Abstract
Objectives:
Fear of cancer recurrence (FCR) is a top concern of breast cancer (BC) survivors and their spouses, yet little is known about responses to FCR triggers in daily life. We examined whether a biologically-based individual difference—threat sensitivity—predicted FCR in couples facing the first post-diagnosis mammogram (MMG). We hypothesized that threat sensitivity would predict greater FCR reactivity before the MMG and higher peak FCR on the MMG day, controlling for global anxiety. We also explored the link between threat sensitivity and FCR recovery after MMG.
Design and Sample:
Fifty-seven early-stage BC patients and their spouses completed cross-sectional measures of threat sensitivity and global anxiety. Couples then reported daily FCR during a three-week diary period that began two weeks before the patient’s MMG appointment.
Methods:
Multilevel actor-partner interdependence modeling was used to estimate within-person random slopes of FCR before (reactivity) and after (recovery) the MMG. Random intercepts captured individual differences in peak FCR on the MMG day. Patient and spouse threat sensitivity and anxiety were entered as predictors of reactivity, peak, and recovery.
Findings:
FCR increased leading to MMG; however, inconsistent with hypotheses, this reactivity was not significantly predicted by threat sensitivity. Actor, but not partner, effects for peak FCR emerged, such that patients and spouses with greater threat sensitivity had greater FCR on the MMG day. FCR decreased after the MMG, and spouse, but not patient, threat sensitivity predicted slower recovery for both partners.
Conclusions:
Findings lend preliminary support for the role of threat sensitivity in the experience of FCR as couples confront threatening events in BC survivorship.
Implications for Psychosocial Providers:
MMGs can be a triggering event for couples. Threat sensitivity may help identify those who are likely to experience elevations in FCR during this stressful period.
Keywords: breast cancer, fear of cancer recurrence, survivorship, close relationships, daily diaries, behavioral inhibition system, personality
An important aspect of psychosocial adjustment in cancer survivors and their significant others is the experience of fear, worry, or concern about the threat of cancer recurrence.1 The majority of survivors and their romantic partners/spouses (hereafter termed spouse) report some degree of fear of cancer recurrence (FCR),2–4 and higher levels have been linked to more psychological distress, greater functional impairment, and lower quality of life.4,5 Several studies have also reported moderate associations between patient and spouse FCR,2,6 suggesting that intimate relationships may be an important context for understanding the experience of FCR. In the current study, we examined daily FCR in breast cancer (BC) patients and their spouses during a daily diary period that overlapped with the first post-diagnosis mammogram (MMG), an event frequently identified by patients as a trigger of FCR.7–9
Theories of cancer anxiety and FCR posit a variety of factors that interact with the inherently threatening cancer context to explain FCR—these include proximal (e.g., everyday triggering events) and distal (e.g., pre-existing and dispositional characteristics) determinants, cognitive and emotional processes, coping efforts, significant other involvement, and mortality salience.10,11 A central feature of survivorship is the ongoing sense of uncertainty and threat triggered by proximal internal (e.g., transient somatic symptoms) and external (e.g., medical appointments) events that serve as reminders that one has survived cancer diagnosis and treatment. Although proximal triggers of FCR are core components of theoretical and conceptual frameworks,10,11 there have been few direct examinations of the response to and recovery from naturalistic triggering events, such as a follow-up MMG (for an exception, see 8). Intensive longitudinal studies12 of these particularly stressful periods during survivorship could provide important insights into individual and dyadic adjustment to cancer-related proximal events and complement findings from retrospective, cross-sectional studies.
Personality-related individual differences are important distal influences on the experience of FCR throughout survivorship.10,11 For example, models of cancer anxiety have pointed to tolerance of uncertainty as an important (but largely unexamined) pre-existing personality vulnerability that may buffer extreme worry and distress.11 Another relevant personality characteristic yet to be examined as a predictor of FCR, to our knowledge, is threat sensitivity. Threat sensitivity reflects individual differences in the general tendency to attend to, behaviorally and emotionally respond to, and avoid threatening negative stimuli.13 Linked to the brain-based behavioral inhibition system (BIS)14 or withdrawal system,15 individual differences in BIS (or threat) sensitivity have been identified by prominent theorists as a fundamental component of personality underlying traits such as neuroticism and negative emotionality.13,16–18 Studies have shown that individuals with greater threat sensitivity experience more anxiety during tasks involving potential punishment13 and heightened reactivity to daily negative events.19 Moreover, threat sensitivity was found to interact with expectancies of cancer recurrence to predict greater global distress and disengagement coping in BC patients.20 FCR is, by definition, a cognitive and emotional response to the threat of cancer recurrence, and thus should be experienced to a greater extent among high threat sensitive patients and spouses when the possibility of recurrence is salient. This notion, however, has not yet been studied empirically.
