Europe will increase by 33% by 2030, and in the USA, 1.2 million people will be affected by 2040.3
PD has major economic impacts on the patients, their families, and societies. Direct (medical, nonmedical) and indirect (loss of productivity) PD costs in Europe reached €7.7 billion in 2010.4 In the USA, each PD patient bears $12,800 more medical expenses, expressed as healthcare visits and hospital inpatient days, and $10,000 more nonmedical costs, including absenteeism/presenteeism of patients and informal caregivers than those with the same characteristics without PD.3 The costs of informal caregivers comprise an important subgroup of the total costs and are often greater than direct costs.5, 6 Furthermore, since the vast majority of PD patients are in need of informal care, these costs pose a significant burden.7
The causes of PD remain unknown; however, a combination of genetic and environmental factors possibly plays an important role in its genesis and progress.8 There is no cure for PD and existing treatments mainly relieve symptoms. Also, there is no early diagnostic test for PD, so its diagnosis often occurs late stage, after the symptoms have appeared.9 The most common and effective medication for PD symptomatology is levodopa.10 In its simplest form, oral levodopa has prolonged effect on increasing dopamine levels in the brain and restores movement functions.10 Apart from levodopa, dopamine agonists (DA) or MAO‐B inhibitors (rasagiline and selegiline) can be used during the initial stages of PD.11
Although oral levodopa conduces to long‐lasting, adverse motor and psychological consequences, it could have short‐term efficacy depending on its gastric absorption.12 Thus, oral levodopa can be either replaced by continuous infusion therapies like subcutaneous apomorphine and intraduodenal levodopa/carbidopa (duodopa) or combined with MAO‐B inhibitors and entacapone that can prolong levodopa's short half‐life and boost its effectiveness.10, 12, 13, 14 DA show similar negative side effects only when they are used as an adjunct to levodopa.15, 16
As alternatives to drug therapies, surgical procedures (i.e., deep brain stimulation [DBS]), stimulate the brain to decrease motor fluctuations in advanced PD patients.17 DBS is based on the implantation of a medical device that induces electrical pulses to the subthalamic nucleus (STN) or the globus pallidus internus (GPi) brain sites. The pulses restore activity in neurons and improve patients’ mobility and functionality while reducing medication use.17, 18 Besides the aforementioned interventions, there is also a positive effect of physical activity, occupational therapy and physiotherapy, complementary to drug treatment, on improving the motor and cognitive functions of PD patients in early stages.12, 19, 20, 21, 22 Researchers are interested in interventions that can offer relief of the symptoms and lead to sustainable and long‐lasting health outcomes.2
Every intervention requires the utilization of scarce resources; thus, economic evaluation is an expedient tool that facilitates decision‐making in regards to efficient use of resources.23 The bulk of economic evaluation studies in healthcare evaluate ways of allocating available resources in order to maximize population health.23 There are four main categories of economic evaluations: (1) cost‐effectiveness analysis (CEA) measures outcomes in naturals units and compares the efficiency of alternative interventions targeting the same objective; (2) cost‐utility analysis (CUA) measures outcomes in utility units (QALYs or DALYs), and since it compares the intervention to other interventions, it can be used for optimal spending decisions; (3) cost‐benefit analysis (CBA) presents both outcomes and costs in monetary units, and informs about the number of societal resources needed to achieve a goal; and (4) cost‐minimization analysis (CMA) assumes equal outcomes and compares only the costs.23
Current needs to ascertain PD interventions that provide the most efficient resource utilization (i.e., best outcomes for the occurring costs) imply constant reviews of the relevant economic evaluation studies. The systematic literature review is an effective method to identify the commonalities among the existing studies, highlight the knowledge gaps, and provide recommendations for future research. There are several literature reviews of economic evaluations on PD10, 24, 25, 26, 27, 28 that do not include studies after 2010. The exception is the study by Becerra et al., which does not include all types of interventions simultaneously.28 Thus, this paper has a twofold aim; (1) to update the existing knowledge by appraising economic evaluations of PD interventions, and (2) promote decision making on PD management by assessing the cost‐effectiveness of all types of PD interventions.
Methodology
A systematic literature review was conducted for answering the research question in accordance with the PRISMA guidelines.29 Moreover, the Campbell and Cochrane Economics Methods Group guidelines30 were followed for incorporating economic evidence, including search criteria, data extraction, synthesis, and critical analysis.
Search Strategy
A systematic search was performed to identify relevant articles published in both health economics and biomedical databases from 01.01.2010 till 31.12.2018. The databases searched were Medline (PubMed), Embase and ECONbase, EconLit, and Cumulative Index to Nursing and Allied Health (CINAHL) through Embase. Moreover, one additional database, the Centre for Reviews and Dissemination database was explored. We also searched the reference lists of the selected studies. A detailed search strategy including keywords is presented in the Supporting Information (Supporting Appendix 1) section.
Inclusion and Exclusion Criteria
After each search in the databases, the initial hits were exported into EndNote and duplicates were removed. The exclusion and inclusion of each study were based on the PICOS criteria, which refer to the population, intervention, comparison, outcomes, and study design of an article (Supporting Table 1). The inclusion criteria were referential to all types of economic evaluations (CEA, CUA, CMA, CBA) of any intervention for PD management, including drug therapies, with no limitation regarding the comparator involving PD patients of any severity level. The retrieved studies were assessed in two phases; first, titles and abstracts were checked, according to PICOS, and second, the full text of the remaining articles was screened for final selection.
