Abstract
Background and Purpose:
Sepsis has been identified as a trigger for stroke, but the underlying mechanisms and risk factors that predispose patients with sepsis to increased stroke risk remain unclear. We sought to identify predictors of stroke after sepsis and bloodstream infections.
Methods:
The 2007 – 2009 California State Inpatient Database from the Health Care Utilization Project was used to identify patients over the age of 18 years and hospitalized with sepsis or bloodstream infection defined by International Classification of Diseases (ICD-9) codes. Patients who died during their sepsis hospitalization were excluded. The primary outcome was a primary diagnosis of ischemic or hemorrhagic stroke on a subsequent hospitalization within one year. Associations between risk factors, also defined by ICD-9 codes, and stroke were analyzed using multivariable logistic regression. A composite risk score was generated to predict stroke risk.
Results:
Of 121,947 patients with sepsis, 0.5% (n=613) had a primary diagnosis of stroke within a year of their sepsis hospitalization. Significant predictors for stroke were identified. A score was generated from these risk factors with points assigned based on regression coefficients: valvular heart diseases (1 point), congestive heart failure (1), renal failure (1), lymphoma (2), peripheral vascular diseases (2), pulmonary circulation disorders (2), and coagulopathy (3). The C-statistic for the receiver operating characteristic curve for the score was 0.68. The risk of stroke increased 43% (OR = 1.43, 95% CI: 1.37, 1.48) per point increase in the score. The effect of increase in score was greater among younger patients.
Conclusion:
Risk factors and a composite risk score for stroke may help identify a subpopulation of sepsis patients that could be targeted to reduce the short-term risk of stroke after serious infections.
Indexing Terms: Stroke, Sepsis, Population at Risk, Epidemiology
Introduction
Stroke remains the leading cause of serious long-term adult disability in the US, and incurs a huge cost from both clinical and societal perspectives.1 Though stroke mortality has declined substantially over the past decade due to effective combinations of prevention and intervention programs, the decline in incidence and prevalence of stroke has recently begun to reverse, and there is evidence that stroke is increasing in younger individuals.2,3 Traditional cardiovascular risk factors do not fully account for the risk of stroke, moreover, and they also fail to explain why strokes occur at a particular “vulnerable” point in time. Therefore, it is a high priority to identify novel risk factors of long-term risk, as well as triggers associated with short-term risk, to better prevent stroke.
One such novel risk factor may be sepsis. Recent evidence has suggested that infections, including sepsis, could function as acute triggers for stroke, increasing stroke risk within a relatively short period of time.4,5,6,7,8,9 Sepsis is a leading cause of death in the US, particularly among patients in the Intensive Care Unit.6 Sepsis patients are at long-term increased risk of death and major adverse cardiovascular events.7 Additionally, sepsis is associated with an increased intermediate and long-term risk for stroke.5,8,9 The risk of stroke after sepsis appears to be higher in younger sepsis patients, and in patients who do not have diabetes, but these associations and interactions need further exploration.10 Possible mechanisms linking sepsis to stroke could be atrial fibrillation, hemodynamic instability, coagulopathy, the systemic inflammatory response syndrome, and prolonged inflammation.8 It is therefore important to identify which patients are at highest risk for stroke after sepsis and blood-stream infection for risk management purposes. In this study, we aimed to identify those patients at greatest risk of stroke after their sepsis hospitalization.
Methods
Study Population
The data that support the findings of this study are available through the Healthcare Cost and Utilization Project (HCUP) Central Distributor, maintained by Agency for Healthcare Research and Quality (AHRQ). We used data collected from the 2007 – 2009 California State Inpatient Database (SID) of the HCUP dataset. Within the California SID, all clinical and non-clinical information on patients hospitalized in nonfederal acute care hospitals is covered, regardless of payer status. Demographic information includes age, sex, race, and payment source. Upon every admission, 25 primary and secondary International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9_CM) diagnosis codes, discharge status, length of stay, and AHRQ comorbidity measures were recorded.11 The visit linkage variable (VisitLink)12 allows researchers to study the same patient across multiple hospital admissions over time.
