Abstract
Genetic cardiomyopathies, a group of cardiovascular disorders based on ventricular morphology and function, are amongst the leading causes of morbidity and mortality worldwide. Such genetically-driven forms of hypertrophic (HCM), dilated (DCM) and restrictive (RCM) cardiomyopathies are chronic, debilitating diseases that result from biomechanical defects in cardiac muscle contraction and frequently progress to heart failure (HF). Locus and allelic heterogeneity, as well as clinical variability combined with genetic and phenotypic overlap between different cardiomyopathies, have challenged proper clinical prognosis and provided an incentive for identification of pathogenic variants. This review attempts to provide an overview of inherited cardiomyopathies with a focus on their genetic etiology in myosin regulatory (RLC) and essential (ELC) light chains, which are EF-hand protein family members with important structural and regulatory roles. From the clinical discovery of cardiomyopathy-linked light chain mutations in patients to an array of exploratory studies in animals, reconstituted, and recombinant systems, we have summarized the current state of knowledge on light chain mutations and how they induce physiological disease states via biochemical and biomechanical alterations at the molecular, tissue and organ level. Cardiac myosin RLC phosphorylation and the N-terminus ELC have been discussed as two important emerging modalities with important implications in the regulation of myosin motor function and thus cardiac performance. A comprehensive understanding of such triggers is absolutely necessary for the development of target-specific rescue strategies to ameliorate or reverse the effects of myosin light chain-related inherited cardiomyopathies.
Keywords: Cardiomyopathy mutations, myosin regulatory light chain, myosin essential light chain, human phenotype, transgenic mice
Introduction
Mutations in genes encoding sarcomeric proteins are the most predominant causes of inherited cardiomyopathies. In this review, we report on mutations in myosin regulatory (RLC) and essential (ELC) light chains that are associated with one or more types of cardiomyopathy disease: hypertrophic (HCM), dilated (DCM) and restrictive (RCM) cardiomyopathy 76 (Table 1).
Table 1.
Overview of mutations in myosin light chains associated with cardiomyopathies
| Gene/Protein | Phenotype | Mutation | References to population studies |
|---|---|---|---|
| MYL2/KLC | HCM | A13T, F18L, M20L, E22K, N47K, R58Q, D94A, P95A, K104E, E134A, I158L, G162R, D166A, D166V, IVS6-1, IVS5-2, | Poetter et al.92, Flavigny et. al.26, Kabaeva et al.54, Olivotto et. al.88, Santos et al. 100, Anderson et. al.5, Richard et al.95, Álvarez-Acosta et al.4, Barth et al.11 |
| RCM | G57E | Caleshu et al.16 | |
| DCM | D94A | Huang et al.49, | |
| MYL3/ELC | HCM | E56G, A57G, R63C, V79I, R81H, G128C, M149V, E152K, R154H, H155D, M173V, E177G | Richard et al.95, Lee W. et al.64, Chiou et al.17, Andersen et al.6, Fokstuen et al.28, Garcia-Pavia et al.30, Poetter et al.92, Epstein23, Arad at al.8, Kaski et al.56, Morita et al.83, Jay et al.53 |
| RCM | E143K | Olson et al.89, Caleshu et al.16 | |
| DCM | Not reported |
HCM is a disease of the myocardium characterized by asymmetric hypertrophy of the left ventricle (LV) and impaired diastolic function76, 104. The prevalence of HCM is 1 in 500 of the general population, and occurrences of sudden cardiac death (SCD) are common, especially among young athletes 75. Histopathological features of HCM include myocyte enlargement (hypertrophy) and myofibrillar disarray, changes which are often accompanied by fibrotic depositions 31, 73. Dominant pathogenic variants in HCM genes are the most common causes of HCM 3, and the two predominant genes responsible for approximately half of the patients with familial HCM are MYH7 (β-myosin heavy chain) and MYBPC3 (myosin binding protein C) 73. Mutations in MYL2 or MYL3, encoding the ventricular myosin RLC or ELC, respectively, are rare, but they are often implicated in malignant HCM outcomes 26, 54, 84, 92
In DCM, enlargement of the LV cavity (without a proportional increase of wall thickness) is observed which is followed by systolic dysfunction. Histopathological images of DCM hearts show vacuolated myocytes with myofibrillar loss and interstitial fibrosis 51, 90. The prevalence of DCM in the general population is 1 in 250 19, 43. The majority of familial DCM is caused by genetic mutations with an autosomal dominant trait 78 and 35-40% of these mutations are linked to sarcomeric proteins 41.
RCM is characterized by increased stiffness of the LV wall with no increase in wall thickness and a largely impaired diastolic function with usually normal or near-normal systolic function 63. RCM is less common than HCM and DCM, but the prognosis is poor and the risk for ischemia-related complications and death is high 96. The restrictive phenotype can also be observed in HCM families63, 89. The majority of idiopathic RCM is caused by genetic mutations identified in myosin heavy chain, myosin binding protein C, troponin I and T, tropomyosin and desmin genes 29.
The role of MLCs in cardiac muscle contraction
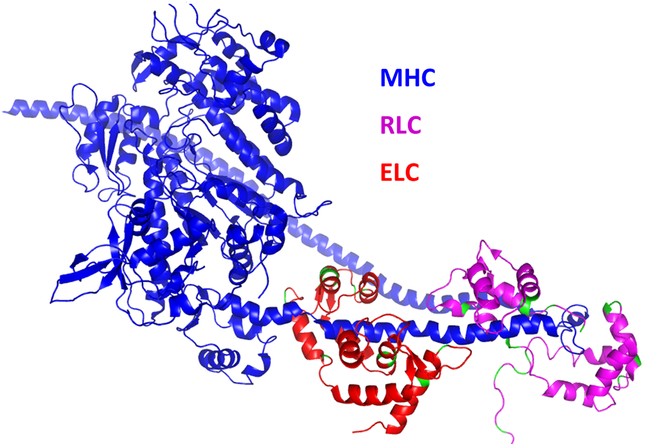
Cardiac muscle contraction involves the ATP-dependent cyclic attachments and detachments of the myosin cross-bridges to the actin-tropomyosin (Tm)-troponin (Tn) filaments 31, 66. The myosin cross-bridge is the molecular motor of the heart; it binds ATP and actin and its neck region (lever arm) amplifies small conformational changes generated in the motor domain into large movements needed to produce force and sarcomere shortening 44. Myosin head domain (S1) contains the ATP and actin binding sites and the lever arm domain where MLCs are attached to their respective IQ motifs94. The myosin regulatory (RLC) and essential (ELC) light chains structurally support the lever arm region of myosin heavy chain (MHC) (Fig. 1)2, but they also modulate myosin motor activity and thus cardiac muscle contraction in light chain-dependent manner (review in 42, 109). Mutations in ventricular myosin heavy chain (MHC) and both MLCs have been associated with different forms of cardiomyopathy 2, 20, 32, 35, 121 and over the last two decades, our lab has generated humanized mouse models of RLC and ELC related HCM/RCM/DCM 59, 60, 85, 111, 132-134 expressing the wild-type/mutant human cardiac isoforms of RLC/ELC.
Figure 1. Tertiary structure of human beta-cardiac heavy meromyosin interacting-heads motif (PDB: 5TBY) obtained by homology modeling (using Swiss-model) of human sequence from aphonopelma homology model (PDB: 3JBH).
The myosin heavy chain (MHC) is indicated with blue ribbon, myosin regulatory light chain (RLC) with magenta ribbon and myosin essential light chain (ELC) in red ribbon.
Striated muscle myosin can be found in three states: an active/force-producing state, a disordered relaxed (DRX) state, in which myosin heads protrude into the interfilament space but are restricted from binding to actin, and a super-relaxed (SRX) state characterized by an ordered head arrangement along the thick filament axis and a highly inhibited ATP turnover rate 45, 79, 107. The presence of the folded state of myosin, where the heads interact with each other and with myosin tail (S2) is hypothesized to be the origin of SRX 7, 120. The cardiac SRX serves as a modulator of cardiac energy utilization, involved in decreasing metabolic rate (load) in both, the normally functioning myocardium and during times of stress, e.g. cardiomyopathy 45, 79. The important question in the area of myosin RLC and ELC research regards the role of MLCs disease-causing mutations in modifying the super-relaxed state of myosin and controlling the SRX ↔ DRX equilibrium 7, 106, as well as contributing to the regulation of myosin’s power stroke, ATP utilization and force production in muscle.
Myosin RLC (MYL2)
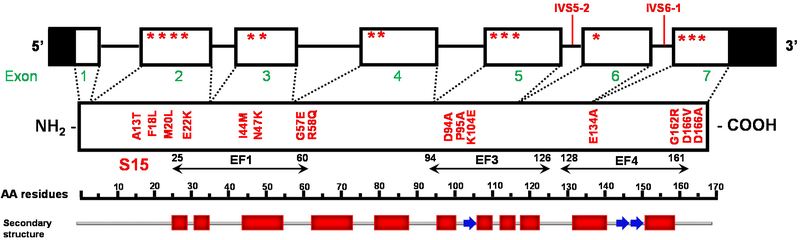
There are two unique features of myosin RLC: the Mg2+ and Ca2+binding site, containing a helix-loop-helix motif (EF1), and the myosin light chain kinase (MLCK) dependent phosphorylation site, both located in the N-terminus of RLC 109 (Fig. 2). Under physiological conditions, in relaxed muscle, it is thought that this site is occupied by Mg2+ and may become partially saturated with Ca2+, depending on the length of the [Ca2+] transient 97, 115. In rodent cardiac RLC, there are two phosphorylation sites, at serine 14 and 15, which can be phosphorylated with cardiac MLCK (cMLCK), but also with zipper kinase (ZIP kinase) 102. Human cardiac RLC has only one phosphorylatable serine 15, but there is also adjacent asparagine 14 that can be deamidated, and both posttranslational modifications increase the negative charge of the RLC 103 (Fig. 2).
Figure 2. Schematic representation of MYL2 (genomic and protein) of the human cardiac myosin regulatory light chain (RLC).
The non-coding regions of MYL2 are highlighted in black, and the location of mutations are indicated with stars. EF-hand domains and the MLCK-phosphorylation site are depicted in the amino-acid sequence. The structural domains are denoted with red blocks (±-helix) and blue arrows (β-strand). Modified from Bonne et al. Circ Res 83: 580–593, 1998.
MYL2 mutations
In 1996, initial RLC exon screening in HCM patients revealed the first three distinct mutations (A13T, E22K, P95A-initially reported as P95R), with A13T and E22K patients showing a particular type of familial HCM with pronounced mid-cavity obstruction (MVC) 92. A later report found the A13T-mutated patients to suffer from profound septal hypertrophy, exertion induced dyspnea and severe cardiac abnormalities, including left ventricular outflow tract (LVOT), which were enhanced by ergonomic exercise 5.
The Ala13Thr (A13T) mutation is located in the N-terminus of the myosin RLC (Fig. 2), which together with the ELC support the neck region of the myosin head attaching to their respective IQ motifs. Recombinant A13T RLC mutant resulted in a large increase in the α-helical content which decreased upon the binding of Ca2+ to A13T mutant and even returned to the level of wild-type (WT) RLC upon phosphorylation. Using flow dialysis, the group showed a decrease in Ca2+ binding affinity compared with human cardiac RLC-WT. The study also showed that phosphorylation of A13T resulted in a large increase (increase in KCa value) in its Ca2+ binding affinity compared with non-phosphorylated A13T. A decrease in calcium sensitivity in rat ventricular RLC-reconstituted skinned rabbit psoas muscle fibers, without any change in maximal tension, was also reported by Roopnarine et al. 98. Transgenic mice expressing human ventricular A13T RLC mutation showed enlarged interventricular septa and profound fibrotic lesions. Cardiac muscle preparations from these mice revealed a 1.4-fold increase in maximal contractile force and ~25% decrease in actin-activated myosin ATPase activity compared to Tg-WT or NTg mice 58. In that study, A13T was also shown to have lower binding affinity for RLC-depleted porcine myosin. It was hypothesized that the A13T mutation affects the myosin power stroke generation by changing the kinetic properties of the cross-bridges, possibly exerting a dominant-negative effect on cardiomyocyte structure and function. A13T mutation differs from other HCM-RLC mutations in that no changes in force/ATPase–pCa relationship as well as endogenous RLC phosphorylation in Tg mice myocardium were observed in the study 58. A13T and F18L also resulted in dramatic reduction in unloaded shortening velocity at saturating myosin surface densities, suggesting that the amino acids around the phosphorylatable serine 15, play an important role in the myosin lever arm stabilization25.