Data Extraction
The data from the selected studies were extracted regarding two dimensions, the study results (empirical evidence) and the methods (methodology). The reporting quality of the studies was assessed by using the CHEERS checklist,31 which consists of 24 items divided into six main categories according to the articles’ structure (title and abstract, introduction, methods, results, discussion, and other). For computing a final score, we assigned one point (1) if the item was complete and zero points (0) if the requirement was not fulfilled. In cases where the requirements were not applicable to the subject or structure of the study, we assigned not applicable (NA). The maximum score for complete reporting of all items was 24 points. Finally, for ease of comparison, the extracted results were converted to US dollars in price‐year 2016,32 and the local currency values are presented in parenthesis as exhibited in the studies. The cost is converted by using the country‐specific gross domestic product (GDP) deflator indices to account for inflation. After that, the price‐year adjusted cost is converted to US dollars using purchasing power parity rates.32
Results
Twenty‐eight studies were identified in the review. The detailed selection process of the studies is presented in Fig. 1. The main characteristics of the selected studies are summarized in Supporting Table 2. The categories and sources of costs, as well as the measures and sources of QALYs (as these were reported in the selected studies), are presented in Supporting Table 3. For ease of presentation and discussion, the subsequent interventions are divided into three main categories: (1) drug treatments; (2) deep brain stimulation (DBS); and (3) other therapies focusing, mostly, on physical activity and occupational therapy. The cost‐effectiveness results in the selected studies were presented either by incremental cost‐effectiveness ratios (ICERs; i.e., the difference in costs between two alternatives divided by the difference in outcomes of the two alternatives), or by reporting costs and outcomes in the intervention and comparator. The interventions are generally implemented according to the severity level of PD. PD severity is evaluated according to the Hoehn and Yahr (H&Y) staging scale that, by categorizing motor and balance/gait dysfunctions into five stages, combines both disability and impairment.33 Stage 1 represents the initial, least severe state and it is assumed that patients, starting from stage 1, progress to stage 5 as their status deteriorates.33
Figure 1.
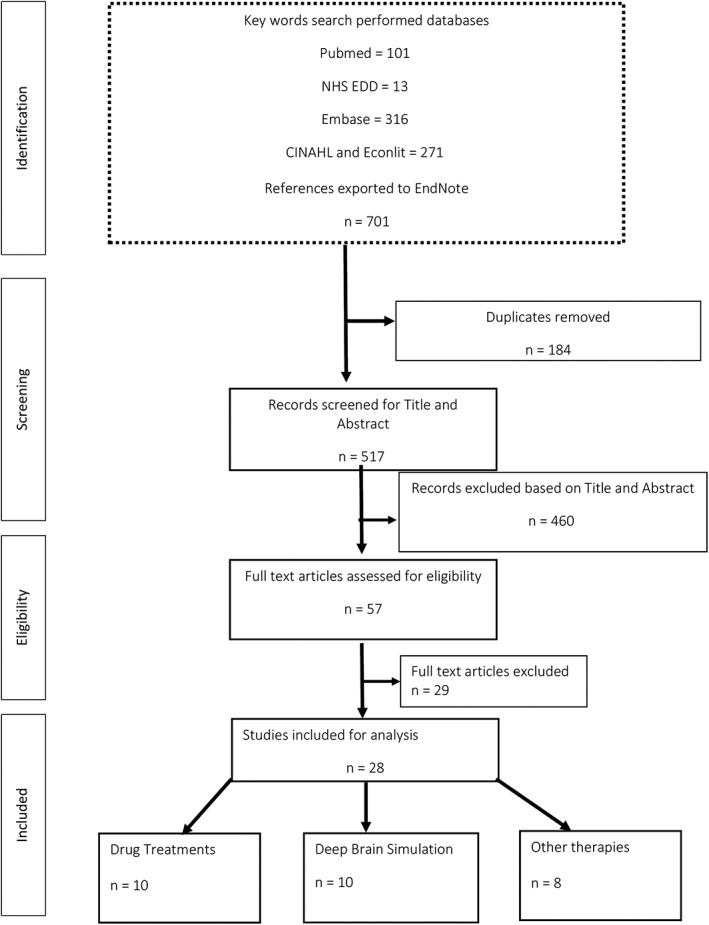
PRISMA flow chart for the selection of studies
Drug Treatments
The most common treatment for counteracting the incipient symptoms of PD is oral monotherapy medication (i.e., levodopa, DA, or rasagiline), which is effective in reducing motor fluctuations. In the USA, researchers compared the cost‐effectiveness of rasagiline versus DA (ropinirole XL, pramipexole, generic ropinirole) or levodopa as initial therapies in PD.34 Treatment with rasagiline led to more QALYs (3.45 vs. 3.34, 3.34, 3.34 for the DAs respectively, and 3.21 for levodopa), fewer patients with dyskinesia (38% and 73% respectively), and lower costs than ropinirole XL, pramipexole, and levodopa, thus dominating the alternatives. Although rasagiline resulted in higher costs compared to generic ropinirole, it generated an ICER of $28,406/QALY ($25,939).
Ten patients in Norway underwent a before–after prospective study and shifted their treatment from oral levodopa to intestinal levodopa.35 The aim was to estimate whether this transition was cost‐effective compared to maintaining the initial oral treatment. Intestinal levodopa had 0.047 higher QALY gain, and approximately $60,533 (472,000 NOK) higher costs compared to oral treatment, leading to $1.18 million/QALY (NOK 9.2 million/QALY).
Levodopa/carbidopa intestinal gel (LCIG) was compared to standard treatment for PD patients from a healthcare perspective in Bulgarian, UK, and Irish settings.36, 37, 38 The 12 PD patients in Bulgaria exhibited improvement in UPDRS scores after the LCIG treatment with an ICER of $3,050/QALY (1,904 BGN). In the UK and Irish study, a Markov model was used, over the lifetime, where patients remained in LCIG for the first five years, then returned back to standard treatment.38 The ICER was estimated at $55,366/QALY (£36,024) in the UK and $34,823/QALY (€26,944/QALY) in the Irish study.36 However, the results of these studies were relatively sensitive to patients’ health state during the treatment initiation and to the duration of the health benefits. In a Swedish study, duodopa's ICER compared to usual care amounted to $776,408/QALY (SEK 6.1 million).39 However, the inclusion of nonmedical costs, such as formal home help and informal care in the societal perspective, reduced the estimated ICER to $54,730/QALY (SEK 430,000).
A Markov model was used to compare continuous subcutaneous apomorphine to adjunct therapies (standard care), and to levodopa/carbidopa intestinal gel (LCIG) in the UK and Germany.40 LCIG dominated adjunct therapies over the lifetime in both countries; however, the ICER of LCIG compared to continuous subcutaneous apomorphine was $355,169/QALY (£244,684) in the UK and $350,000/QALY (€272,914) in Germany. Moreover, apomorphine's ICER over adjunct therapies in the UK was $9,347/QALY (£6,440). Although apomorphine could limit some motor functions for the patients, the researchers suggested that, from a healthcare provider's perspective, it could be used as an alternative to adjunct therapies for patients not eligible to alternative treatments.40
A CEA comparing prolonged release ropinirole (PR) versus immediate‐release ropinirole (IR) was conducted for PD patients in the Netherlands.41 Both drugs were used complementary to levodopa treatment. In the Markov model, the health states were based on the H&Y stages, and the transition among these states could be performed in six‐month cycles. The analyses, both short‐term (five years) and over a patient's lifetime were performed from a healthcare providers’ perspective, and only direct costs were included (i.e., drug costs, costs for elderly care, and hospital care). PR was dominant over IR, with a QALY gain of 0.08 in the short‐term and 0.24 in the long‐term and reduced the costs of $25,444 (€19,700) and $52,050 (€40,300) over seven‐ and 10‐year periods, respectively. However, indirect costs and outcomes were not considered; thus, an intimate apprehension was not possible.
