Abstract
Background:
Leadless pacemakers (LPMs) have been shown to have lower post- operative complications than traditional permanent pacemakers but there have been no studies on the outcomes of LPMs in patients with transcatheter heart valve replacements (THVRs). This study determined outcomes of LPMs compared to single chamber transvenous pacemakers (SCPs) post-THVR.
Methods:
This is a retrospective single-center study including 10 patients who received LPMs post-THVR between February 2017 and August 2018 and a comparison group of 23 patients who received SCP post-THVR between July 2008 and August 2018. LPM or SCP was implanted at the discretion of electrophysiologists for atrial fibrillation with slow ventricular response or sinus node dysfunction with need for single chamber pacing only.
Results:
LPMs were associated with decreased tricuspid regurgitation (p=0.04) and decreased blood loss during implantation (7.5 ± 2.5cc for LPMs versus 16.8 ± 3.2cc for SCPs, p=0.03). Five LPM patients had devices positioned in the right ventricular septum as seen on transthoracic echocardiogram. Frequency of ventricular pacing was similar between LPM and SCP groups. In the LPM group, one case was complicated by a pseudoaneurysm and one death was due to non-cardiac causes. There was one pneumothorax and one pocket infection in the SCP group.
Conclusions:
In this small retrospective study, LPMs were feasible post-THVR and found to perform as well as SCPs, had less intra-procedural blood loss, and were associated with less tricuspid regurgitation. Further larger studies are required to follow longer term outcomes and complications.
Keywords: leadless pacemaker, transcatheter heart valve replacement, tricuspid regurgitation, single chamber pacemaker, permanent pacemaker
INTRODUCTION
Transfemoral replacement of the aortic valve has steadily increased since the pivotal PARTNER trials1–3, which has paved the way for development of new transcatheter heart valve replacement (THVR) techniques for the replacement of the mitral valve and tricuspid valve. Permanent pacemaker (PPM) implantation after transcatheter aortic valve replacement (TAVR) occurs at a rate between 2.3% to 37.7%4 , with trial data showing newer generation valves to have PPM implantation rates between 12 and 20%.5–7 Little has been published for pacemaker implantation after transcatheter mitral valve replacement (TMVR). Traditional PPMs have long been known to have complications, such as surgical pocket infections, lead infections, and worsening tricuspid regurgitation, with short-term complication rates as high as 8 to 12%,.8–10 Since the implantations of THVR continue to increase, these complications rates should not be taken lightly and if there is an alternative to traditional PPMs with lower complication rates, then it should be investigated.
Leadless pacemakers (LPMs) were first approved for use by the United States Food and Drug Administration in April 2016.11 Sitting entirely inside the right ventricle of the heart, LPMs have been shown in initial clinical trials to have excellent performance in terms of device electrical parameters up to 1 year. These initial trials also showed LPMs to have lower lead and pocket complication rates than traditional pacemakers, with rates ranging from 3.4 to 6.5%.12–15 Based on this data, LPMs could be considered a favorable alternative to traditional pacemakers post-THVR, especially since the THVR patient population usually is older and are more likely to have preexisting tricuspid regurgitation.16 However, the use of LPMs in patients who have undergone THVR has yet to be studied systematically. There are a few case reports published that have shown LPM implantation post-TAVR to be successful; however, these are all single cases.17–20
This study included 10 patients who received LPMs post-THVR in order to analyze initial outcomes when compared with patients who received transvenous single-chamber pacemakers (SCPs) post-THVR. This is the largest study on LPMs post-THVR to the authors’ knowledge and is also the first study to compare outcomes between LPMs and traditional SCPs post-THVR. This study will provide much needed data on how well patients do after receiving LPMs post-THVR.
METHODS
Patient Selection
This study was approved by the Columbia University Medical Center Institutional Review Board and was designed as a retrospective chart review. Patients post-THVR underwent implantation of the LPM at the discretion of the electrophysiologists at Columbia University Medical Center. Between February 2017 and August 2018, nine individuals received a LPM following TAVR and one patient received LPM following TMVR. For the comparison group, post-THVR patients who underwent SCP placement at Columbia University Medical Center were identified from the THVR QI database, which includes all patients who had a permanent pacemaker placed after TAVR/TMVR between July 2008 and August 2018. Twenty-three post-THVR patients in this database were identified as having had a SCP placed and included in the study. Patients who required dual chamber pacing were excluded from this study.
