Abstract
Converging lines of evidence suggest that heightened responding to unpredictable threat may be an important neurobiological marker of internalizing psychopathology (IP). Prior data also indicate that aversive responding to uncertainty may be mediated by hyperactivation of several brain regions within the frontolimbic circuit, namely the anterior insula (aINS) and the dorsal anterior cingulate cortex (dACC). To date, however, the majority of this research has been focused on individual diagnoses and it is unclear whether abnormal neural reactivity to unpredictable threat is observed within heterogeneous, transdiagnostic IP patient populations, as theory would suggest. The aim of the current study was to therefore examine the neural correlates of temporally unpredictable (U) and predictable (P) threat in a sample of healthy controls (n=24) and patients with a broad range of IP diagnoses (n=51). We also examined whether symptom severity measures of fear and distress/misery dimensions correlated with neural reactivity to U- and P-threat. All participants completed a modified version of a well-validated threat-of-shock task during functional magnetic resonance imaging (fMRI). Across all participants, U-and P-threat elicited heightened activation in the aINS and brainstem, while P-threat alone also activated the dACC. Relative to healthy controls, patients displayed greater activation in the right aINS during U-threat, and greater right brainstem activation during P-threat. In addition, we found that brainstem activity during U-threat correlated with fear, but not distress/misery, psychopathology. Taken together, these preliminary results suggest that exaggerated aINS reactivity during U-threat and brainstem reactivity during P-threat may have the potential to become important transdiagnostic biomarkers of IP; however, future research efforts are needed to corroborate and expand the present findings.
Keywords: Predictable threat, unpredictable threat, threat-of-shock, fear, distress, neural correlates
1. INTRODUCTION
A wealth of evidence attests to the considerable comorbidity and phenotypic overlap across internalizing psychopathologies (IPs), most notably mood and anxiety disorders (for a review, please see [1]). This overlap has proven a major challenge to the study of underlying disease mechanisms and development of targeted treatments [2]. The field has therefore begun to move away from discrete diagnoses towards a transdiagnostic conceptualization and treatment of mood and anxiety disorders (for a review, please see [3]). Consistent with this mission, the National Institute of Mental Health (NIMH) put forth the Research Domain Criteria (RDoC) initiative [4,5] with the goal of developing a new psychiatric nosology based on empirically-validated neurobiological constructs that cut across multiple IPs.
Two core neurobiological constructs within the RDoC framework are psychophysiological response to predictable (P-) and unpredictable (U-) threat. U-threat is temporally uncertain and of varying intensity, frequency and/or duration. It involves situations where there is a potential for harm, but no immediate threat is present, therefore eliciting a state of generalized apprehension and sustained hypervigilance, also referred to as sustained anxiety [6,7]. P-threat, in contrast, describes situations in which threat of harm is imminent and immediately present, which elicits a phasic ‘fight or flight’ response [6,8]. These aversive states are pharmacologically distinct [9,10], and are mediated by overlapping, but separable, neural circuits [11–13]. Recent theories suggest that response to U-threat, in particular, is at the core of IP [14].
One way response to U-threat and P-threat has been frequently assessed is using the No-Predictable-Unpredictable (NPU) paradigm [15], in which temporal predictability of threat (e.g. mild electric shock, negative emotional images, air puff) is manipulated and startle eye-blink potentiation is recorded as an index of aversive responding. Using the NPU task, separate studies have shown that, relative to healthy controls, patients with panic disorder (PD; [16,17]) and post-traumatic stress disorder (PTSD; [18]) displayed increased startle responding to U-threat but not P-threat. Meanwhile, individuals with major depressive disorder (MDD; [17]) and generalized anxiety disorder (GAD; [18]) showed comparable startle to healthy controls during both forms of threat. A recent study by our lab directly compared response to threat during the NPU task across different IPs and found that individuals with PD, PTSD, and specific phobia (SP) all displayed greater startle reactivity to U-threat, but not P-threat, compared with individuals with MDD, GAD, and healthy controls (who did not differ from each other; [19]). Taken together, these findings suggest that heightened reactivity to U-threat may be a putative transdiagnostic psychophysiological indicator of ‘fear-based’ IPs (e.g. PD, social anxiety disorder [SAD], SP, PTSD), but not ‘distress/misery’ IPs (e.g. MDD, dysthymia, GAD) [20]. Distress/misery IPs reflect pervasive subjective distress, while fear IPs involve intense circumscribed fear of specific stimuli or situations. Several large-factor analytic familial and twin studies have shown that the two dimensions are phenotypically and genotypically distinct [21–24]. In recent years, numerous studies have recognized the above distinction and have started to identify the behavioral and neural correlates of the two dimensions [17,25–27].
In order to elucidate the neurobiology underlying reactivity to U- and P-threat, studies have begun to employ variants of the NPU task during functional magnetic resonance imaging (fMRI). To date, studies have identified a specific frontolimbic circuit that becomes engaged during the negative processing of threatening stimuli [14,28]. This circuit is comprised of affect-generating limbic regions, such as the amygdala, anterior insula (aINS), and bed nucleus of stria terminalis (BNST) [8,29,30], which project to subcortical structures like the brainstem, but also have bidirectional connections with affect-modulating prefrontal regions, such as the dorsolateral, ventrolateral and ventromedial prefrontal cortices, orbitofrontal cortex (OFC), and dorsal anterior cingulate cortex (dACC; [31,32]). Of these regions, aINS and dACC appear to be especially relevant to anxious responding during the anticipation of U-threat. Both regions are involved in interoceptive awareness and the generation of anticipatory emotional responses to future events [33–35]. Hyperactivity of the aINS and dACC during the anticipation of unpredictable aversiveness has been associated with PD [29,36], SP [37], and PTSD [38], but not GAD or MDD [39]. Of note, these studies included presentation of temporally unpredictable aversive emotional images. To our knowledge, no study has extended this line of work to an NPU threat-of-shock paradigm to assess the neural activity elicited by a physical threat. This gap in the literature is noteworthy given that shock has been shown to be a more robust elicitor of defensive reactivity than aversive images [40]. Furthermore, past neuroimaging studies have focused on discrete individual diagnoses and to date, no study has explored the neural correlates of P- and U-threat in a heterogeneous, representative IP patient sample. Finally, no study has moved beyond diagnostic categories and explored how IP symptom dimensions of fear and distress relate to neural activity during U- and P-threat. Given that prior startle studies suggest exaggerated reactivity to U-threat characterizes fear-based psychopathology specifically (e.g., [41]), it is important to assess how individual differences in fear symptom severity map onto neural patterns of activation to better understand the pathophysiology of fear-based IPs.
Therefore, the present study had three main research objectives. First, we examined the neural correlates of U- and P-threat across all participants using an fMRI variant of the NPU threat-of-shock paradigm. Next, we assessed group differences in neural activity during U- and P-threat between clinically representative adult patients and healthy controls. We hypothesized that relative to healthy controls, patients would display greater neural activation within the aINS and dACC during U-threat, but not P-threat. Lastly, in order to more precisely identify the clinical profile associated with the pattern of neural activity during U- and P-threat, we explored the correlations between the neural activity and transdiagnostic symptom dimensions relevant to fear and distress disorders. Research addressing these aims is critically needed to clarify the role of U-threat in psychopathology and elucidate the potential basic, transdiagnostic mechanisms and targets for prevention and treatment of multiple IPs.
