Abstract
Renal allotransplantation clearly offers better survival and quality of life for end-stage renal disease (ESRD) patients than chronic dialysis. The median waiting time for a deceased donor kidney in a suitable ESRD patient is 3.9 years. The initial candidates for pig kidney xenotransplantation will be those with ESRD unlikely to receive an allograft within a reasonable period of time. It is thus reasonable to ascertain whether clinical trials of xenotransplantation might likewise offer superior outcomes. Chronic dialysis in patients with ESRD is associated with poor quality of life, significant morbidity, and relatively high mortality, with only 56% surviving 3 years and 42% at 5 years. However, a significant number of these patients, because of comorbidities, frailty, etc., would not be considered for renal allotransplantation, and likely not for xenotransplantation. As genetically-engineered pig kidneys have satisfactorily supported life in immunosuppressed nonhuman primates for many months or even more than a year, consideration in carefully selected patients could be given to pig kidney xenotransplantation. We suggest that, in order to give a patient the best possible outcome, the pig kidney could be transplanted pre-emptively (before dialysis is initiated). If it fails at any stage, the patient would then begin chronic dialysis, and continue to await an allograft. The present (limited) evidence is that failure of a pig graft would not be detrimental to a subsequent allograft.
Keywords: Cost, Dialysis, chronic, Hemodialysis, Morbidity, Mortality, Quality of life, Xenotransplantation
Introduction
The incidence of end-stage renal disease (ESRD) is increasing worldwide. In the USA, old age, diabetes, hypertension, obesity, and cardiovascular disease all contribute to the development of chronic kidney disease.1 Since the mid-1970s, hemodialysis has been life-sustaining for millions of patients with ESRD.
On December 31, 2015 (the most recent data available to us), in the USA there were almost 500,000 patients undergoing renal replacement therapy (RRT), of whom approximately 90% were receiving hemodialysis and 10% peritoneal dialysis.2 An additional approximate 210,000 patients had a functioning kidney transplant. Many members of the public, as well as some health care2 professionals, are under the impression that, although time-consuming and a nuisance, hemodialysis can maintain a patient with ESRD for many years. However, it is not without significant morbidity and mortality.
As a consequence of comorbidities or socioeconomic instability, most dialysis patients, are not considered candidates for a kidney transplant, though the absolute number is debatable.3 Even so, there are currently >80,000 patients wait-listed for kidney transplantation in the USA.4 The benefit of a kidney transplant, even a high-risk transplant,5 is most evident when the outcome is compared to that of dialysis in wait-listed patients thought reasonable candidates, who did not receive an allograft. It seems likely that the initial patients to be offered pig kidney xenotransplantation will be those on chronic dialysis considered suitable transplant candidates, but unlikely to receive an allograft for some years. On the basis of recent experimental results,6–9 it is reasonable to presume that clinical trials of kidney xenotransplantation will at least offer an outcome competitive with chronic dialysis. Thus, the initial success of the clinical pig kidney xenotransplants will be defined by comparison with chronic dialysis rather than renal allotransplantation.
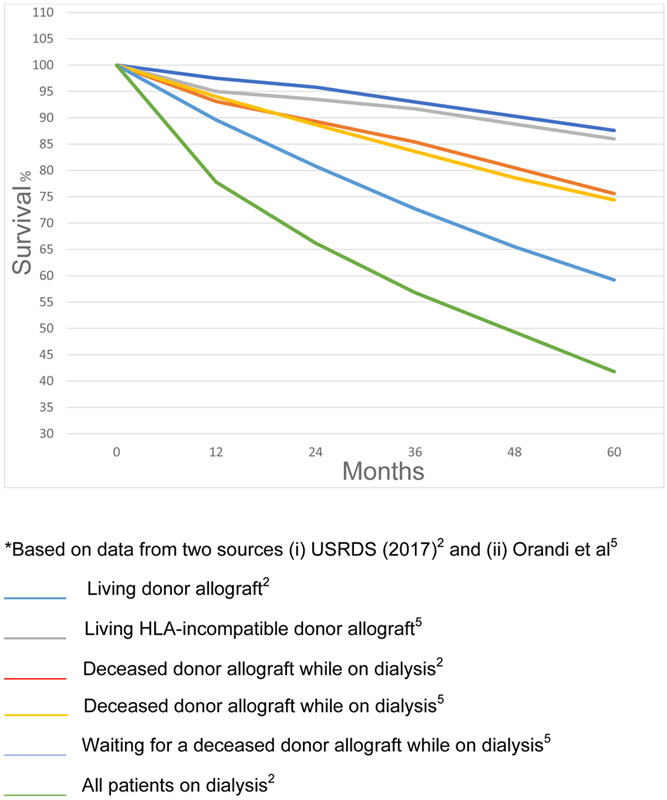
Mortality of patients with ESRD on chronic dialysis
The overall survival of patients on hemodialysis in the USA is 78% at 1 year, 57% at 3 years, and only 42% at 5 years (Figure 1).2 Perhaps surprisingly, the risk of dying is greatest when dialysis is initiated, i.e., within the first two months.2 These deaths are mainly related to comorbidities, e.g., congestive heart failure, previous myocardial infarction, malnutrition, and cancer, accompanied by kidney failure and the initial stress of hemodialysis.10,11 There may even be some under-reporting of deaths in the first two months as some patients who die soon after starting dialysis might not be registered as having ESRD.10 Mortality among patients >65 years of age is higher than in younger patients. Remarkably, patients with ESRD on dialysis have poorer survival and fewer remaining years of life than many with cancer, diabetes, and cardiovascular disease.2
Figure 1: Percentage survival of ESRD patients by treatment modality in 2010*.