The current study aimed to determine whether threat sensitivity predicts trajectories of FCR among early-stage BC patients and their spouses over the course of a daily-diary period beginning about two weeks before and ending about one week after the patient’s first follow-up MMG post-diagnosis. Threat sensitivity was measured cross-sectionally with a brief, global, self-report inventory about a week before the diary period. Patient and spouse ratings of FCR were obtained daily over this 3-week period and were expected to increase over the days leading up to the MMG (reactivity) and decrease over the days after the MMG (recovery). We hypothesized that threat sensitivity would predict greater FCR reactivity before the MMG as well as higher peak FCR on the MMG day. The relevance of threat sensitivity for FCR recovery after the removal of the threatening stimulus (i.e., after patients received negative MMG results) is unclear. Therefore, we explored the link between threat sensitivity and FCR recovery after the MMG, but no specific hypothesis was made for this effect. For these tests, we controlled for patient and spouse global anxiety symptoms, which were measured in the cross-sectional assessment. Threat sensitivity can be viewed as an indicator of anxiety proneness,13 such that individuals who are more threat sensitive are more likely to experience anxiety when confronting threatening stimuli. However, in the absence of such stimuli, these individuals are not expected to be more anxious than those who are less threat sensitive. Thus, controlling for global state anxiety symptoms provides a more precise test of threat sensitivity as a stable individual difference.
Method
Participants and Procedure
Data were drawn from a larger longitudinal IRB-approved study of early-stage BC patients and their spouses. Patients were recruited from a community cancer center if they met the following criteria: diagnosed with Stage 0 (lobular/ductal carcinoma in situ) through IIIA BC, had recent BC surgery, were in a committed romantic relationship with a partner who also agreed to participate, had no prior cancer diagnoses, were English speaking, and lived within an hour from the recruitment site. The electronic medical records of 1161 patients who had a recent positive breast biopsy were reviewed for potential eligibility. Those who appeared eligible (n = 463) were contacted about the study. Of these, 192 declined, 110 were ineligible, 82 were unable to be reached, and 79 provided informed consent and participated. Fifty-seven couples participated in the daily diary period examined in this report, which took place at the end of the year-long study (16 couples withdrew/declined and 6 had scheduling conflicts).
The average age of patients and spouses was 58 (SD = 9) and 60 (SD = 10) years, respectively. Most couples were married (93%) and heterosexual (96%), with an average relationship length of 30 years (SD = 14). All participants were non-Hispanic/Latino, most were Caucasian (87%), and the modal family income was over $100,000. Fifty-three percent of patients were diagnosed with Stage IA, 25% with Stage IIA, 12% with Stage 0, 9% with Stage IIB, and 1% with Stage IIIA. In terms of adjuvant treatment, most patients received radiation (72%) and hormonal therapy (84%), and few received chemotherapy (30%).
A three-week daily diary period was scheduled around each patient’s first MMG, which was on average 12.2 months post-diagnosis (SD = 1.9). The three-week diary period began an average of 12 days (SD = 5.4) before the MMG appointment. During the diary period, partners independently completed a brief online survey each evening within about an hour of going to sleep. The diary compliance rate for patients and spouses was 91% and 86%, respectively. At the cancer center from which patients were recruited, it is standard for current BC patients to receive their MMG results on the same day as their appointment. No patients experienced a cancer recurrence during their participation. A cross-sectional assessment took place about a week before this daily diary period. Separate links to the online cross-sectional surveys were emailed to patients and spouses, who completed the surveys from home.
Measures
Cross-sectional measures.
The 7-item Behavioral Inhibition System (BIS) Scale13 was used to measure threat sensitivity as a stable individual difference or personality characteristic. Example items include “criticism or scolding hurts me quite a bit” and “if I think something unpleasant is going to happen I usually get pretty ‘worked up.’” Items were averaged to create a composite ranging from one (very false for me) to four (very true for me), which demonstrated acceptable reliability (for both partners, Cronbach’s α = .80). The 4-item PROMIS Anxiety Short Form21 was used to measure global anxiety. Items were averaged to create a composite ranging from one (never) to five (always), which also had acceptable reliability (patients: α = .86; spouses: α = .71).
Daily fear of recurrence measure.
In the absence of a validated daily measure of FCR, six of the highest-loading items from the Fear of Cancer Recurrence Inventory7 (FCRI) were selected and adapted for daily use. These included the four items comprising the Distress subscale, one item from the Insight subscale (asked whether one “worried excessively” about recurrence), and one item from the Severity subscale (asked “how much time today did you spend thinking about the possibility of cancer recurrence?”). Each item was rated on a zero (first five items: not at all; sixth item: I didn’t think about it at all) to four (first five items: extremely; sixth item: several hours) scale. Items were summed to create a composite. Because we were interested in modeling within-person trajectories of FCR, coefficient omega was used to estimate reliability of within-person change,22 which was strong (for both partners, ω = .91).