Data Collection
The electronic medical records for each patient were screened for target data. This data included demographic and anthropometric information, comorbidities, echocardiographic, and electrocardiogram data at various time points (pre-THVR and post-PPM), operative data, complications, and device interrogation data post-PPM implantation. Grading of tricuspid regurgitation was based on reporting from board certified echocardiographers. Tricuspid regurgitation severity was converted to a numerical system (trace = 0, mild =1, moderate =2, severe = 3) for the purposes of data analysis. Positioning of LPMs was determined based on review of transthoracic echocardiograms, which were performed on all patients post-LPM placement. Patients were followed-up for an average of 158 ± 58 days post-LPM or post-SCP (range of 1 to 1185 days).
Data Analysis
Data analyses were performed using StatPlus software, v6.4.71. One- and two- sided t-tests for normally distributed variables were performed to assess for difference in means for each quantitative variable between the LPM and TPM groups, and the unpaired Wilcoxon rank sum test was used for non-normally distributed data. Means and standard error of the mean were calculated for continuous variables. Categorical variables were assessed for differences between groups via chi-square tests.
RESULTS
Subject Characteristics
There were no statistically significant differences between the groups who received SCPs compared to LPMs in age, gender, co-morbidities, BMI, or pre-THVR left ventricular ejection fraction (Table 1). The mean timing of transthoracic echocardiograms prior to TAVR was 20.2 ± 3.4 days and 40.9 ± 14.7 days for the SCP and LPM groups, respectively. Among patients who had a bundle branch block, there were more right than left bundle branch blocks for both the LPM and SCP groups (4 vs. 2 in the LPM group, respectively, and 7 vs. 6 in the SCP group, respectively). In both groups, the vast majority of patients underwent THVR via transfemoral approach. Among the patients who received LPMs post-TAVR, the vast majority of them received SAPIEN 3 valves as opposed to other types of valves; more variability was seen in the SCP group, with roughly one third receiving CoreValves, another third receiving SAPIEN 3 valves, and the rest receiving a combination of SAPIEN and SAPIEN XT valves. The patient who underwent LPM placement post-TMVR received a SAPIEN 3 valve as well.
Table 1.
Baseline Demographic and THVR Characteristics
| Leadless Pacemaker (n = 10) | Single Chamber Pacemaker (n = 23) | p-value | |
|---|---|---|---|
| Age (y) | 82.8 (3.1) | 84 (1.4) | 0.67 |
| Gender | |||
| Males | 6 (60%) | 12 (52%) | 0.68 |
| Females | 4 (40%) | 11 (48%) | |
| Body Mass Index | 30.4 (2.5) | 26.9 (1.5) | 0.19 |
| Pre-THVR Left Ventricular Ejection Fraction | 53 (5.2) | 52 (3.8) | 0.92 |
| Approach for THVR | |||
| Transfemoral | 9 (90%) | 20 (87%) | n/a |
| Transaortic | 0 | 2 (9%) | |
| Transapical | 1 (10%) | 1 (4%) | |
| Valve Replaced | |||
| Aortic | 9 (90%) | 23 (100%) | n/a |
| Mitral | 1 (10) | 0 | |
| Type of Valve Placed | |||
| CoreValve | 0 | 7 (30%) | n/a |
| Evolut R | 1 (10%) | 0 | |
| SAPIEN | 0 | 5 (22%) | |
| SAPIEN XT | 0 | 2 (9) | |
| SAPIEN 3 | 9 (90) | 9 (39%) | |
| Post-TAVR EKG | |||
| Right bundle branch block | 4 (40%) | 7 (32%) | 0.59 |
| Left bundle branch block | 2 (20%) | 6 (27%) | 0.70 |
Values are presented as mean (SE) or n (%).
THVR = transcatheter heart valve replacement
Procedural Characteristics
The mean time between TAVR and PPM implantation was 44.7 ± 23.8 days and 15.8 ± 13.2 days for LPMs and SCPs, respectively. Indications for either a LPM or SCP consisted of atrial fibrillation with slow ventricular response or sinus node dysfunction with need for single chamber pacing only. There was no statistically significant difference between the indication for PPM between the two groups, with the majority receiving a PPM for atrial fibrillation with slow ventricular response or high-grade AV block (Table 2). Among the SCP group, 11 out of 23 patients had temporary transvenous pacers (TVPs) left in place post-procedure, compared to the LPM group, where 5 out of 10 patients had TVPs left in place post-procedure. None had documented complications from the TVPs. LPMs were more commonly placed with monitored anesthesia care (MAC), whereas SCPs were more commonly placed with moderate/conscious sedation. LPMs required longer fluoroscopy time, 20.8 minutes, compared to SCPs, which required only 5.3 minutes on average, which is likely due to operator inexperience. Lastly, the average estimated blood loss in LPMs was significantly less than with SCPs.
