Abstract
Physical exercise and chronic social stress are both known to impact general health and hypothalamic-pituitary-adrenal (HPA) axis function, albeit typically in opposing directions. Therefore, the question we investigated in this study was how these two factors – physical exercise and chronic social isolation – would interact when presented simultaneously in a female rodent model. Adult female prairie voles were separated into four experimental groups: 1) isolated without wheel access, 2) isolated with wheel access, 3) paired without wheel access, and 4) paired with wheel access. Plasma, hair, and adrenal glands were sampled to investigate changes in stress physiology. Our results indicate that, when isolated, wheel access had a mitigating effect on HPA activity. However, in paired animals, wheel access had the opposite effect, as both adrenal mass and increase in hair corticosterone concentrations were greater in paired animals with wheel access. Strong correlations were detected between change in hair corticosterone and adrenal mass, while no correlations were found between plasma corticosterone and either of the other markers. These results imply that the HPA axis is highly sensitive to both the social environment and the physical demands placed on the individual, and that when investigating the effects of chronic isolation, both hair corticosterone and adrenal mass may be more reliable markers than a single plasma corticosterone sample.
Keywords: Chronic social isolation, exercise, hair corticosterone, Prairie vole, Microtus ochrogaster, female
1.0. INTRODUCTION
Long-term physical activity boosts metabolism, increases muscle mass, decreases body fat, and improves cardiovascular fitness. Not only does exercise promote physical fitness (Bullo et al., 2015; Deuster and Silverman, 2013; Hills et al., 2015), it has also been shown to improve psychological health (Barbour et al., 2007; Carek et al., 2011; McMahon et al., 2017). Depressive symptoms seem to be particularly responsive to exercise, compared to other psychological disorders (Kenkel and Carter, 2016; Lawlor and Hopker, 2001; Rebar et al., 2015; Wegner et al., 2014). The socioenvironmental component of depression has made alleviating depressive symptoms difficult with pharmacotherapy alone (Netz, 2017; Rosenquist et al., 2011; Slavich and Irwin, 2014). Alternative therapeutic strategies incorporate moderate exercise into patient treatment and often result in similar measures of improvement in alleviating depressive symptoms to those observed following pharmacotherapy (Blumenthal et al., 2007; Brenes et al., 2007; Carneiro et al., 2015; Cooney et al., 2013; Danielsson et al., 2013; Ernst et al., 2006; Hoffman et al., 2011; Kvam et al., 2016). Further, in pharmacotherapy treatment-resistant patients, exercise is a proven strategy for reducing depressive symptoms (Mota-Pereira et al., 2011).
Exercise has been shown to improve several behavioral and cognitive functions, as well as central nervous system processes involved in stress reactivity (Chu et al., 2015; Erickson et al., 2011; Nishijima et al., 2013). For example, lifetime stress level appears to negatively influence the hippocampus, but exercise moderates this effect (Head et al., 2012). These seemingly opposing effects of chronic stress and exercise on psychological health might be predicted to have opposing effects on the hypothalamic-pituitary-adrenal (HPA) axis, the neuroendocrine regulatory system that responds to both energetic demands and perception of stressors. However, both chronic exercise (Gerber et al., 2013; Hill et al., 2008; Skoluda et al., 2012; Tremblay et al., 2004) and chronic stress (Allen et al., 2014; Dickerson and Kemeny, 2004; Ulrich-Lai and Herman, 2009) are associated with activation of the HPA axis in humans. This “exercise-glucocorticoid paradox” has been discussed in detail (Chen et al., 2017), with possible explanations. One explanation is that while exercise increases glucocorticoid release acutely, it also facilitates recovery following a stressor. This is illustrated in rats that either engaged in voluntary wheel running prior to 30-min. restraint stress or not. Those that exercised prior to restraint displayed an earlier peak and earlier recovery of plasma glucocorticoids following restraint stress as compared to controls (Hare et al., 2014). Further, quantification of overall glucocorticoid release was lower in exercised rats, a result of a shortened, and perhaps better regulated, HPA response (Chen et al., 2016).
Stress and exercise also influence paraventricular nucleus (PVN) functions. The PVN integrates information from multiple cortical and limbic structures in the context of stress, ultimately resulting in the release of glucocorticoids (i.e., cortisol, corticosterone) from the adrenal cortex (Herman et al., 2016). While this is an adaptive response to acute stressors, prolonged exposure to stress can result in dysregulation of the HPA axis (e.g., elevation of resting cortisol levels), which may have damaging effects centrally and peripherally. Therefore, exercise may have positive effects on HPA functions to promote more adaptive responses to stress (Hare et al., 2014).
Specific effects of exercise on HPA axis functions are not well elucidated, particularly with respect to exercise potentially mitigating HPA axis effects of psychosocial stressors. One study found that acute treadmill running at a high speed increased cFos expression in corticotropin releasing hormone (CRH) neurons in the PVN in rats (Otsuka et al., 2016). However, no difference was found in oxytocin- and vasopressin-immunoreactive cells in the PVN between sedentary and physically active prairie voles (Kenkel and Carter, 2016). Furthermore, the mechanism underlying the stress-buffering effects of exercise within the PVN remain unclear. On one hand, 6 weeks of wheel running buffered against adrenocortical responses and PVN reactivity to white noise in rats (Campeau et al., 2010), whereas 4 weeks of voluntary exercise buffered against corticosterone response but not PVN reactivity to a forced swim stressor in prairie voles (Watanasriyakul et al., 2018). This complication may be due to the fact that the PVN both initiates HPA stress responses and also integrates autonomic signals triggered by exercise (Evanson and Herman, 2015; Michelini and Stern, 2009).
Exercise has been shown to buffer against some psychological stressors and relieve depressive symptoms (Heaney et al., 2014; Zschucke et al., 2015), providing further evidence that it may have benefits at the level of the HPA axis. For example, an 8-week exercise program significantly improved depressive scores and reduced urinary cortisol levels in depressed female adolescents compared to patients who did not participate in the exercise program (Nabkasorn et al., 2005). Depression also is associated with loneliness and social isolation, and many socially isolated individuals live a sedentary lifestyle (Zhai et al., 2015), suggesting a strong association between social isolation and a lack of physical activity. One study found a negative correlation between loneliness and exercise frequency in college students (Page and Hammermeister, 1995). In addition to the psychological impact of social isolation, lonely individuals may also experience damaging physiological changes such as high blood pressure, elevated plasma cortisol, and weakened immune functions (Cacioppo et al., 2003; Choukèr et al., 2002; Cruces et al., 2014; Doane and Adam, 2010). In prairie voles, 4 weeks of voluntary exercise protected against behavioral and endocrine consequences of social isolation. Specifically, physically active animals displayed significantly less depressive- and anxious-like behaviors compared to animals that remained sedentary (Grippo et al., 2014).