This study included all patients (aged ≥ 18 years) with an existing hospitalization record for sepsis or bloodstream infection in California. The population of those with sepsis and bloodstream infections was defined, as in a prior paper on sepsis and stroke10, as the presence of an admission ICD-9 diagnostic code of 038.xx (septicemia), 020.0 (septicemic), 790.7 (bacteremia), 117.9 (disseminated fungal infection), 112.5 (disseminated candida infection), 112.81 (disseminated fungal endocarditis), 995.91 (sepsis), 995.92 (severe sepsis) or 785.52 (septic shock). Patients that died during their sepsis hospitalization, or had concurrent or prior diagnoses of stroke upon their sepsis admission were excluded. Patients with missing information on age, length of stay, and number of chronic conditions were also excluded based on the assumption that missing values occurred at random in the HCUP dataset.
Exposures, Covariates, and Outcomes
Covariates included demographic characteristics such as age, sex, and race/ethnicity. Exposures of interest were AHRQ co-morbidity measures, as defined by HCUP (Supplemental Table I).13 AHRQ co-comorbidity measures are used by federal agencies as standard categories of disorders and conditions to adjust risk of readmission and mortality in quality assurances programs.
The primary outcome was first hospitalization for stroke after sepsis hospitalization, defined as either ischemic or hemorrhagic stroke occurring in 2009. Ischemic stroke was defined, as in prior studies, by the presence of ICD-9 codes 433.x1 (“x,” the fourth digit, can vary to specify a specific arterial distribution), 434 (excluding 434.x0), or 436 present at any diagnostic position between DX1 and DX1210. Patients with ICD-9 codes for traumatic brain injury (800–804, 850 or 854) or rehabilitation care (V57) as a primary diagnosis upon admission were excluded10. Hemorrhagic stroke was defined by the presence of ICD-9 codes 430–432 at any diagnostic position between the first and twelfth diagnostic code.10
Statistical analyses
Baseline demographic characteristics and comorbid conditions were assessed among post-sepsis patients with versus without stroke. In order to develop and validate a prognostic multivariable regression model for post-sepsis stroke, we used bootstrapping methods with Harrell’s c-statistics. Bootstrap methods, which repeatedly generate small samples from the overall population with replacement, have been considered the most efficient resampling strategy.14,15 All possible combinations of post-sepsis comorbidity measures were taken into consideration during the automated model selection process, and sensitivity analyses of selected models were performed using the bootstrap method. The subsets of comorbidity measures that gave the highest Harrell’s c-statistic were selected into the final multivariable logistic models.16,17
Multivariable logistic regression was used to compute odds ratios and 95% confidence intervals (OR, 95% CI) for exposures of interest for all stroke, and then separately for ischemic or hemorrhagic stroke. A composite risk score for post-sepsis stroke was generated. Effect modification by age in this study was assessed. Since the interaction between age and composite risk score was statistically significant in this study, stratified risk of post-sepsis stroke associated with per-point increase in composite risk score was evaluated. The optimal threshold for age as a continuous variable was determined based on highest area under the Receiver Operating Characteristic (ROC) curve for sensitivity analysis. We also conducted sub-analyses investigating risk factors among ischemic and hemorrhagic stroke separately. Additionally, we collapsed composite risk score into high versus low strata to assess the predictive ability of our model using different score cutoffs. The Institutional Review Board at Columbia University waived the need for full ethics review as all data were de-identified and the study does not constitute human subjects research.