Table 2.
Pacemaker Procedural Characteristics
| Leadless Pacemaker (n = 10) | Single Chamber Pacemaker (n = 23) | p-value | |
|---|---|---|---|
| Indication for pacemaker | |||
| Atrial fibrillation with slow ventricular response | 5 (62.5%) | 10 (43%) | 0.26 |
| High-grade atrioventricular block | 3 (37.5%) | 12 (52%) | |
| Sinus arrest | 0 | 1 (5%) | |
| Temporary transvenous pacing | 5 (50%) | 11 (47.8%) | 0.042* |
| Fluoroscopy time (mins) | 20.8 ±4.7 | 5.3 ±1.1 | <0.01* |
| Estimated blood loss (cc) | 7.5 ± 2.5 | 16.8 ± 3.2 | 0.03* |
| Type of anesthesia | |||
| Moderate/Conscious sedation | 1 (10%) | 18 (78%) | <0.01* |
| Monitored anesthesia care | 9 (90%) | 2 (9%) | |
| General anesthesia | 0 (0) | 3 (13%) | N/A |
The same access site, typically right femoral site, was successfully utilized for both TAVR and LPM placement in 8 out of the 10 patients. There were three individuals who were deemed not candidates for LPM due to vascular access issues, such as the presence of a large hematoma, recent IVC filter, or iliac vein stent of which two of were not included in this study and one who received a SCP. Based on echocardiographic data of the patients who received LPMs, 4 LPMs were placed apically, 5 in the septal position, and one LPM was placed in the apical septum (Figure 1).
Figure 1.
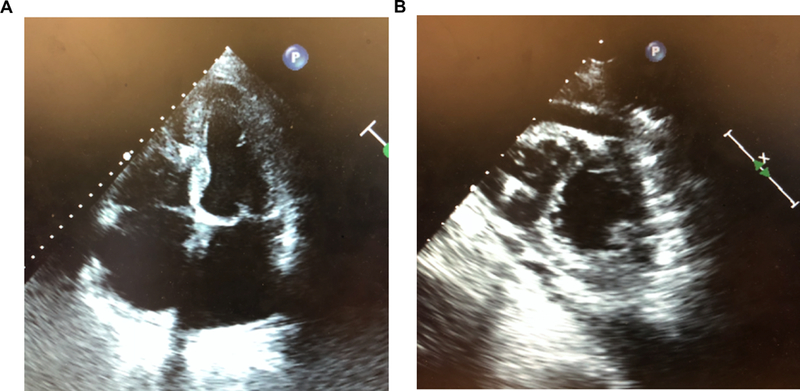
Transthoracic echocardiograms of leadless pacemakers. (a) Apical 4-chamber view of the leadless pacemaker in the right ventricular septal position. (b) Parasternal short-axis view of the leadless pacemaker in the right ventricular apical position.
Outcomes
Follow-up time in this retrospective study was a mean of 199 ± 87.6 days and 84 ± 31.9 days for the SCP and LPM groups, respectively. Patients with LPMs had a statistically significant lower likelihood of worsening tricuspid regurgitation (Table 3). Two post-operative complications in the study occurred in the SCP group: one developed a tension pneumothorax after SCP placement and one individual developed a pocket infection requiring device removal. There was one patient who developed a pseudoaneurysm post LPM, which was later treated with a covered stent due to concern for possible development of high output heart failure. There was no significant difference in frequency of ventricular pacing between LPM and SCP at 1 or 3 months post-PPM, though the LPM group did have lower thresholds and higher impedances. One of 10 patients who received a LPM died of renal failure, as did one of the 23 patients who received a SCP. The one LPM patient who died had chronic renal failure after a failed renal transplant performed over 10 years prior, as well as known long-standing congestive heart failure among other numerous co-morbidities, had been admitted for volume overload and was on dialysis. The TAVR was placed 5 months prior to death, with the LPM implanted 17 days prior to death without any procedure related complications such as cardiac tamponade, perforation, excessive bleeding or contrast induced renal failure.
Table 3.