The current study was designed to further characterize the protective effects of exercise against potentially damaging chronic isolation using the prairie vole model. In this case, the chronic stress came in the form of social isolation, given previous evidence demonstrating the value of the prairie vole model for investigating physiological consequences of social experiences (Grippo, 2011; Sun et al., 2014; Young et al., 2011). The prairie vole model is particularly appropriate for investigating the effects of chronic social stress because this species typically engages in monogamous socioemotional bonds similar to those seen in humans. Because of these unique behavioral characteristics, the prairie vole provides a useful model for studying behavioral and physiological aspects of social partnerships and social stressors as they relate to stress-related disorders (Ahern et al., 2011; Carter, 1998; McNeal et al., 2014). When monogamous pairs or family members are separated, negative effects on behavior, physiology, and the brain are observed (Lieberwirth et al., 2012; McNeal et al., 2014). Specifically, separating two bonded, opposite-sex prairie voles, or two sibling prairie voles increases depression- and anxiety-relevant behaviors (McNeal et al., 2014; Grippo et al., 2007). Further, these animals exhibit increased corticosterone and adrenocorticotropic hormone (ACTH) following acute stress when compared to paired animals (McNeal et al., 2014). In addition, the presence of an opposite-sex partner buffers against the negative consequences of chronic mild stress (McNeal et al., 2017). Together, these findings support the utility of this species as a model for the behavioral and physiological consequences of social stress in humans.
In the present study, stress effects were measured by quantifying corticosterone concentrations in both hair and plasma samples, and protective effects of exercise were measured by comparing corticosterone concentrations across experimental groups that either had or did not have access to an exercise wheel. Generally, we predicted that isolation would be associated with several markers of increased HPA activity, and that exercise would mitigate that increase. Based on previous work in rodents (Ferland and Schrader, 2011), including prairie voles (Bosch et al., 2009; McNeal et al., 2014; Pournajafi-Nazarloo et al., 2011), we predicted that animals experiencing social isolation would exhibit elevated corticosterone concentrations in plasma samples. Based on negative effects of social instability in female mice (Jarcho et al., 2016), we also predicted that social isolation would be associated with increases in hair corticosterone concentrations. We further predicted that effects of isolation would be blunted or eliminated in animals that had access to an exercise wheel (Starzec et al., 1983; Watanasriyakul et al., 2018). Lastly, we predicted that certain physiological measures would be systematically correlated to one another. For example, increases in both hair corticosterone and adrenal weight are indicators of long-term HPA axis hyperactivity (Brain and Nowell, 1971; Weiss et al., 2004), and were expected to be affected in a similar pattern in this study.
2.0. METHODS
2.1. Animals
Sixty-two adult female prairie voles, descendants of a wild stock caught near Champaign, Illinois, were used as experimental subjects in this protocol (each first housed with an unstudied female sibling; n = 62 siblings). Females were chosen for the current investigation for several reasons. Previous work with females of this model species has demonstrated behavioral and physiological consequences of depression, anxiety, and increased stress following chronic social isolation (Grippo et al., 2007; Grippo et al., 2008). Further, in humans, loneliness (i.e., the perception of being alone) is reported more frequently by women than men (Prince et al., 1997), and that loneliness is a significant predictor of depression (Cacioppo et al., 2006; Prince et al., 1997). Finally, females are an understudied population (both in human and animal studies; Beery and Zucker, 2011; Klein et al., 2015; Prendergast et al., 2014).
Experimental animals had a mean (± standard error of the mean; SEM) age of 111± 3.4 days, and a body weight of 33.82 ± 0.55 grams. All animals were maintained on a 14/10 h light/dark cycle (lights on at 0630h), with a mean ± SEM ambient temperature of 25 ± 2°C and relative humidity of 40 ± 5%. Animals were allowed food (Purina rabbit chow) and water ad libitum. Offspring were removed from breeding pairs at 21 days of age and housed in same-sex sibling pairs until the commencement of the experimental procedures. For all procedures described here, only one animal from each sibling pair was studied for physiological responses to social isolation and/or exercise (the other animal in the cage was defined as the “unstudied” sibling). All procedures were conducted according to the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals and approved by the Northern Illinois University Institutional Animal Care and Use Committees.
2.2. Study outline
All experimental subjects received an ear punch (in both ears) to for identification purposes, to denote that this was the animal in the cage that would be studied for physiological responses to social isolation and/or exercise (the unstudied sibling of each animal was not marked). Six weeks prior to any experimental manipulation, experimental animals were shaved in order to ensure that hair samples collected later reflected only the time period relevant to the study. During the initial six weeks (baseline period), all experimental animals remained housed with their unstudied siblings. At the end of the baseline period, experimental animals were again shaved and hair samples were collected. Experimental animals were then randomly assigned to one of the following experimental groups for five weeks: 1) remained paired with the respective unstudied sibling, with access to an exercise wheel (n=16), 2) remained paired with the respective unstudied sibling, without a wheel (n=15), 3) isolated from the unstudied sibling, with access to a wheel (n=16), and 4) isolated from the unstudied sibling, without a wheel (n=15) (stressor period). These conditions resulted in all groups receiving a change in environmental conditions (either social isolation, addition of a running wheel, or both), with the exception of the paired/no wheel group, which represented the continuous, basal control condition. Experimental animals in the isolated groups were housed individually, without olfactory, auditory, or visual cues from the previous sibling. Previous work with this species has demonstrated that this duration of isolation is sufficient to induce behavioral and physiological consequences (Grippo et al., 2007; Grippo et al., 2008; McNeal et al., 2014; Peuler et al., 2012), and that access to exercise can mitigate these effects (Watanasriyakul et al., 2018). At the end of the five-week stressor period, a second hair sample was collected from each experimental animal, plasma samples were collected, and adrenal and body weights were recorded.
2.3. Experimental conditions
Experimental conditions in which an exercise wheel was made available included continuous access to a running wheel (4.5 in diameter; Super Pet Mouse Silent Spinner Mini Exercise Wheel, Model #100079369, Elk Grove Village, IL) for the five-week stressor period, to allow for voluntary physical activity. Only one wheel was available, regardless of whether there was a single isolated or two paired animals in the cage. Daily distance traveled and daily maximum speed were monitored via an odometer adapted for use with the running wheel (Bell F12 Cyclocomputer, Model # 7001115, Van Nuys, CA). Sedentary paired and isolated animals were housed in a standard cage without a running wheel for the five-week period.
2.4. Hair collection and analysis
To ensure that hair corticosterone samples represented HPA activity during the study, all experimental subjects (but not their unstudied siblings) were shaved at the start of the baseline period, during the light period (between 10am and 12pm). This hair was not collected or analyzed for corticosterone. All subjects remained in standard housing for 6 weeks (with the unstudied sibling in the same cage), at which point another fur sample (baseline) was collected during the light period (between 10am and 12pm) and stored for corticosterone assay. Fur samples were collected by shaving 4cm x 4cm section of fur on the subjects’ dorsal rear surfaces. The razor (Andis Pivot Pro PMT-1, Model 23475; Andis Co., Sturtevant, WI) was cleaned with 100% ethanol (and allowed to completely dry) before and after hair sample collection from each subject. Samples were then placed via forceps (also cleaned with 100% ethanol) into an Eppendorf tube. Fur samples were stored at −80° C until assayed for corticosterone. Immediately following baseline hair sample collection, experimental subjects were moved to the assigned experimental housing condition (described above). At the end of the 5-week manipulation period a final hair sample (post-stress) was collected during the light period (between 10am and 12pm).