2. METHODS
2.1. Participants
Participants were recruited from the community as a part of a larger investigation on predictors of IP treatment outcomes. A variety of advertisements were used to recruit different populations (e.g., depression and/or anxiety disorder patients, healthy controls) in an effort to obtain a sample with a broad range of symptom severity and functioning. In line with the aims of the larger study, participants were included if they either (1) had anxiety or depressive symptoms severe enough to warrant treatment and consented to treatment with pharmacotherapy (selective serotonin reuptake inhibitors/SSRIs) or cognitive behavioral therapy (CBT) (i.e., patients), or (2) had no lifetime history of psychopathology (i.e., healthy controls). Diagnoses were assessed using the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders (DSM-5; [42]), and the primary diagnosis warranting treatment was assigned to participants by a group of three clinicians/study staff. Participants were required to be between the ages of 18 and 65 years. Exclusionary criteria for the overall study included any ongoing psychiatric treatment, a major active medical or neurological illness, lifetime history of manic/psychotic symptoms or suicidal ideation, contraindications to SSRI treatment, traumatic brain injury, alcohol and substance dependence (current or in the past six months), contraindications for fMRI, positive urine drug screen or breathalyzer test, and pregnancy. For the purposes of the current study, only data collected at baseline (i.e., prior to treatment randomization) was examined. A total of 98 individuals met inclusionary criteria; however, 23 were excluded due to poor quality fMRI data (i.e. excessive motion and/or artifact). The final sample included 24 healthy controls and 51 patients. Of note, no differences were found between included and excluded subjects on age (F(1,96) = .309, p = .579), sex (χ2 = .017, df =1, p = .898) and IDAS-II panic subscale scores (F(1,95) = .034, p = .855). The groups did differ on IDAS-II depression subscale scores (F(1,96) = 4.343, p = .040) such that excluded participants endorsed higher levels of depressive symptoms (M = 56.70, SD = 16.92) compared with included participants (M = 47.64, SD = 18.60). All study procedures were approved by University of Illinois at Chicago Institutional Review Board. At the end of each study session participants received cash as compensation for their time.
2.2. Self-report measures
Symptoms of anxiety and depression within the past two weeks were assessed using the well-validated Inventory of Depression and Anxiety Symptoms (IDAS-II) [43] – a 99-item self-report measure. Participants responded to each item using a 5-point Likert-type scale ranging from 1 (not at all) to 5 (extremely). The IDAS-II yields 17 empirically derived and symptom-specific scales (Suicidality, Lassitude, Insomnia, Appetite Loss, Appetite Gain, Ill-Temper, Being, Panic, Social Anxiety, Traumatic Intrusions, Traumatic Avoidance, Mania, Euphoria, Claustrophobia, Checking, Ordering and Cleaning) and two, higher-order scales (General Depression and Dysphoria). Given our interest in the potential distinction of fear versus distress/misery disorders, we chose to focus on two, specific IDAS-II scales – Panic and General Depression. Large-scale factor-analytic studies show that panic is the ‘quintessential’ fear disorder as it loads most highly onto the broader fear dimension [44]. The same is true for depression in relation to distress. Thus, panic and depression serve as useful representations of fear and distress, respectively. Research has shown excellent convergent and discriminant validity of IDAS-II scales relative to other self-report measures of depression and anxiety [45]. In the present study we employed the following two scales: General Depression and Panic. Both scales demonstrated strong internal consistency within our study sample (General Depression: α = .91; Panic: α = .86).
2.3. Study Procedure and fMRI Threat Task
After providing written informed consent, participants completed a set of screening assessments including a comprehensive medical history, a dimensional variant of the structured clinical interview for DSM-5, a battery of self-report questionnaires and a set of neurocognitive tasks. Approximately one-week after the screen, participants underwent an fMRI scan, during which they completed a modified version of the NPU threat task (described below). In preparation for this task, each participant had two shock electrodes placed on their left foot. Participants then completed a shock work-up procedure by which they were administered increasing levels of shock intensity until they reached a level that they described as “highly annoying but not painful”. Ideographic shock levels were used to ensure equality in perceived shock aversiveness [46].
The threat task used in the scanner is a modified version of the NPU startle task [15] and is described in detail elsewhere [36]. Briefly, the task consists of three within-subject conditions – predictable (P), unpredictable (U) and no-shock (N) (see Figure 1). Discrete images of different rooms were used to signal each condition (i.e., N, P, U; counterbalanced across participants). Each room included a lamp that served as a cue and turned on once for 2s during each trial. During P trials, participants were shocked only when the light was on and thus the timing of the threat was fully predictable. During U trials, participants were shocked at any time and the timing of the threat was therefore unpredictable. During N trials, participants never received a shock. Light onset occurred either at 0s (for one third of trials), 2s (for one third of trials) or 4s (for one third of trials) across all conditions. Prior research has shown that administering shocks during every trial (100% reinforcement) can lead to faster rates of habituation. Therefore, having fewer shocks and a reinforcement rate around 60% is ideal. With that in mind, we designed our task such that during the P condition, individuals were consistently shocked during the cue only for 60% of the trials, resulting in 18 shocks during P. Our U condition was matched with P on total number of shocks and therefore also included 18 shocks. Each participant was therefore administered a total of 36 electric shocks (18 during P- and 18 during U-condition). This design is notably consistent with the startle version of the NPU task designed by Grillon and colleagues [47]. The task consisted of 90 trials, with 30 trials in each condition. Each trial was 6s long and was followed by a fixation cross presented with varying duration, ranging from one to 8s (M = 4.3s).
Figure 1.
Illustration of the threat task administered during scan. (a) Discrete images of different rooms, counterbalanced across participants, used to signal three within-subject conditions – predictable, unpredictable and safe. (b) An example of each condition, including cues (light on) and shocks, followed by a fixation cross.
2.4. Acquisition and Analysis of Neuroimaging Data
Participants were scanned at the UIC Center for Magnetic Resonance Research using a 3.0 Tesla GE scanner with an 8-channel phased-array radio frequency head coil. Functional images were acquired using gradient-echo echo-planar imaging (EPI; 2s TR, 25ms TE, 82° flip, 64 × 64 matrix, 200mm FOV, 3mm slice thickness, 0mm gap, with 44 axial slices). Imaging data were inspected for high quality and scan stability; any individual with >3mm displacement in any direction was excluded from the analysis.1 fMRI data processing was carried out using Statistical Parametric Mapping software (SPM8, Wellcome Department of Imaging Neuro-Science, London, UK). Images were spatially realigned to correct for head motion, slice-time corrected (44 slices, TR = 2, TA = 2, slice order: ascending interleaved, reference slice 21), warped to standardized Montreal Neurological Institute (MNI) space using the participants’ mean functional image, resampled to 2mm3 voxels, and smoothed with an 8mm3 kernel to minimize noise and residual differences in gyral anatomy. The general linear model was applied to the time series, convolved with the canonical hemodynamic response function and with a 128s high-pass filter. Condition effects (N, P, and U) were separately estimated at each voxel for each subject. Each trial was a total of 6s long and the shock occurred between 1.7 and 5.6s. For the trials that included a shock, only the data points prior to the shock were included in the first-level model (1.7–5.6s of data). For the trials that did not include a shock, the entire trial was modeled. Given that this results in more N trial data than U or P trial data, an equivalent random number of N trials were also excluded to ensure that data across the threat and no-threat trials was balanced. Total seconds modeled per each condition (N, P, and U) were approximately 137 seconds. Individual movement parameters obtained during realignment were included in the first-level models as regressors-of-no-interest to account for motion-related effects on BOLD. Individual contrast maps for U threat > No threat (and P threat > No threat) were created for each person.
These contrast maps were then entered into second-level, one-sample t-tests in SPM, in order to examine main task effects. To confirm that the task successfully activated regions implicated in threat responding, we examined task activation across all subjects. We considered activations that survived family-wise error (FWE) whole-brain correction at p < .05, with a cluster size greater than 20 contiguous voxels (volume > 160mm3), as significant. We then also extracted BOLD parameter estimates (i.e. β weights [arbitrary units]) from 5mm (radius) spheres surrounding significant peak activations across both models in order to perform region-of-interest (ROI) analyses (detailed below).