In one CUA, three different treatment combinations were compared to standard care (levodopa monotherapy) in the USA.42 The treatments were rasagiline + levodopa (RAS+LD), entacapone + levodopa (ENT + LD), and levodopa/carbidopa/entacapone (LCE). A two‐year Markov model was used where patients moved to different health states every four months. The transition probabilities, cost, and health utilities were derived from various clinical trials, and the cost‐effectiveness was investigated from both the societal and payer perspectives. In both perspectives, RAS+LD and LCE had greater effectiveness compared to levodopa. From a societal perspective, ENT + LD led to $13,340/QALY ($12,031), compared to levodopa. The indirect costs, caregiver's costs, as well as patients’ heterogeneity, were not included. Furthermore, no sensitivity analysis was conducted. Francois et al.43 used a Markov model to explore the cost‐effectiveness of droxidopa (six months) followed by standard care (six months) versus 12 months of standard care, from a payer perspective in the USA. The ICER was $47,528/QALY ($47,001/QALY).
Deep Brain Stimulation
A Markov model was used to determine cost‐effectiveness of DBS plus best medical treatment compared to best/standard medical treatment alone in two studies in the UK,44, 45 two studies in Germany,46 and one studies in the USA.47 The time frame for the studies was five years, 15 years,45 lifetime,46 and 10 years.47 All the studies defined patients’ health state according to the H&Y stages, and the cycle lengths were one year44, 45 and six months.46, 47 Costs of surgery, battery replacement, and the cost of the adverse events were the main contributors to the total costs. The ICERs for the treatment options were $31,780/QALY (£20,678),44 $28,867/QALY (£19,887/QALY),45 $9,333/QALY (€6,700/QALY),46 and $23,870/QALY ($23,404/QALY).47 The results were sensitive to the patients’ H&Y stage. This was further elaborated in a study from Japan where CUA was performed considering different PD stages of the patients (early, intermediate, late).48 Using a Markov model, they showed that the ICER varied from $29,442/QALY ($70,200/QALY) in the early, increased to $26,110/QALY ($25,600/QALY) in the intermediate and dropped to $27,742/QALY ($27,200/QALY) in the late over the 10 years from a healthcare perspective.
A similar study was conducted in Hong Kong, where DBS was compared with standard medical treatment by following 13 patients having DBS surgery over two years.49 The standard care cost was estimated before the surgery. For the two‐year period, the ICER was $134,821/QALY ($123,110) in the first year and $68,824/QALY ($62,846) in the second year. The cost was higher in the first year due to DBS surgery, but in the next year, reduced substantially. The authors suggested that the procedure might have been even more cost‐effective during the following years; however, the sample size was relatively small.49 DBS was reported cost‐effective compared to standard care,40 but dominated by continuous subcutaneous apomorphine in both the UK and Germany,40 as described in the drug treatment section.
In two studies, the cost‐effectiveness of DBS procedure in two different sites, globus pallidus internus (GPi) and subthalamic nucleus (STN), was compared.50, 51 The first study was a CMA that compared the medication costs before and after the surgery, and between the GPi and STN approaches. The medication costs were significantly lower for both sites compared to best medical treatment and STN had significantly lower costs compared to GPi.50 In the second study, the medication costs of STN were also lower compared to GPi. The ICER of GPi versus STN stimulation was $109,901/QALY ($100,355) from a provider's perspective and $59,280/QALY ($54,129) from a societal perspective.51 Using a previously used Markov model,44 Dams et al.52 showed that of STN DBS plus BMT had ICER $30,316.81/QALY (€22,710/QALY) comparing to BMT alone in Germany over the lifetime of 251 young PD patients. Only one study was performed alongside an RCT, the PD SURG trial in the UK.53 The ICER was $735,200/QALY (£468,528/ QALY) at year one and was interpreted as not cost‐effective from a health and social care perspective, but the extrapolation of costs and outcomes in the DSA over five years resulted in a lower ICER $91,272/QALY (£45,180/QALY).
Other Therapies
Cost‐effectiveness of a physical exercise program was explored compared to usual care in Australia from a health system's perspective.54 Both CEA and CUA were conducted where the outcomes for CEA were fall prevention and prevention of mobility deterioration, and for the CUA, the outcome was QALYs. Fall rates had decreased among patients six months post‐intervention. The ICERs were $408/fall prevented (AUD 574), $6,810/person avoiding mobility deterioration (AUD 9,570), and $241,097/QALY (AUD 338,800). In a UK study, no differences in effect (fall prevention) or costs were observed for an exercise trial. The duration was 20 weeks, and the comparator was usual care.55
Fall prevention was also evaluated in an American study of Ti Ji Quan for PD patients.56 The secondary outcome was QALY. Ti Ji Quan is a balance‐based exercise; this was compared to both resistance training and stretching. During the 9‐month period, the Ti Ji Quan participants had a lower number of falls and significantly higher QALY than both the resistance training and stretching groups. The calculated ICER of Ti Ji Quan was $3,641/QALY ($3,394); however, the long‐term cost‐effectiveness of the intervention was not observed, and informal caregivers’ costs were not included.56 In a Dutch setting, an evidence‐based physiotherapy community program was assessed and compared to usual physiotherapy as a cοmplementary treatment to drug therapy.57 Main outcomes were patients’ improvement in mobility and mobility‐related quality of life. Although no differences were observed between the two groups in terms of health outcomes, the total costs were $939 (€727) lower in the intervention group. The largest cost saving in the intervention group was due to reduced informal care costs $404 (€313).