Hair samples were prepared following a modified previously published protocol (Davenport et al., 2006; Jarcho et al., 2016). Briefly, weighed samples were washed with isopropanol to remove debris. Samples were then chopped into fine pieces with a razor blade to facilitate steroid extraction (Yu et al., 2015). Steroids were then extracted from the hair by incubating the samples in methanol for 48 hours. Finally, the steroid-containing methanol solution was purified by passing the solution through Supelco-select HLB SPE tubes (Sigma-Aldrich). Purified extracts were reconstituted with assay buffer (Arbor Assays, Ann Arbor, MI). Reconstituted samples were assayed in duplicate for corticosterone via commercially available enzyme immunoassay kits (Arbor Assays, Ann Arbor, MI). The detectable range of corticosterone for these kits was 78.125–10,000 pg/ml, and the intra-assay and inter-assay coefficients of variance were 6.36 and 7.75, respectively. Corticosterone concentrations as detected by enzyme immunoassay were then matched with the original weight of the hair collected in order to account for minor variations in hair quantity collected. Corticosterone concentrations are, therefore, expressed in pg/mg of hair.
2.5. Blood collection and analysis
All experimental subjects were anesthetized with a mixture of ketamine (67 mg/kg, sc; NLS Animal Health, Owings Mills, MD) and xylazine (13.33 mg/kg, sc; NLS Animal Health), during the light period (between 10am and 12pm). Blood was sampled within two minutes of the anesthetic injection, from the periorbital sinus via a heparanized capillary tube, and was collected during a period not exceeding 1.5 minutes. The blood was placed immediately on ice, and then centrifuged at 4°C at 3500 rpm for 15min to obtain plasma. Plasma aliquots were stored at −80°C until assayed for circulating corticosterone. Plasma concentrations of corticosterone were measured using a commercial enzyme-linked immunosorbent assay kit, according to the kit instructions (Enzo Life Sciences, ADI-900–097, Farmingdale, NY). Plasma was diluted in assay buffer as necessary (1:500) to yield results reliably within the linear portion of the standard curve. The minimum detection limit of this kit is 0.027 ng/ml. Inter- and intra-assay coefficients of variation are <5% (according to both manufacturer specifications and confirmed by multiple in-house assays). Cross-reactivity with other steroids or peptides is <1.7%.
2.6. Adrenal gland collection and analysis
Immediately after the collection of blood, each animal was euthanized under anesthesia. Adrenal glands were immediately dissected and weighed. Adrenal weight is expressed both as an absolute measure (g) and as a relative weight to animal body mass.
2.7. Statistical analyses
Data are presented as means ± SEM for all analyses and figures. A value of p < 0.05 was considered to be statistically significant. When comparing groups within a given data set (e.g., paired v. isolated on hair corticosterone) the data were analyzed with single-factor or two-factor independent-groups analyses of variance (ANOVA) to compare group (i.e., paired or isolated) and manipulation (i.e., wheel or no wheel) effects, followed by a priori Student’s t tests with Bonferroni correction for multiple comparisons. In order to assess synchronicity across physiological responses to chronic social isolation (e.g., association between hair and plasma corticosterone), we calculated Pearson product moment correlations across all biomarkers (i.e., both measures of adrenal weight, plasma corticosterone, and hair corticosterone), and Bonferroni correction was used for multiple comparisons (n=4), resulting in an alpha level of 0.0125 (i.e., 0.05/4).
3.0. RESULTS
3.1. Body weight
Body weight did not differ between experimental groups at either the start or end of the study (Table 1). A two-factor ANOVA yielded no significant main effect of wheel or pairing on body weight at either the start (wheel: F1, 58=0.13, p=0.72; pairing: F1, 58=0.90, p=0.35) or end (wheel: F1, 58=0.11, p=0.74; pairing: F1, 58=0.63, p=0.43) of the study. Nor was there a significant wheel by pairing interaction at either the start (F1, 58=0.56, p=0.46) or the end (F1, 58=0.52, p=0.47) of the study. No follow-up tests were conducted.
Table 1.
Animal weights and physical activity characteristics (mean ± SEM).
| Group | Body mass (g) | Running (km) | |||
|---|---|---|---|---|---|
| Start | End | Change | Distance (km/day) |
Max speed (km/hr) |
|
| Isolated | 33.9±1.3 | 36.5±1.6 | 2.6±0.9 | — | — |
| Isolated, wheel | 32.7±1.1 | 35.1±1.6 | 2.4±0.8 | 2.7±0.6 | 1.07±0.1 |
| Paired | 34.1±1.1 | 36.6±1.1 | 2.5±1.1 | — | — |
| Paired, wheel | 34.6±1.3 | 37.1±1.1 | 2.6±0.7 | 3.8±0.5 | 2.2±0.5 |
3.2. Physical activity
Animals with access to running wheels (both paired and isolated) ran a mean (±SEM) distance of 3.22 ± 0.39 km/day with a mean (±SEM) maximum speed of 1.65 ± 0.27 km/hr. No difference was detected between paired and isolated groups in the daily distance traveled (paired mean ± SEM: 2.67 ± 0.60 km/day isolated mean ± SEM: 3.77 ± 0.47 km/day; t30 = 1.45, p = 0.16; Table 1), but paired animals reached a faster maximum speed (paired mean ± SEM: 2.23 ± 0.49 km/hr; isolated mean ± SEM: 1.07 ± 0.14 km/hr; t30 = 2.29, p = 0.03).
3.3. Hair corticosterone
Hair samples were collected at the beginning (baseline) and end (post-stressor) of the study to quantify corticosterone as a global measure of HPA axis activity (Table 2). Two-factor ANOVA was conducted to assess hair corticosterone at baseline, and found that neither wheel availability, nor housing status predicted corticosterone concentrations (wheel: F1, 58 = 2.76, p = 0.10; pairing: F1, 58 = 0.04, p = 0.84). However, the interaction between these factors was significant (F1, 58 = 18.04, p < 0.01), and post-hoc t tests revealed that within isolated animals, those with later access to a wheel had higher corticosterone concentrations at baseline (t31 = 4.05, p < 0.01). The same analyses were used to assess hair corticosterone at the end of the study, and found that neither wheel availability, nor pairing status, nor the interaction term predicted corticosterone concentration (wheel: F1, 58 = 0.47, p = 0.50; pairing: F1, 58 = 0.02, p = 0.88; interaction: F1, 58 = 0.03, p = 0.86).
Table 2.