These mortality rates, however, largely reflect outcomes among those who are not candidates for renal transplantation, as most of those who initiate dialysis in the USA are not considered suitable for a transplant. Only approximately 20% of the prevalent dialysis population is currently on transplant waiting lists. However, Schold and colleagues suggested that any ESRD patient with a projected lifespan on dialysis of >5 years might be considered an acceptable transplant candidate.3 If this criterion were applied, adding those patients to the pool would approximately double the size of the waiting list, but would still leave almost two-thirds of those on dialysis as unacceptable or poor transplant candidates.
Approximately 25 patients are removed from the kidney waiting list each day, either because they die (approximately 15%) or because they become too sick to tolerate the transplant procedure.4 Current data from the Scientific Registry of Transplant Recipients (SRTR) indicate the 3-year mortality of wait-listed candidates as 8%, with a further 11% removed from the list for other reasons. There is a great deal of geographic disparity, with some centers demonstrating greater wait-list mortality than transplant rates. Importantly, in one analysis, Casingal et al found that most of those who died on the wait-list had been excellent candidates at the time of listing.12
The median waiting period for a patient with ESRD to obtain a deceased human donor kidney is 3.9 years,2 by which time approximately 20% of transplant candidates may have died or been removed from the wait-list rather than undergone transplantation. (We would emphasize that 3.9 years is the median waiting period, indicating that 50% of the patients wait for a significantly longer period, sometimes extending over several more years.) Approximately 9% and 16% of patients wait for 4–5 years or >5 years, respectively.4 In particular, those of blood group B or O may experience a longer waiting period,2 with an average wait-time of almost 5 years, even when the patient has no anti-HLA antibodies (Table1), and this factor must be taken into consideration.
Table 1:
Average wait time (in years) for a deceased human kidney transplant by blood type and percentage panel-reactive antibodies (PRA), 1998-20102
| PRA (%) | Blood group | |||
|---|---|---|---|---|
| A | B | AB | O | |
| 0 | 2.9 | 4.8 | 2.0 | 4.7 |
| >0<20 | 2.5 | 4.8 | 1.4 | 4.7 |
| >20<80 | 2.5 | 3.7 | 1.4 | 4.3 |
| >80<98 | 3.7 | * | 3.2 | 4.8 |
| >98<100 | * | 5.9 | * | * |
As the estimated time to the transplant probability had not reached 50% (median) at the end of follow-up, the median waiting time could not be calculated.
Traditionally, allocation was based on ABO- and MHC-compatibility. In the new kidney allocation system (KAS) implemented in the USA in 2014, other variables play a larger role, with a goal of improving allocation to patients sensitized to human leukocyte antigens (HLA), and linking projected longevity of grafts to projected longevity of candidates. The net result has been dramatically improved access to transplantation for highly-sensitized patients, but with a 21% reduction in access, with greater dependence on lower quality kidneys (i.e., those with a high kidney donor profile index [KDPI]), for wait-list candidates >65 years of age.13 Thus, there are significant numbers of suitable transplant candidates for whom early transplantation would offer significant survival benefit but, due to a relative shortage of transplantable kidneys, are not afforded the opportunity.
Because they are at greater risk of dying before a suitable renal allograft becomes available, we would suggest that older patients, e.g., 65 or older, particularly if of blood group B or O, would be candidates who could be considered for pig kidney transplantation.
Quality of life of patients with ESRD on chronic dialysis
ESRD is associated with many comorbidities, and both hemo- and peritoneal dialysis contribute further complications and adverse effects (Tables 2 and 3). Medications aimed to correct one abnormality can contribute side-effects of their own, often compounding the patient’s problems.2,14–20
Table 2:
Complications and morbidity associated with advanced CKD (Stages 4-5) and chronic hemodialysis
| Chronic kidney disease | Chronic hemodialysis | |
|---|---|---|
| Malnutrition17 | Dietary restriction regimens •Low potassium •Low phosphate Uremic toxicity Insulin resistance Growth hormone resistance Increased levels and sensitivity of glucagon Hyperparathyroidism Acidemia and metabolic acidosis |
Hypercatabolism of comorbid illness |
| Inflammation17 | Due to decreased glomerular filtration rate •Decreased clearance of pro-inflammatory cytokines •Volume overload** •Oxidative stress** •Carbonyl stress** •Decreased antioxidants** Coexistence of comorbid conditions •Inflammatory diseases, e.g., systemic lupus erythematosus, cardiovascular disease, diabetes mellitus, advanced age |
•Exposure to dialysis tubing •Less biocompatible dialysis membrane (e.g., cuprophane) •Impurities related to dialysate •Back-filtration and back-diffusion of contaminants •Foreign bodies in dialysis-access grafts •Intravenous catheters Beta-2-microglobulin accumulation63 |
| Infections | Increased susceptibility to certain infections, e.g., influenza, pneumococcal pneumonia64 | Bacteremia and sepsis65
Increased prevalence of hepatitis B (2.4%), hepatitis C (7.4%), hepatitis G, HIV (1.1%)]63 Cellulitis |
| Anemia66 | Decreased production of erythropoietin | Uremic platelet dysfunction*67 |
| Vascular access-related 2,68 | N/A | Thrombosis Infection Distal ischemia Aneurysms / pseudoaneurysms Nerve injuries |
| Cardiovascular | Hypertension (83%) Left ventricular hypertrophy** (34%) Accelerated atherosclerosis** Accelerated vascular and valvular calcification**69 |
•Arrythmia /cardiac arrest •Myocardial ischemia and acute myocardial infarction* •Atherosclerotic heart disease (50%)* •Congestive heart failure* •Cardiomyopathy*2,70-73 Underlying pathophysiological derangements •Systolic and diastolic dysfunction* •Electrolyte shifts74 •Intradialytic hypotension •Autonomic dysfunction •Endothelial dysfunction*75 •Interstitial fibrosis* •Decreased perfusion reserve76 •Diminished ischemia tolerance Cerebrovascular (18%) and peripheral arterial diseases (26%) Transient ischemic disease and stroke •Ischemia* •Peripheral arterial occlusive disease* •Gangrene (11%) and amputation24,77 •Calciphylaxis*20 •Deep venous thrombosis (3%) |