The cost‐effectiveness of a home‐based occupational therapy program for PD patients and their caretakers in the Netherlands was compared with a control group receiving usual care. The CUA was conducted alongside a randomized controlled trial.58 There were insignificant differences in costs between the two groups irrespectively of inclusion or exclusion of informal care. The only significant difference was the lower institutional costs for the intervention group ($1,516/€1,458). The intervention group of patients and caregivers gained 0.02 and 0.04 QALYs respectively over a six‐month trial period. Occupational therapy combined with physiotherapy was also compared with no therapy in the UK.59 The CUA was performed alongside RCT in patients with moderate PD (H&Y 3). The ICER was $5,282.64/QALY (£3,493/QALY).
In a Dutch study, 301 PD patients either participated in a multidisciplinary intervention or served as control following their usual care.60 The intervention included an assessment from a multidisciplinary team and guidance on both pharmacological and non‐pharmacological therapies. Over the eight‐month follow‐up, the results showed that activities of daily living and quality of life (QoL) were slightly higher in the intervention group (1.3 and 3.0 points, respectively) while no differences were noted for motor outcomes or overall caregivers’ burden. No statistically significant differences in costs were observed.
A CEA alongside RCT examined the cost‐effectiveness of home‐based motor monitoring plus standard in‐office visits (HBMM) compared to in‐office visits alone for advanced PD patients in Spain.61 The outcomes were UPDRS (I, II, III, IV) and QALYs, and were examined from a healthcare perspective throughout one year. The HBMM was cost‐effective considering UPDRS outcome (i.e., $191.20/UPDRS unit; €126.72/UPDRS unit), but not cost‐effective considering QALY.
Discussion
This systematic literature review evaluated all available evidence on the cost‐effectiveness of treatment/interventions for PD patients to enrich the existing literature. Although the cost‐effectiveness of all types of PD interventions was evaluated, our findings regarding the key role of time horizon in defining the cost‐effectiveness, as well as the importance of early treatment initiation, coincide with previous reviews.10, 28
Although the interventions included in this review were very heterogeneous, the comparability of cost‐effectiveness results across the three categories of PD interventions was determined by various key factors (i.e., the types of analyses and comparators of treatments, the efficacy of interventions, the perspectives, the existing reimbursement mechanisms, and the diverse instruments for assessing effectiveness). Taking into account the existing diversification in acceptable willingness‐to‐pay threshold ranges; NICE's threshold varies from £20,000 to £30,000/QALY gained;51, 52 the American literature mentions $50,000/QALY;62 and in Australian studies, AUD50,000/DALY is used;54 the cost‐effectiveness results may differ in terms of generalizability and applicability across settings. In this review, NICE's threshold is considered for determining the cost‐effectiveness of PD interventions ($33,022–$49,533).
For ease of discussion, we categorize PD management in two main tiers as identified by NICE, (1) management of early PD (initial functional effects of the disease) and (2) management of late PD (motor implications).63 Standard care or best medical treatment, including mostly oral levodopa, was used as the main comparator across interventions, which eased the comparability of the outcomes. However, there were studies that compared the results within the same category of interventions.34, 35, 37, 38, 40, 41, 42, 50, 51, 56
Management of Early PD
Initial drug treatments and physical and occupational therapy were used in this stage. The contribution of physical activity and occupational therapy, in addition to the usual treatment, is limited, as most differences between the intervention and the control group in costs and outcomes were statistically insignificant.55, 58, 59 When QALYs were measured by the EQ‐5D scale for physical exercise, there were no significant differences in the outcomes,55, 59 and although a positive effect was noted when measured by the SF‐6D scale, the intervention was not cost‐effective (ICER $241,097/QALY).54 It is known that SF‐6D is more sensitive in detecting differences in patients’ health status, disability, and medication use than EQ‐5D.64
Ti Ji provided better results compared to other types of physical activity despite a variation in its cost‐effectiveness. The variation derives from the use of different types of analysis, CEA that used natural units (falls prevented) to measure the outcome, and CUA that used the utility measure of QALYs for the outcome. It is widely argued that CEA is more relevant to clinicians since the preferred types of outcome measure are therapeutic units.65 CUA is preferred to facilitate decision‐making and increase the comparability of results.66 Nevertheless, the QALY underestimates the gains of short‐term palliative care interventions and is not well suited to capture symptom improvements in elderly PD populations with a short lifespan.67 Hence, QALY‐based results should be treated with caution since they could facilitate poor decision‐making that would only serve the needs of younger populations in the early stages of the disease. Aimed at obtaining more robust findings, we need to further explore long‐term cost‐effectiveness of Ti Ji in correlation to the duration, intensity, and frequency of the activities.68, 69
Multidisciplinary interventions of combined drug treatments and nonmedical therapy when practiced in early stages led to minor improvements in QoL with the same costs.60 Indeed, multidisciplinary therapies help patients remain functional in their everyday life for a longer period, thus not needing institutionalization or informal care.70 Institutional costs, transport, and caregivers’ time are important categories of expenses for this type of interventions.70
Management of Late PD
PD management in the advanced stages prioritizes adjunct or continuous infusion drug therapies and surgery for soothing patients’ motor impairments. Continuous subcutaneous apomorphine was more cost‐effective from the healthcare perspective, and adjunct treatments appeared more cost‐effective in the societal perspective. In the UK, non‐oral treatments in advanced PD patients led to reduced healthcare costs compared to oral therapy, expressed as a decrease of 28% in non‐elective admissions to the hospital.71 From a healthcare perspective, we find that apomorphine is dominant among non‐oral treatments;40 however, when apomorphine was compared to levodopa/carbidopa intestinal gel, the results varied according to the setting, as the ICER was lower in the UK ($9,350) than in Germany ($108,423).40 A possible explanation is that PD drug costs per patient in Germany amount to approximately €1,520 whereas in the UK drug costs are considered the smallest component of the total cost of the disease.72, 73 Adjunct treatments can relieve motor symptoms and reduce adverse side effects, leading to cost‐savings and long‐term improvements in patients’ QoL.7 Prolonged time without motor symptoms lengthens patients’ mobility and lightens the burden on informal caregivers.74 While patients could also enjoy the benefits of this moderate symptom progression earlier by initiating adjunct treatments in the initial stages, prescription rates of single‐drug therapies continue to be higher than those of adjunct drug treatment in early PD. The official guidelines of NICE and the Canadian Guidelines on Parkinson's Disease still suggest monotherapy as the initial pharmacological therapy in PD patients.75, 76 Accordingly, prescription patterns in the USA show that almost 80% of newly treated PD patients receive single drug treatment.77 Adjunct treatments were cost‐effective from a societal perspective; however, informal caregivers’ costs and QoL were wrongly excluded from one analysis,42 which if avoided, might have indicated greater societal benefits.