Data Analytic Plan
We used multilevel actor-partner interdependence modeling in Mplus23 to estimate parameters of interest and conduct inferential tests of our hypotheses. Negative binomial hurdle models were estimated to account for the count distribution of daily FCR and high frequency of zero scores (patients: 62.4%, spouses: 77.1%).24 Piecewise growth curve modeling was used to estimate within-person random slopes of FCR before (reactivity) and after (recovery) the MMG. Time was centered on the MMG day, such that random intercepts captured individual differences in peak FCR. Patient and spouse threat sensitivity and global anxiety were grand mean-centered and entered as between-person predictors of individual differences in FCR reactivity (i.e., within-person change in FCR before the MMG), peak (i.e., model-implied level of FCR on the MMG day), and recovery (i.e., within-person change in FCR after the MMG). Both the effect of one partner’s threat sensitivity on his/her own FCR (actor effects) and the effect of one partner’s threat sensitivity on his/her partner’s FCR (partner effects) were examined for each outcome of interest. Satorra-Bentler χ2 difference tests were conducted to compare fit of nested models.25
Results
Descriptive statistics are shown in Table 1. First, we examined paired differences between patients’ and spouses’ scores on raw versions of the focal variables. On average across the diary period, patients reported significantly higher FCR scores than spouses (mean difference = 0.74, t(56) = 2.71, p < .01), corresponding to a medium sized effect (d = 0.45). Patients also reported greater peak levels of FCR on the day of the MMG compared to spouses (mean difference = 3.21, t(56) = 3.63, p < .01), with a medium sized effect (d = 0.71). Patients reported greater threat sensitivity than spouses, with a mean difference of 0.31, t(56) = 3.28, p < .01, which is a medium effect size (d = 0.61). Patients also endorsed greater global anxiety than spouses, with a mean difference of 0.31, t(56) = 3.13, p < .01, also a medium sized effect (d = 0.59). Thus, compared to spouses, patients reported greater average daily FCR across the MMG period and peak FCR on the MMG day as well as greater threat sensitivity and global anxiety.
Table 1.
Descriptive statistics
| 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|
| 1. Patient fear of recurrence | - | |||||
| 2. Spouse fear of recurrence | .29* | - | ||||
| 3. Patient threat sensitivity | .26† | −.06 | - | |||
| 4. Spouse threat sensitivity | .07 | .14 | .19 | - | ||
| 5. Patient global anxiety | .24† | .03 | .43* | .35* | - | |
| 6. Spouse global anxiety | −.03 | .39* | .02 | .07 | −.02 | - |
| Mean | 1.70 | 0.96 | 2.91 | 2.59 | 1.55 | 1.24 |
| Standard deviation | 2.67‡ | 1.78‡ | 0.53 | 0.51 | 0.64 | 0.40 |
| Intraclass correlation | .28 | .45 | - | - | - | - |
Note. N = 57 couples. Correlations are based on between-person averages of fear of recurrence (FCR) across diary period. The possible range of FCR is 0-24, threat sensitivity is 1-4, and global anxiety is 1-5.
Within-person standard deviation.
p < .10.
p < .05.
Reactivity
Both patient and spouse FCR exhibited notable increases during the days leading up to the MMG, and thus this effect was constrained to be equal across partners, which resulted in similar model fit, χ2 (1) = 0.24, p = .62. The model-predicted reactivity slopes for patient and spouse are shown in Figure 1. The rate ratio associated with the fixed reactivity slope indicated that patient and spouse FCR scores increased by 2.4% each day as they each approached the MMG day. Results of the focal analysis testing the effects of threat sensitivity on FCR reactivity are shown in Table 2. Neither patient nor spouse threat sensitivity emerged as a significant predictor of FCR reactivity, thus failing to support our hypothesis that threat sensitivity would predict greater FCR reactivity for patients and spouses before the MMG.2
Figure 1.