| Acid-base and electrolyte disturbances 78-81 | Metabolic acidosis and hyperkalemia | |
| Endocrine and musculoskeletal14 | Secondary hyperparathyroidism Decreased synthesis of calcitriol Hyperphosphatemia due to impaired excretion Increased levels of FGF-2382 |
Muscular weakness*# Tendon rupture Bone pain*# Extra skeletal calcifications* Skeletal deformities*83,84 Thyroid and growth hormone dysregulation, *# Impaired gluconeogenesis and triglyceride metabolism*# Sexual dysfunction*85 |
| Pulmonary (13.0%)86 | Dyspnea** (30%) | Pulmonary hypertension Pulmonary fibrosis Hypoxemia and regional ventilation-perfusion mismatch Bronchospasm and airway hyper-responsiveness |
| Neurologic (12%) and psychiatric (24%) effects 87 | Depression Suicidal behavior Delirium Anxienty and panic symptoms Restless leg syndrome**88 |
Similar effects can be seen |
| Gastrointestinal symptoms89 | Nausea** Dyspepsia** Anorexia Abdominal pain** |
Peptic ulcer disease* (16.2%) Chronic constipation* Chronic diarrhea* Irritable bowel syndrome* |
| Skin90 | Pruritus**
Hyperpigmentation** Ecchymoses** Xerosis** |
Nephrogenic systemic fibrosis Calcinosis cutis |
| Malignancy (10%)91 | Increased risk for malignancy92 | Increased risks of all cancers and site-specific cancers (i.e., oral, liver, breast, colorectal, kidneys, bladder and blood cancers) |
| Miscellaneous63 | Carpal tunnel syndrome* (5%) |
These complications can be related to both ESRD and dialysis, but are more commonly seen when chronic dialysis is ongoing.
Can also be associated with chronic dialysis.
Related to acid-base imbalance
Values in bracket indicate prevalence63
N/A = not applicable
Table 3:
Complications related to peritoneal dialysis
| Infections93,94 | Peritonitis Catheter site infections Abdominal wall cellulitis |
| Catheter-related95 | Perioperative bowel injury / hemorrhage Obstruction to flow Exit site or concealed leakage Pain (on infusion or drainage) |
| Related to increased intra-abdominal pressure95 | Hernia Pleural leak (hydrothorax) Back pain |
| Metabolic95 | Hyperglycemia Hypertriglyceridemia Hyperinsulinemia Hyperleptinemia |
| Gastrointestinal96 | Gastric reflux Eating dysfunction |
| Neurological97 | Convulsions Toxic encephalopathy |
| Miscellaneous | Encapsulating peritoneal sclerosis98
Nutrient through peritoneal dialysate |
The very poor quality of life of some of these patients is illustrated by the fact that a significant percentage of them choose withdrawal of dialysis.21 In the USA in1994, 19% of patients supported by dialysis chose to discontinue it. In 2004, 24% discontinued dialysis, and in 2014, approximately 13–17% did so. Remarkably, in New England, almost every third patient chooses to withdraw from treatment.2,22 The percentage of patients (or their responsible family members or guardians) choosing withdrawal of dialysis and all other treatment is therefore high, and has not declined in recent years. Patients who withdraw from dialysis usually die within 10 days (median 8 days).22,23 However, it should again be emphasized that these patients are mainly not those who are on the waiting list for a kidney transplant.
Comparison with outcome after kidney allotransplantation
Kidney allotransplantation can ameliorate many of the problems associated with ESRD and chronic dialysis,24–33 addressing both survival and quality of life issues. Survival of patients with renal allotransplants from deceased donors is 85% after 3 years, and rises to 93% when a living donor kidney has been transplanted. At 5 years, survival in these two groups is 76% and 88%, respectively.2 Many studies have demonstrated that the quality of life in patients with kidney transplants is better than in those on dialysis.34–37 However, if the patient is not carefully selected, renal allotransplantation can be associated with serious complications that impact the patient’s quality of life. This could be even more so in those undergoing the initial renal xenotransplants, and so very careful patient selection will be essential.
There were (and possibly still are) some hospitals that refused to undertake kidney allotransplantation in patients >65 years of age, citing the adverse effect on survival of frailty and comorbidities. However, survival of these patients on dialysis was 81% at 1 year, but only 30% at 5 years, and 15% at 7 years, whereas after renal allotransplantation, survival was 93%, 70%, and 46% at the same time intervals.38,39 More recent data continue to indicate survival of patients >65 years of age after renal allotransplantation of approximately 80–90%, 80–90%, and 70–80% at 1, 3, and 5 years, respectively.4,40,41 Even patients >75 years of age at the time of renal transplantation did relatively well, with 81% alive at 1 year, 69% at 3 years, and 58% at 5 years.42
In a highly relevant study, Heldal et al compared survival after renal allotransplantation with that of patients on the waiting list for an allograft.43 At 1, 3, and 5 years, survival was 89% (after transplantation) vs 98% (on the waitlist), 74% vs 56%, and 64% vs 33%, suggesting that, if the patient survived the initial post-transplant period, transplantation offered better long-term outcome. Advancing age, therefore, should not be an absolute contraindication to kidney xenotransplantation.
The average remaining lifespan for a patient aged 20–24 on dialysis is 18.8 years, whereas after kidney allotransplantation it is 43.4 years, which is only 11 years less than in the general population. The average remaining lifespan for a patient aged 65–69 years on dialysis is only 4.6 years,44 whereas it is 11.4 years after a kidney transplant, approximately 5 years less than the general population.2 Once again, however, the patients on dialysis include many who have comorbidities rendering them unsuitable for transplantation, and therefore they cannot be compared directly with those with functioning renal allografts.