Outcomes after pacemaker implantation.
| Leadless Pacemaker (n = 10) | Single Chamber Pacemaker (n = 23) | p-value | |
|---|---|---|---|
| Change in tricuspid regurgitation grade pre-THVR to post-PPM* | −0.4 ±0.5 | 0.11 ±0.17 | 0.04 |
| Post-operative complications | |||
| Pseudoaneurysm | 1 (4.5%) | 0 | N/A |
| Pneumothorax | 0 | 1 (4.5%) | |
| Pocket infection | 0 | 1 (4.5%) | |
| PPM Interrogation | |||
| Follow-up post-PPM (d) | 84 ± 32 | 199 ± 87 | 0.36 |
| Frequency of ventricular pacing at 1 month post-PPM (%) | 50.9 ± 19.4 (n = 5/10) | 59.2 ± 9.4 (n = 14/23) | 0.46 |
| Frequency of ventricular pacing at 3 months post-PPM (%) | 29.9 ± 24.2 (n = 2/10) | 46.8 ± 12.1 (n = 4/23) | 0.64 |
| Ventricular capture threshold (V) | 0.48 ± 0.07 | 0.70 ± 0.05 | <0.01 |
| Impedance (ohm) | 652 ± 56 | 544 ± 34 | 0.02 |
| Sensed ventricular amplitude (mV) | 8.6 ± 2.9 | 9.5 ± 1.9 | 0.01 |
| Deaths | |||
| PPM-related | 0 | 0 | N/A |
| Not PPM-related | 1 (12.5%) | 1 (4.3%) | 0.51 |
Values are presented as mean (SE) or n (%).
Change in tricuspid regurgitation was graded as 0=none, 1=trace, 2=mild, 3=moderate, 4=severe; PPM = permanent pacemaker; THVR = transcatheter heart valve replacement
DISCUSSION
While LPMs are still relatively new in use, these devices are a promising option for patients with new arrhythmias after THVR requiring PPMs. In this study that included 10 patients receiving LPM post-THVR, which is not only the largest study on LPMs post-THVR to the authors’ knowledge but also the first study to compare outcomes between LPMs and traditional SCPs post-THVR, LPMs were found to be associated with less tricuspid regurgitation and less blood loss than SCPs with only one complication associated with LPMs.
Although placement of LPM through the left femoral vein access is possible, when placing LPM in our electrophysiology laboratory, the right femoral vein is preferred. Even though the 27 Fr Micra delivery system is hydrophilic, it is rigid and maneuvering from the left femoral vein challenging, especially in an elderly population with tortuous and angulated venous anatomy. In our electrophysiology laboratory, we now use ultrasound guided for every LPM to minimize vascular complications, especially after the access complication of a pseudoaneurysm in one of our early cases. Prior to LPM placement, we also use femoral vein contrast venography after access with an 8 Fr sheath to roadmap the vein and help the operator decide whether the femoral vein can accommodate the Micra delivery system.
Our finding of less tricuspid regurgitation post-LPM than post-SCP is consistent with prior studies that have shown LPMs to lead to less tricuspid regurgitation than traditional PPMs. 12–15 This is an important consideration, especially in the THVR population, as this population tends to be older with a higher prevalence of tricuspid regurgitation at baseline.16 The decreased blood loss seen with LPMs as compared with SCPs is another important consideration that is particularly relevant to the THVR population, as patients post-THVR will typically be on some form of anti-platelet therapy. Also, over 35% of patients who undergo THVR have pre-existing atrial fibrillation,21 a population requiring therapeutic anticoagulation. It was also noted in this study that LPMs could be placed via the ipsilateral access site as THVR without notable complications while on systemic anticoagulation for atrial fibrillation. Lastly, while a significantly longer fluoroscopy time was noted for those undergoing LPM, this time decreased substantially with increased operator experience. The complications of pneumothorax and pocket infection in the SCP group were notable. These are all important factors that should prompt electrophysiologists to consider placement of a LPM in patients who develop bradyarrhythmias requiring a PPM post-THVR. Overall, these devices are most suitable for patients at high risk of infection with adequate vascular access and if performed by a knowledgeable/experienced operator.