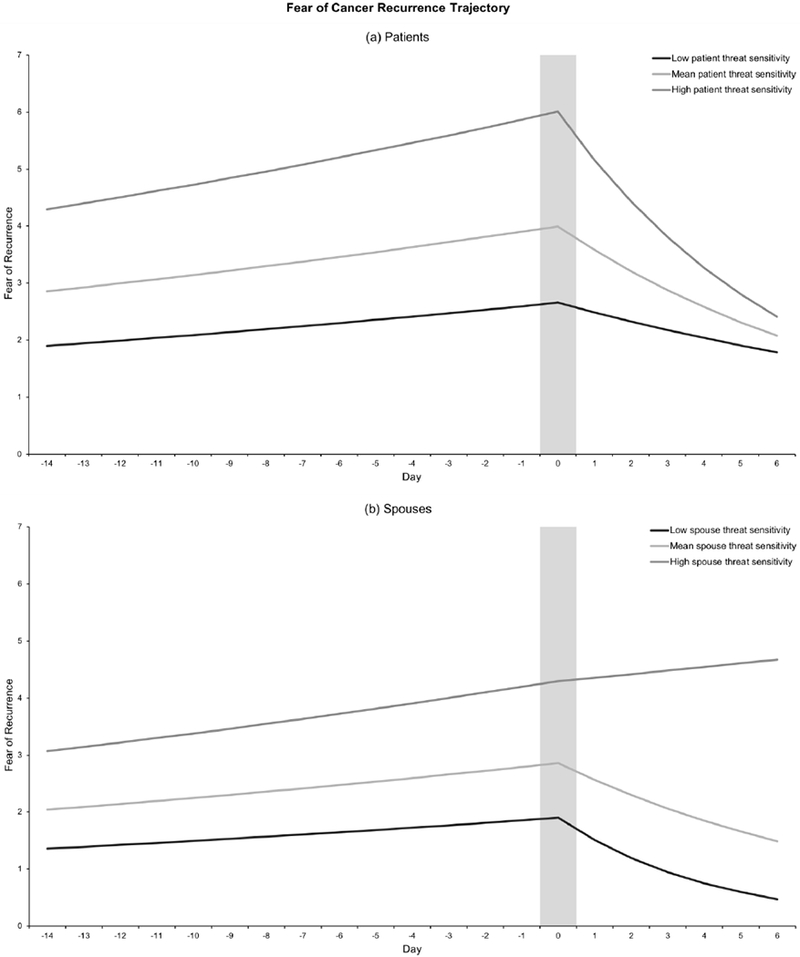
Predicted fear of cancer recurrence (FCR) trajectories over the three-week daily diary period for low, mean, and high levels of threat sensitivity. Low and high threat sensitivity is defined as minus and plus two standard deviations (i.e., one unit), respectively. Zero on the x-axis represents the day of the mammogram (peak FCR; marked by gray vertical bar). Panel A (top) depicts predicted values of patient FCR at mean levels of spouse threat sensitivity. Panel B (bottom) depicts predicted values of spouse FCR at mean levels of patient threat sensitivity. Patient threat sensitivity is a statistically significant predictor of greater patient peak FCR but is not significantly related to patient FCR recovery (Panel A). Spouse threat sensitivity is significantly associated with both greater spouse peak FCR and slower spouse FCR recovery (Panel B). Predicted values are based on estimates from the focal analysis, results of which are shown in Table 2.
Table 2.
Results of dyadic multilevel path modeling of threat sensitivity and fear of recurrence
| Effect | Estimate | SE | Rate ratio§ | p |
|---|---|---|---|---|
| Fear of Recurrence: Intercept and Linear Slopes Before and After Mammogram | ||||
| Patient | ||||
| Reactivity slope‡ | 0.024 | 0.006 | 1.024 | < .001 |
| Peak (intercept) | 1.385 | 0.106 | 3.995 | < .001 |
| Recovery slope‡ | −0.109 | 0.024 | 0.897 | < .001 |
| Spouse | ||||
| Reactivity slope‡ | 0.024 | 0.006 | 1.024 | < .001 |
| Peak (intercept) | 1.050 | 0.119 | 2.858 | < .001 |
| Recovery slope‡ | −0.109 | 0.024 | 0.897 | < .001 |
| Outcome: Patient and Spouse Reactivity a | ||||
| Patient threat sensitivity‡ | 0.008 | 0.023 | 1.008 | .715 |
| Spouse threat sensitivity‡ | −0.012 | 0.027 | 0.988 | .664 |
| Patient global anxiety‡ | −0.006 | 0.019 | 0.994 | .742 |
| Spouse global anxiety‡ | −0.005 | 0.024 | 0.995 | .840 |
| Outcome: Patient Peak Fear of Recurrence | ||||
| Patient threat sensitivity‡ | 0.408** | 0.147 | 1.504 | .006 |
| Spouse threat sensitivity | −0.231 | 0.191 | 0.794 | .228 |
| Patient global anxiety | 0.189 | 0.156 | 1.208 | .226 |
| Outcome: Spouse Peak Fear of Recurrence | ||||
| Spouse threat sensitivity‡ | 0.408** | 0.147 | 1.504 | .006 |
| Patient threat sensitivity | 0.067 | 0.229 | 1.069 | .769 |
| Spouse global anxiety | 0.448 | 0.287 | 1.565 | .118 |
| Outcome: Patient and Spouse Recovery | ||||
| Patient threat sensitivity‡ | −0.043 | 0.049 | 0.958 | .374 |
| Spouse threat sensitivity‡ | 0.123* | 0.048 | 1.131 | .011 |
| Patient global anxiety‡ | −0.077† | 0.045 | 0.926 | .087 |
| Spouse global anxiety‡ | −0.129† | 0.077 | 0.879 | .095 |
Note. N = 57 couples. Peak (intercept) refers to fear of recurrence on the day of the mammogram.