2.5. Data Analysis Plan
Participants’ demographic and clinical variables were compared at baseline across the two groups using one-way ANOVA or Pearson Chi-Square tests as appropriate. To test our primary hypothesis, we first conducted an independent sample t-test to examine main task effects during U threat > No threat and P threat > No threat. Next, we assessed the group differences between healthy controls and patients using extracted BOLD parameter estimates from peak activations during either contrast as independent variables. Lastly, to test the link between symptom severity measures and neural activity, we carried out bivariate Pearson correlations between extracted BOLD parameter estimates from peak activations within frontolimbic regions (only) and IDAS-II scales (General Depression and Panic) in patients. All tests were two-tailed and were deemed statistically significant at α < .05. All statistical analyses were performed using SPSS software (Version 24.0, SPSS, Inc.) and results were graphed using GraphPad Prism (Version 7.0, GraphPad Software, La Jolla, CA, United States).
3. RESULTS
3.1. Baseline demographics and clinical characteristics
Please see Table 1 for participants’ descriptives. The groups were comparable in age, sex and ethnicity; however, the patient group was comprised of more “White” individuals than the healthy control group (χ2 = 5.74, df =1, p = .017). The groups also differed on IDAS-II depression and panic scores, as expected (F(1,73) = 202.73, p < .001, and F(1,73) = 35.52, p < .001, respectively).
Table 1.
Participant Demographics and Baseline Clinical Characteristics
| Controls (n=24) | Patients (n=51) | Statistic | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 27.0 (12.3) | 25.4 (7.8) | F(1,73) = .478, p = .492 |
| Sex (% female) | 54.2% | 72.5% | χ2 = 2.48, df =1, p = .115 |
| Ethnicity (% Hispanic) | 16.7% | 19.6% | χ2 = .093, df =1,p = .760 |
| Race | |||
| White | 29.2% | 58.8% | χ2 = 5.74, df =1, p = .017 |
| Black | 25.0% | 9.8% | χ2 = 3.01, df =1, p = .083 |
| Asian | 37.5% | 21.6% | χ2 = 2.12, df =1, p = .146 |
| American Indian or Alaskan Native | 4.2% | 0.0% | p = .320 (Fisher’s exact) |
| Other or Unknown | 4.2% | 9.8% | p = .657 (Fisher’s exact) |
| Clinical variables | |||
| IDAS-II General Depression | 24.5 (2.8) | 58.5 (11.5) | F(1,73) = 202.73, p < .001 |
| IDAS-II Panic | 8.0 (0.2) | 14.5 (5.3) | F(1,73) = 35.52, p < .001 |
| Current major depressive disorder | - | 39.2% | - |
| Current generalized anxiety disorder | - | 29.4% | - |
| Current social anxiety disorder | - | 39.2% | - |
| Current panic disorder | - | 19.6% | - |
| Current post-traumatic stress disorder | - | 9.8% | - |
| Lifetime major depressive disorder | - | 21.6% | - |
| Lifetime social anxiety disorder | - | 3.9% | - |
| Lifetime panic disorder | - | 7.8% | - |
| Lifetime post-traumatic stress disorder | - | 17.6% | - |
Note. All values are means, standard deviations, unless otherwise noted. IDAS-II = Inventory for Depression and Anxiety Symptoms-II.
3.2. Neuroimaging results
3.2.1. Main Effects of the fMRI Threat Task, Whole-Brain Corrected
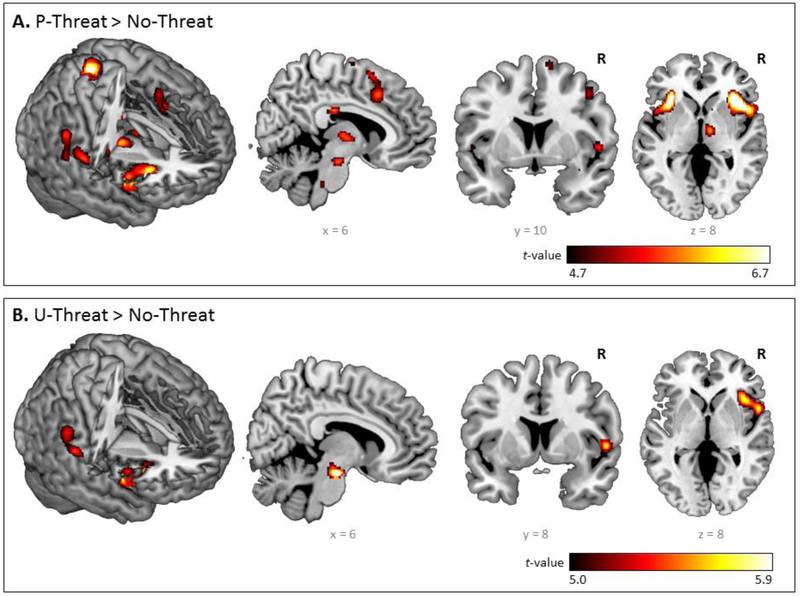
Detailed neural activation elicited by U- and P-threat is presented in Figure 2 and Table 2. U-threat significantly activated the right brainstem, anterior insula and supramarginal gyrus (p < .05corrected) across all participants. For P-threat, whole-brain results indicated significant activation in bilateral anterior insula and thalamus, right postcentral and supramarginal gyrus, right dACC and brainstem (p < .05corrected). No other significant whole-brain task effects were found.2
Figure 2.
Whole-brain voxel-wise statistical t maps overlaid on a canonical brain, displaying significant activations at p<0.05, family-wise error corrected (FWE), with a cluster size of 20 or more contiguous voxels, during (a) predictable threat > no threat and (b) unpredictable threat > no threat, across all participants. Color bars represent statistical t-scores.
Table 2.
Main Effects of the Threat Task, Whole-Brain Corrected
| MNI coordinates | ||||||
|---|---|---|---|---|---|---|
| Region | x | y | z | Cluster (voxels) | Volume (mm3) | Z score |
| U threat > Fix | ||||||
| R brainstem (midbrain) | 6 | −18 | −18 | 490 | 3920 | 7.71 |
| L hippocampus | −22 | −26 | −8 | 159 | 1272 | 7.11 |
| R anterior insula | 38 | 22 | −2 | 1200 | 9600 | 6.66 |
| R middle frontal gyrus | 48 | 44 | −4 | 436 | 3488 | 6.25 |
| L anterior insula | −34 | 20 | 4 | 206 | 1648 | 5.64 |
| R supramarginal gyrus | 58 | −40 | 32 | 81 | 648 | 4.94 |
| P threat > Fix | ||||||
| R anterior insula | 36 | 24 | 0 | 1147 | 9176 | 6.52 |
| L frontal middle gyrus | −46 | 44 | 20 | 437 | 3496 | 6.13 |
| L anterior insula | −34 | 20 | 4 | 464 | 3712 | 6.11 |
| R frontal middle gyrus | 44 | 46 | −18 | 379 | 3032 | 5.57 |
| R precentral | 50 | 8 | 48 | 89 | 712 | 5.02 |
| R brainstem | 10 | −16 | −16 | 100 | 800 | 4.87 |
| R thalamus | 12 | −10 | 2 | 100 | 800 | 4.87 |
| R supramarginal gyrus | 58 | −40 | 26 | 55 | 440 | 4.64 |
| No threat > Fix | ||||||
| L frontal middle gyrus | −38 | 56 | 12 | 654 | 5,232 | 5.55 |
| R frontal middle gyrus | 54 | 44 | 0 | 158 | 1,264 | 5.14 |
| U threat > No threat | ||||||
| R brainstem (midbrain) | 6 | −16 | −20 | 149 | 1,192 | 5.60 |
| R anterior insula | 40 | 18 | −4 | 623 | 4,984 | 5.32 |
| R supramarginal gyrus | 58 | −28 | 22 | 258 | 2,064 | 5.06 |
| P threat > No threat | ||||||
| L anterior insula | −32 | 18 | 4 | 425 | 3,400 | 5.94 |
| R anterior insula | 36 | 22 | 2 | 851 | 6,808 | 5.87 |
| R postcentral gyrus | 18 | −40 | 74 | 204 | 1,632 | 5.51 |
| R thalamus | 10 | −12 | 4 | 158 | 1.264 | 5.09 |
| R supramarginal gyrus | 58 | −22 | 20 | 207 | 1,656 | 5.05 |
| R brainstem (midbrain) | 8 | −18 | −18 | 33 | 264 | 4.96 |
| R medial frontal gyrus/dorsal anterior cingulate cortex | 4 | 18 | 44 | 159 | 1,272 | 4.93 |
| L thalamus | −8 | −10 | 6 | 24 | 192 | 4.72 |
Note. Reporting of all significant peak voxels at p< .05, family-wise error corrected (FWE), with a cluster size of >20 contiguous voxels. R = Right; L = Left; MNI = Montreal Neurologic Institute.