Soon after Poetter’s discovery, two novel missense mutations, Phe18Leu (F18L) in exon 2 and Arg58Gln (R58Q) in exon 4 (Fig. 2) were identified in three unrelated families with a typical form of HCM, with increased left ventricular wall thickness and abnormal echocardiography with no mid-left ventricular obstruction 26, 95. The mutated F18L residue is located in a protein domain in which two previously identified mutations (A13T and E22K) were shown to be responsible for the HCM phenotype 92. F18L was also shown to decrease calcium affinity of RLC compared with human cardiac RLC-WT and no effect of phosphorylation was observed on calcium binding. Ca2+ binding to F18L produced a slight increase in the α-helix but no change in α-helical content was observed for phosphorylated F18L-RLC109. A slight decrease in calcium sensitivity of myofibrillar ATPase was shown in reconstituted porcine cardiac myofibrils 110 F18L was found to decrease tension, Ca2+ sensitivity, and cooperativity in reconstituted rat ventricular RLC system 98.
Met20Leu (M20L) (along with E134A and G162R) (Fig. 2) was identified as a novel mutation in a cohort of 203 unrelated patients with HCM in a study to determine the influence of a positive genetic test for HCM on clinical outcome 88. Single molecule measurements in mutant RLC reconstituted porcine cardiac fibers by Burghardt et al. found M20L fibers to produce maximal isometric force with below normal lever-arm stiffness 12. Using TIRF, lever-arm orientation was measured in rigor, isometric contraction, and relaxation. It was noted that radical reconfiguration in M20L reinforces the suggestion that light chain modification near the RLC phosphorylation site at Ser15 affects function, linking the M20L phenotype to lower efficiency for ATP free-energy conversion to mechanical work 12.
In addition to Poetter’s 1996 discovery of Glu22Lys (E22K) (Fig. 2) in three patients from two unrelated families with MVC phenotype, the mutation was later associated with moderate septal hypertrophy, late onset of clinical manifestation, and benign disease course and prognosis54. Similarly, this mutation was also reported homozygously in two Spanish patients by Garcia-Pavia et al. during the genetic screening of a cohort of 26 patients, transplanted for end-stage HCM 30. In addition, Claes et al. reported E22K mutation in 14 apparently unrelated HCM families and suggested that the mutation, on its own, was incapable of triggering clinical HCM and might require the presence of an additional risk factor for hypertrophy, particularly hypertension 18. Interestingly, E22K mouse model from Robbins group poorly recapitulated in vivo alterations found in human as transgenic mice carrying E22K RLC failed to exhibit either overt hypertrophy or mid-ventricular cavity obstruction despite changes at the myofilament and cellular levels, with the myofibrils showing increased Ca2+ sensitivity and significant deficits in relaxation in a transgene dose-dependent manner 99. Similarly, echocardiography abnormalities seen in most of the human patients with the E22K mutation were not detected in transgenic mice expressing mutant human ventricular E22K RLC, despite visibly enlarged inter-ventricular septa as well as papillary muscles seen in H&E staining 111. Skinned cardiac muscle preparation showed an increase in calcium sensitivity of myofibrillar ATPase activity and force development, leading to the idea that E22K-mediated HCM may be mediated via Ca2+dependent levels 111. Likewise, E22K was found to increase Ca2+ sensitivity of force in reconstituted rat ventricular RLC system 98. Such increase in calcium sensitivity of force development was also shown in slow skeletal muscle fibers, containing same RLC isoform as that of cardiac, from biopsy samples from patients with inherited HCM carrying the E22K RLC mutation 65. In in vitro studies, E22K mutation was shown to render the protein non-phosphorylatable, increase α-helical content, decrease calcium binding affinity (by ~20 fold) to isolated RLC 114. No effect of the E22K mutation on the kinetics of mutated myosin cross-bridges in its ATP-powered interaction with fluorescently labeled single actin filaments was observed by Dumka et al. 22. However, maximal force and ATPase were found to be ~20% decreased in Tg-E22K skinned papillary muscle fibers from mice compared with Tg-WT animals 112. In addition, intracellular [Ca2+] and force transients were significantly faster in intact papillary muscle fibers from Tg-E22K compared to Tg-WT cardiac mice preparations 112.
Santos et al. identified a novel Ile44Met (I44M) MYL2 mutation (Fig. 2) in Portuguese population presenting with familial history of HCM or clinical diagnosis using High Resolution Melting technology by means of analyzing 28 HCM-associated genes, including the most frequent 4 HCM-associated sarcomere genes, as well as 24 genes with lower reported HCM-phenotype association 100. further experimental studies are needed to investigate the potential mechanisms for I44M-induced HCM.
The Asn47Lys (N47K) mutation in MYL2 (Fig. 2) was discovered during screening of 68 Danish and 130 South African HCM patients and was found to be associated with a mid-ventricular hypertrophy (MVH) phenotype in a Danish patient, found to cause a late onset of the disease with a rapidly progressing MVH phenotype and a significant increase in the size of the papillary muscles, but with an absence of SCD 5. Previous studies have determined the N47K-mediated depression of cardiac function in transgenic mice and in β-MHC reconstituted porcine cardiac muscle preparations, without affecting the MLCK-dependent phosphorylation of RLC in the ventricles of Tg mice 1, 36, 37, 55, 124. N47K substitution in the second from last coordinating position of the Ca2+ binding loop of human cardiac RLC was shown to abolish its Ca2+ binding and increase the Ca2+ sensitivity of myofibrillar ATPase, without any change in the calcium sensitivity of force 110. N47K both showed a reduction in isometric force with an increase in duty cycle, meaning that the attachment time would be longer, increasing the probability of “dragging heads” 36. The N47K mutation also caused a reduction in isometric force compared with native and wild-type myosin and appeared to cause a reduction in the peak power output as well as the load at which peak power occurs 37. In addition, N47K mutation resulted in prolonged [Ca2+] transients assessed in intact papillary muscle fibers with no change in calcium sensitivity of force or ATPase in transgenic N47K muscle fibers 124. Cardiac output, cardiac work, and cardiac power were significantly decreased in Tg-N47K aerobically perfused and spontaneously beating hearts compared with controls 1. N47K mutant showed slower cross-bridge cycles, higher actin-activated ATPase activity and exhibited larger tension than WT; however, no changes in rigor acto-myosin binding were observed and a significant decrease in dP/dtmax-EDV relationship, indicative of depressed ventricular contractility in N47K mice was reported 123.
Caleshu et al. discovered another point mutation, Gly57Glu (G57E) (Fig. 2), in MYL2 along with previously reported homozygous E143K ELC mutation in a 22-year-old patient with restrictive cardiomyopathy (RCM) presenting with class III/IV heart failure and requiring heart transplantation. Her mother was a double heterozygote for these mutations, with no evidence of cardiomyopathy 16.
Single-strand polymorphism analysis of exon 4 in two unrelated probands of families revealed the Arg58Gln (R58Q) mutation (Fig. 2), responsible for “classic” HCM phenotype with no mid-ventricular hypertrophy 26. The R58Q mutation showed moderate septal hypertrophy, but in contrast, it was associated with an early onset of clinical manifestation and premature sudden cardiac death 54. The R58Q mutant did not bind Ca2+ and interestingly, Ca2+ binding to the R58Q-RLC was restored upon phosphorylation. In addition, the α-helical content of R58Q was greatly increased upon calcium binding 114. No change in α-helical content was observed for the phosphorylated R58Q in the apo-state 114. Echocardiographic evaluations of transgenic R58Q mice showed significant alterations in diastolic transmitral velocities and deceleration time and isolated myofibrils exhibited changes in Ca2+ sensitivity, cooperativity, and an increased level of ATPase activity at low [Ca2+] along with reduced endogenous RLC phosphorylation in Tg ventricles 1. R58Q mutation exhibited much more severe changes in cardiac function, including more reduced cardiac work as well as cardiac power compared with control hearts 1. Simultaneous measurements of the ATPase and force in skinned papillary muscle fibers obtained from transgenic R58Q mice showed an increase in the Ca2+ sensitivity of ATPase and steady-state force as well as manifested prolonged force and [Ca2+] transients in electrically stimulated intact papillary muscles 124. A significant increase in ATPase was observed for R58Q myosin that was hypothesized to offset part of the increase in observed duty cycle, thereby imparting R58Q an increased sliding velocity in the in vitro motility assays on myosin isolated from transgenic mice 36. R58Q was also shown to reduce isometric force compared to the WT which could lead to compensatory hypertrophy. Consistent with this, fiber studies of the R58Q mutation done previously in skinned muscle fibers also showed a shift towards submaximal calcium activation of ATPase compared to WT 124. In addition, R58Q-exchanged myosins show reductions in force and power output compared with WT or native myosin and the changes in loaded kinetics were thought to result from mutation-induced loss of myosin strain sensitivity of ADP affinity37. Yadav et al., using a phosphomimic recombinant RLC variant where the phosphorylation site Ser-15 was substituted with aspartic acid (S15D) and placed in the background of R58Q, showed a multitude of rescue effects of R58Q-exerted adverse phenotypes in S15D-R58Q-reconstituted porcine cardiac muscle preparations 131. A low level of maximal isometric force observed for R58Q- vs. WT-reconstituted fibers was restored by S15D-R58Q. The authors also report that R58Q promotes the OFF state of myosin, both in reconstituted porcine fibers and in transgenic mouse papillary muscles, thereby stabilizing the super-relaxed state (SRX) of myosin, characterized by a very low ATP turnover rate 131. Experiments in S15D-R58Q-reconstituted porcine fibers showed a mild destabilization of the SRX state, suggesting an S15D-mediated shift in disordered-relaxed (DRX) ↔ SRX equilibrium towards the DRX state of myosin. The study concluded that S15D-phosphomimic can be used as a potential rescue strategy to abrogate/alleviate the RLC mutation-induced phenotypes 131. Interestingly, it was recently shown that R58Q-mutant mice showed functional similarities between the cardiac papillary muscles and slow-twitch soleus skeletal muscle, as evident by lower contractile force in both papillary and soleus muscles compared to fast-twitch extensor digitorum longus muscles of R58Q vs. wild-type–RLC mice 61. This coincided a decreased proportion of fiber type I/type II only in SOL muscles but not in the extensor digitorum longus muscles, as well as in differential regulation of proteins between the heart and SOL muscles of R58Q mice. These data suggest that MYL2 models of HCM may be used as a model to study the molecular, structural, and energetic mechanisms of RLC mutation-induced cardioskeletal myopathy 61.
Huang et al. described a novel Asp94Ala (D94A) DCM mutation in MYL2 (Fig. 2), in which aspartic acid at position 94 is replaced by alanine (D94A) 49. It was identified by Dr. Hershberger group using exome sequencing in a pedigree with familial DCM. Recombinant D94A protein induced a reduction in the α-helical content of the RLC, without any change in its ability to be phosphorylated by Ca2+/calmodulin-activated MLCK 49. The mutation was shown to impair binding to the myosin heavy chain and had significantly higher actin-activated ATPase activity. D94A reconstituted porcine papillary muscles exhibited slightly reduced maximal tension with no change in the calcium sensitivity of force 49. Transgenic D94A mice, generated by Yuan et al. 134 exhibited a significant reduction in the ejection fraction, with younger male D94A mice showing a more pronounced LV chamber dilation compared with female counterparts. Papillary muscles isolated from transgenic D94A mice showed a rightward shift of the force–pCa dependence and decreased actin-activated myosin ATPase activity. Small-angle X-ray diffraction study at submaximal Ca2+ concentrations revealed repositioning of cross-bridge mass toward the thick-filament backbone, supporting the hypocontractile state of D94A myosin motors 134.
Pro95Ala (P95A) (originally mislabeled as P95R) was identified along with A13T and E22K, with pronounced mid-ventricular obstruction 92. Flow dialysis experiments with recombinant P95A revealed a decrease in calcium binding affinity, with an increase in the α-helical content upon subsequent calcium binding 114 P95A-reconstituted porcine cardiac myofibrils showed the lowest ATPase activity with no change in calcium sensitivity of myofibrillar ATPase activity 110. Experiments in reconstituted skinned rabbit psoas muscle fibers showed no change in maximal tension, calcium sensitivity of force, or cooperativity of activation of force generation 98.