Another novel aspect of this study is the analysis of the post-operative imaging of the positioning of LPMs post-THVR. The goal position for LPMs is the septum of the right ventricle.22 In this study, 5 out of the 10 patients who received LPMs post-TAVR had LPMs positioned in the septal position. This could be due to left ventricular hypertrophy that is commonly seen in patients with severe aortic stenosis, which can cause bowing of the septum such that the apex of the right ventricle may be more difficult to reach. There were no adverse outcomes associated with the septal positioning of LPMs and a previous study on Micra LPMs also found about 50% of LPMs to be placed in the septal position, similar to our study.23 Also, in traditional PPMs, studies have shown that septal positioning of leads may actually be preferred to apical positioning as this leads to reduced ventricular dyssynchrony and significantly preserved left ventricular ejection fraction.24,25 Longer follow-up, however, will be needed to determine whether the same benefit would be seen with LPMs as with traditional PPMs.
Limitations
The main limitations of this study are 1) the small sample size and 2) its retrospective nature, with some patients having short follow up times. That being said, this is the largest group of LPM patients post-THVR identified in the literature to date. Further studies with longer follow-up times and larger sample sizes will be needed to determine if the results found in this study are seen long-term. Additionally, as this is a retrospective study so no causal effects can be claimed.
CONCLUSIONS
In this study, which is the largest study on LPMs post-THVR to date and also the first study to compare outcomes between LPMs and traditional SCPs post-THVR, LPMs were found to perform as well as SCPs and were found to have less tricuspid regurgitation as well as decreased blood loss during the procedure. Based on these results, LPMs should be considered for patients with new pacemaker-requiring bradyarrhythmias following THVR. Larger studies should be conducted to further investigate longer term outcomes of LPMs placed post-THVR.
Acknowledgments
FUNDING
E.Y.W. was supported by NIH K08HL122526, the Louis V. Gerstner, Jr. Scholars Program, the Esther Aboodi Endowed Professorship at Columbia University and the M. Iréne Ferrer Scholar Award from the Foundation of Gender Specific Medicine.
Footnotes
Disclosures: None
REFERENCES
- 1.Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, Thourani VH, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. The New England journal of medicine 2010;363:1597–607. [DOI] [PubMed] [Google Scholar]
- 2.Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. The New England journal of medicine 2011;364:2187–98. [DOI] [PubMed] [Google Scholar]
- 3.Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, Thourani VH, et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. New England Journal of Medicine 2016;374:1609–20. [DOI] [PubMed] [Google Scholar]
- 4.van Rosendael PJ, Delgado V, Bax JJ. Pacemaker implantation rate after transcatheter aortic valve implantation with early and new-generation devices: a systematic review. European heart journal 2018;39:2003–13. [DOI] [PubMed] [Google Scholar]
- 5.Popma JJ, Reardon MJ, Khabbaz K, Harrison JK, Hughes GC, Kodali S, George I, et al. Early Clinical Outcomes After Transcatheter Aortic Valve Replacement Using a Novel Self-Expanding Bioprosthesis in Patients With Severe Aortic Stenosis Who Are Suboptimal for Surgery. Results of the Evolut R US Study. JACC Cardiovasc Interv 2017;10:268–75. [DOI] [PubMed] [Google Scholar]
- 6.Thourani VH, Kodali S, Makkar RR, Herrmann HC, Williams M, Babaliaros V, Smalling R, et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: a propensity score analysis. Lancet 2016;387:2218–25. [DOI] [PubMed] [Google Scholar]
- 7.Herrmann HC, Thourani VH, Kodali SK, Makkar RR, Szeto WY, Anwaruddin S, Desai N, et al. One-Year Clinical Outcomes With SAPIEN 3 Transcatheter Aortic Valve Replacement in High-Risk and Inoperable Patients With Severe Aortic Stenosis. Circulation 2016;134:130–40. [DOI] [PubMed] [Google Scholar]
- 8.Tjong FV, Reddy VY. Permanent Leadless Cardiac Pacemaker Therapy: A Comprehensive Review. Circulation 2017;135:1458–70. [DOI] [PubMed] [Google Scholar]
- 9.Udo EO, Zuithoff NP, van Hemel NM, de Cock CC, Hendriks T, Doevendans PA, Moons KG et al. Incidence and predictors of short- and long-term complications in pacemaker therapy: the FOLLOWPACE study. Heart rhythm 2012;9:728–35. [DOI] [PubMed] [Google Scholar]
- 10.Lin G, Nishimura RA, Connolly HM, Dearani JA, Sundt TM 3rd, Hayes DL. Severe symptomatic tricuspid valve regurgitation due to permanent pacemaker or implantable cardioverter-defibrillator leads. Journal of the American College of Cardiology 2005;45:1672–5. [DOI] [PubMed] [Google Scholar]
- 11.FDA approves first leadless pacemaker to treat heart rhythm disorders 2016. (Accessed August 25, 2018, at https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm494417.htm.)