The advantages of ‘pre-emptive’ kidney transplantation
The complications (and costs) associated with dialysis are so significant that, in 2007, the National Kidney Foundation/Kidney Disease Outcomes Quality Initiative (NKF/KDOQI™) Conference decided to promote pre-emptive kidney allotransplantation, in which a patient would proceed directly to renal transplantation instead of dialysis, thus avoiding dialysis-related morbidity and mortality, with substantial benefit in terms of quality of life.
In the current era, pre-emptive transplantation is largely limited to those who can receive a graft from a living donor.45 One problem with this approach, however, is that the potential living donor is often not identified until the respective patient is close to the need for renal replacement therapy, and yet full assessment of the potential donor may take many months. Indeed, a recent report from Canada indicated that the mean donor evaluation time was 10.6 months for pre-emptive kidney transplantation. There is, however, considerable variation depending on the transplant center. For example, evaluation took so long in approximately one-third of the cases that the recipients had had to progress to dialysis while their donors were being evaluated (with a mean evaluation time in these cases of a remarkable 22 months).46,47In contrast to human living donors, xenotransplantation would not be delayed due to assessment of the pig.
The results of pre-emptive kidney transplantation are superior to those of transplantation after dialysis is underway.48,49 More than 50% of patients return to some form of paid work after pre-emptive kidney transplantation, whereas only approximately 25% re-join the workforce if hemodialysis precedes transplantation.45 All pig kidney xenotransplants could be pre-emptive.
Nevertheless, as the results of the clinical transplantation of a pig kidney are unknown, the patient might require subsequent allotransplantation (or the transplantation of a second pig graft). There are additional risks associated with retransplantation with allograft survival ranging from approximately 60–90% at 5 years,50–54 but in our opinion an initial pig renal transplant could be justified because, in its absence, an older patient may not survive until an allograft becomes available.
Expenditure related to renal replacement therapy
According to data from the USRDS, in the USA in 2015, expenditure on patients with ESRD was $33.8 billion,2 and accounted for 7.1 % of the overall costs of Medicare. In the same year, the total cost of care of patients with chronic kidney disease or ESRD was $98 billion. The cost of hemodialysis was $88,750 per person per year, and of peritoneal dialysis was $75,140. In contrast, the cost after kidney allotransplantation was $34,084 per person per year.44
In a detailed analysis of costs, Held and colleagues provided slightly different data (Table 4).55 From their data, it could be concluded that, if a patient with a functioning renal allograft survives for longer than two years, the procedure has been cost-effective.
Table 4:
Costs related to (i) management of a patient developing renal failure, (ii) chronic dialysis, and (ii) kidney allotransplantation*
| Item | Approximate cost (US$) |
|---|---|
| Chronic dialysis (per year) | 120,000a |
| Chronic dialysis total over 5 years | 600,000 |
|
Kidney allotransplantation (deceased human donor) Procurement of deceased donor kidney (from OPO) |
33,000b |
| Renal transplantation (initial hospitalization) | 145,000a |
| Maintenance immunosuppressive therapy (per year) | 32,000a |
| Renal allotransplantation total over 5 years | 338,000 |
Whether the costs of pig kidney xenotransplantation will be comparable to, or greater than, those associated with allotransplantation remains unknown. Many factors have to be taken into consideration. If the immunosuppressive therapy required is comparable (which is not yet certain), then the only major difference in costs may be the acquisition of the kidney. In the USA, the costs of acquisition of organs from deceased human donors are not insubstantial, with the cost of a single kidney varying from approximately $25,000 to $40,000 (mean $33,000),56 which is passed on to the recipient. When genetically-engineered pig organs become commercially available, it could well be that a pig kidney will be priced significantly higher than this, in part to defray the very considerable research and development expenditure that has been incurred during the past 20–30 years. However, against this expense, savings in the cost of caring for a patient with kidney failure, including dialysis (Table 4), convenience of ready availability of the organ, etc., will need to be considered.
The problem of the HLA-sensitized patient
Some HLA-sensitized wait-list patients have anti-HLA antibodies that cross-react with swine leukocyte antigens (SLA),57–59 thus placing them at a greater risk of rejection of a pig kidney graft, but there are other HLA-sensitized patients who do not appear to have antibodies that cross-react with SLA.57,60 Highly HLA-sensitized patients who do not have anti-SLA antibodies may therefore be very suitable candidates for kidney xenotransplantation. However, to avoid any increased risk of rejection, we suggest it might be wise to avoid any patient with anti-HLA antibodies in the first clinical trials of pig kidney xenotransplantation. Accepting highly HLA-sensitized patients for the first clinical trial may be adding an unnecessary risk, which may impact the outcome and complicate interpretation of the results of the trial. Once xenotransplantation is established, then it is likely that carefully-selected HLA highly-sensitized patients will become leading candidates for pig grafts.
Instead, selection should possibly be directed to non-sensitized older ESRD patients (i.e., those >65 years of age), otherwise in good health, but who are likely to wait (on dialysis) many years for a human donor organ. It is these patients who might benefit most from undergoing pig kidney xenotransplantation, even if only to delay the need for chronic dialysis for a significant period of time while they await allotransplantation.
Of importance in this respect is that the current (admittedly limited) evidence is that sensitization to pig antigens, if it occurred after a pig kidney transplant, would not reduce the possibility of the patient obtaining an allograft, nor be detrimental to the outcome of allotransplantation.61
Immunosuppression for xenotransplantation
It is not yet clear whether conventional immunosuppressive therapy (as administered to a patient with a renal allograft) will be fully successful after xenotransplantation. Novel co-stimulation blockade agents may be required.6–8,62
Conclusions
Chronic dialysis in patients with ESRD is associated with high costs, relatively high mortality, a poor quality of life, and significant morbidity, though those selected as candidates for kidney transplantation are generally healthier than others. Nevertheless, the median wait-time for a patient with ESRD on dialysis to obtain a kidney from a deceased donor is almost 4 years, and may be significantly longer. During these long periods of time a significant number of excellent transplant candidates will have died. Any procedure that might (i) delay the need for dialysis and (ii) reduce the period during which the patient is on dialysis while awaiting an allograft would therefore be worthwhile.