p <.10
p < .05
p < .01
p < .001.
Corresponding coefficients constrained to be equal across partners.
Coefficient estimates are exponentiated for interpretation as rate ratios and intercepts as model-implied fear of recurrence counts on the mammogram day.
To achieve model convergence, random reactivity slopes were fixed to zero for both partners.
Peak Fear of Recurrence on Mammogram Day
Also detailed in Table 2, the model-implied average peak level of FCR on the MMG day was 4.00 for patients and 2.86 for spouses. Actor effects of threat sensitivity on peak FCR were positive and statistically significant for both patients and spouses, and constraining these effects across partners resulted in similar model fit, χ2 (1) = 0.79, p = .37. A one-unit (roughly two standard deviations) increase in patients’ or spouses’ threat sensitivity was associated with a 50% increase in their own FCR on the day of the MMG. Neither patient nor spouse partner effects of threat sensitivity were significant predictors of peak FCR. Taken together, these results indicate that threat sensitivity is predictive of one’s own FCR on the day of the MMG, but not that of one’s partner. The significant actor effects for peak FCR are depicted in Figure 1.
Recovery
Results are shown in Table 2. The fixed and random recovery slopes were constrained to be equal for patients and spouses, which did not significantly alter model fit, χ2 (2) = 1.97, p = .37. Patient and spouse FCR significantly decreased after the MMG day, with an estimated 10% decrease in FCR scores for each passing day. Spouse, but not patient, threat sensitivity emerged as a significant predictor of both patient and spouse rates of FCR recovery. A one-unit increase in spouse threat sensitivity was associated with a 13% increase in both patient and spouse FCR recovery slopes. This suggests that for spouses who are more threat sensitive, they and their partners (i.e., patients) exhibit slower decreases in FCR after the stressful event of the MMG has passed. The significant spouse actor effect and the non-significant patient actor effect on FCR recovery are displayed in Figure 1.
Discussion
FCR is a critical aspect of psychosocial adjustment in cancer survivorship for not only patients, but also their spouses. Despite the prominence of responses to triggering events in theoretical models of FCR,10,11 scant research has directly studied these particularly stressful periods of survivorship (for an exception, see 8). In this study, we examined daily FCR in early-stage BC patients and their spouses two weeks before and one week after the patient’s first follow-up MMG post-diagnosis—an event frequently described by patients as a trigger of FCR7,9). We hypothesized that threat sensitivity would predict greater FCR reactivity over the period leading up to the MMG and higher peak FCR on the MMG day, controlling for global anxiety symptoms. An exploratory aim was to examine the association between threat sensitivity and FCR recovery after the MMG had passed.
As expected, patient and spouse FCR increased over the days leading up to the MMG and decreased after patients received negative MMG results on the day of their appointment. These findings are consistent with one other prior intensive longitudinal study that measured FCR one month, one week, and immediately before and after a follow-up MMG in BC patients.8 Our use of a three-week daily diary design provides even finer temporal resolution of couples’ response to and recovery from this triggering event. The present study complements and builds on this past work by demonstrating that this event triggers an increase in FCR not only for BC patients, but for their spouses as well. One alternative explanation for the significant increase in FCR before the MMG is measurement reactivity bias (i.e., repeatedly assessing daily FCR may cause a systematic increase or decrease in FCR over the entire daily diary period); however, the finding that FCR then significantly decreased after the MMG is consistent with our hypothesis that this event triggered an increase in FCR.
We found that patients experienced significantly more FCR than their spouses on average across the diary period and greater peak FCR on the day of the MMG. This finding is inconsistent with most prior work2–4,6,26–28—a systematic review found that nearly all reviewed studies that tested differences between patient and spouse/caregiver FCR found spouses to have higher FCR than patients.4 Conversely, our finding is consistent with meta-analytic data suggesting that differences in distress by role (patient versus spouse) disappear after accounting for gender, and that females experience more distress than males regardless of role.29 In addition, to our knowledge, FCR has not been assessed daily prior to the current study. While one may expect accumulated daily reports to be equivalent to a single global report, a variety of self-report biases associated with global, retrospective reports can lead to discrepancies in these measures.30 This underscores the importance and value of applying intensive longitudinal methods to the study of FCR in daily life.