3.2.2. Group Differences in Neural Activity
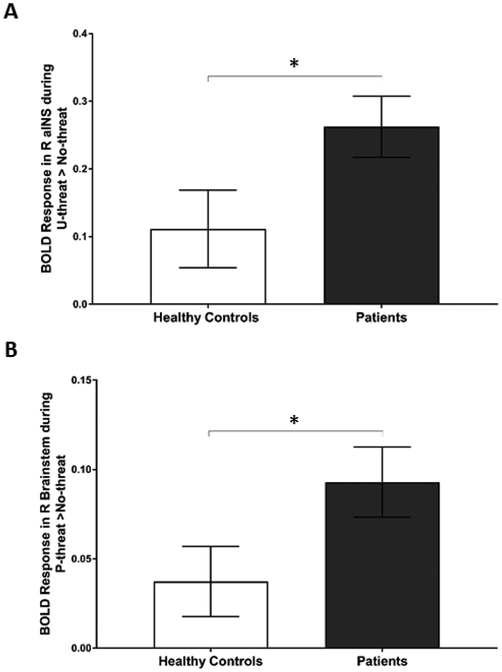
Direct between group comparison of healthy controls and patients revealed no differences at the whole-brain level. Between groups analyses using BOLD parameter estimates extracted from peak activations in either contrast revealed that, relative to healthy controls, patients exhibited significantly greater activation in the right aINS during U-threat > No-threat (t = −2.07, df = 51.24, p = .043; Figure 3A) and the right brainstem during P-threat > No-threat (t = −2.01, df = 63.24, p = .049; Figure 3B). No other differences were observed between patients and healthy controls. The group comparisons do not survive a Bonferroni correction (p > 0.005).
Figure 3.
Bar graphs show mean extracted parameter estimate β weights in arbitrary units (±SEM) within each group from 5 mm spherical region of interest surrounding peak, during (a) unpredictable threat > no threat and (b) predictable threat > no threat. *p < 0.05. aINS = anterior insula.
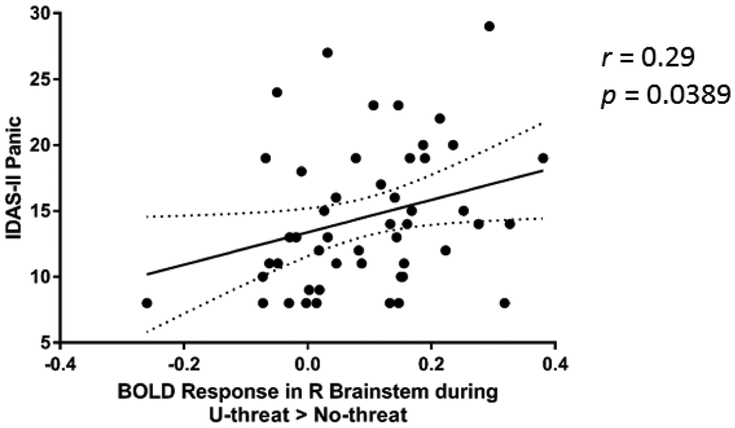
3.3. Symptom Severity Correlations with Neural Activity
In addition to between group analyses, we examined the correlations between symptom severity measures and BOLD parameter estimates extracted from peak activations within frontolimbic regions during both U- and P-threat. As illustrated in Figure 4, greater panic symptoms were associated with greater right brainstem activation during U-threat (r = 0.29, p = 0.0389)3. The correlation does not survive a Bonferroni correction (p > 0.005). No other significant associations were found between panic or depressive symptoms and neural activity during either contrast (Supplementary Figure 1). We formally compared the correlations between panic (or depressive) symptoms and the right brainstem activation during U-threat and found that they were not significantly different (Fisher’s z = .884, p = .377).
Figure 4.
Scatter plot depicting the significant correlation between IDAS-II panic scores and extracted BOLD parameter estimate for right brainstem during unpredictable threat relative to no threat across participants with current psychopathology.
4. DISCUSSION
Heightened reactivity to U-threat has emerged as a potential transdiagnostic neurobiological marker of IP as evidenced by increased startle responding [48,49]. Studies have also used fMRI to identify the neural correlates of heightened responding to U-threat across IP. However, these studies included presentation of temporally unpredictable negative emotional images, as opposed to physical threat. Furthermore, the majority of this research has been focused on individual diagnoses and no study to date has extended this line of research in a transdiagnostic IP sample using dimensional symptom assessments. Therefore, the present study examined the neural correlates of reactivity to U-threat in a heterogeneous, transdiagnostic IP sample using an fMRI variant of the well-validated NPU threat-of-shock paradigm.
Results of the present study showed that the pattern of neural activation on a whole-brain level during U-threat and P-threat across all participants was similar, with both types of threat eliciting activation in the right brainstem, anterior insula and supramarginal gyrus, while P-threat also elicited neural activation in bilateral thalamus, right postcentral gyrus, and right dACC. Specifically, within the frontolimbic circuit, both U- and P-threat elicited activation in the aINS and brainstem, while P-threat alone also activated dACC. Although we did not have hemisphere-specific hypotheses, it is notable that much of the brain activation we observed is right lateralized. A review of the literature suggests that left and right hemispheres of the brain may have differential roles in approach/avoidance behaviors. Specifically, prior work purports that positive affect may be differentially lateralized to the left hemisphere, while negative affect may be differentially lateralized to the right hemisphere [50,51]. The relative nature of specialization in approach versus avoidance motivation of the two hemispheres (also known as the “valence hypothesis”) may be particularly evidenced by abnormal right aINS activation, which has often been reported in the literature during the processing of temporally unpredictable negative emotional stimuli [29,38,52,53]. Together these studies propose aINS as a neural correlate of altered threat processing shared across multiple IPs.