Hair corticosterone concentrations at baseline and post-stressor (mean ± SEM).
| Group | Corticosterone (pg/mg hair) | |||
|---|---|---|---|---|
| Baseline | Post-stressor | Changea | pb | |
| Isolated | 14.7±1.2 | 30.4±5.2 | 16.5±5.3 | 0.008 |
| Isolated, wheel | 30.5±3.5 | 36.1±5.5 | 5.9±4.2 | 0.182 |
| Paired | 23.8±3.0 | 30.2±4.6 | 7.5±3.5 | 0.049 |
| Paired, wheel | 19.4±2.4 | 35.7±5.7 | 16.5±5.3 | 0.006 |
Change calculated as average of difference scores (i.e., post-stressor — baseline) for all individuals in an experimental group.
p value corresponds to t test comparison of baseline and post-stressor within an experimental group.
To evaluate the effect of the manipulation, difference scores in hair corticosterone were calculated by subtracting baseline from post-stress, and these scores were analyzed with two-factor ANOVA. Neither main effect predicted corticosterone concentration (wheel: F1, 58 = 0.06, p = 0.81; pairing: F1, 58 = 0.03, p = 0.86), but the interaction between these factors significantly predicted hair corticosterone concentration (F1, 58 = 4.52, p = 0.04; Fig. 1). Post-hoc t tests comparing means across either pairing status or wheel availability did not detect significant differences (all ps > 0.10).
Figure 1.
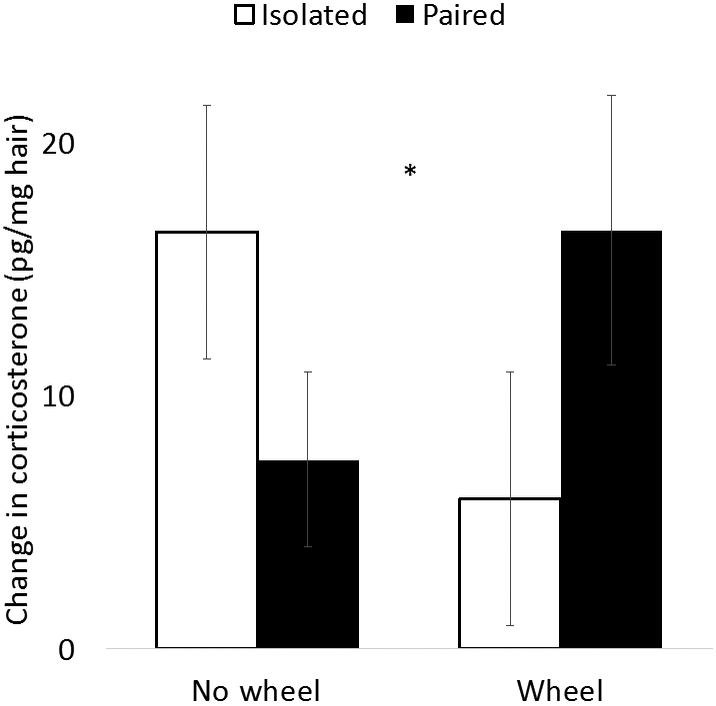
Change in hair corticosterone concentrations (post-manipulation – baseline) as a function of social isolation and wheel access. Social isolation and wheel access interact such that among isolated animals wheel access results in a mitigated increase in hair corticosterone, whereas among paired animals the opposite pattern was observed. * indicates ANOVA interaction p < 0.05.
3.4. Plasma corticosterone
Plasma samples were collected at the end of the study and corticosterone concentrations were analyzed with two-factor ANOVA. Analyses revealed that both main effects predicted plasma corticosterone (wheel: F1, 58 = 4.56, p = 0.04; pairing: F1, 58 = 7.22, p < 0.01; Fig. 2), but the interaction term did not (F1, 58 = 3.44, p = 0.07). Post-hoc t tests revealed isolated animals without wheel access had significantly higher plasma corticosterone than any other experimental group (isolated v. isolated with wheel: t29 = 2.68, p = 0.01; isolated v. pooled paired: t44 = 3.91, p < 0.01), and that no differences existed among the other three groups (all ps > 0.5).
Figure 2.
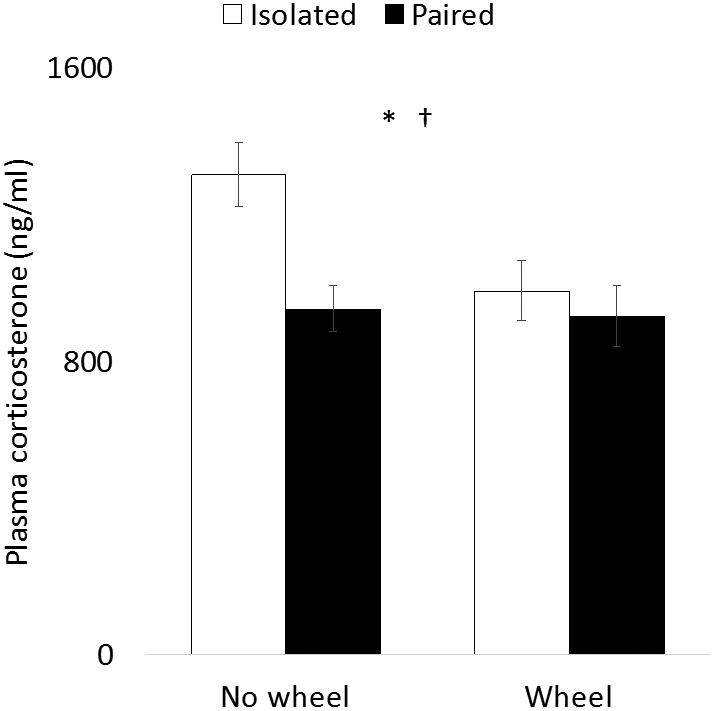
Plasma corticosterone concentrations as a function of social isolation and wheel access. Elevated plasma corticosterone was observed only in animals that experienced social isolation and did not have access to an exercise wheel. * indicates condition (wheel access or sedentary) p < 0.05; † indicates group (paired or isolated) p < 0.01.
3.5. Adrenal weight
Adrenal glands were collected and weighed at the end of the study. Two-factor ANOVA was used to evaluate both absolute adrenal weight and adrenal:body weight ratio. For absolute adrenal weight, there was a significant interaction (F1, 55 = 4.25, p = 0.04; Fig. 3a), but neither main effect predicted adrenal weight (wheel: F1, 55 = 0.59, p = 0.48; pairing: F1, 55 = 0.14, p = 0.71). For adrenal:body weight ratio, a similar pattern was observed, with a significant interaction (F1, 56 = 4.89, p = 0.03; Fig. 3b), and no significant main effects (wheel: F1, 56 = 1.78, p = 0.19; pairing: F1, 56 = 0.06, p = 0.81). For both measures, no post-hoc t tests between experimental groups revealed significant differences (all ps > 0.05).
Figure 3.