- 12.Reddy VY, Knops RE, Sperzel J, Miller MA, Petru J, Simon J, Sediva L, et al. Permanent leadless cardiac pacing: results of the LEADLESS trial. Circulation 2014;129:1466–71. [DOI] [PubMed] [Google Scholar]
- 13.Knops RE, Tjong FV, Neuzil P, Sperzel J, Miller MA, Petru J, Simon J, et al. Chronic performance of a leadless cardiac pacemaker: 1-year follow-up of the LEADLESS trial. Journal of the American College of Cardiology 2015;65:1497–504. [DOI] [PubMed] [Google Scholar]
- 14.Reddy VY, Exner DV, Cantillon DJ, Doshi R, Bunch TJ, Tomassoni GF, Friedman PA, et al. Percutaneous Implantation of an Entirely Intracardiac Leadless Pacemaker. The New England journal of medicine 2015;373:1125–35. [DOI] [PubMed] [Google Scholar]
- 15.Reynolds D, Duray GZ, Omar R, Soejima K, Neuzil P, Zhang S, Narasimhan C, et al. A Leadless Intracardiac Transcatheter Pacing System. The New England journal of medicine 2016;374:533–41. [DOI] [PubMed] [Google Scholar]
- 16.Barbanti M, Binder RK, Dvir D, Tan J, Freeman M, Thompson CR, Cheung A, et al. Prevalence and impact of preoperative moderate/severe tricuspid regurgitation on patients undergoing transcatheter aortic valve replacement. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions 2015;85:677–84. [DOI] [PubMed] [Google Scholar]
- 17.Shikama T, Miura M, Shirai S, Hayashi M, Morita J, Nagashima M, Ando K Leadless Pacemaker Implantation Following Transcatheter Aortic Valve Implantation Using SAPIEN 3. Korean Circ J 2018;48:534–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fudim M, Fredi JL, Ball SK, Ellis CR. Transcatheter Leadless Pacemaker Implantation for Complete Heart Block Following CoreValve Transcatheter Aortic Valve Replacement. Journal of cardiovascular electrophysiology 2016;27:125–6. [DOI] [PubMed] [Google Scholar]
- 19.Kypta A, Blessberger H, Lichtenauer M, Steinwender C. Dawn of a new era: the completely interventionally treated patient. BMJ case reports 2016;2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tyler Bloomer CE. Pacing-induced cardiomyopathy from a transcatheter leadless pacemaker following transcatheter aortic valve replacement. American College of Cardiology Annual Scientific Sessions. Washington, DC: 2017. [Google Scholar]
- 21.Tarantini G, Mojoli M, Windecker S, Wendler O, Lefevre T, Saia F, Walther T, et al. Prevalence and Impact of Atrial Fibrillation in Patients With Severe Aortic Stenosis Undergoing Transcatheter Aortic Valve Replacement: An Analysis From the SOURCE XT Prospective Multicenter Registry. JACC Cardiovascular interventions 2016;9:937–46. [DOI] [PubMed] [Google Scholar]
- 22.El-Chami MF, Roberts PR, Kypta A, Omdahl P, Bonner MD, Kowal RC, Duray GZ, et al. How to Implant a Leadless Pacemaker With a Tine-Based Fixation. Journal of cardiovascular electrophysiology 2016;27:1495–501. [DOI] [PubMed] [Google Scholar]
- 23.Roberts PR, Clementy N, Al Samadi F, Garweg C, Martinez-Sande JL, Iacopino S, Johansen JB, et al. A leadless pacemaker in the real-world setting: The Micra Transcatheter Pacing System Post-Approval Registry. Heart rhythm 2017;14:1375–9. [DOI] [PubMed] [Google Scholar]
- 24.Yu Z, Chen R, Su Y, Chen X, Qin S, Li M, Han F, et al. Integrative and quantitive evaluation of the efficacy of his bundle related pacing in comparison with conventional right ventricular pacing: a meta-analysis. BMC cardiovascular disorders 2017;17:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Catanzariti D, Maines M, Cemin C, Broso G, Marotta T, Vergara G. Permanent direct his bundle pacing does not induce ventricular dyssynchrony unlike conventional right ventricular apical pacing. An intrapatient acute comparison study. Journal of interventional cardiac electrophysiology : an international journal of arrhythmias and pacing 2006;16:81–92. [DOI] [PubMed] [Google Scholar]