With regard to group differences, we found significantly greater activation in the right aINS during U-threat in patients relative to controls. Consistent with this finding, previous fMRI studies have found greater aINS activation in individuals with PD [29], PTSD [38] or SP [37], relative to healthy controls. At the functional connectivity level, prior studies have also shown that in individuals with PTSD there is increased connectivity between aINS and other regions within insula-based networks (such as the salience network) both at rest and during threat [54]. Individuals who report higher levels of intolerance of uncertainty have also demonstrated exaggerated aINS reactivity to U-threat [52]. The current preliminary results show for the first time that exaggerated aINS reactivity to U-threat has the potential to become an important transdiagnostic biomarker that may relate to multiple forms of IP, suggesting they have a shared vulnerability. The aINS is known to play an important role in interoceptive awareness during uncertainty by encoding internal and external state signals in order to predict future subjective feelings about possible threat outcomes (i.e. to help answer the question “how will it feel?”) [33,55,56]. In the anticipation of an aversive stimulus, aINS engages adaptive preparatory cognitive and behavioral resources that help an individual avoid, minimize, and cope with possible negative consequences. Dysfunction of aINS may lead to negatively biased perception of U-threat [57], regardless of its true potential to confer harm. The worry and avoidance behaviors that are hallmarks of anxiety disorders may therefore be a result of this overestimation mediated by aINS hyperactivity. Given its well-established role in the development and maintenance of anxiety disorders, aINS may constitute an important treatment target, particularly for anxiety disorders characterized by heightened reactivity to U-threat (e.g. panic disorder [41]).
Results of the present study also showed that patients exhibited greater brainstem activation during P-threat compared with healthy controls. This finding is noteworthy for two reasons. First, only a few psychophysiological startle studies have reported differences in P-threat responding across IPs [17,58] and thus, it has been proposed that response to P-threat does not characterize individuals with IP. Second, the role of the brainstem in psychopathology has been relatively understudied in comparison to aINS and other frontolimbic regions, which garnered more interest due to their involvement in higher order functions such as thinking, decision-making or emotional processing [59]. Nevertheless, the brainstem and midbrain structures, namely the pons and periaqueductal gray (PAG), play an important role in rapid modulation of respiration, heart rate and blood pressure [60] during defensive responding to threats [14,61,62]. Furthermore, prior studies have shown that electrical stimulation of PAG in humans can result in elevated fear and anxiety levels [63,64], particularly among PD [65] as well as PTSD patients after being exposed to a trauma reminder [66]. Akin to hyperactive aINS during U-threat, exaggerated brainstem reactivity to P-threat may therefore be an important transdiagnostic biomarker of IP. Our current observations during U- and P-threat, though preliminary, concur to an extent with prior research, which seems to suggest that anticipation of U-threat implicates higher cortical systems; however, once the threat is imminent (i.e. predictable), there is a shift in neural activity to more phylogenetically primitive brain structures, such as the PAG [62]. Though further research is needed to corroborate this hypothesis, diffusion tensor imaging (DTI) and tracing studies suggest that forebrain-to-midbrain switch is anatomically plausible [67–69].
Although we did not see group-level differences in brainstem activation between patients and controls during U-threat, we did find that right brainstem activity during U-threat correlated with symptom severity measure of fear (e.g. panic), but not distress disorders (e.g. depression). This finding is consistent with previous studies, which have shown that, across species, stimulation of dorsal and ventrolateral PAG can induce panic-like behavior [70–72]. In light of these findings, a recent comprehensive review of the available neuroimaging studies assessing the involvement of brainstem in PD reported that the “fear network” model of PD, which currently implicates limbic system primarily, should be revised to include other brain areas such as the brainstem and the insula [73]. Broader literature also suggests that heightened threat sensitivity, particularly under uncertain conditions, may be more relevant to the clinical picture of fear-based disorders relative to distress disorders [17,74–76]. In fact, prior work has shown that, relative to non-anxious group, individuals with panic and other anxiety disorders were more likely to appraise naturally occurring life-events as overly negative or threatening in the six month period preceding the onset of disorder [77]. In line with previous evidence, the results of the present study tentatively suggest that elevated threat sensitivity during U-threat may reflect a dispositional vulnerability of PD and fear-based IPs relative to MDD and other distress disorders. Therefore, therapeutic interventions that include strategies to help correct faulty threat appraisals in patients with fear disorders may be more effective at improving treatment outcomes [78]. However, further research is needed to corroborate the present findings and examine their potential to reflect a transdiagnostic IP treatment targets.
Our results so far have been consistent with prior findings and theoretical models of psychopathology; however, a few hypotheses were unsupported. First, we hypothesized that dACC, another key node implicated in interoceptive awareness, would be hyperactivated during the processing of U-threat since dACC and aINS are highly interconnected and are a part of a larger affective salience network [14]. We also hypothesized that panic symptoms would be associated with both dACC and aINS activity during U-threat. There are several possible explanations for the unsupported hypotheses including a small sample size and a highly comorbid patient sample (see limitation section below). Another possible explanation for the observed inconsistencies is that some abnormalities may manifest at the level of interaction between different brain regions rather than their activation in isolation [79]. Although we found no evidence to support this specific hypothesis, future studies should continue to investigate fMRI connectivity measures and the potential correlation between the dACC and aINS functioning and panic symptoms.
The current study had several strengths including the use of an fMRI variant of the NPU threat-of-shock paradigm and inclusion of multiple diagnostic groups. However, the present findings should be interpreted in light of several limitations. First, the sample size was relatively small, particularly for the group cells, which likely reduced our power to detect additional group differences, including detecting the group differences at the whole-brain level. Given the small sample size the findings do not withstand correction for multiple comparisons (e.g. Bonferroni correction) and thus, are preliminary and require replication. Second, the cue (light-on image) is likely more threatening during P-threat condition than the light-off images. However, the task design did not allow us to model the cue separately in a first level model for P-threat given that its timing is short (2s) and no fixation cross followed the cue to allow for the BOLD signal to decrease. Third, the high levels of comorbidity in our study sample, particularly between the distress and fear disorders, can be seen as both a potential strength and limitation. Although it enhances the external validity of the present findings, the potential impact of co-occurring psychopathology on the pattern of results is unclear. Additional studies are needed to replicate and extend the current findings. Lastly, given the highly comorbid nature of the sample, we did not have the power to test differences in the association between brainstem activity during U-threat and IDAS-II panic across discrete IP diagnoses. This was never the aim of the present study, but it may be useful if future studies examined the association between neural activity during U- and P-threat and symptom severity measures both dimensionally and categorically.
In conclusion, the present study marks the first investigation of the neural correlates of predictable and unpredictable threat in a heterogeneous, transdiagnostic IP sample using an fMRI variant of the well-validated NPU threat-of-shock paradigm. We found that across all participants, U- and P-threat elicited heightened activation in the aINS and brainstem, while P-threat alone also activated the dACC. Follow up analyses revealed that patients displayed greater activation in the right aINS during U-threat, and greater right brainstem activation during P-threat, compared with healthy controls. In addition, brainstem activity during U-threat correlated with fear, but not distress/misery, psychopathology. Taken together, these preliminary results suggest that exaggerated aINS reactivity during U-threat and brainstem reactivity during P-threat have the potential to become important transdiagnostic biomarkers of IP. In addition, heightened brainstem reactivity to U-threat may track fear-based anxiety symptoms, though more research is needed to further examine its potential to reflect a transdiagnostic IP treatment target. Finally, future studies should continue to probe the neural and behavioral processes that mediate reactivity to P- and U-threats in highly comorbid samples and examine whether those processes are shared across IPs.
Supplementary Material
Highlights.
Examined neural response to predictable (P) and unpredictable (U) threat using fMRI
Those with internalizing disorders had heightened insula activation to U-threat
Patients also had heightened brainstem activation to P-threat
Brainstem activation during U-threat correlated with fear-based anxiety symptoms
Exaggerated limbic reactivity to threat may characterize internalizing disorders
Funding
Research reported in this publication was supported by the National Institute of Mental Health (NIMH) of the National Institute of Health (NIH) under Award R01MH101497 (PI: K.L.P.). Other support for this work was provided by the University of Illinois at Chicago (UIC) Center for Clinical and Translational Science (CCTS) Award UL1RR029879 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding sources.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Footnotes
Patients and healthy controls did not differ in head motion during fMRI (translation: t = 1.41, df = 32.89, p = .168, rotation: t = .55, df = 69.59, p = .585).