Results
Study Population Characteristics
Of the 121,947 eligible patients with a hospitalization record of sepsis or bloodstream infection in 2009, 613 (0.5%) also had either ischemic or hemorrhagic stroke within one year and 121,334 did not have any stroke. Among stroke cases, 490 (80%) were ischemic and 123 (20%) were hemorrhagic. Baseline demographic characteristics and AHRQ co-morbidity measures were presented in Table 1. Post-sepsis patients who later had a stroke were slightly older on average (66.1 vs.64.4 years) than those patients without. Neither sex nor race/ethnicity characteristics differed significantly between the two groups. Hypertension was the most prevalent comorbid condition for all patients. Atrial fibrillation, valvular heart disease, renal failure, congestive heart failure, coagulopathy, peripheral vascular disorders, pulmonary circulation disorders, and lymphoma were more prevalent among patients with stroke.
Table 1.
Baseline Characteristics of California Healthcare Cost and Utilization Project (HCUP) Patients Post Sepsis or Bloodstream Infection, Stratified by Stroke Status (N = 121,947)
| Variable | With Stroke Within One Year (N=613) |
|---|---|
| Age (years) | 66.1 (19.2) |
| Women, No. (%) | 306 (49.92) |
| Black, No. (%) | 58 (9.86) |
| AHRQ Comorbidities (%) | |
| Valvular heart disease | 73 (11.91) |
| Congestive heart failure | 195 (31.81) |
| Peripheral vascular disorders | 91 (14.85) |
| Coagulopathy | 194 (31.65) |
| Rheumatoid arthritis/collagen vascular diseases | 30 (4.89) |
| Pulmonary circulation disorders | 49 (7.99) |
| Chronic pulmonary disease | 125 (20.39) |
| Hypertension | 378 (61.66) |
| Diabetes with chronic complications | 76 (12.40) |
| Diabetes, uncomplicated | 153 (24.96) |
| Atrial fibrillation | 134 (21.9) |
| Obesity | 52 (8.48) |
| Lymphoma | 20 (3.26) |
| Acquired immune deficiency syndrome | <10 (0.00) |
| Solid tumor without metastasis | 15 (2.45) |
| Metastatic cancer | 17 (2.77) |
| Renal failure | 228 (37.19) |
| Alcohol abuse | 35 (5.71) |
| Drug abuse | 22 (3.59) |
| Depression | 44 (7.18) |
| Psychoses | 34 (5.55) |
Risk Factors and Composite Risk Score for Post-Sepsis Stroke
After stepwise model selection and internal validation, an adjusted multivariable logistic model was developed, including valvular heart diseases, congestive heart failure, coagulopathy, lymphoma, peripheral vascular diseases, pulmonary circulation disorders, and renal failure as covariates (Table 2). Definitions of these AHRQ comorbidity measures using ICD-9 codes are included in the supplement (Supplemental Table I). All these risk factors were independently associated with an increased risk for stroke post sepsis.
Table 2.
Association between Risk Factors and Composite Risk Score for Stroke after Sepsis or Bloodstream Infection
| Risk of Stroke after Hospitalization for Sepsis or Bloodstream Infection | ||
|---|---|---|
| Predictor | Odds Ratio (95% CI) | Point Contribution to Risk Score |
| Valvular Heart Diseases | 1.53 (1.18,1.98) | 1 |
| Congestive Heart Failure | 1.51 (1.26, 1.81) | 1 |