DBS was dominated by apomorphine but was more cost‐effective than adjunct and standard drug treatments from a healthcare perspective, leading to decreased medication use and a prolonged state of mild motor symptoms.40, 44, 45, 46, 52 According to McIntosh, a latent cost‐saving effect of DBS derives from the subsequent reduction of medication use among patients.78 Time was an integral factor in the cost‐effectiveness of DBS. First, taking into account the progressive nature of PD, there is a clear association between undertaking DBS in early age (60 years) and greater cost‐effectiveness ($4,740/QALY).46, 48 Second, the costs were particularly bound to the time horizon followed, with greater reductions observed after the first year of surgery.49, 53 The findings from CUA alongside RCT stated that DBS was not considered cost‐effective at first year $735,200/QALY (£468,528/ QALY), which is also confirmed by extrapolation analysis, but its ICER was expected to fall under accepted thresholds in a five‐year timespan.53 This variation could be attributed to expensive medical equipment, maintenance costs and hospitalization due to surgery.28, 78 However, it is worth mentioning that the QALY information measured by EQ‐5D for DBS patients are limited in many studies. Only one study (the PD SURG trial) had patient‐level data but was limited to only the first year.53 One‐year QALY data were extrapolated for 10 years in this study.
The ICER of GPi versus STN is higher than the acceptable WTP range, but STN DBS was cost‐effective comparing to BMT.52 In terms of the health outcome, there is no conclusive evidence for the optimal site; thus, researchers suggested a patient‐tailored evaluation by a multidisciplinary professional team for choosing the DBS site.79 Nevertheless, STN had comparatively lower costs than GPi.50
Disease Severity and Funding Source
The observed trends of costs and outcomes showed that the costs ascended and QoL descended sequentially at the severe state of PD (H&Y 4.0‐5.0),38, 41, 44, 48 which is in line with previous studies.6, 25, 80, 81 Therefore, greater cost‐effectiveness can be achieved in interventions that are initiated at an early stage than later. Furthermore, the majority of studies referring to drug treatments and surgery were funded by pharmaceutical companies, and only those including physical activity, occupational therapy, and multidisciplinary interventions were funded by the government or non‐governmental organizations (Supporting Table 2). Generally, caution is advised in the interpretation of studies funded by industry, as these studies have been shown to be more prone to report favorable cost‐effectiveness ratios,82 and in the case of model‐based studies, the findings tend to be even more problematic.83
Reporting Quality Assessment
The quality of reporting was insufficient for several articles despite the fact that guidelines for conducting economic evaluations are available. Several items were partially reported or missing in some articles, including a proper description of costing methods such as unit costs, sources of costs items (registers or data from other countries), the timing of the cost collection (prospectively or retrospectively), and methods to transform the costs from one country to another country. Taking into account that the CHEERS guidelines were published in 2013, studies published earlier than 2013 had a lower mean score (19.33) than those published in 2013 and later (20.33). It is possible that the CHEERS statement has improved the reporting quality and we suggest that it should be habitually employed for further improvements in reporting.
Limitations
This study is not free of limitations. In this review, we investigated all types of intervention that were provided to PD patients. On the one hand, the reader is presented with a comprehensive overview of drug interventions, DBS, and other intervention types, which could be seen as a strength of the review. On the other hand, methodological differences between the interventions may have prevented, in a precise way, which types of interventions are most cost‐effective. Thus, our broad approach could also be seen as a weakness. Moreover, as the reporting quality of the articles according to CHEERS was based on personal interpretations, disagreements may arise about each study's score. A quality assessment of modeling studies using different checklists would have been interesting.84 Furthermore, we have not assessed the methodological quality of the articles, especially for the simulation models, and we did not perform a systematic quantitative assessment to identify key drivers of the cost‐effectiveness.
Conclusions
Tailoring PD management according to the subsequent cost‐effectiveness of PD interventions should consider the absence of the cure and the progressive nature of the disease. Under certain restrictions, Ti Ji and multidisciplinary interventions seem to be more cost‐effective for early PD management. In advanced PD, apomorphine was considered cost‐effective from a healthcare perspective and adjunct treatments from a societal perspective. DBS presented cost‐effectiveness in the long‐term; however, PD progression differs depending on patients’ characteristics, levels, and quality of informal care as well as disease severity. Hence, further research on the cost‐effectiveness of individually‐tailored combinations of existing PD interventions, concerning patients’ circumstances, is needed in order to draw more robust conclusions about optimal PD management.
Author Roles
1. Research Project: A. Conception, B. Organization, C. Execution; 2. Statistical Analysis: A. Design, B. Execution, C. Review and Critique; 3. Manuscript Preparation: A. Writing the First Draft, B. Review and Critique.
N.A.: 2A, 2B, 3A
U.G.: 3B
J.J.: 3B
S.S.: 2C, 3B
Disclosures
Ethical Compliance Statement: The authors confirm that the approval of an institutional review board and patient consent were not required for this work. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflict of Interest: The authors declare that there are no conflicts of interest relevant to this work. U.G. received governmental funding by ALF funding from Region Skåne (grant number Dnr F: 2014/354) for conducting research. The funding organization had no role in the design and conduct of the study, as well as the preparation, review, or approval of the manuscript.
Financial Disclosures for the preceding 12 months: The authors declare that there are no additional disclosures to report.
Supporting information
Supplemental Table 1. PICOS criteria for the economic review.
Supplemental Table 2. Characteristics of the selected studies.
Supplemental Table 3. Costs and QALYs reported in the selected studies.