Direct comparison of U- and P-threat revealed no differences at the whole-brain level.
The correlation remains significant after controlling for head motion (r = 0.27, p = 0.021).
Declaration of Conflicting Interests
The authors declared that they had no conflicts of interest with respect to their authorship or the publication of this article.
REFERENCES
- [1].Mineka S, Watson D, Clark LA, Comorbidity of Anxiety and Unipolar Mood Disorders, Annu. Rev. Psychol 49 (1998) 377–412. doi: 10.1146/annurev.psych.49.1.377. [DOI] [PubMed] [Google Scholar]
- [2].Harvey R, Allison G, Watkins, Edward, Mansell, Warren, and Shafran, Cognitive behavioural processes across psychological disorders : a transdiagnostic approach to research and treatment, Oxford University Press, 2004. https://global.oup.com/academic/product/cognitive-behavioural-processes-across-psychological-disorders-9780198528883?cc=us&lang=en& (accessed June 7, 2018). [Google Scholar]
- [3].Newby JM, McKinnon A, Kuyken W, Gilbody S, Dalgleish T, Systematic review and meta-analysis of transdiagnostic psychological treatments for anxiety and depressive disorders in adulthood, Clin. Psychol. Rev 40 (2015) 91–110. doi: 10.1016/J.CPR.2015.06.002. [DOI] [PubMed] [Google Scholar]
- [4].Cuthbert BN, The RDoC framework: facilitating transition from ICD/DSM to dimensional approaches that integrate neuroscience and psychopathology., World Psychiatry. 13 (2014) 28–35. doi: 10.1002/wps.20087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P, Research Domain Criteria (RDoC): Toward a New Classification Framework for Research on Mental Disorders, Am. J. Psychiatry 167 (2010) 748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- [6].Barlow DH, Unraveling the mysteries of anxiety and its disorders from the perspective of emotion theory, Am. Psychol . 55 (1986) 1247–1263. [DOI] [PubMed] [Google Scholar]
- [7].Davis M, Are different parts of the extended amygdala involved in fear versus anxiety?, Biol. Psychiatry 44 (1998) 1239–1247. doi: 10.1016/S0006-3223(98)00288-1. [DOI] [PubMed] [Google Scholar]
- [8].Davis M, Walker DL, Miles L, Grillon C, Phasic vs Sustained Fear in Rats and Humans: Role of the Extended Amygdala in Fear vs Anxiety, Neuropsychopharmacology. 35 (2010) 105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Grillon C, Baas JMP, Pine DS, Lissek S, Lawley M, Ellis V, Levine J, The Benzodiazepine Alprazolam Dissociates Contextual Fear from Cued Fear in Humans as Assessed by Fear-potentiated Startle, Biol. Psychiatry 60 (2006) 760–766. doi: 10.1016/j.biopsych.2005.11.027. [DOI] [PubMed] [Google Scholar]
- [10].Grillon C, Heller R, Hirschhorn E, Kling MA, Pine DS, Schulkin J, Vythilingam M, Acute hydrocortisone treatment increases anxiety but not fear in healthy volunteers: a fear-potentiated startle study., Biol. Psychiatry 69 (2011) 549–55. doi: 10.1016/j.biopsych.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Alvarez RP, Chen G, Bodurka J, Kaplan R, Grillon C, Phasic and sustained fear in humans elicits distinct patterns of brain activity., Neuroimage. 55 (2011) 389–400. doi: 10.1016/j.neuroimage.2010.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Davis M, Neural Systems Involved in Fear and Anxiety Measured With Fear-Potentiated Startle, J. Neurosci 22 (2006) 2343–2351. [DOI] [PubMed] [Google Scholar]
- [13].Tovote P, Fadok JP, Lüthi A, Neuronal circuits for fear and anxiety, Nat. Rev. Neurosci 16 (2015) 317–331. doi: 10.1038/nrn3945. [DOI] [PubMed] [Google Scholar]
- [14].Grupe DW, Nitschke JB, Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective., Nat. Rev. Neurosci 14 (2013) 488–501. doi: 10.1038/nrn3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Schmitz A, Grillon C, Assessing fear and anxiety in humans using the threat of predictable and unpredictable aversive events (the NPU-threat test), Nat. Protoc 7 (2012) 517–531. doi: 10.1038/nprot.1011.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Grillon C, Lissek S, Rabin S, McDowell D, Dvir S, Pine DS, Increased Anxiety During Anticipation of Unpredictable But Not Predictable Aversive Stimuli as a Psychophysiologic Marker of Panic Disorder, Am. J. Psychiatry 165 (2008) 898–904. doi: 10.1176/appi.ajp.1007.07101581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Shankman SA, Nelson BD, Sarapas C, Robison-Andrew EJ, Campbell ML, Altman SE,McGowan SK, Katz AC, Gorka SM, A psychophysiological investigation of threat and reward sensitivity in individuals with panic disorder and/or major depressive disorder., J. Abnorm. Psychol 111 (2013) 311–338. doi: 10.1037/a0030747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Grillon C, Pine DS, Lissek S, Rabin S, Bonne O, Vythilingam M, Increased anxiety during anticipation of unpredictable aversive stimuli in posttraumatic stress disorder but not in generalized anxiety disorder., Biol. Psychiatry 66 (2009) 47–53. doi: 10.1016/j.biopsych.1008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gorka SM, Lieberman L, Shankman SA, Phan KL, Association between neural reactivity and startle reactivity to uncertain threat in two independent samples., Psychophysiology. 54 (2017) 651–661. doi: 10.1111/psyp.11819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Clark D, Lee A and Watson, Distress and fear disorders: an alternative empirically based taxonomy of the “mood” and “anxiety” disorders, Br. J. Psychiatry 189 (2006) 481–483. [DOI] [PubMed] [Google Scholar]
- [21].Hettema JM, Prescott CA, Myers JM, Neale MC, Kendler KS, The Structure of Genetic and Environmental Risk Factors for Anxiety Disorders in Men and Women, Arch. Gen. Psychiatry 61 (2005) 181. doi: 10.1001/archpsyc.61.1.181. [DOI] [PubMed] [Google Scholar]
- [22].Vollebergh WAM, Iedema J, Bijl RV, de Graaf R, Smit F, Ormel J, The Structure and Stability of Common Mental Disorders, Arch. Gen. Psychiatry 58 (2001) 597. doi: 10.1001/archpsyc.58.6.597. [DOI] [PubMed] [Google Scholar]
- [23].Kendler KS, Prescott CA, Myers J, Neale MC, The Structure of Genetic and Environmental Risk Factors for Common Psychiatric and Substance Use Disorders in Men and Women, Arch. Gen. Psychiatry 60 (2003) 919. doi: 10.1001/archpsyc.60.9.919. [DOI] [PubMed] [Google Scholar]
- [24].Brown TA, Campbell LA, Lehman CL, Grisham JR, Mancill RB, Current and Lifetime Comorbidity of the DSM-IV Anxiety and Mood Disorders in a Large Clinical Sample, J. Abnorm. Psychol. Brown Barlow 110 (2001) 585–599. doi: 10.1037//0011-843X.110.4.585. [DOI] [PubMed] [Google Scholar]