| Coagulopathy | 3.48 (2.93, 4.14) | 3 |
| Lymphoma | 1.60 (1.02, 2.51) | 2 |
| Peripheral Vascular Diseases | 1.60 (1.27, 2.01) | 2 |
| Pulmonary Circulation Disorders | 1.65 (1.22, 2.24) | 2 |
| Renal Failure | 1.39 (1.18, 1.65) | 1 |
| Odds Ratio (95% CI) Per Point Increase in Composite Risk Score | ||
| Entire Study Population | 1.43 (1.37, 1.48) | |
| Adults between 18 and 45 years old | 1.86 (1.62, 2.14) | |
| Adults above 45 years old | 1.39 (1.33, 1.45) | |
CI indicates Confidence Interval
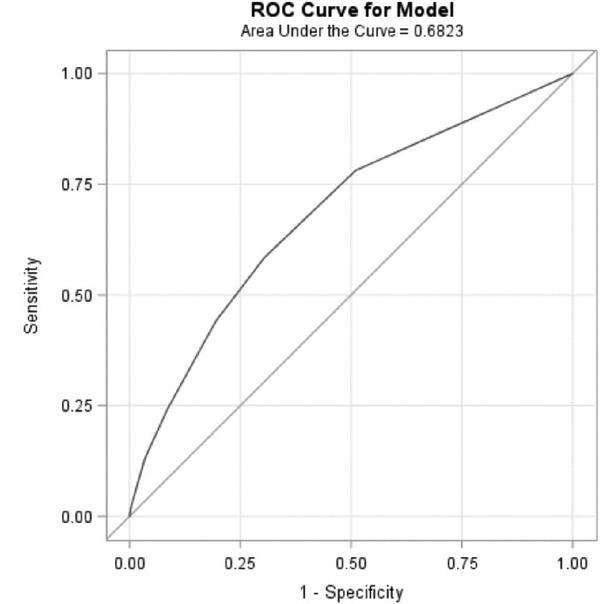
The odds ratio for each predictor was converted to an integer risk score, and a composite risk score was developed. One point was assigned if the OR was between 1 and 1.50. Two points were assigned if OR was between 1.59 and 2.50. Three points were assigned if OR was greater than 2.50. Based on this method, one point was assigned to valvular diseases, congestive heart failure, and renal failure; two points to lymphoma, peripheral vascular disorders and pulmonary circulation disorders; and three points to coagulopathy. For every one-point increase in the composite risk score, the odds of having a stroke post-sepsis increased by 43% (OR= 1.43, 95% CI 1.37–1.48; Table 2; Figure 1) with an area under the curve (AUC) equal to 0.68. For every point increase in risk score, the odds of developing stroke post-sepsis was slightly higher for patients aged 18 to 45 years old (OR= 1.86, 95% CI 1.62–2.14) than for patients over 45 years old (OR=1.39, 95% CI 1.33–1.45; Table 2).
Figure 1.
Receiver Operating Characteristics (ROC) Curve Analysis for Composite Risk Score Predicting Stroke after Sepsis or Bloodstream Infection
The absolute risk of stroke increased as the composite risk score increased. The highest absolute risk of stroke was observed among patients with a score between 7 and 11 (Table 3). Based on increments in absolute risk of stroke, we collapsed the composite risk score into high versus low strata, using 4, 5, 6, and 7 as cutoffs. The predictive ability of our models decreased, however, using these strata, (Supplemental Table II) likely due to the low incidence of stroke among patients scoring above 4 (Table 3). Therefore, the validity of evaluating patients’ stroke risk based on a binary scoring system needs to be assessed in a larger study.
Table 3.
Absolute Risk of Stroke after Sepsis or Bloodstream Infection, Based on Composite Risk Score Stratum
| Composite Risk Score Stratum | Number of Post-Sepsis Patients
(%) (N=121,947) |
Number of Stroke Cases per Stratum | Absolute Risk of Stroke within One Year |
|---|---|---|---|
| 0 – 3 | 111,479 (91.42) | 465 | 0.004 |
| 4 – 6 | 9,861 (8.01) | 131 | 0.013 |
| 7 – 11 | 606 (0.50) | 17 | 0.028 |
Risk of Stroke Subtypes after Sepsis or Bloodstream Infection
Independent risk factors that predicted ischemic stroke are shown in Supplemental Table III. Risk factors included age >45 years old, valvular heart diseases, coagulopathy, hypertension, peripheral vascular diseases, pulmonary circulation disorders, renal failure, and rheumatoid arthritis/collagen vascular diseases.