Lys104Glu (K104E) and IVS6-1 were initially identified in an MYL2 mutational screening that identified SSCP conformers in both exon 5 and exon 7 (Fig. 2) in the Danish proband presenting with pronounced proximal septal hypertrophy, and diastolic filling abnormality as evidenced by an inverted transmitral flow pattern 5. Transgenic mice expressing the Lys104Glu mutation did not show major histopathological abnormalities or myofilament disarray, except for in older animals, but showed early signs of diastolic disturbance with significantly reduced E/A transmitral velocities ratio along with a borderline significant prolonged isovolumic relaxation time (Tau) and a tendency for slower rate of pressure decline as evident in invasive hemodynamics studies. K104E hearts also showed higher mitochondrial content which was supported by higher ATPase activity and skinned papillary muscles showed a reduction in maximal tension and slower muscle relaxation rates 48. Mutated cross-bridges were significantly better ordered during steady-state contraction and during rigor, but the mutation had no effect on the degree of order in relaxed myofibrils. The K104E mutation increased the rate of XB binding to thin filaments and the rate of execution of the power stroke. Stopped-flow experiments revealed a significantly faster dissociation rate observed in Tg-K104E vs. Tg-wild-type (WT) myosin and a smaller second-order ATP-binding rate for the K104E compared with WT myosin 21. Barth et al. reported a new skeletal muscle fiber type-I myopathy in three unrelated Dutch families with progressive cardiomyopathy and early infant deaths due to cardiac failure 11, and the genetic cause was only recently identified by Wetermen et al. to be a homozygous mutation in the last acceptor splice site of the myosin regulatory light chain 2 gene which results in use of a cryptic splice site upstream of the last exon causing a frameshift and replacement of the last 32 codons by 20 different codons 129 (Fig. 2).
Recombinant IVS6-1-RLC showed decreased binding to the MHC while IVS6-1-reconstituted porcine myosin displayed reduced binding to actin in rigor and demonstrated a significantly lower Vmax of the actin-activated myosin ATPase activity 135. IVS6-1-reconstituted myosin also showed slower kinetics of the ATP-induced dissociation of the acto-myosin complex and a significantly reduced slope of the kobc-[MgATP] relationship as observed by stopped-flow fast kinetic studies 135. In addition, a significantly decreased maximal contractile force and a significantly increased Ca2+ sensitivity, which are both hallmarks of HCM-associated mutations, were observed in skinned papillary muscles reconstituted with IVS6-1-RLC 135. DNA SSCP analysis of exon and flanking intronic regions, followed by sequencing of an abnormal pattern on a capillary DNA sequencer, identified a splice acceptor site mutation (IVS5-2) in the European population. One donor-site splice mutation (IVS5-1:A>G) was predicted to lead to a premature termination codon95.
Olivotto et al. discovered novel missense MYL2 mutations, Glu134Ala (E134A), in Exon 6 and Gly162Arg (G162R) in Exon 7 (Fig. 2), while determining the influence of a positive genetic test for HCM on clinical outcome in a cohort of 203 unrelated patients with HCM. They showed that patients with myofilament-positive HCM exhibited an increased risk of the combined end-points of cardiovascular death, nonfatal stroke, or progression to New York Heart Association class III or IV 88. Burghardt et al. assessed human cardiac RLC for sensitivity to mutation with M20L, E134A, and G162R. Measurements using photoactivatable GFP tagged RLC (RLC-PAGFP) exchanged into permeabilized papillary muscle fibers showed that disease-linked mutants invert intermediate state occupation favoring lower free-energy in the active cycle when compared to WT. Lower free-energy intermediate occupancy led to lower energy conversion efficiency in the fiber, demonstrating a myosin functional modification characterized at the single molecule level, and in the context of the crowded muscle fiber, linking the HCM phenotype to lower efficiency for ATP free-energy conversion to mechanical work. M20L and G162R are able to produce maximal isometric force with below normal lever-arm stiffness by radically or modestly reconfiguring sub-state populations while E134A cannot maintain normal maximal force/stiffness, thereby compromising actin binding 12.
Alvarez-Acosta et al. reported on two novel MYL2 mutations in a cohort of 124 consecutive HCM index patients: Ile158Leu (I158L) causing severe hypertrophy with atrial fibrillation and likely pathogenic but related to a good prognosis, and Asp166Ala (D166A), pathogenic and related to obstructive septal asymmetrical hypertrophy with a good prognosis 4. No in vitro or in vivo studies exist reporting on these two new MYL2 mutations (Fig. 2).
This is in contrast to a thoroughly studied Asp166Val (D166V) mutation that occurs at the last amino acid residue of the human cardiac RLC similar to D166A (Fig. 2). It was identified by Richard et al. where it was mistakenly labeled as D166L 95. Transgenic D166V mice hearts exhibited fibrotic lesions, reduced level of in situ RLC phosphorylation and skinned papillary muscle fibers from transgenic D166V mouse revealed a large increase in the Ca2+ sensitivity of contractile force, decreased maximal ATPase and force, profoundly decreased kinetics of force-generating myosin cross-bridges 62. Muthu et al. tested the hypothesis that an ex vivo phosphorylation of Tg-D166V cardiac muscle may rescue the detrimental contractile phenotypes observed at the level of single myosin molecules and in Tg-D166V papillary muscle fibers 86. They showed that MLCK-induced phosphorylation of Tg-D166V cardiac myofibrils and muscle fibers was able to increase the reduced myofibrillar ATPase and reverse an abnormally increased Ca2+ sensitivity of force, but not for the maximal force which decreased upon phosphorylation 86. This was followed by subsequent experiments to test whether pseudo-phosphorylation (S15D) of cardiac RLC could be used as a rescue method to alleviate a cardiomyopathy phenotype brought about by a disease-causing mutation in the myosin RLC. The S15D substitution at the phosphorylation site of RLC was inserted into the recombinant human cardiac WT and D166V mutant to mimic constitutively phosphorylated RLC proteins 87. They showed an S15D-induced rescue of both the enzymatic and binding properties of D166V-myosin to actin, along with a significant increase in force production capacity in the in vitro motility assays for S15D-D166V vs. D166V reconstituted myosin as well as a rescue in D166V-elicited abnormal Ca2+ sensitivity of force in porcine papillary muscle strips 87. The group subsequently tested the S15D-RLC rescue hypothesis in vivo Tg-S15D-D166V mice where the human cardiac S15D-D166V construct was substituted for mouse cardiac RLC and compared to WT and D166V mice in functional, structural, and morphological assessments 132. Echocardiography and invasive hemodynamic studies demonstrated significant improvements of intact heart function in S15D-D166V mice compared with D166V, with the systolic and diastolic indices reaching those monitored in WT mice. A largely reduced maximal tension and abnormally high myofilament Ca2+ sensitivity observed in D166V-mutated hearts were reversed in S15D-D166V mice. Low-angle X-ray diffraction study revealed that altered myofilament structures present in HCM-D166V mice were mitigated in S15D-D166V rescue mice 132.
Population studies show most HCM-associated RLC mutations to cause hypertrophied left ventricles and diastolic abnormalities. Biophysical and biochemical characterizations of mutated RLC indicate that depending upon the location and the nature of the mutation, stochastic changes in RLC sequence may render changes in the structure, as well as affect enzymatic, binding, and force production capability of myosin. Despite the progress in the identification of RLC pathogenic variants, genotype-phenotype correlations for HCM are incompletely defined which limits the use of genetic test results to guide clinical management.
Myosin ELC (MYL3)
The human heart contains two ELC isoforms: the atrial isoform (ELCa), encoded by the MYL4 gene located on chromosome 17q21-qter, and the ventricular isoform (ELCv), encoded by the MYL3 gene localized on chromosome 3p21.3-p21.2 which also encodes for the slow-twitch skeletal ELC 27. Striated muscle ELC belongs to the EF-hand family of Ca2+ binding proteins, but evolutionarily it lost the ability to bind Ca2+ 34. The ELC protein is an integral structural component of the actomyosin cross-bridge; however, little is known about the functional significance of the cardiac-specific isoform, containing a unique ~43 amino acids / 91Å long N-terminus (N-ELC) 10. The N-ELC is comprised of a lysine-rich actin binding region that was shown by many to make direct molecular contacts with actin during actin-myosin interaction and muscle contraction 40, 57, 81, 82, 108, 117-119, 128, 130. The N-ELC was also proposed to interact with actin by binding to the SH3 domain of myosin heavy chain during the ATPase cycle67, 70 and to modulate the weak-to-strong structural transition 38. Regardless of whether the effect of the ELC/actin interaction on the actomyosin ATPase cycle proceeds through the SH3 domain or through a direct N-ELC-actin binding, the finding that the N-ELC may bind to SH3 suggests that the conformation, and thus the function of N-ELC may be highly sensitive to any structural modifications (e.g. due to mutation) in the entire myosin molecule. It was proposed to function as a tether between the myosin head and actin capable of regulating the thick and thin filament interactions and force production in the heart 57. It was also proposed to use a novel mechanism of step frequency modulation to control myosin power output and cardiac myosin power generation in vivo 13, 127, 128. The C-terminal region of ELC interacts with the MHC and possibly with the RLC 46. The C-terminus of ELC contains Ser195, a potential phosphorylation site on the cardiac ELC 9, 50, but the role of ELC phosphorylation in heart function is totally unknown 113. Studies in zebrafish model suggested that ELC phosphorylation may have a regulatory role in the heart as the inability of ELC to be phosphorylated at Ser195 in the laz+/− mutant led to contractile defects and cardiac death 101.
MYL3 mutations
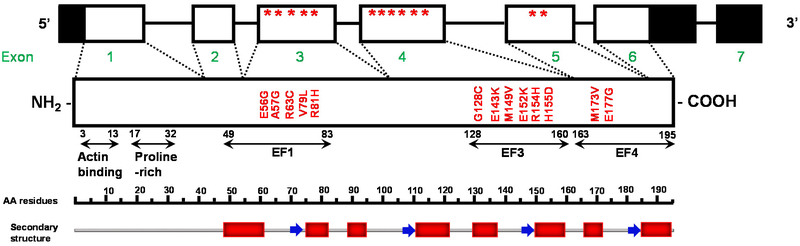
Similar to other sarcomeric proteins, mutations in the ventricular ELC (encoded by MYL3) are implicated in cardiomyopathy diseases. Compared to β-MHC or myosin binding protein-C (MyBP-C), mutations in the ELC are quite rare, but they are also associated with malignant outcomes 56, 64, 83, 89, 92, 95, and they represent a largely understudied area of research. To date, 13 mutations in MYL3 have been identified by population studies to cause HCM. Among them, 5 missense mutations are located in exon 3 (E56G, A57G, R63C, V79I, R81H), 6 in exon 4 (G128C, E143K- HCM with restrictive physiology, M149V, E152K, R154H, H155D) and 2 in exon 5 (M173V, E177G) (Fig. 3). Interestingly, all ELC mutations are located in the non-functional EF-hand Ca2+ binding motifs of the ELC molecule with the majority located in the C-terminus ELC (Fig. 3). The mutations have been shown to cause various HCM phenotypes in humans, from asymmetric septal hypertrophy (A57G, V79I, R63C), MVC obstruction (M149V, R154H) to non-symptomatic course of the disease (in cases of patients heterozygous for E143K). They have been mostly associated with adult-onset HCM, but several are also observed as infantile/childhood forms (R154H, E177G, E143K and M173V). Some ELC mutations are also associated with SCD at a young age (A57G, E143K, and M149V).
Figure 3. Schematic representation of MYL3 (genomic and protein) of the human cardiac myosin essential light chain (ELC).
The non-coding regions of MYL2 are highlighted in black, and the location of mutations are indicated with stars. EF-hand domains and the MLCK-phosphorylation site are depicted in the amino-acid sequence. The structural domains are denoted with red blocks (α-helix) and blue arrows (β-strand). Modified from Bonne et al. Circ Res 83: 580–593. 1998.