- [25].Lang PJ, McTeague LM, Bradley MM, RDoC, DSM, and the reflex physiology of fear: A bio-dimensional analysis of the anxiety disorders spectrum 4 An effort to introduce a transdiagnostic dimensional model into DSM was made by the Personality Disorders Workgroup, Psychophysiology. 53 (2016) 336–347. doi: 10.1111/psyp.11461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Mcteague LM, Lang PJ, Wangelin BC, Laplante M-C, Bradley MM, Defensive mobilization in specific phobia: Fear specificity, negative affectivity and diagnostic prominence, Biol Psychiatry. July 1 (2012). doi: 10.1016/j.biopsych.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Nelson BD, Hodges A, Hajcak G, Shankman SA, Anxiety sensitivity and the anticipation of predictable and unpredictable threat: Evidence from the startle response and event-related potentials, J. Anxiety Disord 33 (2015) 62–71. doi: 10.1016/j.janxdis.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bach DR, Dolan RJ, Knowing how much you don’t know: a neural organization of uncertainty estimates, (2012). doi: 10.1038/nrn3289. [DOI] [PubMed] [Google Scholar]
- [29].Gorka SM, Nelson BD, Phan KL, Shankman SA, Insula response to unpredictable and predictable aversiveness in individuals with panic disorder and comorbid depression., Biol. Mood Anxiety Disord 4 (2014) 9. doi: 10.1186/2045-5380-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sarinopoulos I, Grupe DW, Mackiewicz KL, Herrington JD, Lor M, Steege EE, Nitschke JB, Uncertainty during Anticipation Modulates Neural Responses to Aversion in Human Insula and Amygdala, Cereb. Cortex 20 (2010) 929–940. doi: 10.1093/cercor/bhp155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Grupe DW, Oathes DJ, Nitschke JB, Dissecting the anticipation of aversion reveals dissociable neural networks., Cereb. Cortex 23 (2013) 1874–83. doi: 10.1093/cercor/bhs175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Walker DL, Davis M, Double dissociation between the involvement of the bed nucleus of thestria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear., J. Neurosci 17 (1997) 9375–83. doi: 10.1523/jneurosci.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Craig AD, How do you feel — now? The anterior insula and human awareness, Nat. Rev. Neurosci 10 (2009) 59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- [34].Khalsa SS, Rudrauf D, Feinstein JS, Tranel D, The pathways of interoceptive awareness, Nat. Neurosci 12 (2009) 1494–1496. doi: 10.1038/nn.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Etkin A, Egner T, Kalisch R, Emotional processing in anterior cingulate and medial prefrontal cortex, Trends Cogn. Sci 15 (2011) 85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lieberman L, Gorka SM, Shankman SA, Phan KL, Impact of Panic on Psychophysiological and Neural Reactivity to Unpredictable Threat in Depression and Anxiety, Clin. Psychol. Sci 5 (2017) 52–63. doi: 10.1177/2167702616666507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Münsterkötter AL, Notzon S, Redlich R, Grotegerd D, Dohm K, Arolt V, Kugel H, Zwanzger P, Dannlowski U, Spider or no spider? Neural correlates of sustained and phasic fear in spider phobia, Depress. Anxiety 32 (2015) 656–663. doi: 10.1002/da.22382. [DOI] [PubMed] [Google Scholar]
- [38].Simmons AN, Flagan TM, Wittmann M, Strigo IA, Matthews SC, Donovan H, Lohr JB, Paulus MP, The effects of temporal unpredictability in anticipation of negative events in combat veterans with PTSD, (2012). doi: 10.1016/j.jad.2012.08.006. [DOI] [PubMed] [Google Scholar]
- [39].Schlund MW, Verduzco G, Cataldo MF, Hoehn-Saric R, Generalized anxiety modulates frontal and limbic activation in major depression, Behav. Brain Funct 8 (2012) 8. doi: 10.1186/1744-9081-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Nelson BD, Hajcak G, Defensive motivation and attention in anticipation of different types of predictable and unpredictable threat: A startle and event-related potential investigation, Psychophysiology. 54 (2017) 1180–1194. doi: 10.1111/psyp.12869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Gorka SM, Lieberman L, Shankman SA, Phan KL, Startle potentiation to uncertain threat as a psychophysiological indicator of fear-based psychopathology: An examination across multiple internalizing disorders., J. Abnorm. Psychol 126 (2017) 8–18. doi: 10.1037/abn0000233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].First MB SR, Williams JBW, Karg RS, Structured Clinical Interview for DSM-5-Research Version (SCID-5 for DSM-5, Research Version; SCID-5-RV), American Psychiatric Association, Arlington, VA, 2015. [Google Scholar]
- [43].Watson D, O’Hara MW, Naragon-Gainey K, Koffel E, Chmielewski M, Kotov R, Stasik SM, Ruggero CJ, Development and Validation of New Anxiety and Bipolar Symptom Scales for an Expanded Version of the IDAS (the IDAS-II), Assessment. 19 (2012) 399–420. doi: 10.1177/1073191112449857. [DOI] [PubMed] [Google Scholar]
- [44].Ofrat S, Krueger RF, How research on the meta-structure of psychopathology aids in understanding biological correlates of mood and anxiety disorders, Biol. Mood Anxiety Disord 2 (2012) 1. doi: 10.1186/2045-5380-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Watson D, O’Hara MW, Simms LJ, Kotov R, Chmielewski M, McDade-Montez EA, Gamez W, Stuart S, Development and validation of the Inventory of Depression and Anxiety Symptoms (IDAS)., Psychol. Assess 19 (2007) 253–268. doi: 10.1037/1040-3590.19.3.253. [DOI] [PubMed] [Google Scholar]
- [46].Rollman GB, Harris G , The detectability, discriminability, and perceived magnitude of painful electrical shock, Percept. Psychophys 42 (1987) 257–268. doi: 10.3758/BF03203077. [DOI] [PubMed] [Google Scholar]
- [47].Schmitz A, Grillon C, Assessing fear and anxiety in humans using the threat of predictable and unpredictable aversive events (the NPU-threat test)., Nat. Protoc 7 (2012) 527–32. doi: 10.1038/nprot.2012.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Gorka SM, Nelson BD, Shankman SA, Startle response to unpredictable threat in comorbid panic disorder and alcohol dependence., Drug Alcohol Depend. 132 (2013) 216–22. doi: 10.1016/j.drugalcdep.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Nelson BD, Liu H, Sarapas C, Shankman SA, Intolerance of Uncertainty Mediates the Relationship between Panic and the Startle Reflex in Anticipation of Unpredictable Threat, J. Exp. Psychopathol 7 (2016) jep.048115. doi: 10.5127/jep.048115. [DOI] [Google Scholar]