Supplemental Appendix 1. Details of search in the databases including keywords.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. de Lau LML, Breteler MMB. Epidemiology of Parkinson's disease. The Lancet Neurol 2006;5(6):525–535. [DOI] [PubMed] [Google Scholar]
- 2. Tinelli, Kanavos P, Grimaccia F. The value of early diagnosis and treatment in parkinson's disease: a literature review of the potential clinical and socioeconomic impact of targeting unmet needs in Parkinson's disease. London: The London School of Economics and Political Science; 2016. [Google Scholar]
- 3. Kowal SL, Dall TM, Chakrabarti R, Storm MV, Jain A. The current and projected economic burden of Parkinson's disease in the United States. Mov Disord 2013;28(3):311–318. [DOI] [PubMed] [Google Scholar]
- 4. Gustavsson A, Svensson M, Jacobi F, et al. Cost of disorders of the brain in Europe 2010. Eur Neuropsychopharmacol 2011;21:718–779. [DOI] [PubMed] [Google Scholar]
- 5. Céu M, Coloma J. Health economics and cost of illness in Parkinson's disease. Mov Disord 2013;8(1):6–9. [Google Scholar]
- 6. McCrone P, Allcock LM, Burn DJ. Predicting the cost of Parkinson's disease. Mov Disord 2007;22(6):804–812. [DOI] [PubMed] [Google Scholar]
- 7. von Campenhausen S, Winter Y, Rodrigues e Silva A, et al. Costs of illness and care in Parkinson's disease: an evaluation in six countries. Eur Neuropsychopharmacol 2011;21(2):180–191. [DOI] [PubMed] [Google Scholar]
- 8. Wirdefeldt K, Adami H‐O, Cole P, Trichopoulos D, Mandel J. Epidemiology and etiology of Parkinson's disease: a review of the evidence. Eur J Epidemiol 2011;26(1):1. [DOI] [PubMed] [Google Scholar]
- 9. Pahwa R, Lyons K. Early diagnosis of Parkinson's disease: recommendations from diagnostic. Am J Manag Care 2010:S94–99. [PubMed] [Google Scholar]
- 10. Cubo E. Pharmacotherapy in the management of early Parkinson's disease: cost‐effectiveness and patient acceptability. Clinicoecon Outcomes Res 2010;2:127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Riederer P, Laux G. MAO‐inhibitors in Parkinson's disease. Exp Neurobiol 2011;20(1):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Clarke CE, Worth P, Grosset D, Stewart D. Systematic review of apomorphine infusion, levodopa infusion and deep brain stimulation in advanced Parkinson's disease. Parkinsonism Relat Disord 2009;15(10):728–741. [DOI] [PubMed] [Google Scholar]
- 13. Rascol O, Brooks DJ, Melamed E, et al. Rasagiline as an adjunct to levodopa in patients with Parkinson's disease and motor fluctuations (LARGO, lasting effect in adjunct therapy with rasagiline given once daily, study): a randomised, double‐blind, parallel‐group trial. Lancet 2005;365(9463):947–954. [DOI] [PubMed] [Google Scholar]
- 14. Zhuo C, Ji F, Zhu X, et al. Comparison for efficacy and tolerability among ten drugs for treatment of Parkinson's disease: a network meta‐analysis. Sci Rep 2017;8:45865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Markham C, Diamond S. Long‐term follow‐up of early dopa treatment in Parkinson's disease. Annals Neurol 1986:365–372. [DOI] [PubMed] [Google Scholar]
- 16. Brooks DJ. Dopamine agonists: their role in the treatment of Parkinson's disease. J Neurol Neurosurg Psychiatry 2000;68(6):685–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ludovico I, Damborská A. Deep brain stimulation in Parkinson's disease: Act Nerv Super 2017:4–11. [Google Scholar]
- 18. Perestelo‐Pérez L, Rivero‐Santana A, Pérez‐Ramos J, Serrano‐Pérez P, Panetta J, Hilarion P. Deep brain stimulation in Parkinson's disease: meta‐analysis of randomized controlled trials. J Neurol 2014;261(11):2051–2060. [DOI] [PubMed] [Google Scholar]
- 19. Lauzé M, Daneault J‐F, Duval C. The effects of physical activity in Parkinson's disease: a review. J Parkinsons Dis 2016;6(4):685–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Goodwin VA, Richards SH, Taylor RS, Taylor AH, Campbell JL. The effectiveness of exercise interventions for people with Parkinson's disease: a systematic review and meta‐analysis. Mov Disord 2008;23(5):631–640. [DOI] [PubMed] [Google Scholar]
- 21. Tomlinson CL, Patel S, Meek C, et al. Physiotherapy intervention in Parkinson's disease: systematic review and meta‐analysis. BMJ 2012;345:e5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shen X, Wong‐Yu ISK, Mak MKY. Effects of exercise on falls, balance, and gait ability in Parkinson's disease. Neurorehabil Neural Repair 2016;30(6):512–527. [DOI] [PubMed] [Google Scholar]
- 23. Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press, 2015. [Google Scholar]
- 24. Rubenstein LM, de Leo A, Chrischilles EA. Economic and health‐related quality of life considerations of new therapies in Parkinson's disease. PharmacoEconomics 2001;19(7):729. [DOI] [PubMed] [Google Scholar]
- 25. Dowding CH, Shenton CL, Salek SS. A review of the health‐related quality of life and economic impact of Parkinson's disease. Drugs Aging 2006;23(9):693–721. [DOI] [PubMed] [Google Scholar]
- 26. Eggert KM, Reese JP, Oertel WH, Dodel R. Cost effectiveness of pharmacotherapies in early Parkinson's disease. CNS Drugs 2008;22(10):841–860. [DOI] [PubMed] [Google Scholar]
- 27. Siderowf AD, Holloway RG, Stern MB. Cost‐effectiveness analysis in Parkinson's disease: determining the value of interventions. Mov Disord 2000;15(3):439–445. [DOI] [PubMed] [Google Scholar]
- 28. Becerra JE, Zorro O, Ruiz‐Gaviria R, et al. Economic Analysis of Deep Brain Stimulation in Parkinson Disease: Systematic Review of the Literature. World Neurosurg 2016;93:44–49. [DOI] [PubMed] [Google Scholar]
- 29. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Chichester (UK): John Wiley & Sons; 2008. [Google Scholar]
- 31. Husereau D. et al. Consolidated health economic evaluation reporting standards (CHEERS)—explanation and elaboration: a report of the ISPOR health economic evaluation publication guidelines good reporting practices task force. Value Health 2013:231–250. [DOI] [PubMed] [Google Scholar]
- 32. Shemilt I, Thomas J, Morciano M. A web‐based tool for adjusting costs to a specific target currency and price year. Evid Policy 2010;6(1):51–59. [Google Scholar]
- 33. Goetz CG, Poewe W, Rascol O, et al. Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: status and recommendations. Mov Disord 2004;19(9):1020–1028. [DOI] [PubMed] [Google Scholar]
- 34. Farkouh RA, Wilson MR, Tarrants ML, Castelli‐Haley J, Armand C. Cost‐effectiveness of rasagiline compared with first‐line early Parkinson disease therapies. Am J Pharm Benefits 2012;4(3):99–107. [Google Scholar]