As genetically-engineered pig kidneys have satisfactorily supported life in immunosuppressed nonhuman primates for many months or even more than a year, with apparent low (or no) risk of developing allosensitization, a patient population amenable to participating in clinical trials of xenotransplantation clearly exists, and may be substantial. Patients >65 years of age with no major comorbidities, who are not sensitized to HLA (to avoid any possibility of antibodies cross-reacting with SLA) might be suitable candidates. In order to offer a potential candidate the best possible outcome, he or she should be added to the waiting-list for an allograft, with a pig kidney transplanted pre-emptively (before initiating dialysis). If the pig kidney graft fails at any stage, the patient would then begin chronic dialysis, and continue to await an allograft. The present evidence is that failure of a pig graft would not be detrimental to a subsequent allograft.61
Acknowledgements
Work by our group on xenotransplantation at UAB is supported in part by NIH grant #U19 AI090959/08.
Abbreviations
- ESRD
end-stage renal disease
- HLA
human leukocyte antigens
- SLA
swine leukocyte antigens
Footnotes
Conflict of interest
Robert Gaston is an employee of CTI Clinical Trials and Consulting, Inc., Covington, KY. The other authors report no conflict of interest.
References
- 1.Levey AS, Coresh J. Chronic kidney disease. Lancet. 2012;379:165–180. [DOI] [PubMed] [Google Scholar]
- 2.USRDS. Annual data report 2017. [cited 2018 3/30/2018]; Available from: https://www.usrds.org/adr.aspx.
- 3.Schold JD, Meier-Kriesche HU. Which renal transplant candidates should accept marginal kidneys in exchange for a shorter waiting time on dialysis? Clin J Am Soc Nephrol. 2006;1:532–538. [DOI] [PubMed] [Google Scholar]
- 4.SRTR. Annual Data Report 2016. [cited 2018 7/13/2018]; Available from:https://www.srtr.org/.
- 5.Orandi BJ, Luo X, Massie AB, et al. Survival benefit with kidney transplants from HLA-incompatible live donors. N Engl J Med. 2016;374:940–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Higginbotham L, Mathews D, Breeden CA, et al. Pre‐transplant antibody screening and anti‐CD154 costimulation blockade promote long‐term xenograft survival in a pig‐to‐primate kidney transplant model. Xenotransplantation. 2015;22:221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwase H, Liu H, Wijkstrom M, et al. Pig kidney graft survival in a baboon for 136 days: longest life-supporting organ graft survival to date. Xenotransplantation. 2015;22:302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwase H, Hara H, Ezzelarab M, et al. Immunological and physiological observations in baboons with life‐supporting genetically engineered pig kidney grafts. Xenotransplantation. 2017;24:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martens GR, Estrada J, Sidner RA, et al. Porcine GGTA1/B4GalNT2 gene knockout reduces antibody binding and achieves one-year life-supporting renal xenograft in pig-to-rhesus model. Xenotransplantation. 2017;24:12–15. [Google Scholar]
- 10.Foley RN, Chen S-C, Solid CA, Gilbertson DT, Collins AJ. Early mortality in patients starting dialysis appears to go unregistered. Kidney Int. 2014;86:392–398. [DOI] [PubMed] [Google Scholar]
- 11.Bae EH, Kim HY, Kang YU, Kim CS, Ma SK, Kim SW. Risk factors for in-hospital mortality in patients starting hemodialysis. Kidney Res Clin Pract. 2015;34:154–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casingal V, Glumac E, Tan M, Sturdevant M, Nguyen T, Matas A. Death on the kidney waiting list—good candidates or not? Am J Transplant. 2006;6:1953–1956. [DOI] [PubMed] [Google Scholar]
- 13.Stewart D, Kucheryavaya A, Klassen D, Turgeon N, Formica R, Aeder M. Changes in deceased donor kidney transplantation one year after KAS implementation. Am J Transplant. 2016;16:1834–1847. [DOI] [PubMed] [Google Scholar]
- 14.Llach F, Forero FV. Secondary hyperparathyroidism in chronic renal failure: pathogenic and clinical aspects. Am J Kidney Dis. 2001;38:S20–S33. [DOI] [PubMed] [Google Scholar]
- 15.Kalantar-Zadeh K, Kopple JD. Relative contributions of nutrition and inflammation to clinical outcome in dialysis patients. Am J Kidney Dis. 2001;38:1343–1350. [DOI] [PubMed] [Google Scholar]
- 16.Pecoits-Filho R, Lindholm B, Stenvinkel P. The malnutrition, inflammation, and atherosclerosis (MIA) syndrome–the heart of the matter. Nephrol Dial Transplant. 2002;17:28–31. [DOI] [PubMed] [Google Scholar]
- 17.Kalantar-Zadeh K, Ikizler TA, Block G, Avram MM, Kopple JD. Malnutrition inflammation complex syndrome in dialysis patients: causes and consequences. Am J Kidney Dis. 2003;42:864–881. [DOI] [PubMed] [Google Scholar]
- 18.Hauser AB, Stinghen AE, Kato S, et al. Characteristics and causes of immune dysfunction related to uremia and dialysis. Perit Dialysis Int. 2008;28:S183–S187. [PubMed] [Google Scholar]