Glu56Gly (E56G) was identified together with other HCM-linked casual mutations in the nine genes encoding for sarcomeric proteins including ELC 95. The MYL3 gene accounted for < 0.5% of all analyzed cases with only one E56G mutation in ELC 95. Six families presented with more than one mutation (double heterozygous, compound heterozygous, and homozygous mutations), and phenotypic analyses suggested a gene dose effect in these patients 95. The E56G mutation is located in exon 3 of ELC (Fig. 3). A comparison of various ELC sequences demonstrated that the glutamic acid in position 56 is highly conserved across species and tissues 42. Biophysical studies using time-resolved FRET measurements by Thomas group showed that the E56G mutation may alter the distribution of structural states of the actin-myosin complex, perturbing the weak to strong cross-bridge transition, which is a primary component of force generation 39. To gain a better understanding on pathological changes leading to the development of HCM by E56G, Lossie and colleagues generated two mouse models with cardiomyocyte-specific overexpression of the non- (TgMhVLC-1) and E56G- mutated (TgME56G) human ventricular ELC 69. Echocardiography and gene expression assessment of hearts of 3 month-old TgME56G showed no signs of cardiac hypertrophy and no changes in hypertrophic markers. Maximal left ventricular pressure development of isolated perfused hearts in vitro prepared from TgME56G was significantly lower compared with TgMhVLC-1. Differences between mutant and normal mice were also observed at the sarcomere level - in experiments on skinned fibers and myosin. Laser trap and in vitro motility assays demonstrated reduced stiffness, force generation and actin sliding velocity of myosin molecules prepared from E56G mice compared with control myosin. Maximal isometric force obtained at the pCa 4.5 of skinned fibers was significantly lower for TgME56G mice compared with control mice while Ca2+ sensitivity was not affected. Observed deleterious effects of the E56G mutated ELC on the myosin function suggested that E56G mutation may cause HCM by means of weakened myosin-lever arm affinity, reduced stiffness and force generation, slowed actin filament sliding velocity and the depressed cardiac performance. The resulting cardiac hypocontractility could be activating hypertrophic pathways responsible for the development of E56G-induced HCM phenotype 69.
Ala57Gly (A57G) was discovered in two unrelated Korean families and one Japanese man diagnosed with HCM 64. The mutation is located in exon 3 of ELC (Fig. 3) and similar to the E56 residue, it is highly conserved in other ELC isoforms across species 42. The phenotype associated with this mutation entailed classic asymmetric septal hypertrophy and SCD, with pathology and disease progression varying among siblings and other family members 64. A study by Lossie et al. using protein-binding experiments by surface plasmon resonance showed the A57G-mediated disturbed affinities to myosin heavy chain (significantly lower binding to head-rod fragment reflected by 2-fold higher KD) 68. The A57G mutation was associated with higher myofilament Ca2+-sensitivity in mice causing pathological cardiac remodeling and increased cardiac output and stroke work 59. Using particle tracking of Qdot-labeled actin in the standard in vitro motility assay 127, and measuring force-velocity for single and ensemble myosins 14, 15, 127, it was shown that compared to WT, the A57G and E143K ELC mutations had high and rising duty ratio with increasing load implying that the fraction of force producing myosin is higher and growing during contraction phase 128. Interestingly, force-velocity measurements demonstrated an upregulation of power for A57G myosin and downregulation for E143K 128. Ensemble motility and single myosin mechanical characteristics were shown to be consistent with A57G that impairs the N-ELC - actin binding 128. In transgenic animals, A57G mutation led to pathological cardiac morphology, including extensive disorganization of myocytes and substantial interstitial fibrosis, most likely due to accumulation of collagen aggregates, as well as led to a decrease in lattice spacing in small angle X-ray diffraction study in Tg-A57G papillary muscle fibers but had no effect on the crossbridge mass distribution 85. The structural alterations were paralleled by mechanical changes resulting in increased stiffness/passive tension, increased Ca2+ sensitivity of force and decreased maximal tension in skinned papillary muscle from Tg-A57G mice 59. In addition, Tg-A57G animals showed a significant upregulation of hypertrophic markers (ANP, BNP, collagen VIIIa) that were activated upon exercise, along with a visible increase in heart size and occurrences of fibrosis, especially in the interventricular septum 60. Proteomic analysis of Tg-A57G hearts demonstrated alterations in Ca2+ homeostasis along with fatty acid metabolism, suggesting these may be important in the pathological cardiac remodeling observed in Tg-A57G myocardium 33.
Arg63Cys (R63C) mutation was identified during a systematic mutation screening of 38 Taiwanese patients diagnosed with HCM 17. It was found in a 32-year-old proband with the phenotype of asymmetric septal hypertrophy with LV outflow tract pressure gradient of 52 mmHg (obstructive HCM), intraventricular conduction defects and dyspnea. Additional screening revealed other 2 out of 7 members of the family carrying the mutation and experiencing ECG abnormalities. The R63C mutation is located in the α-helix of the first EF-hand ELC domain (Fig. 3), and the amino acid sequence alignment shows a high degree of conservation of Arg63 across species. No in vitro or in vivo studies report on this new MYL3 mutation.
Val79Ile (V79I) mutation was identified in 38-year-old asymptomatic HCM patient referred for clinical evaluation due to a cardiac murmur 6. Echocardiography analysis showed classic asymmetric ventricular hypertrophy. Additional screening revealed that 9 members of the family were positive for V79I mutation, with three displaying borderline HCM phenotype (ECG and/or echocardiographic abnormalities) but they did not fulfill diagnostic criteria for HCM. V79I is located in the region of contact between the ELC and the myosin heavy chain in the first IQ motif on MHC lever arm (Figs. 3 and 4). Similar to other ELC mutations, Val79 is highly conserved across species and tissues.
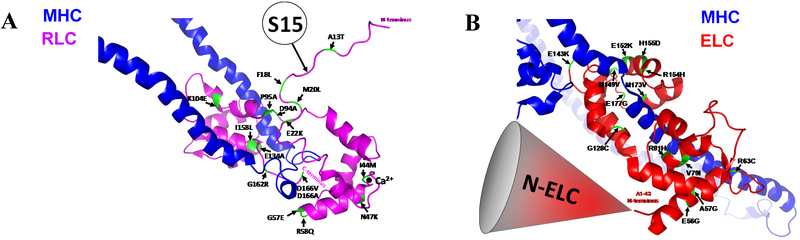
Figure 4. Mutations in myosin MYL2 (A) and MYL3 (B) in the background of human beta-cardiac heavy meromyosin interacting-heads motif (PDB: 5TBY).
Cardiac MLCK-dependent phosphorylation site at Ser-15 (S15) is emphasized in myosin RLC (A) and the N-ELC region in myosin ELC (B).
Arg81His (R81H) mutation was found during a genetic screening of 122 unrelated patients with HCM (64 with a positive family history and 58 with unremarkable or unknown family history), but the clinical information on the specific disease phenotype is not available 28. No research has been performed on this ELC mutation.
Gly128Cys (G128C) mutation was found during a genetic screening of 10 HCM-causing genes of 26 patients transplanted for end-stage HCM 30. Out of all the patients, one had a novel missense mutation located in the exon 4 of MYL3. However, no further phenotypic information was provided in this study. No research findings are available for this ELC mutation.
Glu143Lys (E143K) mutation was found in a young proband homozygous for the mutation who was undergoing cardiac evaluation administered after the premature death of his two younger siblings 89. An incidence of early death, at 7 years of age, from unknown causes, was reported in a distant relative 89. The proband demonstrated a phenotype of LV hypertrophy and ECG abnormalities. An unusual variant of HCM was identified, characterized by LV hypertrophy with mild dynamic obstruction in systole 89. The medical records for deceased brothers indicated they had cardiomyopathy with dilated atria, suggesting restrictive heart disease. The proband’s sister and parents, who were heterozygous for the E143K mutation were asymptomatic and their ECG findings were normal 89. In contrast, family members homozygous for E143K manifested severe HCM in childhood. In another study, 22-year-old female carrying E143K mutation was diagnosed with RCM and class III/IV heart failure while awaiting heart transplantation 16. Electrocardiogram and transthoracic echocardiogram revealed severe biatrial enlargement with preserved biventricular systolic function and no LV hypertrophy, while the Doppler assessment indicated advanced LV diastolic dysfunction, all features reminiscent of RCM 16. In addition, elevated right- and left-sided filling pressures and myofibrillar disarray with interstitial fibrosis were found. E143K mutation is the only one in the group of HCM ELC mutations showing restrictive heart physiology and therefore classifying it as an RCM mutation. The E143K occurs at an evolutionarily conserved residue across species and in various ELC isoforms 42. The mutation is found in the exposed loop of the EF3-hand motif of ELC 46, 94 (Fig. 3). In the studies by Lossie et al, E143K mutant showed significantly weaker binding to the myosin lever arm (2-fold higher KD) 68. Studies using transgenic E143K mice showed diastolic dysfunction, histopathological changes, fibrosis and sarcomeric irregularities with Z-lines streaming and M-lines vanishing 133. Gene expression profiles showed upregulation of hypertrophic markers (collagen I and III and ANF). Data obtained from skinned papillary muscles showed an increased ability of myosin to generate maximal force, increased Vmax of actin-activated ATPase, suggesting that E143K may lead to hypercontractile myosin motor behavior 133. Particle tracking Qdot in vitro motility assays showed high duty ratio for E143K but force-velocity measurements demonstrated downregulation of power, opposite to what was observed for A57G ELC mutation 128. However, single-molecule assessments of myosin power in both A57G and E143K were in accordance with the in vivo hemodynamic measurements 59, 133. In addition, decreased RLC phosphorylation in myofibrils from E143K-ELC hearts was thought to contribute to the E143K-RCM phenotype 133.
Met149Val (M149V) mutation was the first mutation in the myosin ELC shown to cause HCM 23, 92. Six of the thirteen family members who were positive for the mutation had a rare phenotype of mid-left ventricular chamber thickening due to papillary muscles and ventricular hypertrophy 23, 92. Studies suggested that the stretch-activation response of the papillary muscles in the M149V patients might be impaired 24, 92. A similar phenotype associated with the M149V mutation in myosin ELC was also reported by Arad et al. 8. Apical or mid-ventricular hypertrophic cardiomyopathy and multiple cases of SCD at young ages were found in patients harboring this mutation 8, 74 The M149V mutation like E143K is located in exon 4 of ELC (Fig. 3) and is shown to be highly conserved across species and tissues 42. According to the crystal structure (1WDC) by Houdusse et al. 47, M149V is found in the β-sheet end of the exposed loop region of the EF3 motif of ELC (Fig. 3). M149V mutant showed significantly weaker binding to the myosin lever arm in the studies by Lossie et al. (2-fold higher KD) 68. Several transgenic animal models of M149V mutation were created 52, 99, 122. Older animals (1-1.5 years of age) replicated the phenotype present in patients carrying this mutation and showed hypertrophied papillary muscles and adjacent ventricular tissue resulting in profound mid-left ventricular cavity obstruction 122. Interestingly, stretch-activation of response for the papillary muscles of the 3-5 month-old M149V mutant compared to control was distorted. Therefore, it was suggested that molecular defects caused by M149V mutation affect myosin function by disrupting the stretch-activation response of the cardiac papillary muscles and adjacent ventricular tissue through a change that they produce in the elasticity of the neck region of the myosin molecule 122. Sanbe et al. 99 showed mild fibrosis, myocyte disarray and loss of sarcomere organization as well as mechanical abnormalities in skinned papillary muscles including increased Ca2+ sensitivity and lower maximal force in M149V mice compared with controls. But their animals did not develop mid-ventricular obstruction or hypertrophy, which was the main phenotypic feature of the M149V mutation in the study by Poetter et al. 92. Another attempt to generate transgenic M149V model was made using rabbits 52. However, similar to transgenic mice expressing mouse isoform of ventricular ELC, transgenic M149V rabbits did not develop HCM at the structural or functional levels at either the neonatal, juvenile or adult stages 52.
Glu152Lys (E152K) and His155Asp (H155D) mutations were first identified in 2009 by detailed clinical and genetic analysis of 79 patients diagnosed with HCM 56. The genetic screening led to the identification of novel missense mutations located in exon 4 of MYL3. No clinical information was available for these mutations. Likewise, no research findings are available for these ELC mutations.