- 35. Lundqvist C, Beiske AG, Reiertsen O, Kristiansen IS. Real life cost and quality of life associated with continuous intraduodenal levodopa infusion compared with oral treatment in Parkinson patients. J Neurol 2014;261(12):2438–2445. [DOI] [PubMed] [Google Scholar]
- 36. Lowin J, Sail K, Baj R, et al. The cost‐effectiveness of levodopa/carbidopa intestinal gel compared to standard care in advanced Parkinson's disease. J Med Econ 2017;20(11):1207–1215. [DOI] [PubMed] [Google Scholar]
- 37. Kamusheva MS, Gerasimov N, Petrova GI. Intestinal gel levodopa + carbidopa in Parkinson's patients with frequent and prolonged akinesia ‐ an economic evaluation. Int J Pharm Sci Rev Res 2013;22(1):244–246. [Google Scholar]
- 38. Lowin J, Bergman A, Chaudhuri KR, et al. A cost‐effectiveness analysis of levodopa/carbidopa intestinal gel compared to standard care in late stage Parkinson's disease in the UK. J Med Econ 2011;14(5):584–593. [DOI] [PubMed] [Google Scholar]
- 39. Willis M, Persson U, Zoellner Y, Gradl B. Reducing uncertainty in value‐based pricing using evidence development agreements: the case of continuous intraduodenal infusion of levodopa/carbidopa (duodopa) in Sweden. Appl Health Econ Health Policy 2010;8(6):377–386. [DOI] [PubMed] [Google Scholar]
- 40. Walter E, Odin P. Cost‐effectiveness of continuous subcutaneous apomorphine in the treatment of Parkinson's disease in the UKand Germany. J Med Econ 2015;18(2):155–165. [DOI] [PubMed] [Google Scholar]
- 41. van Boven JFM, Novak A, Driessen MT, Boersma C, Boomsma MM, Postma MJ. Economic evaluation of ropinirole prolonged release for treatment of Parkinson's disease in The Netherlands. Drugs Aging 2014;31(3):193–201. [DOI] [PubMed] [Google Scholar]
- 42. Groenendaal H, Tarrants ML, Armand C. Treatment of advanced parkinson's disease in the United States a cost‐utility model. clinical drug investigation 2010;30(11):789–798. [DOI] [PubMed] [Google Scholar]
- 43. Francois C, Hauser RA, Aballea S, Dorey J, Kharitonova E, Hewitt LA. Cost‐effectiveness of droxidopa in patients with neurogenic orthostatic hypotension: post‐hoc economic analysis of Phase 3 clinical trial data. J Med Econ 2016;19(5):515–525. [DOI] [PubMed] [Google Scholar]
- 44. Eggington S, Valldeoriola F, Chaudhuri KR, Ashkan K, Annoni E, Deuschl G. The cost‐effectiveness of deep brain stimulation in combination with best medical therapy, versus best medical therapy alone, in advanced Parkinson's disease. J Neurol 2014;261(1):106–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fundament T, Eldridge PR, Green AL, et al. Deep brain stimulation for Parkinson's disease with early motor complications: a UKcost‐effectiveness analysis. PLoS One 2016;11(7):e0159340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dams J, Siebert U, Bornschein B, et al. Cost‐effectiveness of deep brain stimulation in patients with Parkinson's disease. Mov Disord 2013;28(6):763–771. [DOI] [PubMed] [Google Scholar]
- 47. Pietzsch JB, Garner AM, Marks WJ, Jr. Cost‐effectiveness of deep brain stimulation for advanced Parkinson's disease in the United States. Neuromodulation 2016;19(7):689–697. [DOI] [PubMed] [Google Scholar]
- 48. Kawamoto Y, Mouri M, Taira T, Iseki H, Masamune K. Cost‐effectiveness analysis of deep brain stimulation in patients with Parkinson's disease in Japan. World Neurosurg 2016;89:628–635. [DOI] [PubMed] [Google Scholar]
- 49. Zhu XL, Chan DTM, Lau CKY, et al. Cost‐effectiveness of subthalmic nucleus deep brain stimulation for the treatment of advanced Parkinson disease in Hong Kong: a prospective study. World Neurosurg 2014;82(6):987–993. [DOI] [PubMed] [Google Scholar]
- 50. Weaver FM, Stroupe KT, Cao L, et al. Parkinson's disease medication use and costs following deep brain stimulation. Mov Disord 2012;27(11):1398–1403. [DOI] [PubMed] [Google Scholar]
- 51. Stroupe KT, Weaver FM, Cao L, et al. Cost of deep brain stimulation for the treatment of Parkinson's disease by surgical stimulation sites. Mov Disord 2014;29(13):1666–1674. [DOI] [PubMed] [Google Scholar]
- 52. Dams J, Balzer‐Geldsetzer M, Siebert U, et al. Cost‐effectiveness of neurostimulation in Parkinson's disease with early motor complications. Mov Disord 2016;31(8):1183–1191. [DOI] [PubMed] [Google Scholar]
- 53. McIntosh E, Gray A, Daniels J, et al. Cost‐utility analysis of deep brain stimulation surgery plus best medical therapy versus best medical therapy in patients with Parkinson's: Economic evaluation alongside the PD SURG trial. Mov Disord 2016;31(8):1173–1182. [DOI] [PubMed] [Google Scholar]
- 54. Farag I, Sherrington C, Hayes A, et al. Economic evaluation of a falls prevention exercise program among people with Parkinson's disease. Mov Disord 2015;31(1):53–61. [DOI] [PubMed] [Google Scholar]
- 55. Fletcher E, Goodwin VA, Richards SH, Campbell JL, Taylor RS. An exercise intervention to prevent falls in Parkinson's: an economic evaluation. BMC Health Serv Res 2012;12:426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Li F, Harmer P. Economic evaluation of a Tai Ji Quan intervention to reduce falls in people with parkinson disease, Oregon, 2008–2011. Prev Chronic Dis 2015;12:E120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Munneke M, Nijkrake MJ, Keus SH, et al. Efficacy of community‐based physiotherapy networks for patients with Parkinson's disease: a cluster‐randomized trial. Lancet Neurol 2010;9(1):46–54. [DOI] [PubMed] [Google Scholar]
- 58. Sturkenboom IHWM, Hendriks JCM, Graff MJL, et al. Economic evaluation of occupational therapy in Parkinson's disease: a randomized controlled trial. Mov Disord 2015;30(8):1059–1067. [DOI] [PubMed] [Google Scholar]
- 59. Clarke CE, Patel S, Ives N, et al. Clinical effectiveness and cost‐effectiveness of physiotherapy and occupational therapy versus no therapy in mild to moderate Parkinson's disease: a large pragmatic randomised controlled trial (PD REHAB). Health Technol Assess 2016;20(63):1–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. van der Marck MA, Munneke M, Mulleners W, et al. Integrated multidisciplinary care in Parkinson's disease: a non‐randomised, controlled trial (IMPACT). Lancet Neurol 2013;12(10):947–956. [DOI] [PubMed] [Google Scholar]
- 61. Cubo E, Mariscal N, Solano B, et al. Prospective study on cost‐effectiveness of home‐based motor assessment in Parkinson's disease. J Telemed Telecare 2017;23(2):328–338. [DOI] [PubMed] [Google Scholar]
- 62. Weinstein MC. How much are Americans willing to pay for a quality‐adjusted life year? Med Care 2008;46(4):343–345. [DOI] [PubMed] [Google Scholar]
- 63. NICE . Parkinson's disease in adults‐NICE guideline. https://www.nice.org.uk/guidance/NG712017. Accessed August 15, 2018.