- 19.Cengic B, Resic H. Depression in hemodialysis patients. Bosn J Basic Med Sci. 2010;10:S73–S78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khan IH. Comorbidity: the major challenge for survival and quality of life in endstage renal disease. Nephrol Dial Transplant. 1998;13 Suppl1:76–79. [DOI] [PubMed] [Google Scholar]
- 22.Germain MJ, Cohen LM, Davison SN. Withholding and withdrawal from dialysis: what we know about how our patients die. Semin Dial. 2007;20:195–199. [DOI] [PubMed] [Google Scholar]
- 23.Chater S, Davison S, Germain M, Cohen L. Withdrawal from dialysis: a palliative care perspective. Clin Nephrol. 2006;66:364–372. [DOI] [PubMed] [Google Scholar]
- 24.Kawamura M, Fijimoto S, Hisanaga S, Yamamoto Y, Eto T. Incidence, outcome, and risk factors of cerebrovascular events in patients undergoing maintenance hemodialysis. Am J Kidney Dis. 1998;31:991–996. [DOI] [PubMed] [Google Scholar]
- 25.La Rocca E, Fiorina P, Di Carlo V, et al. Cardiovascular outcomes after kidney–pancreas and kidney–alone transplantation1. Kidney Int. 2001;60:1964–1971. [DOI] [PubMed] [Google Scholar]
- 26.Oliveras A, Roquer J, Puig JM, et al. Stroke in renal transplant recipients: epidemiology, predictive risk factors and outcome. Clin Transplant. 2003;17:1–8. [DOI] [PubMed] [Google Scholar]
- 27.Seliger SL, Gillen DL, Tirschwell D, Wasse H, Kestenbaum BR, Stehman-Breen CO. Risk factors for incident stroke among patients with end-stage renal disease. J Am Soc Nephrol. 2003;14:2623–2631. [DOI] [PubMed] [Google Scholar]
- 28.Snyder JJ, Kasiske BL, Maclean R. Peripheral arterial disease and renal transplantation. J Am Soc Nephrol. 2006;17:2056–2068. [DOI] [PubMed] [Google Scholar]
- 29.Aull-Watschinger S, Konstantin H, Demetriou D, et al. Pre-transplant predictors of cerebrovascular events after kidney transplantation. Nephrol Dial Transpl. 2007;23:1429–1435. [DOI] [PubMed] [Google Scholar]
- 30.Kunzendorf U, Krämer BK, Arns W, et al. Bone disease after renal transplantation. Nephrol Dial Transplant. 2008;23:450–458. [DOI] [PubMed] [Google Scholar]
- 31.Lentine KL, Rey LAR, Kolli S, et al. Variations in the risk for cerebrovascular events after kidney transplant compared with experience on the waiting list and after graft failure. Clin J Am Soc Nephrol. 2008;3:1090–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zolty R, Hynes P, Vittorio T. Severe left ventricular systolic dysfunction may reverse with renal transplantation: uremic cardiomyopathy and cardiorenal syndrome. Am J Transplant. 2008;8:2219–2224. [DOI] [PubMed] [Google Scholar]
- 33.Willicombe M, Kumar N, Goodall D, et al. Incidence, risk factors, and outcomes of stroke post‐transplantation in patients receiving a steroid sparing immunosuppression protocol. Clin transplant. 2015;29:18–25. [DOI] [PubMed] [Google Scholar]
- 34.Ware JE Jr. SF-36 Health Survey Update. Spine. 2000;25:3130–3139. [DOI] [PubMed] [Google Scholar]
- 35.Matas AJ, Halbert R, Barr ML, et al. Life satisfaction and adverse effects in renal transplant recipients: a longitudinal analysis. Clin Transplant. 2002;16:113–121. [DOI] [PubMed] [Google Scholar]
- 36.Ogutmen B, Yildirim A, Sever MS, et al. Health-related quality of life after kidney transplantation in comparison intermittent hemodialysis, peritoneal dialysis, and normal controls. Transplant Proc. 2006;38:419–421. [DOI] [PubMed] [Google Scholar]
- 37.Liem YS, Bosch JL, Arends LR, Heijenbrok-Kal MH, Hunink M. Quality of life assessed with the medical outcomes study Short Form 36‐Item Health Survey of patients on renal replacement therapy: a systematic review and meta‐analysis. Value Health. 2007;10:390–397. [DOI] [PubMed] [Google Scholar]
- 38.Mallick N, El Marasi A. Dialysis in the elderly, to treat or not to treat? Nephrol Dial Transplant. 1999;14:37–39. [DOI] [PubMed] [Google Scholar]
- 39.Oniscu GC, Brown H, Forsythe JL. How great is the survival advantage of transplantation over dialysis in elderly patients? Nephrol Dial Transplant. 2004;19:945–951. [DOI] [PubMed] [Google Scholar]
- 40.Rao PS, Merion RM, Ashby VB, Port FK, Wolfe RA, Kayler LK. Renal transplantation in elderly patients older than 70 years of age: results from the Scientific Registry of Transplant Recipients. Transplantation. 2007;83:1069–1074. [DOI] [PubMed] [Google Scholar]
- 41.Huang E, Poommipanit N, Sampaio MS, et al. Intermediate-term outcomes associated with kidney transplantation in recipients 80 years and older: an analysis of the OPTN/UNOS database. Transplantation. 2010;90:974–979. [DOI] [PubMed] [Google Scholar]
- 42.Sener A, Schweitzer EJ, Munivenkatappa R, et al. Deceased-donor renal transplantation in the geriatric population demonstrates equal graft survival compared with younger recipients. Transplantation. 2009;87:1549–1554. [DOI] [PubMed] [Google Scholar]
- 43.Heldal K, Hartmann A, Grootendorst DC, et al. Benefit of kidney transplantation beyond 70 years of age. Nephrol Dial Transplant. 2010;25:1680–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.USRDS. Annual Data Report 2015. [cited 2018 3/30/2018]; Available from: https://www.usrds.org/adr.aspx.