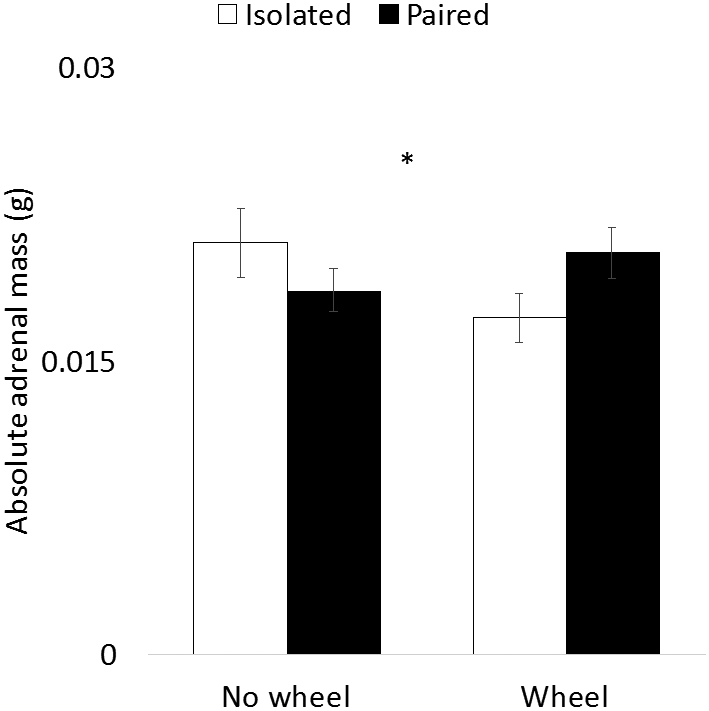
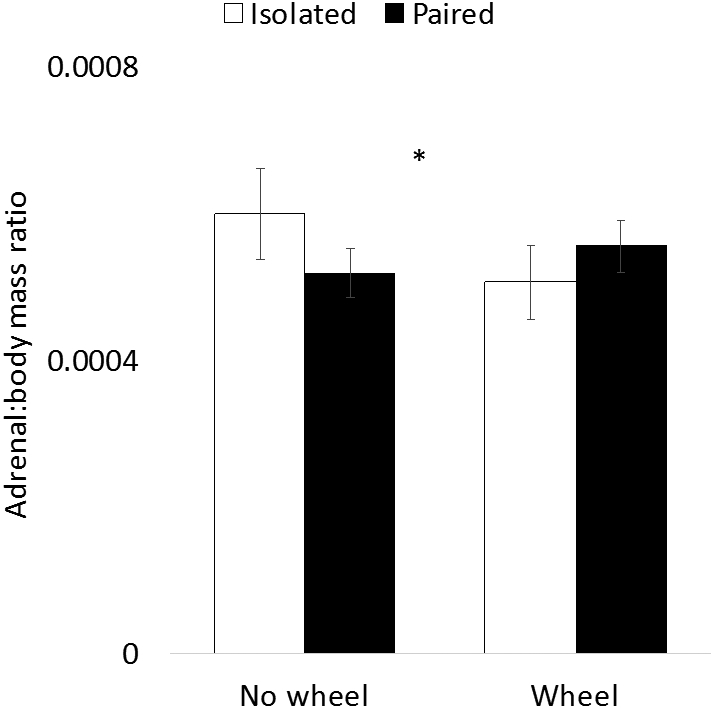
Adrenal gland mass as a function of social isolation and wheel access. Absolute adrenal mass (a) and adrenal mass relative to body mass (b) are predicted by the interaction between social isolation and wheel access. In both adrenal measures, among isolated animals, wheel access is associated with lower adrenal mass than no wheel access. However, among paired animals the opposite pattern is observed. * indicates ANOVA interaction p < 0.05.
3.6. Correlations between physiological measures
Pearson product moment correlation analyses were conducted to evaluate associations across physiological measures. Associations were found between the change in hair corticosterone and both adrenal measures (absolute adrenal weight: r59 = 0.43, p = 0.001; adrenal:body weight ratio: r59 = 0.42, p = 0.001; Fig. 4). Plasma corticosterone was not associated with either measure of adrenal mass (both ps > 0.15).
Figure 4.
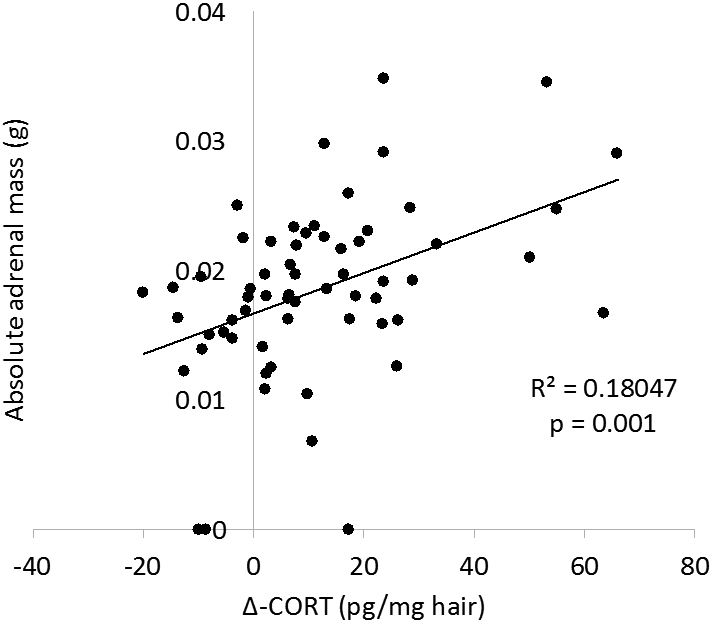
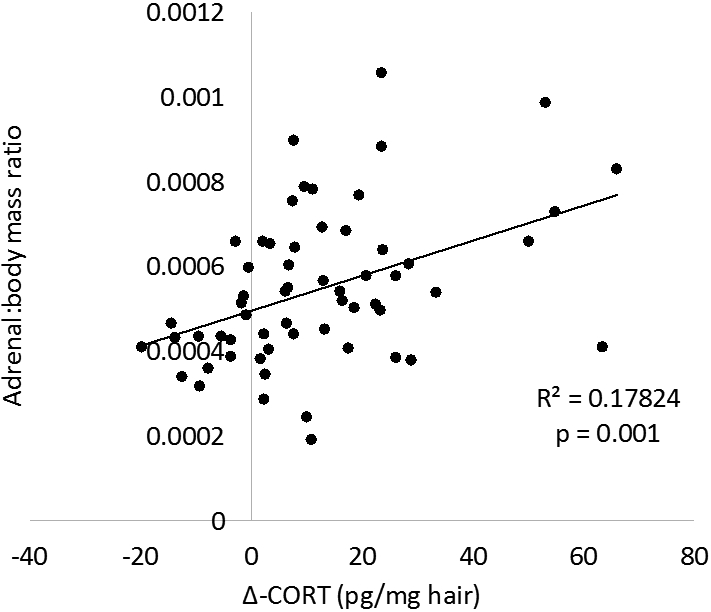
Association between change in hair corticosterone and adrenal mass. Absolute adrenal mass (a) and adrenal mass relative to body mass (b) are positively associated with change in hair corticosterone from baseline to post-manipulation.