For hemorrhagic stroke, predictors were age < 65 years old, pulmonary circulation disorders, lymphoma, coagulopathy, and congestive heart failure (Supplemental Table IV). We observed a protective effect against post-sepsis hemorrhage for those above 65 years old (OR= 0.46, 95% CI 0.31 – 0.69). This finding could be a result of survivor bias in this study.
Discussion
We found that patients with valvular heart diseases, congestive heart failure, lymphoma, coagulopathy, peripheral vascular diseases, pulmonary circulation disorders, and renal failure were at increased risk for stroke in the year after sepsis or bloodstream infection. A combination of these independent risk factors increases the risk for stroke, particularly among younger patients. We created a composite stroke risk score for sepsis patients and illustrated the predictive ability of our composite stroke risk score. According to these models, risk factors predicting stroke after sepsis hospitalization vary for the outcomes of different stroke subtypes. However, the risk of stroke is highest for post-sepsis patients with the AHRQ co-morbidity code of coagulopathy across all groups. Our results provide a possible framework to dissect the mechanisms for post-sepsis stroke among different sub-populations. More importantly, our findings may be useful to help identify post-sepsis patients at highest risk for stroke, whether for clinical practice or for the design of future trials in the prevention of stroke after sepsis.
In our study, the AHRQ co-morbidity of coagulopathy was associated with a three-fold increased odds for post-sepsis ischemic stroke, and a seven-fold increased odds of hemorrhagic stroke. Coagulopathy was defined in this analysis according to AHRQ standards, which includes primarily antithrombotic coagulation defects, qualitative platelet defects, and thrombocytopenia. Some of these abnormalities, however, may also be associated with thrombotic tendencies. Notably, previous reports identified several vascular biological pathways by which sepsis may cause stroke, and abnormal coagulation played a key role in many these proposed pathophysiological mechanisms.18,19 The activation of inflammatory responses and the hemostatic system during sepsis might lead to hemodynamic collapse and coagulopathy as well as potential embolism.20 Previous studies estimated that more than 80% of sepsis patients have either clinical or subclinical coagulopathy, increasing risks of both thrombosis and hemorrhage.21 Often, patients with sepsis present with abnormal platelet-leukocyte aggregates in blood circulation and are more prone to develop disseminated intravascular coagulation (DIC).21 DIC involves the activation of the clotting cascade, which could lead to thrombus formation in vasculature and hypercoagulability, resulting in embolism or ischemic stroke.21,22 Moreover, sepsis-related coagulopathy such as DIC can also cause consumption of platelets and coagulation factors, resulting in severe bleeding events including hemorrhagic stroke.21,23 Our finding provides additional support for the biological plausibility that coagulopathy may play an important role in linking sepsis to stroke. More importantly, if this finding is confirmed in future studies, antithrombotic therapy might be an effective strategy for stroke prevention among sepsis patients.
Peripheral vascular disease (PVD) and congestive heart failure (CHF) were also associated with an increased stroke risk after sepsis in our model. PVD involves damage to peripheral arteries and veins.24 The presence of elevated plasma indices of thrombosis and inflammation in PVD is associated with future vascular events such as stroke24,25 and progression of vascular disease.26 Moreover, high levels of cytokines generated during sepsis, such as tumor necrosis factor (TNF)-α and interleukin (IL)-1, also promote intravascular coagulation, ventricular dysfunction, and endothelial dysfunction during CHF pathogenesis.27 The additive adverse effects of sepsis, PVD, and CHF may explain the increased risk of stroke. However, CHF was not among the risk factors for post-sepsis ischemic stroke. Rather, it accounted for an almost two-fold increase in risk for post-sepsis hemorrhagic stroke. This result could be due to failure to capture hemorrhagic conversion from ischemic stroke cases in the administrative dataset, to medications including antithrombotic agents used in patients with CHF, or to chance.