- 45.Abecassis M, Bartlett ST, Collins AJ, et al. Kidney transplantation as primary therapy for end-stage renal disease: a National Kidney Foundation/Kidney Disease Outcomes Quality Initiative (NKF/KDOQI™) conference. Clin J Am Soc Nephrol. 2008;3:471–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gourlay W Preemptive kidney transplantation: what’s the hold up? (Commentary) Transplantation. 2018;102:1035–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Habbous S, Dixon S, Lam N, et al. Initiiating maintenance dialysis prior ro living kidney donor transplantation when a donor candidate evaluation is well underway. Transplantation. 2018;102:e345–e353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kasiske BL, Snyder JJ, Matas AJ, Ellison MD, Gill JS, Kausz AT. Preemptive kidney transplantation: the advantage and the advantaged. J Am Soc Nephrol. 2002;13:1358–1364. [DOI] [PubMed] [Google Scholar]
- 49.Innocenti GR, Wadei HM, Prieto M, et al. Preemptive living donor kidney transplantation: do the benefits extend to all recipients? Transplantation. 2007;83:144–149. [DOI] [PubMed] [Google Scholar]
- 50.Coupel S, Giral-Classe M, Karam G et al. Ten-year survival of second kidney transplants: impact of immunologic factors and renal function at 12 months. Kidney Int. 2003;64:674–680. [DOI] [PubMed] [Google Scholar]
- 51.Pour-Reza-Gholi F, Nafar M, Saeedinia A et al. Kidney retransplantation in comparison with first kidney transplantation. Transplant proc. 2005;37:2962–2964. [DOI] [PubMed] [Google Scholar]
- 52.Surga N, Viart L, Wetzstein M, et al. Impact of renal graft nephrectomy on second kidney transplant survival. Int Urol Nephrol. 2013;45:87–92. [DOI] [PubMed] [Google Scholar]
- 53.Dinis P, Nunes P, Marconi L et al. Kidney retransplantation: removal or persistence of the previous failed allograft? Transplant Proc. 2014;46:1730–1734. [DOI] [PubMed] [Google Scholar]
- 54.Fadli SE, Pernin V, Nogue E et al. Impact of graft nephrectomy on outcomes of second kidney transplantation. Int J Urol. 2014;21:797–802. [DOI] [PubMed] [Google Scholar]
- 55.Held PJ, McCormick F, Ojo A, Roberts JP. A cost-benefit analysis of government compensation of kidney donors. Am J Transplant. 2016;16:677–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saari R, Cooper DKC Financial aspects of organ procurement from deceased donors in the USA – relevance to xenotransplantation. Xenotransplantation 2017;24:e12322 doi: 10.1111/xen.12322. [DOI] [PubMed] [Google Scholar]
- 57.Cooper D, Tseng Y, Saidman S. Alloantibody and xenoantibody cross-reactivity in transplantation. Transplantation. 2004;77:1–5. [DOI] [PubMed] [Google Scholar]
- 58.Martens GR, Reyes LM, Butler JR, et al. Humoral reactivity of renal transplant waitlisted patients to cells from GGTA1/CMAH/B4GalNT2, and SLA class I knockout pigs. Transplantation. 2017;101:e86–e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ladowski JM, Reyes LM, Martens GR, et al. Swine leukocyte antigen Class II Is a xenoantigen. Xenotransplantation. 2018;102:249–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang Z, Hara Z, Long C, et al. Immune responses of HLA-highly-sensitized and non-sensitized patients to genetically engineered pig cells. Transplantation. 2018;102:e195–e204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qi Li, Hara H Breimer ME, Wang Y Cooper DKC. Is sensitization to pig antigens detrimental to subsequent allotransplantation? Xenotransplantation. 2018;25:e12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kannan S, Butler J, Li P, Cooper D, Ekser B. The role of costimulation blockade in solid organ and islet xenotransplantation. J Immunol res. 2017;2017:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goodkin DA, Bragg-Gresham JL, Koenig KG, et al. Association of comorbid conditions and mortality in hemodialysis patients in Europe, Japan, and the United States: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Clin J Am Soc Nephrol. 2003;14:3270–3277. [DOI] [PubMed] [Google Scholar]
- 64.Dalrymple LS, Go AS. Epidemiology of acute infections among patients with chronic kidney disease. Clin J Am Soc Nephrol. 2008;3:1487–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Naqvi SB, Collins AJ. Infectious complications in chronic kidney disease. Adv Chronic Kidney Dis. 2006;13:199–204. [DOI] [PubMed] [Google Scholar]
- 66.Pisoni RL, Bragg-Gresham JL, Young EW, et al. Anemia management and outcomes from 12 countries in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2004;44:94–111. [DOI] [PubMed] [Google Scholar]
- 67.Cheung J, Yu A, LaBossiere J, Zhu Q, Fedorak RN. Peptic ulcer bleeding outcomes adversely affected by end-stage renal disease. Gastrointest Endosc. 2010;71:44–49. [DOI] [PubMed] [Google Scholar]
- 68.Roy-Chaudhury P, Kelly BS, Melhem M, et al. Vascular access in hemodialysis: issues, management, and emerging concepts. Clin Cardiol. 2005;23:249–273. [DOI] [PubMed] [Google Scholar]
- 69.Schiffrin EL, Lipman ML, Mann JF. Chronic kidney disease: effects on the cardiovascular system. Circulation. 2007;116:85–97. [DOI] [PubMed] [Google Scholar]
- 70.Herzog CA. Cardiac arrest in dialysis patients: approaches to alter an abysmal outcome. Kidney Int. 2003;63:S197–S200 [DOI] [PubMed] [Google Scholar]