Arg154His (R154H) mutation of ELC was identified in parallel to M149Vby Poetter et al. 92. Likewise, this mutation also caused an atypical phenotype of hypertrophic cardiomyopathy 23. A young boy was diagnosed with massive mid-left ventricular chamber thickening obstruction; a phenotype that occurs sporadically in children 23, 92. As with the other ELC mutations, the R154H is highly conserved in different ELC isoforms and across species 42. As shown in the crystal structure of scallop myosin (1WDC) 47, this mutation is located in the exiting helix of the EF3 motif of ELC (Fig. 3). R154H mutant showed significantly weaker binding to the myosin lever arm in the studies by Lossie et al. (3-fold higher KD) using protein-binding experiments by surface plasmon resonance 68.
Met173Val (M173V) mutation was identified in an adult proband who had childhood-onset cardiac hypertrophy, but the clinical information on the specific disease phenotype is very limited 83. In the absence of animal models of heart disease for M173V, the studies were performed in ELC mutant-exchanged porcine cardiac muscle preparations 50. Data for M173V-ELC exchanged muscles (~60 %) were compared with ~50 % WT-ELC exchanged porcine cardiac preparations. The M173V mutation was observed to decrease the actin-activated mutant-exchanged porcine myosin ATPase activity and increased calcium sensitivity of force compared with WT-ELC myosin 50. Interestingly, the S195D-M173V-ELC phosphomimetic protein was able to partially restore the low level of actin-activated myosin ATPase activity that was observed for M173V-ELC myosin 50. The authors suggested that this C-terminal M173V-ELC mutation may play a critical role in the interaction of the ELC with the C-terminal lever arm region of the myosin cross-bridge and its interaction with actin during force generation and that the C-terminus of ELC is important for the formation of stable ELC-MHC structures and sarcomere assembly 91. Further studies are needed to interrogate the role of S195D-phosphomimic ELC in abrogating the ELC mutation-induced phenotypes.
Glu177Gly (E177G) mutation similar to M173V is located in the EF4-hand motif of ELC in exon 5 of the MYL3 gene. Amino acid sequence alignment shows that like all other ELC mutations this residue is highly conserved across species. The E177G mutation was identified in a 3 month-old infant with severe progressive HCM, associated with a paternally inherited mutation in MYL3, and leading to his death at 6-month old 53. Pathological features of this patient’s phenotype included left ventricular concentric hypertrophy and systolic dysfunction even though the father of the infant was entirely asymptomatic. No research findings are available for this E177G mutation in ELC.
In summary, all discussed ELC mutations are located in the non-functional EF-hand Ca2+ binding motifs of the myosin ELC (Fig. 3). The majority is located in the N and C-terminus ELC, known to make contact with actin and MHC respectively. The associated phenotypes vary by mutation and the limited availability of animal models carrying these mutations have made it difficult to further characterize the molecular mechanisms underlying these phenotypes.
MLC variants of unknown significance
A pathogenic mutation bears the following criteria: co-segregation with the HCM phenotype (such as LVH) in family members; previously reported or identified as a cause of HCM; absent from unrelated and ethnic-matched normal controls; protein structure and function is importantly altered (for example, frame shift with truncation); and amino acid sequence change in a region of the protein otherwise highly conserved through evolution (no variation observed) among species 77. There are many substitutions in the DNA sequence that do not actually cause disease and are therefore regarded as benign polymorphisms. And hence, despite applying all these pathogenicity criteria, the relevance of such minority of variants for causing disease remains unclear. Such mutations are designated into an ambiguous category, variants of uncertain significance (VUS), and have virtually no clinical utility for family screening. Unlike MYH7 and MYBPC3, HCM linked ELC and RLC mutations are quite rare and therefore not many studies are available. However, there are studies showing rare or novel variants for which disease pathogenicity have not yet been demonstrated (and hence classified as VUS). Ma et al. described a missense VUS in ELC with contradictory findings with the goal of determining the pathogenicity of this variant using a combined CRISPR/Cas9-iPSC approach 72. The group screened for any VUS in a dozen of individuals and found one individual with one variant (A57D) annotated as “likely pathogenic” but did not find any abnormalities in gene expression or morphological phenotypes associated with HCM. Other such MYL3 variants found in HCM phenotypes include splice acceptor variants (such as c.560-2delA), intron variants (c.560-3C>T, c.482-6T>C, c.481+5G>A), missense variants (Glu177Gly, Met173Thr, Arg154Cys, Lys142Glu), and synonymous variant (c.219C>T). Similarly, MYL2 variants in HCM phenotype with uncertain significance include missense variants (Asn101Ser, Ala127Val, Ala140Thr Ala141Thr, Pro143Leu, Pro143Thr, Glu163Gln), synonymous variants (Gly148=), intron variants (c.402+6G>C, c.353+6T>A) (ClinVar). Many such variants need to be further studied in order to establish any meaningful disease relevance and clinical utility.
Potential mechanisms of MLCs mutation elicited heart remodeling and failure
The advantage of using transgenic animal models of cardiomyopathy lies in the ability to explore the disease phenotypes across multiple scales of organization, ranging from single molecules, myofilament ensemble, skinned and intact muscle fibers and the whole heart. One has to integrate the molecular defects at the level of sarcomeres with the hemodynamic, contractile and energetic responses of the heart. This complex data integration would not be possible without the use of genetic models of hypertrophic, dilated or restrictive cardiomyopathy that recapitulate clinical phenotypes of heart disease. Transgenic mouse models of MYL2 (RLC) and MYL3 (ELC)-related cardiomyopathy provide invaluable insights into molecular mechanisms of MLC mutant-dependent alterations in myosin motor function that can ultimately lead to the development of light chain-specific therapeutic modalities. Two latent mechanisms associated with MLC mutation-induced alterations of myosin motor function in heart disease are related to the role of myosin RLC phosphorylation at Ser15 (Fig. 4A) and the N-ELC (Fig. 4B) in modifying the myosin-actin interaction and heart contractility. Research reports demonstrate that maintaining the physiological levels of RLC phosphorylation is critical for the normal function of the heart and suggest that the myocardium containing dephosphorylated myosin has a reduced ability to produce force and maintain cardiac function at physiological levels. Phosphorylation of myosin RLC has been proposed to play a potential protective role in cardiomyopathy disease that involves alterations of the SRX state and the phosphorylation-induced shift in the SRX ↔ DRX equilibrium toward the DRX state in which myosin heads can readily interact with thin filaments and produce force 45. The mechanism underlying myosin ELC function builds on the studies from our and other laboratories demonstrating that the cardiac-specific N-ELC may play important roles in regulating myosin motor function and force production in muscle 38, 57, 70, 80, 85, 125–127, 133, including mutant-mediated regulation of the SRX ↔ DRX equilibrium 7, 106. It would be interesting to test whether mutations in MLC that are associated with HCM 59, 62, 132 and RCM 133 shift the equilibrium from a myosin OFF-state (SRX)45 to the myosin ON state (DRX) 7, explaining their hypercontractile phenotypes while those associated with DCM 134 favor the OFF-state explaining their clinical hypo-contractility.
Conclusions
The goal of this review is to summarize up-to-date studies on the pathophysiology of HCM, RCM and DCM linked with RLC/ELC mutations. Due to genetic heterogeneity as well as phenotypic variability in HCM, there is no clear distinction/correlation between the location of the mutation on the proteins and the consequent phenotypes. In general, HCM mutations are known to induce a hypercontractility phenotype 105 (with exceptions such as R58Q that shows a hypocontractile phenotype with SRX stabilization) and are associated with an increase in Ca2+ sensitivity of force 93 and occurrences of fibrosis and myofilament disarray 116. Such an increase in Ca2+ sensitivity of force is observed in RLC- E22K 99, K104E 48 and D166V 62, and ELC-A57G 59 while fibrosis is evident in RLC-A13T 58, N47K and R58Q 124, K104E 48, and D166V 62, and ELC-A57G 59. It is difficult to summarize consistent findings in DCM and RCM models between ELC/RLC mutations as there is only one known DCM mutation in RLC (D94A) and one known RCM associated mutation in RLC (G57E) and ELC (E143K). It is important to note that for many of the mutations described in this review, there is a profound lack of mechanistic, in vitro recombinant or in vivo animal models, making it difficult to summarize consistent findings between ELC/RLC mutations. Creating transgenic animal models in the future would help in understanding the unknown mechanisms behind some of these poorly studied mutations. A potential limitation of using transgenic mouse models may arise from the expression of α-MHC exclusively in the ventricles. Hence, there is a greater interest in using larger animal models (e.g. pigs or rabbits) as they express β-MHC predominantly in the ventricles 71, which may bear more clinical implications for translational therapies.
Future directions
Using different experimental animal models of heart disease, studies can be designed to provide novel insights into complex genotype-phenotype relationships in MLCs-related HCM, DCM, and RCM. Investigations of heart remodeling performed at the level of myosin molecules, myofilaments, and the organ level may reveal the MLC-and mutant-specific disease mechanisms underlying specific cardiomyopathy phenotypes. Understanding the molecular triggers of MLCs-dependent HCM, DCM and RCM is critical for the identification of potential therapeutic measures. Future studies are needed to test whether myosin RLC and possibly ELC phosphorylation and N-ELC related heart remodeling can serve as potential novel therapeutic targets to battle heart disease.
Acknowledgments:
This work was supported by the National Institutes of Health R01-HL123255 (DSC) and the American Heart Association 17PRE33650085 (SY).