Lymphoma was associated with risk of stroke after sepsis in our analysis. Previous studies reported that solid tumors and hematologic cancer increase risk for stroke.28 However, this effect is rather short-lived.29,30 We did not find a significant association between solid tumors and stroke after sepsis. The low prevalence of cancer cases in our study and other limitations of the dataset did not allow exploration of mechanistic hypotheses. Future studies are needed to confirm this association.
This study has potential implications for clinical practice and future research. If risk factors for stroke among patients with sepsis or bloodstream infection can be reliably identified, then clinicians may consider treating those sepsis patients at highest risk with therapies, such as aspirin or statins, that have been proven to prevent cerebrovascular disease in other settings.27,31 An episode of sepsis may provide a particularly appropriate time to calculate a patient’s cardiovascular risk profile. Clinical and translational investigators, moreover, may be inspired both to identify the mechanisms by which sepsis leads to cerebrovascular disease and to conduct clinical trials to test whether the implementation of vasculoprotective strategies among sepsis patients does in fact lead to a reduction in cerebrovascular events.
Our study has limitations. Due to the nature of the dataset, we were not able to obtain detailed clinical information, particularly with regards to the temporal relationship between sepsis and comorbidities. Second, the broad definitions of AHRQ co-morbidity measures in this dataset could lead to covariate misclassification. For example, endocarditis is included as a valvular disease based on AHRQ definitions. However, it is associated with risk for both sepsis and stroke.32,33 Third, we were not able to capture the medication usage history of patients. We observed a relatively higher prevalence of vascular risk factors such as coagulopathy and hypertension among patients with stroke. Patients with these comorbidities, and especially older patients, might have been taking anticoagulant, antiplatelet, or statin therapies prior to their diagnosis of sepsis, or during their hospitalization. This might lead to biased risk estimate towards null among older patients. We also did not have information on the previous infectious conditions of each patient. Since sepsis is a result of a triggered inflammatory response against infection34 and infection can function as trigger for stroke, it might be possible that the increased risk for stroke among post-sepsis patients could be partially attributed to patients’ previous exposure to infection. Therefore, it is hard to parse out the unique pathway linking sepsis to stroke from these data. Another limitation is potential competing risks. As a result, the analysis might be prone to collider bias. For example, we observed a negative association between age greater than 65 and post-sepsis hemorrhagic stroke. This could be due to the fact that we are observing older patients who already survived sepsis hospitalization (survivor bias). Our scoring algorithm needs to be validated in a larger study population before it can be used widely. Finally, the low incidence of stroke in the cohort suggests that stroke after sepsis is uncommon and perhaps suggests limited clinical significance of its occurrence. Our scoring system, however, suggests that at least certain subgroups of patients can be identified in whom the risk of stroke is higher. On the population level, given the high burden of sepsis and other infectious diseases, identification of those high-risk patients could have practical clinical relevance.
Our study also has strengths, moreover. It is one of the first to identify risk factors for stroke among post-sepsis patients. In addition, given the low prevalence of sepsis in the general population, the use of a statewide population dataset allows for inclusion of a large number of post-sepsis patients. The diverse population also increases the generalizability of our study results. Lastly, our findings provide implications for future studies on mechanisms linking sepsis to stroke and effective clinical management strategies for stroke prevention among post-sepsis patients. Considering coagulopathy is a risk factor common to all stroke subtypes, it will be important to further investigate what type of coagulopathy is the main driver for the association so that we could examine the timing and potential therapeutic options among post-sepsis patients.
Conclusion
Patients at increased risk of stroke after sepsis can be identified. Further investigation needs to be carried out to confirm the effects of specific stroke risk factors among patients with sepsis and bloodstream infections as well as dissecting the detailed mechanisms linking each risk factor to post-sepsis stroke. Finally, future trials may be entertained that would focus on sepsis patients at highest risk who could be candidates for treatments to reduce their risk of stroke.
Supplementary Material
Acknowledgements
We thank Jana and Robert Giordano for their support of this study.