- 71.Herzog CA, Mangrum JM, Passman R. Non‐coronary heart disease in dialysis patients: sudden cardiac death and dialysis patients. Semin Dial. 2008;21:300–307. [DOI] [PubMed] [Google Scholar]
- 72.McIntyre CW, Burton JO, Selby NM, et al. Hemodialysis-induced cardiac dysfunction is associated with an acute reduction in global and segmental myocardial blood flow. Clin J Am Soc Nephrol. 2008;3:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Burton JO, Jefferies HJ, Selby NM, McIntyre CW. Hemodialysis-induced cardiac injury: determinants and associated outcomes. Clin J Am Soc Nephrol. 2009;4:914–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Selby NM, McIntyre CW. The acute cardiac effects of dialysis. Semin dial. 2007; 20:220–228. [DOI] [PubMed] [Google Scholar]
- 75.Recio-Mayoral A, Banerjee D, Streather C, Kaski JC. Endothelial dysfunction, inflammation and atherosclerosis in chronic kidney disease–a cross-sectional study of predialysis, dialysis and kidney-transplantation patients. Atherosclerosis. 2011;216:446–451. [DOI] [PubMed] [Google Scholar]
- 76.Tok D, Gullu H, Erdogan D, et al. Impaired coronary flow reserve in hemodialysis patients: a transthoracic Doppler echocardiographic study. Nephron Clin Pract. 2005;101:c200–c206. [DOI] [PubMed] [Google Scholar]
- 77.O’Hare A, Johansen K. Lower-extremity peripheral arterial disease among patients with end-stage renal disease. J Am Soc Nephrol. 2001;12:2838–2847. [DOI] [PubMed] [Google Scholar]
- 78.Kovacic V, Roguljic L, Kovacic V. Metabolic acidosis of chronically hemodialyzed patients. Am J Nephrol. 2003;23:158–164. [DOI] [PubMed] [Google Scholar]
- 79.Kopple JD, Kalantar-Zadeh K, Mehrotra R. Risks of chronic metabolic acidosis in patients with chronic kidney disease. Kidney Int. 2005;67:S21–S27. [DOI] [PubMed] [Google Scholar]
- 80.Kraut JA, Kurtz I. Metabolic acidosis of CKD: diagnosis, clinical characteristics, and treatment. Am J Kdney Dis. 2005;45:978–993. [DOI] [PubMed] [Google Scholar]
- 81.Cibulka R, Racek J. Metabolic disorders in patients with chronic kidney failure. Physiol Res. 2007;56:697–705 [DOI] [PubMed] [Google Scholar]
- 82.Imanishi Y, Inaba M, Nakatsuka K, et al. FGF-23 in patients with end-stage renal disease on hemodialysis. Kidney Int. 2004;65:1943–1946. [DOI] [PubMed] [Google Scholar]
- 83.Bardin T Musculoskeletal manifestations of chronic renal failure. Curr Opin Rheumatol. 2003;15:48–54. [DOI] [PubMed] [Google Scholar]
- 84.Moe S, Drüeke T, Cunningham J, et al. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2006;69:1945–1953. [DOI] [PubMed] [Google Scholar]
- 85.Arslan D, Aslan G, Sifil A, et al. Sexual dysfunction in male patients on hemodialysis: assessment with the International Index of Erectile Function (IIEF). Int J Impot Res. 2002;14:539–542. [DOI] [PubMed] [Google Scholar]
- 86.Salerno FR, Parraga G, McIntyre CW. Why Is your patient still short of breath? understanding the complex pathophysiology of dyspnea in chronic kidney disease. Semin Dial. 2017;30:50–57. [DOI] [PubMed] [Google Scholar]
- 87.De Sousa A Psychiatric issues in renal failure and dialysis. Indian J Nephrol. 2008;18:47–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Murtagh FE, Addington-Hall J, Higginson IJ. The prevalence of symptoms in endstage renal disease: a systematic review. Adv Chronic Kidney Dis. 2007;14:82–99. [DOI] [PubMed] [Google Scholar]
- 89.Cano AE, Neil AK, Kang J-Y, et al. Gastrointestinal symptoms in patients with end stage renal disease undergoing treatment by hemodialysis or peritoneal dialysis. Am J Gastroenterol. 2007;102:1990–1997. [DOI] [PubMed] [Google Scholar]
- 90.Galperin TA, Cronin AJ, Leslie KS. Cutaneous manifestations of ESRD. Clin J Am Soc Nephrol. 2014;9:201–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lin MY, Kuo MC, Hung CC, et al. Association of dialysis with the risks of cancers. PLoS One. 2015;10:e0122856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wong G, Hayen A, Chapman JR, et al. Association of CKD and cancer risk in older people. J Am Soc Nephrol. 2009;20:1341–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Swartz RD. Chronic peritoneal dialysis: mechanical and infectious complications. Nephron. 1985;40:29–37. [DOI] [PubMed] [Google Scholar]
- 94.Van Diepen AT, Tomlinson GA, Jassal SV. The association between exit site infection and subsequent peritonitis among peritoneal dialysis patients. Clin J Am Soc Nephrol. 2012;7:1266–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McCormick BB, Bargman JM. Noninfectious complications of peritoneal dialysis: implications for patient and technique survival. J Am Soc Nephrol. 2007;18:3023–3025. [DOI] [PubMed] [Google Scholar]
- 96.Strid H, Simrén M, Johansson AC, Svedlund J, Samuelsson O, Björnsson ES. The prevalence of gastrointestinal symptoms in patients with chronic renal failure is increased and associated with impaired psychological general well‐being. Nephrol Dial Transplant. 2002;17:1434–1439. [DOI] [PubMed] [Google Scholar]
- 97.Tyler HR. Neurologic disorders in renal failure. Am J Med. 1968;44:734–748. [DOI] [PubMed] [Google Scholar]
- 98.Moinuddin Z, Summers A, Van Dellen D, Augustine T, Herrick SE. Encapsulating peritoneal sclerosis—a rare but devastating peritoneal disease. Front Physiol. 2015;5:470–481. [DOI] [PMC free article] [PubMed] [Google Scholar]