References
- 1.Abraham TP, Jones M, Kazmierczak K, Liang H-Y, Pinheiro AC, Wagg CS, Lopaschuk GD, Szczesna-Cordary D. Diastolic dysfunction in familial hypertrophic cardiomyopathy transgenic model mice. Cardiovasc Res 2009; 82: 84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alamo L, Ware JS, Pinto A, Gillilan RE, Seidman JG, Seidman CE, Padron R. Effects of myosin variants on interacting-heads motif explain distinct hypertrophic and dilated cardiomyopathy phenotypes. Elife 2017; 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alfares AA, Kelly MA, McDermott G, Funke BH, Lebo MS, Baxter SB, Shen J, McLaughlin HM, Clark EH, Babb U, Cox SW, DePalma SR, Ho CY, Seidman JG, Seidman CE, Rehm HL. Results of clinical genetic testing of 2,912 probands with hypertrophic cardiomyopathy: expanded panels offer limited additional sensitivity. Genet Med 2015; 17: 880–888. [DOI] [PubMed] [Google Scholar]
- 4.Alvarez-Acosta L, Mazzanti A, Fernández X, Ortí M, Barriales-Villa R, García D, Maneiro E, Rebolo P, Álvarez E, Monserrat L. Regulatory Light Chain (MYL2) Mutations in Familial Hypertrophic Cardiomyopathy. JCVD 2014; 2: 82–90. [Google Scholar]
- 5.Andersen PS, Havndrup O, Bundgaard H, Moolman-Smook JC, Larsen LA, Mogensen J, Brink PA, BÃglum AD, Corfield VA, Kjeldsen K, Vuust J, Christiansen M. Myosin light chain mutations in familial hypertrophic cardiomyopathy: phenotypic presentation and frequency in Danish and South African populations. J Med Genet 2001; 38: e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andersen PS, Hedley PL, Page SP, Syrris P, Moolman-Smook JC, McKenna WJ, Elliott PM, Christiansen M. A novel Myosin essential light chain mutation causes hypertrophic cardiomyopathy with late onset and low expressivity. Biochem Res Int 2012; 2012: 685108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson RL, Trivedi DV, Sarkar SS, Henze M, Ma W, Gong H, Rogers CS, Gorham JM, Wong FL, Morck MM, Seidman JG, Ruppel KM, Irving TC, Cooke R, Green EM, Spudich JA. Deciphering the super relaxed state of human beta-cardiac myosin and the mode of action of mavacamten from myosin molecules to muscle fibers. Proc Natl Acad Sci U S A 2018; 115: E8143–E8152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arad M, Penas-Lado M, Monserrat L, Maron BJ, Sherrid M, Ho CY, Barr S, Karim A, Olson TM, Kamisago M, Seidman JG, Seidman CE. Gene Mutations in Apical Hypertrophic Cardiomyopathy. Circulation 2005; 112: 2805–2811. [DOI] [PubMed] [Google Scholar]
- 9.Arrell DK, Neverova I, Fraser H, Marban E, Van Eyk JE. Proteomic analysis of pharmacologically preconditioned cardiomyocytes reveals novel phosphorylation of myosin light chain 1. Circ Res 2001; 89: 480–487. [DOI] [PubMed] [Google Scholar]
- 10.Aydt EM, Wolff G, Morano I. Molecular modeling of the myosin-S1(A1) isoform. Journal of Structural Biology 2007; 159: 158–163. [DOI] [PubMed] [Google Scholar]
- 11.Barth PG, Wanders RJ, Ruitenbeek W, Roe C, Scholte HR, van der Harten H, van Moorsel J, Duran M, Dingemans KP. Infantile fibre type disproportion, myofibrillar lysis and cardiomyopathy: a disorder in three unrelated Dutch families. Neuromuscul Disord 1998; 8: 296–304. [DOI] [PubMed] [Google Scholar]
- 12.Burghardt TP, Sikkink LA. Regulatory light chain mutants linked to heart disease modify the cardiac myosin lever arm. Biochemistry 2013; 52: 1249–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burghardt TP, Sun X, Wang Y, Ajtai K. In vitro and in vivo single myosin step-sizes in striated muscle. J Muscle Res Cell Motil 2015; 36: 463–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burghardt TP, Ajtai K, Sun X, Takubo N, Wang Y. In vivo myosin step-size from zebrafish skeletal muscle. Open Biol 2016; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burghardt TP, Sun X, Wang Y, Ajtai K. Auxotonic to isometric contraction transitioning in a beating heart causes myosin step-size to down shift. PLoS One 2017; 12: e0174690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caleshu C, Sakhuja R, Nussbaum RL, Schiller NB, Ursell PC, Eng C, De Marco T, McGlothlin D, Burchard EG, Rame JE. Furthering the link between the sarcomere and primary cardiomyopathies: Restrictive cardiomyopathy associated with multiple mutations in genes previously associated with hypertrophic or dilated cardiomyopathy. Am J Med Genet A 2011; 155: 2229–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiou KR, Chu CT, Charng MJ. Detection of mutations in symptomatic patients with hypertrophic cardiomyopathy in Taiwan. J Cardiol 2015; 65: 250–256. [DOI] [PubMed] [Google Scholar]
- 18.Claes GR, van Tienen FH, Lindsey P, Krapels IP, Helderman-van den Enden AT, Hoos MB, Barrois YE, Janssen JW, Paulussen AD, Sels JW, Kuijpers SH, van Tintelen JP, van den Berg MP, Heesen WF, Garcia-Pavia P, Perrot A, Christiaans I, Salemink S, Marcelis CL, Smeets HJ, Brunner HG, Volders PG, van den Wijngaard A. Hypertrophic remodelling in cardiac regulatory myosin light chain (MYL2) founder mutation carriers. Eur Heart J 2016; 37: 1815–1822. [DOI] [PubMed] [Google Scholar]
- 19.Codd MB, Sugrue DD, Gersh BJ, Melton LJ 3rd. Epidemiology of idiopathic dilated and hypertrophic cardiomyopathy. A population-based study in Olmsted County, Minnesota, 1975-1984. Circulation 1989; 80: 564–572. [DOI] [PubMed] [Google Scholar]
- 20.Debold EP, Schmitt JP, Patlak JB, Beck SE, Moore JR, Seidman JG, Seidman C, Warshaw DM. Hypertrophic and dilated cardiomyopathy mutations differentially affect the molecular force generation of mouse {alpha}-cardiac myosin in the laser trap assay. Am J Physiol Heart Circ Physiol 2007; 293: H284–291. [DOI] [PubMed] [Google Scholar]
- 21.Duggal D, Nagwekar J, Rich R, Huang W, Midde K, Fudala R, Das H, Gryczynski I, Szczesna-Cordary D, Borejdo J. Effect of a myosin regulatory light chain mutation K104E on actin-myosin interactions. Am J Physiol Heart Circ Physiol 2015; 308: H1248–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dumka D, Talent J, Akopova I, Guzman G, Szczesna-Cordary D, Borejdo J. E22K mutation of RLC that causes familial hypertrophic cardiomyopathy in heterozygous mouse myocardium: effect on cross-bridge kinetics. Am J Physiol Heart Circ Physiol 2006; 291: H2098–2106. [DOI] [PubMed] [Google Scholar]
- 23.Epstein ND. The molecular biology and pathophysiology of hypertrophic cardiomyopathy due to mutations in the beta myosin heavy chains and the essential and regulatory light chains. Adv Exp Med Biol 1998; 453: 105–114. [DOI] [PubMed] [Google Scholar]
- 24.Epstein ND, Davis JS. When Is a Fly in the Ointment a Solution and not a Problem? Circ Res 2006; 98:1110–1112. [DOI] [PubMed] [Google Scholar]
- 25.Farman GP. Impact of familial hypertrophic cardiomyopathy-linked mutations in the NH2 terminus of the RLC on beta-myosin cross-bridge mechanics 2014; 117: 1471–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flavigny J, Richard P, Isnard R, Carrier L, Charron P, Bonne G, Forissier JF, Desnos M, Dubourg O, Komajda M, Schwartz K, Hainque B. Identification of two novel mutations in the ventricular regulatory myosin light chain gene (MYL2) associated with familial and classical forms of hypertrophic cardiomyopathy. J Mol Med (Berl) 1998; 76: 208–214. [DOI] [PubMed] [Google Scholar]
- 27.Fodor WL, Darras B, Seharaseyon J, Falkenthal S, Francke U, Vanin EF. Human ventricular/slow twitch myosin alkali light chain gene characterization, sequence, and chromosomal location. J Biol Chem 1989; 264: 2143–2149. [PubMed] [Google Scholar]
- 28.Fokstuen S, Munoz A, Melacini P, Iliceto S, Perrot A, Ozcelik C, Jeanrenaud X, Rieubland C, Farr M, Faber L, Sigwart U, Mach F, Lerch R, Antonarakis SE, Blouin JL. Rapid detection of genetic variants in hypertrophic cardiomyopathy by custom DNA resequencing array in clinical practice. J Med Genet 2011; 48: 572–576. [DOI] [PubMed] [Google Scholar]
- 29.Gallego-Delgado M, Delgado JF, Brossa-Loidi V, Palomo J, Marzoa-Rivas R, Perez-Villa F, Salazar-Mendiguchía J, Ruiz-Cano MJ, Gonzalez-Lopez E, Padron-Barthe L, Bornstein B, Alonso-Pulpon L, Garcia-Pavia P. Idiopathic Restrictive Cardiomyopathy Is Primarily a Genetic Disease. Journal of the American College of Cardiology 2016; 67: 3021–3023. [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Pavia P, Vazquez ME, Segovia J, Salas C, Avellana P, Gomez-Bueno M, Vilches C, Gallardo ME, Garesse R, Molano J, Bornstein B, Alonso-Pulpon L. Genetic basis of end-stage hypertrophic cardiomyopathy. Eur J Heart Fail 2011; 13: 1193–1201. [DOI] [PubMed] [Google Scholar]
- 31.Geeves MA, Holmes KC. The molecular mechanism of muscle contraction. Adv Protein Chem 2005; 71: 161–193. [DOI] [PubMed] [Google Scholar]
- 32.Geisterfer-Low ranee AA, Christe M, Conner DA, Ingwall JS, Schoen FJ, Seidman CE, Seidman JG. A mouse model of familial hypertrophic cardiomyopathy. Science 1996; 272: 731–734. [DOI] [PubMed] [Google Scholar]
- 33.Gomes AV, Kazmierczak K, Cheah JX, Gilda JE, Yuan CC, Zhou Z, Szczesna-Cordary D. Proteomic analysis of physiological versus pathological cardiac remodeling in animal models expressing mutations in myosin essential light chains. J Muscle Res Cell Motil 2015; 36: 447–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grabarek Z. Structural Basis for Diversity of the EF-hand Calcium-binding Proteins. Journal of Molecular Biology 2006; 359: 509–525. [DOI] [PubMed] [Google Scholar]
- 35.Green EM, Wakimoto H, Anderson RL, Evanchik MJ, Gorham JM, Harrison BC, Henze M, Kawas R, Oslob JD, Rodriguez HM, Song Y, Wan W, Leinwand LA, Spudich JA, McDowell RS, Seidman JG, Seidman CE. A small-molecule inhibitor of sarcomere contractility suppresses hypertrophic cardiomyopathy in mice. Science 2016; 351: 617–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greenberg MJ, Watt JD, Jones M, Kazmierczak K, Szczesna-Cordary D, Moore JR. Regulatory light chain mutations associated with cardiomyopathy affect myosin mechanics and kinetics. J Mol Cell Cardiol 2009; 46: 108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greenberg MJ, Kazmierczak K, Szczesna-Cordary D, Moore JR. Cardiomyopathy-linked myosin regulatory light chain mutations disrupt myosin strain-dependent biochemistry. Proc Natl Acad Sci U S A 2010; 107: 17403–17408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guhathakurta P, Prochniewicz E, Thomas DD. Amplitude of the actomyosin power stroke depends strongly on the isoform of the myosin essential light chain. Proc Natl Acad Sci U S A 2015; 112: 4660–4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guhathakurta P, Prochniewicz E, Roopnarine O, Rohde JA, Thomas DD. A Cardiomyopathy Mutation in the Myosin Essential Light Chain Alters Actomyosin Structure. Biophys J 2017; 113: 91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Henry GD, Winstanley MA, Dalgarno DC, Scott GM, Levine BA, Trayer IP. Characterization of the actin-binding site on the alkali light chain of myosin. Biochim Biophys Acta 1985; 830: 233–243. [DOI] [PubMed] [Google Scholar]
- 41.Herman DS, Lam L, Taylor MR, Wang L, Teekakirikul P, Christodoulou D, Conner L, DePalma SR, McDonough B, Sparks E, Teodorescu DL, Cirino AL, Banner NR, Pennell DJ, Graw S, Merlo M, Di Lenarda A, Sinagra G, Bos JM, Ackerman MJ, Mitchell RN, Murry CE, Lakdawala NK, Ho CY, Barton PJ, Cook SA, Mestroni L, Seidman JG, Seidman CE. Truncations of titin causing dilated cardiomyopathy. The New England Journal of Medicine 2012; 366: 619–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hernandez OM, Jones M, Guzman G, Szczesna-Cordary D. Myosin essential light chain in health and disease. Am J Physiol Heart Circ Physiol 2007; 292: H1643–1654. [DOI] [PubMed] [Google Scholar]
- 43.Hershberger RE, Hedges DJ, Morales A. Dilated cardiomyopathy: the complexity of a diverse genetic architecture. Nature Reviews Cardiology 2013; 10: 531–547. [DOI] [PubMed] [Google Scholar]