Footnotes
Disclosures
Dr. Elkind receives compensation for providing consultative services for Abbott and Vascular Dynamics; for providing expert witness testimony for Merck/Organon (Nuvaring and stroke), Auxilium (testosterone and stroke), and Sorin/LivaNova (stroke after cardiac surgery); serves on the National, Founders Affiliate, and New York City chapter boards of the American Heart Association/American Stroke Association; and receives royalties from UpToDate for chapters related to stroke. Dr. Elkind receives research funding from the National Institute of Neurological Disorders and Stroke, and research support from the BMS-Pfizer Alliance for Eliquis® and from Roche, both for a trial of stroke prevention. The other authors report no disclosures.
References:
- 1.Di Carlo A Human and economic burden of stroke. Age Ageing. 2009;38:4–5. [DOI] [PubMed] [Google Scholar]
- 2.Lackland DT, Roccella EJ, Deutsch AF, Fornage M, George MG, Howard G, et al. Factors influencing the decline in stroke mortality a statement from the american heart association/american stroke association. Stroke. 2014;45:315–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MG G, Tong X, BA B. Prevalence of cardiovascular risk factors and strokes in younger adults. JAMA Neurol. 2017;74:695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elkind MS V Why now? Moving from stroke risk factors to stroke triggers. Curr Opin Neurol. 2007;20:51–57. [DOI] [PubMed] [Google Scholar]
- 5.Lee-Iannotti JK, Capampangan DJ, Hoffman-Snyder C, Wellik KE, Patel B, Tondato F, et al. New-onset Atrial Fibrillation in Severe Sepsis and Risk of Stroke and Death. Neurologist. 2012;18:239–243. [DOI] [PubMed] [Google Scholar]
- 6.Mayr Florian B, Yende Sachin & Angus DC. Epidemiology of severe sepsis. Landes Biosci. 2014;5:4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yende S, Linde-Zwirble W, Mayr F, Weissfeld LA, Reis S, Angus DC. Risk of cardiovascular events in survivors of severe sepsis. Am. J. Respir. Crit. Care Med. 2014;189:1065–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goss CH, Carson SS. Is Severe Sepsis Associated With New-Onset Atrial Fibrillation and Stroke? JAMA. 2011;306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walkey a J, Wiener RS, Ghobrial JM, Curtis LH, Benjamin EJ. Incident stroke and mortality associated with new-onset atrial fibrillation in patients hospitalized with severe sepsis. JAMA. 2011;306:2248–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boehme AK, Ranawat P, Luna J, Kamel H, Elkind MSV. Risk of acute stroke after hospitalization for sepsis: A case-crossover study. Stroke. 2017;48:574–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agency for Healthcare Research and Quality. Introduction to the HCUP state inpatient databases (SID). Healthc. Cost Util. Proj 2012;4287. [Google Scholar]
- 12.Healthcare Cost and Utilization Project (HCUP). User Guide: HCUP Supplemental Variables for Revisit Analyses. 2013;36. [Google Scholar]
- 13.Elixhauser A, Steiner C, Kruzikas D. HCUP Methods Series Report # 2004–1. Comorbidity Software Documentation. 2004; [Google Scholar]
- 14.Miao Y, Francisco S, Boscardin WJ, Francisco S, Francisco S. SAS Global Forum 2013: Statistics and Data Analysis. Estimating Harrell ’s Optimism on Predictive Indices Using Bootstrap Samples. 2013;1–12. [Google Scholar]
- 15.Steyerberg EW, Harrell FE Jr, J M Borsboom GJ, C Eijkemans MJ, Vergouwe Y, Dik Habbema JF. Internal validation of predictive models: Efficiency of some procedures for logistic regression analysis. J. Clin. Epidemiol 2001;54:774–781. [DOI] [PubMed] [Google Scholar]