- 64. Petrou S, Hockley C. An investigation into the empirical validity of the EQ‐5D and SF‐6D based on hypothetical preferences in a general population. Health Econ 2005;14(11):1169–1189. [DOI] [PubMed] [Google Scholar]
- 65. Gold M, Siegel J, Russell L, Weinstein M. Cost‐Effectiveness in Health and Medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 66. Jakubiak‐Lasocka J, Jakubczyk M. Cost‐effectiveness versus cost‐utility analyses: what are the motives behind using each and how do their results differ? A Polish example. Value Health Reg Issues 2014;4:66–74. [DOI] [PubMed] [Google Scholar]
- 67. Pettitt D, Raza, S , Naughton, B et al. The limitations of QALY: a literature review. Stem Cell Res Ther 2016;6(4):1–7. [Google Scholar]
- 68. Amano S, Nocera JR, Vallabhajosula S, et al. The effect of Tai Chi exercise on gait initiation and gait performance in persons with Parkinson's disease. Parkinsonism Relat Disord 2013;19(11):955–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ransmayr G. Physical, occupational, speech and swallowing therapies and physical exercise in Parkinson's disease. J Neural Transm (Vienna) 2011;118(5):773–781. [DOI] [PubMed] [Google Scholar]
- 70. Gage H, Kaye J, Owen C, Trend P, Wade D. Evaluating rehabilitation using cost‐consequences analysis: an example in Parkinson's disease. Clin Rehabil 2006. Mar;20(3):232–238. [DOI] [PubMed] [Google Scholar]
- 71. Heald AH, Livingston M, Stedman M, Wyrko Z. Higher levels of apomorphine and rotigotine prescribing reduce overall secondary healthcare costs in Parkinson's disease. Int J Clin Pract 2016;70(11):907–915. [DOI] [PubMed] [Google Scholar]
- 72. Findley LJ, Wood E, Lowin J, Roeder C, Bergman A, Schifflers M. The economic burden of advanced Parkinson's disease: an analysis of a UKpatient dataset. J Med Econ 2011;14(1):130–139. [DOI] [PubMed] [Google Scholar]
- 73. Spottke AE, Reuter M, Machat O, et al. Cost of illness and its predictors for Parkinson's disease in Germany. Pharmacoeconomics 2005;23(8):817–836. [DOI] [PubMed] [Google Scholar]
- 74. Krol M, Papenburg J, van Exel J. Does including informal care in economic evaluations matter? A systematic review of inclusion and impact of informal care in cost‐effectiveness studies. Pharmacoeconomics 2015;33(2):123–135. [DOI] [PubMed] [Google Scholar]
- 75. Canadian Guidelines on Parkinson's Disease Executive Summary. Can J Neurol Sci 2012;39: Suppl 4:S1–S30. [DOI] [PubMed] [Google Scholar]
- 76. National Collaborating Centre for Chronic Conditions (UK) Parkinson's Disease: National Clinical Guideline for Diagnosis and Management in Primary and Secondary Care. London: Royal College of Physicians; 2006. [PubMed] [Google Scholar]
- 77. Huse DM, Castelli‐Haley J, Orsini LS, Lenhart G, Abdalla JA. Patterns of initial pharmacotherapy for Parkinson's disease in the United States. J Geriatr Psychiatry Neurol 2006;19(2):91–97. [DOI] [PubMed] [Google Scholar]
- 78. McIntosh E. Perspective on the economic evaluation of deep brain stimulation. Front Integr Neurosci 2011;5:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mirza S, Yazdani U, Dewey Iii R, et al. Comparison of globus pallidus interna and subthalamic nucleus in deep brain stimulation for Parkinson disease: an institutional experience and review. Parkinsons Dis 2017;2017:3410820. doi: 10.1155/2017/3410820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Keränen T, Kaakkola S, Sotaniemi K, et al. Economic burden and quality of life impairment increase with severity of PD. Parkinsonism Relat Disord 2003;9:163–168. [DOI] [PubMed] [Google Scholar]
- 81. Winter Y, von Campenhausen S, Brozova H, et al. Costs of Parkinson's disease in eastern Europe: a Czech cohort study. Parkinsonism Relat Disord 2010;16(1):51–56. [DOI] [PubMed] [Google Scholar]
- 82. Bell CM, Urbach DR, Ray JG, et al. Bias in published cost effectiveness studies: systematic review. BMJ 2006;332(7543):699–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Garattini L, Koleva D, Casadei G. Modeling in pharmacoeconomic studies: funding sources and outcomes. Int J Technol Assess Health Care 2010;26(3):330–333. [DOI] [PubMed] [Google Scholar]
- 84. Caro JJ, Briggs AH, Siebert U, Kuntz KM. Modeling good research practices—overview a report of the ISPOR‐SMDM modeling good research practices task force–1. Med Decis Making 2012;32(5):667–677. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. PICOS criteria for the economic review.
Supplemental Table 2. Characteristics of the selected studies.
Supplemental Table 3. Costs and QALYs reported in the selected studies.
Supplemental Appendix 1. Details of search in the databases including keywords.