- 44.Holmes KC, Geeves MA. The structural basis of muscle contraction. Philos Trans R Soc Lond B Biol Sci 2000; 355:419–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hooijman P, Stewart MA, Cooke R. A new state of cardiac Myosin with very slow ATP turnover: a potential cardioprotective mechanism in the heart. Biophys J 2011; 100: 1969–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Houdusse A, Cohen C. Structure of the regulatory domain of scallop myosin at 2 A resolution: implications for regulation. Structure 1996; 4: 21–32. [DOI] [PubMed] [Google Scholar]
- 47.Houdusse A, Silver M, Cohen C. A model of Ca2+-free calmodulin binding to unconventional myosins reveals how calmodulin acts as a regulatory switch. Structure 1996; 4: 1475–1490. [DOI] [PubMed] [Google Scholar]
- 48.Huang W, Liang J, Kazmierczak K, Muthu P, Duggal D, Farman GP, Sorensen L, Pozios I, Abraham T, Moore JR, Borejdo J, Szczesna-Cordary D. Hypertrophic Cardiomyopathy Associated Lys104Glu Mutation in the Myosin Regulatory Light Chain Causes Diastolic Disturbance in Mice. J Mol Cell Cardiol 2014; 74: 318–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang W, Liang J, Yuan CC, Kazmierczak K, Zhou Z, Morales A, McBride KL, Fitzgerald-Butt SM, Hershberger RE, Szczesna-Cordary D. Novel familial dilated cardiomyopathy mutation in MYL2 affects the structure and function of myosin regulatory light chain. FEBS J 2015; 282: 2379–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang W, Szczesna-Cordary D. Molecular mechanisms of cardiomyopathy phenotypes associated with myosin light chain mutations. J Muscle Res Cell Motil 2015; 36: 433–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hughes SE, McKenna WJ. New Insights Into the Pathology of Inherited Cardiomyopathy. Heart 2005; 91: 257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.James J, Zhang Y, Wright K, Witt S, Glascock E, Osinska H, Klevitsky R, Martin L, Yager K, Sanbe A, Robbins J. Transgenic rabbits expressing mutant essential light chain do not develop hypertrophic cardiomyopathy. J Mol Cell Cardiol 2002; 34: 873–882. [DOI] [PubMed] [Google Scholar]
- 53.Jay A, Chikarmane R, Poulik J, Misra VK. Infantile hypertrophic cardiomyopathy associated with a novel MYL3 mutation. Cardiology 2013; 124: 248–251. [DOI] [PubMed] [Google Scholar]
- 54.Kabaeva ZT, Perrot A, Wolter B, Dietz R, Cardim N, Correia JM, Schulte HD, Aldashev AA, Mirrakhimov MM, Osterziel KJ. Systematic analysis of the regulatory and essential myosin light chain genes: genetic variants and mutations in hypertrophic cardiomyopathy. Eur J Hum Genet 2002; 10: 741–748. [DOI] [PubMed] [Google Scholar]
- 55.Karabina A, Kazmierczak K, Szczesna-Cordary D, Moore JR. Myosin regulatory light chain phosphorylation enhances cardiac beta-myosin in vitro motility under load. Arch Biochem Biophys 2015; 580: 14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaski JP, Syrris P, Esteban MT, Jenkins S, Pantazis A, Deanfield JE, McKenna WJ, Elliott PM. Prevalence of sarcomere protein gene mutations in preadolescent children with hypertrophic cardiomyopathy. Circ Cardiovasc Genet 2009; 2: 436–441. [DOI] [PubMed] [Google Scholar]
- 57.Kazmierczak K, Xu Y, Jones M, Guzman G, Hernandez OM, Kerrick WGL, Szczesna-Cordary D. The Role of the N-Terminus of the Myosin Essential Light Chain in Cardiac Muscle Contraction. J Mol Biol 2009; 387: 706–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kazmierczak K, Muthu P, Huang W, Jones M, Wang Y, Szczesna-Cordary D. Myosin Regulatory Light Chain Mutation Found In Hypertrophic Cardiomyopathy Patients Increases Isometric Force Production in Transgenic Mice. Biochem J 2012; 442: 95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kazmierczak K, Paulino EC, Huang W, Muthu P, Liang J, Yuan CC, Rojas AI, Hare JM, Szczesna-Cordary D. Discrete effects of A57G-myosin essential light chain mutation associated with familial hypertrophic cardiomyopathy. Am J Physiol Heart Circ Physiol 2013; 305: H575–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kazmierczak K, Yuan C-C, Liang J, Huang W, Rojas AI, Szczesna-Cordary D. Remodeling of the heart in hypertrophy in animal models with myosin essential light chain mutations. Frontiers in Physiology 2014; 5:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kazmierczak K, Liang J, Yuan CC, Yadav S, Sitbon YH, Walz K, Ma W, Irving TC, Cheah JX, Gomes AV, Szczesna-Cordary D. Slow-twitch skeletal muscle defects accompany cardiac dysfunction in transgenic mice with a mutation in the myosin regulatory light chain. FASEB J 2018: fj201801402R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kerrick WGL, Kazmierczak K, Xu Y, Wang Y, Szczesna-Cordary D. Malignant familial hypertrophic cardiomyopathy D166V mutation in the ventricular myosin regulatory light chain causes profound effects in skinned and intact papillary muscle fibers from transgenic mice. FASEB J 2009; 23: 855–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kushwaha SS, Fallon JT, Fuster V. Restrictive cardiomyopathy. The New England Journal of Medicine 1997; 336: 267–276. [DOI] [PubMed] [Google Scholar]
- 64.Lee W, Hwang TH, Kimura A, Park SW, Satoh M, Nishi H, Harada H, Toyama J, Park JE. Different expressivity of a ventricular essential myosin light chain gene Ala57Gly mutation in familial hypertrophic cardiomyopathy. Am Heart J 2001; 141: 184–189. [DOI] [PubMed] [Google Scholar]
- 65.Levine RJ, Yang Z, Epstein ND, Fananapazir L, Stull JT, Sweeney HL. Structural and functional responses of mammalian thick filaments to alterations in myosin regulatory light chains. J Struct Biol 1998; 122: 149–161. [DOI] [PubMed] [Google Scholar]
- 66.Lin BL, Song T, Sadayappan S. Myofilaments: Movers and Rulers of the Sarcomere. Compr Physiol 2017; 7: 675–692. [DOI] [PubMed] [Google Scholar]
- 67.Logvinova DS, Levitsky DI. Essential Light Chains of Myosin and Their Role in Functioning of the Myosin Motor. Biochemistry (Mosc) 2018; 83: 944–960. [DOI] [PubMed] [Google Scholar]
- 68.Lossie J, Ushakov DS, Ferenczi MA, Werner S, Keller S, Haase H, Morano I. Mutations of ventricular essential myosin light chain disturb myosin binding and sarcomeric sorting. Cardiovasc Res 2012; 93: 390–396. [DOI] [PubMed] [Google Scholar]
- 69.Lossie J, Kohncke C, Mahmoodzadeh S, Steffen W, Canepari M, Maffei M, Taube M, Larcheveque O, Baumert P, Haase H, Bottinelli R, Regitz-Zagrosek V, Morano I. Molecular mechanism regulating myosin and cardiac functions by ELC. Biochem Biophys Res Commun 2014; 450: 464–469. [DOI] [PubMed] [Google Scholar]
- 70.Lowey S, Saraswat LD, Liu H, Volkmann N, Hanein D. Evidence for an Interaction between the SH3 Domain and the N-terminal Extension of the Essential Light Chain in Class II Myosins. Journal of Molecular Biology 2007; 371: 902–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lowey S, Bretton V, Joel PB, Trybus KM, Gulick J, Robbins J, Kalganov A, Cornachione AS, Rassier DE. Hypertrophic cardiomyopathy R403Q mutation in rabbit beta-myosin reduces contractile function at the molecular and myofibrillar levels. Proc Natl Acad Sci U S A 2018; 115: 11238–11243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ma N, Zhang J, Itzhaki I, Zhang SL, Chen H, Haddad F, Kitani T, Wilson KD, Tian L, Shrestha R, Wu H, Lam CK, Sayed N, Wu JC. Determining the Pathogenicity of a Genomic Variant of Uncertain Significance Using CRISPR/Cas9 and Human-Induced Pluripotent Stem Cells. Circulation 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Marian AJ, Braunwald E. Hypertrophic Cardiomyopathy: Genetics, Pathogenesis, Clinical Manifestations, Diagnosis, and Therapy. Circ Res 2017; 121: 749–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maron BJ, Bonow RO, Seshagiri TNR, Roberts WC, Epstein SE. Hypertrophic cardiomyopathy with ventricular septal hypertrophy localized to the apical region of the left ventricle (apical hypertrophic cardiomyopathy). The American Journal of Cardiology 1982; 49: 1838–1848. [DOI] [PubMed] [Google Scholar]
- 75.Maron BJ, Gardin JM, Flack JM, Gidding SS, Kurosaki TT, Bild DE. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA Study. Coronary Artery Risk Development in (Young) Adults. Circulation 1995; 92: 785–789. [DOI] [PubMed] [Google Scholar]
- 76.Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, Moss AJ, Seidman CE, Young JB, American Heart A, Council on Clinical Cardiology HF, Transplantation C, Quality of C, Outcomes R, Functional G, Translational Biology Interdisciplinary Working G, Council on E, Prevention. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation 2006; 113: 1807–1816. [DOI] [PubMed] [Google Scholar]
- 77.Maron BJ, Maron MS, Semsarian C. Genetics of hypertrophic cardiomyopathy after 20 years: clinical perspectives. J Am Coll Cardiol 2012; 60: 705–715. [DOI] [PubMed] [Google Scholar]
- 78.McNally EM, Golbus JR, Puckelwartz MJ. Genetic mutations and mechanisms in dilated cardiomyopathy. The Journal of Clinical Investigation 2013; 123: 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McNamara JW, Li A, Dos Remedios CG, Cooke R. The role of super-relaxed myosin in skeletal and cardiac muscle. Biophys Rev 2015; 7: 5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Miller MS, Palmer BM, Ruch S, Martin LA, Farman GP, Wang Y, Robbins J, Irving TC, Maughan DW. The Essential Light Chain N-terminal Extension Alters Force and Fiber Kinetics in Mouse Cardiac Muscle. J Biol Chem 2005; 280: 34427–34434. [DOI] [PubMed] [Google Scholar]
- 81.Milligan RA, Whittaker M, Safer D. Molecular structure of F-actin and location of surface binding sites. Nature 1990; 348: 217–221. [DOI] [PubMed] [Google Scholar]
- 82.Morano I, Ritter O, Bonz A, Timek T, Vahl CF, Michel G. Myosin light chain-actin interaction regulates cardiac contractility. Circ Res 1995; 76: 720–725. [DOI] [PubMed] [Google Scholar]
- 83.Morita H, Rehm HL, Menesses A, McDonough B, Roberts AE, Kucherlapati R, Towbin JA, Seidman JG, Seidman CE. Shared genetic causes of cardiac hypertrophy in children and adults. N Engl J Med 2008; 358: 1899–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Morner S, Richard P, Kazzam E, Hellman U, Hainque B, Schwartz K, Waldenstrom A. Identification of the genotypes causing hypertrophic cardiomyopathy in northern Sweden. J Mol Cell Card 2003; 35: 841–849. [DOI] [PubMed] [Google Scholar]
- 85.Muthu P, Wang L, Yuan CC, Kazmierczak K, Huang W, Hernandez OM, Kawai M, Irving TC, Szczesna-Cordary D. Structural and functional aspects of the myosin essential light chain in cardiac muscle contraction. FASEB J 2011; 25: 4394–4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Muthu P, Kazmierczak K, Jones M, Szczesna-Cordary D. The effect of myosin RLC phosphorylation in normal and cardiomyopathic mouse hearts. J Cell Mol Med 2012; 16: 911–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Muthu P, Liang J, Schmidt W, Moore JR, Szczesna-Cordary D. In Vitro Rescue Study of a Malignant Familial Hypertrophic Cardiomyopathy Phenotype by Pseudo-Phosphorylation of Myosin Regulatory Light Chain. Arch Biochem Biophys 2014; 552-553: 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Olivotto I, Girolami F, Ackerman MJ, Nistri S, Bos JM, Zachara E, Ommen SR, Theis JL, Vaubel RA, Re F, Armentano C, Poggesi C, Torricelli F, Cecchi F. Myofilament protein gene mutation screening and outcome of patients with hypertrophic cardiomyopathy. Mayo Clin Proc 2008; 83: 630–638. [DOI] [PubMed] [Google Scholar]
- 89.Olson TM, Karst ML, Whitby FG, Driscoll DJ. Myosin Light Chain Mutation Causes Autosomal Recessive Cardiomyopathy With Mid-Cavitary Hypertrophy and Restrictive Physiology. Circulation 2002; 105: 2337–2340. [DOI] [PubMed] [Google Scholar]