Results supported our hypothesis that threat sensitivity would predict greater peak FCR on the day of the MMG. We found that individuals (patient or spouse) who are more sensitive to threat tended to experience more FCR on the day that patients had their follow-up MMGs. There was no effect of an individual’s (patient or spouse) threat sensitivity on their partner’s peak FCR. Thus, as couples directly confront the threat of the MMG, each partner’s own threat sensitivity, but not that of their partner, is related to a heightened fear response.
We explored whether threat sensitivity predicted the recovery response after the MMG had passed and patients received negative test results. No specific hypotheses were made with regard to FCR recovery. Here, results indicated that patient threat sensitivity was not a significant predictor of patient or spouse FCR recovery. Because threat sensitivity characterizes individual differences in response to threatening stimuli, it is not necessarily surprising that, for patients, threat sensitivity predicted greater FCR on the most threatening day but was unrelated to decreases in FCR after the removal of the threatening stimulus (i.e., possibility of positive MMG results). Interestingly, however, spouse threat sensitivity was positively related to both patient and spouse recovery. The FCR of spouses with greater threat sensitivity remained higher for a longer period of time after the MMG than spouses with lower threat sensitivity, and the same was true for patients of spouses with greater threat sensitivity.
Results failed to provide strong support for our hypothesis that threat sensitivity would predict greater FCR reactivity leading up to the MMG (for either partner). However, these null results should be interpreted with caution—after initial problems with model non-convergence, the random reactivity slopes were fixed to zero in order to test our hypothesis (see footnote 2). Model convergence difficulties may have resulted from a relatively small sample size, non-continuous, zero-inflated count outcomes, and model complexity. It is also possible that FCR reactivity before the MMG does not substantially vary from person-to-person, making the small random effect difficult to estimate. Future research with larger samples is needed to test these complex models of change over time, interdependence between partners, and cross-level interactions. Future work should also address this study’s limitations, including the lack of information about family history of BC or objective recurrence risk and relatively homogeneous sample in terms of race/ethnicity, income, and fairly low levels of distress. It also bears noting that due to our focus on female BC patients, role and gender effects are confounded. We are aware of no prior studies of gender differences in threat sensitivity, and studies of gender differences in FCR have produced mixed results.4 Studies of large samples of mixed cancer patients are still needed to elucidate the intertwined effects of role and gender.29 Finally, the BIS scale is one of several viable operationalizations of threat sensitivity—based on evidence that BIS, neuroticism, and negative emotionality load on the same underlying latent factor, we would expect similar results had we used a measure of neuroticism or negative emotionality instead of the BIS scale.31 However, future research is needed to confirm this hypothesis.
Taken together, our results suggest that the personality characteristic of threat sensitivity is a significant predictor of fear, anxiety, and distress related to the possibility of cancer recurrence in BC patients and their spouses during a routine follow-up MMG appointment. Other medical appointments and screening procedures may similarly trigger FCR in couples, and more research involving direct study of these contexts and time periods is needed to achieve an enhanced understanding of psychosocial needs throughout cancer survivorship. The role of threat sensitivity in patient and spouse FCR appeared more interdependent in nature as couples recovered from the MMG—while neither patient nor spouse threat sensitivity impacted their partner’s peak FCR on the MMG day, spouse threat sensitivity predicted a slower recovery for patients afterward. These unexpected but interesting changes in the interdependent structure of these effects over time, as the couples confronted and recovered from the threat, highlight the need for more longitudinal, real-world research to enhance our understanding of mechanisms of dyadic adjustment over the course of survivorship. In closing, we wish to note that our focus on the role of threat sensitivity in FCR is not meant to pathologize those with high levels of this personality characteristic—we were simply interested in whether this biologically-based, stable, individual difference in reactivity to threats mattered for couples during this critical time in survivorship. Our findings suggest that it does. While threat sensitivity is considered to be relatively immutable, those who are high on this characteristic may benefit from interventions aimed at increasing tolerance to cancer-related threats and uncertainty as well as fostering an acceptance-based style of behavioral coping.
Implications for Psychosocial Oncology Practice.
Breast cancer (BC) survivors and their significant others experience routine MMGs as stressful events, evidenced by increases in patient and spouse fear of cancer recurrence (FCR) at least during the two weeks before the appointment.
Patients and spouses who demonstrate greater threat sensitivity—a personality characteristic—may be particularly vulnerable to experiencing heightened FCR on the day of the MMG appointment. In the current report, threat sensitivity emerged as a significant risk factor above and beyond the global tendency to experience anxiety.