- 16.Newson RB. Comparing the predictive power of survival models using Harrell’s c or Somers’ D. Stata J. 1983;10:1–19. [Google Scholar]
- 17.Steyerberg EW, Vickers AJ, Cook NR, Gerds T, Obuchowski N, Pencina MJ, et al. Assessing the performance of prediction models : A framework for some traditional and novel measures. Epidemiology. 2010;21:128–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lupu F, Keshari RS, Lambris JD, Coggeshall KM. Crosstalk between the coagulation and complement systems in sepsis. Thromb. Res 2014;133:S28–S31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levi M, Schultz M, van der Poll T. Sepsis and Thrombosis. Semin Thromb Hemost. 2013;39:559–566. [DOI] [PubMed] [Google Scholar]
- 20.Caplan LR, Ka SW, Gao S, Hennerici MG. Is hypoperfusion an important cause of strokes? If so, how? Cerebrovasc. Dis 2006;21:145–153. [DOI] [PubMed] [Google Scholar]
- 21.Ishikura H, Nishida T, Murai A, Nakamura Y, Irie Y, Tanaka J, et al. New diagnostic strategy for sepsis-induced disseminated intravascular coagulation: a prospective single-center observational study. Crit. Care 2014;18:R19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Polito A, Eischwald F, Maho ALL, Polito A, Azabou E, Annane D, et al. Pattern of Brain Injury in the Acute Setting of Human Septic Shock. Crit. Care 2013;17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saracco P, Vitale P, Scolfaro C, Pollio B, Pagliarino M, Timeus F. The coagulopathy in sepsis: Significance and implications for treatment. Pediatr. Rep 2011;3:119–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Makin A Peripheral vascular disease and Virchow’s triad for thrombogenesis. QJM. 2002;95:199–210. [DOI] [PubMed] [Google Scholar]
- 25.Rabkin SW. The role of interleukin 18 in the pathogenesis of hypertension-induced vascular disease. Nat Clin Pr. Cardiovasc Med 2009;6:192–199. [DOI] [PubMed] [Google Scholar]
- 26.Vanden Berghe T, Demon D, Bogaert P, Vandendriessche B, Goethals A, Depuydt B, et al. Simultaneous targeting of IL-1 and IL-18 is required for protection against inflammatory and septic shock. Am. J. Respir. Crit. Care Med. 2014;189:282–291. [DOI] [PubMed] [Google Scholar]
- 27.Collaboration AT. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–LP86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Navi BB, Reiner AS, Kamel H, Iadecola C, Elkind MS V, Panageas KS, et al. Association between incident cancer and subsequent stroke. Ann. Neurol 2015;77:291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Navi BB, Reiner AS, Kamel H, Iadecola C, Okin PM, Elkind MS V, et al. Risk of Arterial Thromboembolism in Patients With Cancer. J. Am. Coll. Cardiol 2017;70:926–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murthy SB, Karanth S, Shah S, Shastri A, Rao CPV, Bershad EM, et al. Thrombolysis for acute ischemic stroke in patients with cancer: A population study. Stroke. 2013;44:3573–3576. [DOI] [PubMed] [Google Scholar]
- 31.Investigators TSP by AR in CL (SPARCL). High-Dose Atorvastatin after Stroke or Transient Ischemic Attack. N. Engl. J. Med 2006;355:549–559. [DOI] [PubMed] [Google Scholar]
- 32.Grecu N, Tiu C, Terecoasa E, Bajenaru O. Endocarditis and stroke. Maedica. 2014;9:375–81. [PMC free article] [PubMed] [Google Scholar]
- 33.Werdan K, Dietz S, Löffler B, Niemann S, Bushnaq H, Silber R-E, et al. Mechanisms of infective endocarditis: pathogen–host interaction and risk states. Nat. Rev. Cardiol 2013;11:35–50. [DOI] [PubMed] [Google Scholar]
- 34.Jacobi J Pathophysiology of sepsis. Am. J. Heal. Pharm 2002;59:1435–1444. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.