- 90.Parvari R, Levitas A. The Mutations Associated with Dilated Cardiomyopathy. Biochemistry Research International 2012; 2012: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Petzhold D, Lossie J, Keller S, Werner S, Haase H, Morano I. Human essential myosin light chain isoforms revealed distinct myosin binding, sarcomeric sorting, and inotropic activity. Cardiovasc Res 2011; 90: 513–520. [DOI] [PubMed] [Google Scholar]
- 92.Poetter K, Jiang H, Hassanzadeh S, Master SR, Chang A, Dalakas MC, Rayment I, Sellers JR, Fananapazir L, Epstein ND. Mutations in either the essential or regulatory light chains of myosin are associated with a rare myopathy in human heart and skeletal muscle. Nat Genet 1996; 13: 63–69. [DOI] [PubMed] [Google Scholar]
- 93.Poggesi C, Ho CY. Muscle dysfunction in hypertrophic cardiomyopathy: what is needed to move to translation? J Muscle Res Cell Motil 2014; 35: 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rayment I, Rypniewski WR, Schmidt-Base K, Smith R, Tomchick DR, Benning MM, Winkelmann DA, Wesenberg G, Holden HM. Three-dimensional structure of myosin subfragment-1: a molecular motor. Science 1993; 261: 50–58. [DOI] [PubMed] [Google Scholar]
- 95.Richard P, Charron P, Carrier L, Ledeuil C, Cheav T, Pichereau C, Benaiche A, Isnard R, Dubourg O, Burban M, Gueffet J-P, Millaire A, Desnos M, Schwartz K, Hainque B, Komajda M, EUROGENE Heart Failure Project. Hypertrophic cardiomyopathy: Distribution of disease genes, spectrum of mutations, and implications for a molecular diagnosis strategy. Circulation 2003; 107: 2227–2232, and erratum (2004), 2109(2225), p.3258 [DOI] [PubMed] [Google Scholar]
- 96.Rivenes SM, Kearney DL, Smith EOB, Towbin JA, Denfield SW. Sudden Death and Cardiovascular Collapse in Children With Restrictive Cardiomyopathy. Circulation 2000; 102: 876–882. [DOI] [PubMed] [Google Scholar]
- 97.Robertson SP, Johnson JD, Potter JD. The time-course of Ca2+ exchange with calmodulin, troponin, parvalbumin, and myosin in response to transient increases in Ca2+. Biophysical Journal 1981; 34: 559–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Roopnarine O. Mechanical Defects of Muscle Fibers with Myosin Light Chain Mutants that Cause Cardiomyopathy. Biophys J 2003; 84: 2440–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sanbe A, Nelson D, Gulick J, Setser E, Osinska H, Wang X, Hewett TE, Klevitsky R, Hayes E, Warshaw DM, Robbins J. In vivo analysis of an essential myosin light chain mutation linked to familial hypertrophic cardiomyopathy. Circ Res 2000; 87: 296–302. [DOI] [PubMed] [Google Scholar]
- 100.Santos S, Marques V, Pires M, Silveira L, Oliveira H, Lanca V, Brito D, Madeira H, Fonseca E, Freitas A, Carreira I, Gaspar I, Monteiro C, Fernandes A. High Resolution Melting: improvements in the genetic diagnosis of Hypertrophic Cardiomyopathy in a Portuguese cohort. BMC Med Gen 2012; 13: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Scheid LM, Mosqueira M, Hein S, Kossack M, Juergensen L, Mueller M, Meder B, Fink RH, Katus HA, Hassel D. Essential light chain S195 phosphorylation is required for cardiac adaptation under physical stress. Cardiovasc Res 2016; 111: 44–55. [DOI] [PubMed] [Google Scholar]
- 102.Scruggs SB, Hinken AC, Thawornkaiwong A, Robbins J, Walker LA, de Tombe PP, Geenen DL, Buttrick PM, Solaro RJ. Ablation of ventricular myosin regulatory light chain phosphorylation in mice causes cardiac dysfunction in situ and affects neighboring myofilament protein phosphorylation. The Journal of Biological Chemistry 2009; 284: 5097–5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Scruggs SB, Reisdorph R, Armstrong ML, Warren CM, Reisdorph N, Solaro RJ, Buttrick PM. A novel, insolution separation of endogenous cardiac sarcomeric proteins and identification of distinct charged variants of regulatory light chain. Molecular & Cellular Proteomics : MCP 2010; 9: 1804–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Seidman CE, Seidman JG. Molecular genetic studies of familial hypertrophic cardiomyopathy. Basic Res Cardiol 1998; 93: 13–16. [DOI] [PubMed] [Google Scholar]
- 105.Spudich James A. Hypertrophic and Dilated Cardiomyopathy: Four Decades of Basic Research on Muscle Lead to Potential Therapeutic Approaches to These Devastating Genetic Diseases. Biophysical Journal 2014; 106: 1236–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Spudich JA, Aksel T, Bartholomew SR, Nag S, Kawana M, Yu EC, Sarkar SS, Sung J, Sommese RF, Sutton S, Cho C, Adhikari AS, Taylor R, Liu C, Trivedi D, Ruppel KM. Effects of hypertrophic and dilated cardiomyopathy mutations on power output by human beta-cardiac myosin. J Exp Biol 2016; 219: 161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stewart MA, Franks-Skiba K, Chen S, Cooke R. Myosin ATP turnover rate is a mechanism involved in thermogenesis in resting skeletal muscle fibers. Proc Natl Acad Sci U S A 2010; 107: 430–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sutoh K. An actin-binding site on the 20K fragment of myosin subfragment 1. Biochemistry 1982; 21: 4800–4804. [DOI] [PubMed] [Google Scholar]
- 109.Szczesna-Cordary D. Regulatory light chains of striated muscle myosin. Structure, function and malfunction. Curr Drug Targets Cardiovasc Haematol Disord 2003; 3: 187–197. [DOI] [PubMed] [Google Scholar]
- 110.Szczesna-Cordary D, Guzman G, Ng SS, Zhao J. Familial hypertrophic cardiomyopathy-linked alterations in Ca2+ binding of human cardiac myosin regulatory light chain affect cardiac muscle contraction. J Biol Chem 2004; 279: 3535–3542. [DOI] [PubMed] [Google Scholar]
- 111.Szczesna-Cordary D, Guzman G, Zhao J, Hernandez O, Wei J, Diaz-Perez Z. The E22K mutation of myosin RLC that causes familial hypertrophic cardiomyopathy increases calcium sensitivity of force and ATPase in transgenic mice. J Cell Sci 2005; 118: 3675–3683. [DOI] [PubMed] [Google Scholar]
- 112.Szczesna-Cordary D, Jones M, Moore JR, Watt J, Kerrick WGL, Xu Y, Wang Y, Wagg C, Lopaschuk GD. Myosin regulatory light chain E22K mutation results in decreased cardiac intracellular calcium and force transients. FASEBJ 2007; 21: 3974–3985. [DOI] [PubMed] [Google Scholar]
- 113.Szczesna-Cordary D, de Tombe PP. Myosin light chain phosphorylation, novel targets to repair a broken heart? Cardiovasc Res 2016; 111: 5–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Szczesna D, Ghosh D, Li Q, Gomes AV, Guzman G, Arana C, Zhi G, Stull JT, Potter JD. Familial hypertrophic cardiomyopathy mutations in the regulatory light chains of myosin affect their structure, Ca2+ binding, and phosphorylation. J Biol Chem 2001; 276: 7086–7092. [DOI] [PubMed] [Google Scholar]
- 115.Szczesna D, Zhao J, Jones M, Zhi G, Stull J, Potter JD. Phosphorylation of the regulatory light chains of myosin affects Ca2+ sensitivity of skeletal muscle contraction. Journal of Applied Physiology 2002; 92: 1661–1670. [DOI] [PubMed] [Google Scholar]
- 116.Teekakirikul P, Padera RF, Seidman JG, Seidman CE. Hypertrophic cardiomyopathy: translating cellular cross talk into therapeutics. J Cell Biol 2012; 199: 417–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Timson DJ, Trayer HR, Trayer IP. The N-terminus of A1-type myosin essential light chains binds actin and modulates myosin motor function. Eur J Biochem 1998; 255: 654–662. [DOI] [PubMed] [Google Scholar]
- 118.Trayer HR, Trayer IP. Differential binding of rabbit fast muscle myosin light chain isoenzymes to regulated actin. FEBS Lett 1985; 180: 170–173. [DOI] [PubMed] [Google Scholar]
- 119.Trayer IP, Trayer HR, Levine BA. Evidence that the N-terminal region of A1-light chain of myosin interacts directly with the C-terminal region of actin. A proton magnetic resonance study. Eur J Biochem 1987; 164: 259–266. [DOI] [PubMed] [Google Scholar]
- 120.Trivedi DV, Adhikari AS, Sarkar SS, Ruppel KM, Spudich JA. Hypertrophic cardiomyopathy and the myosin mesa: viewing an old disease in a new light. Biophys Rev 2018; 10: 27–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tyska MJ, Hayes E, Giewat M, Seidman CE, Seidman JG, Warshaw DM. Single-molecule mechanics of R403Q cardiac myosin isolated from the mouse model of familial hypertrophic cardiomyopathy. Circ Res 2000; 86: 737–744. [DOI] [PubMed] [Google Scholar]
- 122.Vemuri R, Lankford EB, Poetter K, Hassanzadeh S, Takeda K, Yu ZX, Ferrans VJ, Epstein ND. The stretch-activation response may be critical to the proper functioning of the mammalian heart. Proc Natl Acad Sci U S A 1999; 96: 1048–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang L, Kazmierczak K, Yuan CC, Yadav S, Kawai M, Szczesna-Cordary D. Cardiac contractility, motor function, and cross-bridge kinetics in N47K-RLC mutant mice. FEBS J 2017; 284: 1897–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang Y, Xu Y, Kerrick WGL, Wang Y, Guzman G, Diaz-Perez Z, Szczesna-Cordary D. Prolonged Ca2+ and force transients in myosin RLC transgenic mouse fibers expressing malignant and benign FHC mutations. J Mol Biol 2006; 361: 286–299. [DOI] [PubMed] [Google Scholar]
- 125.Wang Y, Ajtai K, Burghardt TP. The Qdot-labeled actin super-resolution motility assay measures low-duty cycle muscle myosin step size. Biochemistry 2013; 52: 1611–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang Y, Ajtai K, Burghardt TP. Ventricular myosin modifies in vitro step-size when phosphorylated. J Mol Cell Cardiol 2014; 72: 231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang Y, Ajtai K, Kazmierczak K, Szczesna-Cordary D, Burghardt TP. N-Terminus of Cardiac Myosin Essential Light Chain Modulates Myosin Step-Size. Biochemistry 2016; 55: 186–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang Y, Yuan CC, Kazmierczak K, Szczesna-Cordary D, Burghardt TP. Single cardiac ventricular myosins are autonomous motors. Open Biol 2018; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Weterman MA, Barth PG, van Spaendonck-Zwarts KY, Aronica E, Poll-The BT, Brouwer OF, van Tintelen JP, Qahar Z, Bradley EJ, de Wissel M, Salviati L, Angelini C, van den Heuvel L, Thomasse YE, Backx AP, Nurnberg G, Nurnberg P, Baas F. Recessive MYL2 mutations cause infantile type I muscle fibre disease and cardiomyopathy. Brain 2013; 136: 282–293. [DOI] [PubMed] [Google Scholar]
- 130.Winstanley MA, Trayer HR, Trayer IP. Role of the myosin light chains in binding to actin. FEBS Lett 1977; 77: 239–242. [DOI] [PubMed] [Google Scholar]
- 131.Yadav S, Kazmierczak K, Liang J, Sitbon YH, Szczesna-Cordary D. Phosphomimetic-mediated in vitro rescue of hypertrophic cardiomyopathy linked to R58Q mutation in myosin regulatory light chain. FEBS J 2019; 286: 151–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yuan CC, Muthu P, Kazmierczak K, Liang J, Huang W, Irving TC, Kanashiro-Takeuchi RM, Hare JM, Szczesna-Cordary D. Constitutive phosphorylation of cardiac myosin regulatory light chain prevents development of hypertrophic cardiomyopathy in mice. Proc Natl Acad Sci U S A 2015; 112: E4138–4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yuan CC, Kazmierczak K, Liang J, Kanashiro-Takeuchi R, Irving TC, Gomes AV, Wang Y, Burghardt TP, Szczesna-Cordary D. Hypercontractile mutant of ventricular myosin essential light chain leads to disruption of sarcomeric structure and function and results in restrictive cardiomyopathy in mice. Cardiovasc Res 2017; 113: 1124–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yuan CC, Kazmierczak K, Liang J, Zhou Z, Yadav S, Gomes AV, Irving TC, Szczesna-Cordary D. Sarcomeric perturbations of myosin motors lead to dilated cardiomyopathy in genetically modified MYL2 mice. Proc Natl Acad Sci U S A 2018; 115: E2338–E2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhou Z, Huang W, Liang J, Szczesna-Cordary D. Molecular and Functional Effects of a Splice Site Mutation in the MYL2 Gene Associated with Cardioskeletal Myopathy and Early Cardiac Death in Infants. Front Physiol 2016; 7: 240. [DOI] [PMC free article] [PubMed] [Google Scholar]