- [50].Fetterman AK, Ode S, Robinson MD, For which side the bell tolls: The laterality of approach-avoidance associative networks, Motiv. Emot 37 (2013) 33–38. doi: 10.1007/s11031-012-9306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Killgore WDS, Yurgelun-Todd DA, The right-hemisphere and valence hypotheses: could they both be right (and sometimes left)?, Soc. Cogn. Affect. Neurosci 2 (2007) 240–50. doi: 10.1093/scan/nsm020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Shankman SA, Gorka SM, Nelson BD, Fitzgerald DA, Phan KL, O’Daly O, Anterior insula responds to temporally unpredictable aversiveness: an fMRI study., Neuroreport. 25 (2014) 596–600. doi: 10.1097/wnr.0000000000000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Avery JA, Drevets WC, Moseman SE, Bodurka J, Barcalow JC, Simmons WK, Major depressive disorder is associated with abnormal interoceptive activity and functional connectivity in the insula., Biol. Psychiatry 76 (2014) 258–66. doi: 10.1016/j.biopsych.2013.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Akiki TJ, Averill CL, Abdallah CG, A Network-Based Neurobiological Model of PTSD: Evidence From Structural and Functional Neuroimaging Studies., Curr. Psychiatry Rep 19 (2017) 81. doi: 10.1007/s11920-017-0840-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Sarinopoulos I, Grupe DW, Mackiewicz KL, Herrington JD, Lor M, Steege EE, Nitschke JB, Uncertainty during anticipation modulates neural responses to aversion in human insula and amygdala., Cereb. Cortex 20 (2010) 929–40. doi: 10.1093/cercor/bhp155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Singer T, Critchley HD, Preuschoff K, A common role of insula in feelings, empathy and uncertainty, Trends Cogn. Sci 13 (2009) 334–340. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- [57].Paulus MP, Stein MB, An Insular View of Anxiety, Biol. Psychiatry 60 (2006) 383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- [58].Grillon C, O’Connell K, Lieberman L, Alvarez G, Geraci M, Pine DS, Ernst M, Distinct Responses to Predictable and Unpredictable Threat in Anxiety Pathologies: Effect of Panic Attack, Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2 (2017) 575–581. doi: 10.1016/j.bpsc.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Perna G, Guerriero G, Brambilla P, Caldirola D, Panic and the Brainstem: Clues from Neuroimaging Studies, CNS Neurol. Disord. Drug Targets (2014). http://www.sequentialpsychotherapy.com/assets/panic-and-the-brainstem-final.pdf (accessed June4, 2018). [DOI] [PubMed] [Google Scholar]
- [60].Nicholls JG, Paton JFR, Brainstem: neural networks vital for life., Philos. Trans. R. Soc. Lond. B. Biol. Sci 364 (2009) 2447–51. doi: 10.1098/rstb.2009.0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Kincheski GC, Mota-Ortiz SR, Pavesi E, Canteras NS, Nio A, Carobrez P, The Dorsolateral Periaqueductal Gray and Its Role in Mediating Fear Learning to Life Threatening Events, PLoS One. 7 (2012). doi: 10.1371/journal.pone.0050361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Mobbs CD, Petrovic D, Marchant P, Hassabis JL, Weiskopf D, Seymour N, Dolan B, Frith RJ, When Fear is Near: Threat Imminence Elicits Prefrontal-Periaqueductal Gray Shifts in Humans, Science (80-.). 317 (2007) 1079–1083. doi: 10.1126/science.1144504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Nashold BS, Wilson WP, Slaughter DG, Sensations Evoked by Stimulation in the Midbrain of Man, J. Neurosurg 30 (1969) 14–24. doi: 10.3171/jns.1969.30.1.0014. [DOI] [PubMed] [Google Scholar]
- [64].Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LLB, Parvizi J, Hichwa RD, Subcortical and cortical brain activity during the feeling of self-generated emotions, Nat. Neurosci 3 (2000) 1049–1056. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- [65].Graeff FG, Serotonin, the periaqueductal gray and panic, Neurosci. Biobehav. Rev 28 (2004) 239–259. doi: 10.1016/j.neubiorev.2003.12.004. [DOI] [PubMed] [Google Scholar]
- [66].Pissiota A, Frans Ö, Fernandez M, von Knorring L, Fischer H, Fredrikson M, Neurofunctional correlates of posttraumatic stress disorder: a PET symptom provocation study, Eur. Arch. Psychiatry Clin. Neurosci 252 (2002) 68–75. doi: 10.1007/s004060200014. [DOI] [PubMed] [Google Scholar]
- [67].Hadjipavlou G, Dunckley P, Behrens TE, Tracey I, Determining anatomical connectivities between cortical and brainstem pain processing regions in humans: A diffusion tensor imaging study in healthy controls, Pain. 123 (2006) 169–178. doi: 10.1016/j.pain.2006.02.027. [DOI] [PubMed] [Google Scholar]
- [68].Floyd NS, Price JL, Ferry AT, Keay KA, Bandler R, Orbitomedial prefrontal cortical projections to distinct longitudinal columns of the periaqueductal gray in the rat., J. Comp. Neurol 422 (2000) 556–78. [DOI] [PubMed] [Google Scholar]
- [69].Bandler R, Keay KA, Floyd N, Price J, Central circuits mediating patterned autonomic activity during active vs. passive emotional coping., Brain Res. Bull 53 (2000) 95–104. [DOI] [PubMed] [Google Scholar]
- [70].Deakin JFW, Graeff FG, 5-HT and mechanisms of defence, J. Psychopharmacol 5 (1991) 305–315. doi: 10.1177/026988119100500414. [DOI] [PubMed] [Google Scholar]
- [71].Jenck F, Moreau JL, Martin JR, Dorsal periaqueductal gray-induced aversion as a simulation of panic anxiety: elements of face and predictive validity., Psychiatry Res. 57 (1995) 181–91. http://www.ncbi.nlm.nih.gov/pubmed/7480384 (accessed June 5, 2018). [DOI] [PubMed] [Google Scholar]
- [72].Schenberg LC, Bittencourt AS, Sudré EC, Vargas LC, Modeling panic attacks., Neurosci. Biobehav. Rev 25 (2001) 647–59. http://www.ncbi.nlm.nih.gov/pubmed/11801290 (accessed June 5, 2018). [DOI] [PubMed] [Google Scholar]
- [73].Dresler T, Guhn A, Tupak SV, Ehlis A-C, Herrmann MJ, Fallgatter AJ, Deckert J, Domschke K, Revise the revised? New dimensions of the neuroanatomical hypothesis of panic disorder, J. Neural Transm 120 (2013) 3–29. doi: 10.1007/s00702-012-0811-1. [DOI] [PubMed] [Google Scholar]
- [74].Gorman JM, Kent J, Martinez J, Browne S, Coplan J, Papp LA, Physiological changes during carbon dioxide inhalation in patients with panic disorder, major depression, and premenstrual dysphoric disorder: evidence for a central fear mechanism., Arch. Gen. Psychiatry 58 (2001) 125–31. http://www.ncbi.nlm.nih.gov/pubmed/11177114 (accessed June 22, 2018). [DOI] [PubMed] [Google Scholar]
- [75].Nelson BD, McGowan SK, Sarapas C, Robison-Andrew EJ, Altman SE, Campbell ML,Gorka SM, Katz AC, Shankman SA, Biomarkers of threat and reward sensitivity demonstrate unique associations with risk for psychopathology., J. Abnorm. Psychol 122 (2013) 662–71. doi: 10.1037/a0033982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Gorka SM, Liu H, Sarapas C, Shankman SA, Time course of threat responding in panic disorder and depression., Int. J. Psychophysiol 98 (2015) 87–94. doi: 10.1016/j.ijpsycho.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Rapee RM, Litwin EM, Barlow DH, Impact of life events on subjects with panic disorder and on comparison subjects, Am. J. Psychiatry 147 (1990) 640–644. doi: 10.1176/ajp.147.5.640. [DOI] [PubMed] [Google Scholar]
- [78].Cho Y, Smits JAJ, Powers MB, Telch MJ, Cho Y, Smits JAJ, Powers MB, Telch MJ, Do Changes in Panic Appraisal Predict Improvement in Clinical Status Following Cognitive-Behavioral Treatment of Panic Disorder?, Cogn Ther Res. 31 (2007) 695–707. doi: 10.1007/s10608-006-9068-z. [DOI] [Google Scholar]
- [79].Gard AM, Waller R, Swartz JR, Shaw DS, Forbes EE, Hyde LW, Amygdala functional connectivity during socioemotional processing prospectively predicts increases in internalizing symptoms in a sample of low-income, urban, young men, Neuroimage. 178 (2018) 562–573. doi: 10.1016/j.neuroimage.2018.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.