Associations were further investigated by evaluating the above correlations within experimental groups. In paired animals with wheel access, significant associations were detected between both measures of adrenal mass and hair corticosterone (absolute adrenal weight: r16 = 0.65, p = 0.007; adrenal:body weight ratio: r16 = 0.70, p = 0.003). No relationship was found between either adrenal measure and plasma corticosterone (both ps > 0.3) for paired animals with wheel access. When assessing within all other experimental groups, no significant associations were found between either measure of adrenal mass and either measure of corticosterone (all ps > 0.06).
4.0. DISCUSSION
Using the socially monogamous prairie vole model, this study investigated the interaction between two factors known to affect HPA axis activity: chronic social isolation and exercise. Our general prediction was that isolated animals would exhibit physiological markers of being chronically stressed, given the disruption of an established social bond, and that access to an exercise wheel would mitigate those physiological markers. Specifically, we predicted elevated corticosterone concentrations in both hair and plasma and increased adrenal weight in isolated animals; and among isolated animals, those with access to an exercise wheel would show lower hair and plasma corticosterone and lower adrenal weight, with these responses being comparable to paired animals.
Social isolation is known to activate the HPA axis [reviewed in (Cacioppo et al., 2015; Sandi and Haller, 2015)] and result in elevated glucocorticoid concentrations (Hawkley et al., 2012). This response tends to be particularly pronounced among species that typically engage in robust social bonds (Hawkley et al., 2012) and in female individuals as compared to males (Dadomo et al., 2018; Haller et al., 1999; Herzog et al., 2009; Iñiguez et al., 2018). Exercise is also known to activate the HPA axis and increase circulating glucocorticoid levels (Gerber et al., 2013; Hill et al., 2008; Skoluda et al., 2012; Tremblay et al., 2004). However, under conditions of chronic stress, exercise mitigates glucocorticoid levels and reduces depression- and anxiety-like behaviors (Campeau et al., 2010; Grippo et al., 2014; Sasse et al., 2008; Watanasriyakul et al., 2018). Parts of the current study align well with these previously observed patterns. First, we observed elevated plasma corticosterone in animals that were isolated and without access to an exercise wheel, as compared to either animals who were paired or those who had access to a wheel, or both. Further, among isolated animals, we observed elevated hair corticosterone concentrations and elevated adrenal size in animals without wheel access. However, our results do not align with the previous patterns entirely. For example, although plasma corticosterone levels were lower in paired animals (versus isolated animals), both hair corticosterone concentration and adrenal size were higher in paired animals with wheel access (versus paired sedentary animals). This pattern suggests that stressors might not affect long-term HPA activity in an additive way, but rather, that HPA activity is quite sensitive to specific social and environmental stimuli. That is, exercise might be effective at mitigating some negative HPA consequences associated with social isolation, but likely does not simply down-regulate all HPA axis responses.
We observed differing patterns between our various measures of physiological responses to stress as a function of social housing and access to a running wheel. Namely, increased concentrations of plasma corticosterone were observed only in animals that were isolated without access to an exercise wheel. For hair corticosterone and adrenal size the pattern was different, with isolated animals expressing indicators of chronic stress (i.e., elevated hair corticosterone and increased adrenal size) when wheel access was denied, whereas paired animals expressed those same indicators when they had wheel access. These differences across physiological measures are likely due to differences in what exactly is being measured. That is, plasma corticosterone is a point measure, and is useful for quantifying corticosterone at the exact time that the blood is collected. It is an indicator of HPA activity at the time of sampling. Hair corticosterone and adrenal size are indicators of more long term HPA activity. In the case of hair corticosterone, the concentration reflects the amount of hormone deposited along the hair shaft for the entire period that the hair has been growing (i.e., 5 or 6 weeks, in this study), and adrenal size, presumably, also reflects HPA activity during the entire period that a stressor is present. Previous work in rats has demonstrated the utility of quantifying plasma corticosterone for assessing stress over short time periods (e.g., minutes to hours; Stalder and Kirschbaum, 2012), while acknowledging the sensitivity of this measure to various environmental factors (e.g., time of day, time since exercise, time since ingestion of food, etc.; D’Agostino et al., 1982; Girard and Garland, 2002; Heiderstadt et al., 2000; Starzec et al., 1983; Stupnicki and Obminski, 1992). Measuring corticosterone in hair provides a noninvasive method for assessing HPA activity over a longer period of time, and has been shown to effectively detect exposure to chronic stressors (Heiderstadt et al., 2000; Meyer and Novak, 2012; Russell et al., 2012; Scorrano et al., 2015). Our data indicate that hair corticosterone reflected the expected pattern in isolated animals (i.e., elevated when wheel access was denied), but not in paired animals. Interestingly, a similar pattern was observed in adrenal size, another physiological indicator of chronic stress (Gamallo et al., 1986). Unfortunately, the pattern observed in hair corticosterone is limited by the fact that our experimental groups differed at baseline. Prior to manipulation, hair corticosterone was lower in animals that were randomly assigned to the isolation without wheel access experimental group. Additionally, animals that would be isolated with wheel access had higher hair corticosterone concentrations than animals that would remain paired with wheel access. Although change scores were evaluated in an attempt to statistically account for baseline differences, these pre-existing differences may limit the interpretation of hair corticosterone data.
Additional evidence supporting hair corticosterone and adrenal mass as indicators of long-term HPA axis activity is the highly correlated nature of these measures, both when adrenal size was expressed in absolute mass or when expressed as a percent of body mass. Importantly, these associations were strongest when animals experienced environments (i.e., either social or environmental) typically thought of as beneficial to health. Specifically, when animals were with their sibling and had access to the exercise wheel, the correlations between change in hair corticosterone and adrenal mass were strong. When animals were either isolated or without wheel access, the correlations were weak. These findings support a hypothesis that under favorable conditions, the HPA axis is well regulated and physiological sequelae are in line with one another. However, under chronically stressful conditions, the HPA axis may become dysregulated, and the consequences on various organs and tissues may differ. Neither hair corticosterone nor adrenal mass were associated with plasma corticosterone. These patterns indicate that hair corticosterone and adrenal mass, but possibly not plasma corticosterone, are closely related, and that both hair corticosterone and adrenal mass are reliable measures of chronic social isolation. Plasma corticosterone, on the other hand, while highly effective for detecting acute physiological responses to stress, may be less reliable for detecting accumulated changes associated with chronic stressors, unless multiple samples are collected (Meyer and Novak, 2012). Although we attempted to minimize the influence of short-term influences on plasma corticosterone levels in the current design, it is possible that environmental confounds unsystematically influenced these levels. Further detailed investigations directly comparing repeated measures of hair corticosterone, adrenal function, and plasma corticosterone will provide additional insight into these relationships.