When spouses are more threat sensitive, patients and spouses both may take longer to recover, in terms of their FCR, after the MMG appointment.
While threat sensitivity is considered to be relatively immutable, patients and spouses who are high on this characteristic may benefit from interventions aimed at increasing tolerance to cancer-related threats and uncertainty as well as fostering an acceptance-based style of coping.
Acknowledgments
Author note: Work presented in this manuscript was generously supported by the National Cancer Institute. Address correspondence to Emily C. Soriano, M.A. or J.-P. Laurenceau, Ph.D., 108 Wolf Hall, Department of Psychological & Brain Sciences, University of Delaware, Newark, DE 19716; telephone: (302) 831-8188; fax: (302) 831-3645; esoriano@psych.udel.edu or jlaurenceau@psych.udel.edu.
Contributor Information
Emily C. Soriano, University of Delaware
Christine Perndorfer, University of Delaware
Scott D. Siegel, Helen F. Graham Cancer Center & Research Institute
Jean-Philippe Laurenceau, University of Delaware
References
- 1.Lebel S, Ozakinci G, Humphris G, et al. From normal response to clinical problem: Definition and clinical features of fear of cancer recurrence. Support Care Cancer. 2016;24(8):3265–3268. doi: 10.1007/s00520-016-3272-5 [DOI] [PubMed] [Google Scholar]
- 2.Boehmer U, Tripodis Y, Bazzi AR, Winter M, Clark MA. Fear of cancer recurrence in survivor and caregiver dyads: differences by sexual orientation and how dyad members influence each other. J Cancer Surviv. 2016;10(5):802–813. doi: 10.1007/s11764-016-0526-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mellon S, Kershaw TS, Northouse LL, Freeman-Gibb L. A family-based model to predict fear of recurrence for cancer survivors and their caregivers. Psycho-Oncol. 2007;16(3):214–223. doi: 10.1002/pon.1074 [DOI] [PubMed] [Google Scholar]
- 4.Simard S, Thewes B, Humphris G, et al. Fear of cancer recurrence in adult cancer survivors: A systematic review of quantitative studies. J Cancer Surviv. 2013;7(3):300–322. doi: 10.1007/s11764-013-0272-z [DOI] [PubMed] [Google Scholar]
- 5.Koch L, Jansen L, Brenner H, Arndt V. Fear of recurrence and disease progression in long-term (≥5 years) cancer survivors-a systematic review of quantitative studies: Fear of recurrence and disease progression in long-term cancer survivors. Psycho-Oncol. 2013;22(1):1–11. doi: 10.1002/pon.3022 [DOI] [PubMed] [Google Scholar]
- 6.Kim Y, Carver CS, Spillers RL, Love-Ghaffari M, Kaw C- K. Dyadic effects of fear of recurrence on the quality of life of cancer survivors and their caregivers. Qual Life Res. 2012;21(3):517–525. doi: 10.1007/s11136-011-9953-0 [DOI] [PubMed] [Google Scholar]
- 7.Simard S, Savard J. Fear of Cancer Recurrence Inventory: development and initial validation of a multidimensional measure of fear of cancer recurrence. Support Care Cancer. 2009;17(3):241–251. doi: 10.1007/s00520-008-0444-y [DOI] [PubMed] [Google Scholar]
- 8.McGinty HL, Small BJ, Laronga C, Jacobsen PB. Predictors and patterns of fear of cancer recurrence in breast cancer survivors. Health Psychol. 2016;35(1):1–9. doi: 10.1037/hea0000238 [DOI] [PubMed] [Google Scholar]
- 9.Gil KM, Mishel MH, Belyea M, et al. Triggers of uncertainty about recurrence and long-term treatment side effects in older African American and Caucasian breast cancer survivors. Oncol Nurs Forum. 2004;31(3):633–639. doi: 10.1188/04.ONF.633-639 [DOI] [PubMed] [Google Scholar]
- 10.Lee-Jones C, Humphris G, Dixon R, Bebbington Hatcher M. Fear of cancer recurrence: A literature review and proposed cognitive formulation to explain exacerbation of recurrence fears. Psycho-Oncol. 1997;6:95–105. [DOI] [PubMed] [Google Scholar]
- 11.Curran L, Sharpe L, Butow P. Anxiety in the context of cancer: A systematic review and development of an integrated model. Clin Psychol Rev. 2017;56:40–54. doi: 10.1016/j.cpr.2017.06.003 [DOI] [PubMed] [Google Scholar]
- 12.Bolger N, Laurenceau J- P. Intensive Longitudinal Methods: An Introduction to Diary and Experience Sampling Research. New York, NY: Guilford Press; 2013. [Google Scholar]