An alternative explanation for the differential pattern of hair corticosterone and adrenal mass in the present study is that increased glucocorticoid production in paired animals with wheel access was a result of increased energy expenditure. That is, elevated corticosterone concentrations in the hair may have represented increased metabolic demands, and not increased stress. Indeed, previous work in rats and mice has demonstrated a strong association between exercise and plasma corticosterone (Coleman et al., 1998; Girard and Garland, 2002; Sipp et al., 1993), a pattern that is also well documented in humans (Tharp, 1975). It may be the case that exercise only has a mitigating effect on HPA activity when individuals are already experiencing some form of psychological stress. In the current study, among paired animals we observed a greater increase in hair corticosterone within animals with wheel access. Further, the only experimental group that did not display a significant increase in hair corticosterone was isolated animals with wheel access. To further support this hypothesis, data from exercise studies in rats housed under different social conditions suggest that the effects of exercise are independent from those of social housing, and that voluntary exercise indeed has stress-buffering effects (Greenwood and Fleshner, 2011).
One additional explanation for increased hair corticosterone and adrenal mass in paired animals with wheel access is competition over wheel access. That is, the exercise wheel may be thought of as a limited resource that the subject animal competed over with their sibling. Indeed, only one wheel was placed in each cage, whether the cage had one (isolated) or two (paired) animals. It is also notable the mean distance traveled in the paired group was similar to that of the isolated group, despite the fact that paired animals shared a wheel with a sibling in the same cage. A reasonable prediction might be that the paired group should have traveled approximately twice the distance than that of the isolated group, similar to what is observed in single- vs. pair-housed rats (Greenwood and Fleshner, 2011). Therefore, among paired animals, the limited availability of an exercise wheel might have represented a stressor (Liesenjohann et al., 2013). On the other hand, for isolated animals that did not have a sibling to compete with, the exercise wheel would not represent a stressor. On the contrary, among these isolated animals, the opportunity to exercise might be a coping mechanism to mitigate the stress of being isolated (Campeau et al., 2010; Greenwood and Fleshner, 2011; McNeal et al., 2017; Sasse et al., 2008; Watanasriyakul et al., 2018).
Taken together, the results from the present study indicate that isolation is a potent psychosocial stressor in the monogamous prairie vole, activating the HPA axis. Further, these results indicate that exercise mitigates the physiological reactivity to social isolation. These results have important implications for treatment strategies for patients suffering from depressive symptoms or other consequences of social stress, especially those patients whose symptoms are not entirely mitigated through pharmacological means. This study provides a foundation for additional investigation of the benefits of exercise and other environmental factors in mediating behavioral and neurobiological consequences of social stressors. Lastly, the differing physiological patterns from this study support the use of hair corticosterone and adrenal size as indicators of long-term HPA hyperactivity.
7.0. ACKNOWLEDGEMENTS
We would like to thank the following individuals for their contributions to this work: Harley Bradley, Miranda Cox, Ashley Dagner, Blessy Johnson, Elliott Ihm, Tanzania Thomas, Erin Weaver. This research was supported in part by NIH HL 112350 (AJG).
Footnotes
CONFLIFT OF INTEREST
None declared.
REFERENCES
- Ahern TH, Hammock EAD, Young LJ, 2011. Parental division of labor, coordination, and the effects of family structure on parenting in monogamous prairie voles (Microtus ochrogaster). Dev. Psychobiol 53, 118–131. doi: 10.1002/dev.20498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen AP, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, 2014. Biological and psychological markers of stress in humans: Focus on the Trier Social Stress Test. Neurosci. Biobehav. Rev 38, 94–124. doi: 10.1016/J.NEUBIOREV.2013.11.005 [DOI] [PubMed] [Google Scholar]
- Barbour KA, Edenfield TM, Blumenthal JA, 2007. Exercise as a Treatment for Depression and Other Psychiatric Disorders. J. Cardiopulm. Rehabil. Prev 27, 359–367. doi: 10.1097/01.HCR.0000300262.69645.95 [DOI] [PubMed] [Google Scholar]
- Beery AK, Zucker I, 2011. Sex bias in neuroscience and biomedical research. Neurosci. Biobehav. Rev 35, 565–572. doi: 10.1016/J.NEUBIOREV.2010.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal JA, Babyak MA, Doraiswamy PM, Watkins L, Hoffman BM, Barbour KA, Herman S, Craighead WE, Brosse AL, Waugh R, Hinderliter A, Sherwood A, 2007. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom. Med 69, 587–96. doi: 10.1097/PSY.0b013e318148c19a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ, Nair HP, Ahern TH, Neumann ID, Young LJ, 2009. The CRF System Mediates Increased Passive Stress-Coping Behavior Following the Loss of a Bonded Partner in a Monogamous Rodent. Neuropsychopharmacology 34, 1406–1415. doi: 10.1038/npp.2008.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain PF, Nowell NW, 1971. Isolation versus grouping effects on adrenal and gonadal function in albino mice II. The female. Gen. Comp. Endocrinol 16, 155–159. doi: 10.1016/0016-6480(71)90218-8 [DOI] [PubMed] [Google Scholar]
- Brenes GA, Williamson JD, Messier SP, Rejeski WJ, Pahor M, Ip E, Penninx BWJH, 2007. Treatment of minor depression in older adults: A pilot study comparing sertraline and exercise. Aging Ment. Health 11, 61–68. doi: 10.1080/13607860600736372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullo V, Bergamin M, Gobbo S, Sieverdes JC, Zaccaria M, Neunhaeuserer D, Ermolao A, 2015. The effects of Pilates exercise training on physical fitness and wellbeing in the elderly: A systematic review for future exercise prescription. Prev. Med. (Baltim). 75, 1–11. doi: 10.1016/j.ypmed.2015.03.002 [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Cacioppo S, Capitanio JP, Cole SW, 2015. The neuroendocrinology of social isolation. Annu. Rev. Psychol 66, 733–67. doi: 10.1146/annurev-psych-010814-015240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC, Kiecolt-Glaser JK, 2003. Social Isolation and Health, with an Emphasis on Underlying Mechanisms. Perspect. Biol. Med 46, S39–S52. doi: 10.1353/pbm.2003.0063 [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Hughes ME, Waite LJ, Hawkley LC, Thisted RA, 2006. Loneliness as a Specific Risk Factor for Depressive Symptoms: Cross-Sectional and Longitudinal Analyses. doi: 10.1037/0882-7974.21.1.140 [DOI] [PubMed] [Google Scholar]