- 13.Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. J Pers Soc Psychol. 1994;67:319–333. [Google Scholar]
- 14.Gray JA. Personality dimensions and emotion systems In: Ekman P, Davidson RJ, eds. The Nature of Emotion: Fundamental Questions. New York: Oxford University Press; 1994:329–331. [Google Scholar]
- 15.Davidson RJ. Emotion and affective style: Hemispheric substrates. Psychol Sci 1992;3:39–43. [Google Scholar]
- 16.Carver CS, Sutton SK, Scheier MF. Action, emotion, and personality: Emerging conceptual integration. Pers Soc Psychol Bull. 2000;26(6):741–751. [Google Scholar]
- 17.Elliot AJ, Thrash TM. Approach-avoidance motivation in personality: Approach and avoidance temperaments and goals. J Pers Soc Psychol. 2002;82(5):804–818. doi: 10.1037//0022-3514.82.5.804 [DOI] [PubMed] [Google Scholar]
- 18.Elliot AJ, Thrash TM. Approach and Avoidance Temperament as Basic Dimensions of Personality: Approach and Avoidance Temperament. J Pers. 2010;78(3):865–906. doi: 10.1111/j.1467-6494.2010.00636.x [DOI] [PubMed] [Google Scholar]
- 19.Gable SL, Reis HT, Elliot AJ. Behavioral activation and inhibition in everyday life. J Pers Soc Psychol. 2000;78(6):1135–1149. doi: 10.1037//0022-3514.78.6.1135 [DOI] [PubMed] [Google Scholar]
- 20.Carver CS, Meyer B, Antoni MH. Responsiveness to threats and incentives, expectancy of recurrence, and distress and disengagement: Moderator effects in women with early stage breast cancer. J Consult Clin Psychol. 2000;68(6):965–975. doi: 10.1037//0022-006X.68.6.965 [DOI] [PubMed] [Google Scholar]
- 21.Cella D, Riley W, Stone A, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J Clin Epidemiol. 2010;63(11):1179–1194. doi: 10.1016/j.jclinepi.2010.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cranford JA, Shrout PE, Iida M, Rafaeli E, Yip T, Bolger N. A Procedure for Evaluating Sensitivity to Within-Person Change: Can Mood Measures in Diary Studies Detect Change Reliably? Pers Soc Psychol Bull. 2006;32(7):917–929. doi: 10.1177/0146167206287721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muthén LK, Muthén BO. Mplus User’s Guide, eighth ed Muthén & Muthén, Los Angeles, CA: 1998–2017. [Google Scholar]
- 24.Atkins DC, Baldwin SA, Zheng C, Gallop RJ, Neighbors C. A tutorial on count regression and zero-altered count models for longitudinal substance use data. Psychol Addict Behav. 2013;27(1):166–177. doi: 10.1037/a0029508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Satorra A, Bentler PM. A scaled difference chi-square test statistic for moment structure analysis. Psychometrika. 2001;66(4):507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohee AA, Adams RN, Johns SA, et al. Long-term fear of recurrence in young breast cancer survivors and partners: Long-term fear of recurrence in breast cancer survivors and partners. Psycho-Oncol. 2017;26(1):22–28. doi: 10.1002/pon.4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hodges LJ, Humphris GM. Fear of recurrence and psychological distress in head and neck cancer patients and their carers. Psychooncology. 2009;18(8):841–848. doi: 10.1002/pon.1346 [DOI] [PubMed] [Google Scholar]
- 28.Schmid-Büchi S, Halfens RJ, Dassen T, van den Borne B. A review of psychosocial needs of breast-cancer patients and their relatives. J Clin Nurs. 2008;17(21):2895–2909. doi: 10.1111/j.1365-2702.2008.02490.x [DOI] [PubMed] [Google Scholar]
- 29.Hagedoorn M, Sanderman R, Bolks HN, Tuinstra J, Coyne JC. Distress in couples coping with cancer: A meta-analysis and critical review of role and gender effects. Psychological Bulletin. 2008;134(1):1–30. doi: 10.1037/0033-2909.134.1.1 [DOI] [PubMed] [Google Scholar]
- 30.Schwarz N Why researchers should think “real-time”: A cognitive rationale In: Mehl MR, Conner TS, Csikszentmihalyi M, eds. Handbook of Research Methods for Studying Daily Life. Paperback; ed. New York, NY: Guilford; 2012:22–42. [Google Scholar]
- 31.Elliot AJ, Thrash TM. Approach-avoidance motivation in personality: Approach and avoidance temperaments and goals. Journal of Personality and Social Psychology. 2002;82(5):804–818. doi: 10.1037//0022-3514.82.5.804 [DOI] [PubMed] [Google Scholar]