- Campeau S, Nyhuis TJ, Sasse SK, Kryskow EM, Herlihy L, Masini CV, Babb JA, Greenwood BN, Fleshner M, Day HEW, 2010. Hypothalamic Pituitary Adrenal Axis Responses to Low Intensity Stressors are reduced following Voluntary Wheel Running in Rats. J. Neuroendocrinol. 22, 872–888. doi: 10.1111/j.1365-2826.2010.02007.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carek PJ, Laibstain SE, Carek SM, 2011. Exercise for the Treatment of Depression and Anxiety. Int. J. Psychiatry Med 41, 15–28. doi: 10.2190/PM.41.1.c [DOI] [PubMed] [Google Scholar]
- Carneiro L, Fonseca A, Vieira-Coelho M, Mota M, Vasconcelos-Raposo J, 2015. Effects of structured exercise and pharmacotherapy vs. pharmacotherapy for adults with depressive symptoms: A randomized clinical trial. J. Psychiatr. Res 71, 48–55. doi: 10.1016/J.JPSYCHIRES.2015.09.007 [DOI] [PubMed] [Google Scholar]
- Carter CS, 1998. NEUROENDOCRINE PERSPECTIVES ON SOCIAL ATTACHMENT AND LOVE. Psychoneuroendocrinology 23, 779–818. doi: 10.1016/S0306-4530(98)00055-9 [DOI] [PubMed] [Google Scholar]
- Chen C, Nakagawa S, An Y, Ito K, Kitaichi Y, Kusumi I, 2017. The exercise-glucocorticoid paradox: How exercise is beneficial to cognition, mood, and the brain while increasing glucocorticoid levels. Front. Neuroendocrinol. 44, 83–102. doi: 10.1016/j.yfrne.2016.12.001 [DOI] [PubMed] [Google Scholar]
- Chen C, Nakagawa S, Kitaichi Y, An Y, Omiya Y, Song N, Koga M, Kato A, Inoue T, Kusumi I, 2016. The role of medial prefrontal corticosterone and dopamine in the antidepressant-like effect of exercise. Psychoneuroendocrinology 69, 1–9. doi: 10.1016/J.PSYNEUEN.2016.03.008 [DOI] [PubMed] [Google Scholar]
- Choukèr A, Smith L, Christ F, Larina I, Nichiporuk I, Baranov V, Bobrovnik E, Pastushkova L, Messmer K, Peter K, Thiel M, 2002. Effects of confinement (110 and 240 days) on neuroendocrine stress response and changes of immune cells in men. J. Appl. Physiol 92. [DOI] [PubMed] [Google Scholar]
- Chu C-H, Chen A-G, Hung T-M, Wang C-C, Chang Y-K, 2015. Exercise and fitness modulate cognitive function in older adults. Psychol. Aging 30, 842–848. doi: 10.1037/pag0000047 [DOI] [PubMed] [Google Scholar]
- Coleman MA, Garland T, Marler CA, Newton SS, Swallow JG, Carter PA, Garland T, Marler CA, Newton SS, Swallow JG, CARTER PA, 1998. Glucocorticoid Response to Forced Exercise in Laboratory House Mice (Mus domesticus). Physiol. Behav 63, 279–285. [DOI] [PubMed] [Google Scholar]
- Cooney GM, Dwan K, Greig CA, Lawlor DA, Rimer J, Waugh FR, McMurdo M, Mead GE, 2013. Exercise for depression. Cochrane Database Syst. Rev doi: 10.1002/14651858.CD004366.pub6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruces J, Venero C, Pereda-Peeez I, De la Fuente M, 2014. The Effect of Psychological Stress and Social Isolation on Neuroimmunoendocrine Communication. Curr. Pharm. Des 20, 4608–4628. [DOI] [PubMed] [Google Scholar]
- D’Agostino J, Vaeth GF, Henning SJ, 1982. Diurnal rhythm of total and free concentrations of serum corticosterone in the rat. Acta Endocrinol. (Copenh). 100, 85–90. doi: 10.1530/ACTA.0.1000085 [DOI] [PubMed] [Google Scholar]
- Dadomo H, Gioiosa L, Cigalotti J, Ceresini G, Parmigiani S, Palanza P, 2018. What is stressful for females? Differential effects of unpredictable environmental or social stress in CD1 female mice. Horm. Behav 98, 22–32. doi: 10.1016/J.YHBEH.2017.11.013 [DOI] [PubMed] [Google Scholar]
- Danielsson L, Noras AM, Waern M, Carlsson J, 2013. Exercise in the treatment of major depression: A systematic review grading the quality of evidence. Physiother. Theory Pract 29, 573–585. doi: 10.3109/09593985.2013.774452 [DOI] [PubMed] [Google Scholar]
- Davenport MD, Tiefenbacher S, Lutz CK, Novak MA, Meyer JS, 2006. Analysis of endogenous cortisol concentrations in the hair of rhesus macaques. Gen. Comp. Endocrinol 147, 255–261. doi: 10.1016/j.ygcen.2006.01.005 [DOI] [PubMed] [Google Scholar]
- Deuster PA, Silverman MN, 2013. Physical fitness: a pathway to health and resilience. US. Army Med. Dep. J 24–35. [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME, 2004. Acute Stressors and Cortisol Responses: A Theoretical Integration and Synthesis of Laboratory Research. Psychol. Bull 130, 355–391. [DOI] [PubMed] [Google Scholar]
- Doane LD, Adam EK, 2010. Loneliness and cortisol: Momentary, day-to-day, and trait associations. Psychoneuroendocrinology 35, 430–441. doi: 10.1016/j.psyneuen.2009.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF, 2011. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. U. S. A. 108, 3017–22. doi: 10.1073/pnas.1015950108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst C, Olson AK, Pinel JPJ, Lam RW, Christie BR, 2006. Antidepressant effects of exercise: Evidence for an adult-neurogenesis hypothesis? J. Psychiatry Neurosci 31, 84–92. [PMC free article] [PubMed] [Google Scholar]
- Evanson NK, Herman JP, 2015. Role of Paraventricular Nucleus Glutamate Signaling in Regulation of HPA Axis Stress Responses. Interdiscip. Inf. Sci 21, 253–260. doi: 10.4036/iis.2015.B.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferland CL, Schrader LA, 2011. Cage mate separation in pair-housed male rats evokes an acute stress corticosterone response. Neurosci. Lett 489, 154–158. doi: 10.1016/J.NEULET.2010.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamallo A, Villanua A, Trancho G, Fraile A, 1986. Stress adaptation and adrenal activity in isolated and crowded rats. Physiol. Behav 36, 217–221. doi: 10.1016/0031-9384(86)90006-5 [DOI] [PubMed] [Google Scholar]
- Gerber M, Jonsdottir IH, Kalak N, Elliot C, Pühse U, Holsboer-Trachsler E, Brand S, 2013. Objectively assessed physical activity is associated with increased hair cortisol content in young adults. Stress 16, 593–599. doi: 10.3109/10253890.2013.823599 [DOI] [PubMed] [Google Scholar]
- Girard I, Garland T, 2002. Plasma corticosterone response to acute and chronic voluntary exercise in female house mice. J. Appl. Physiol 92. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Fleshner M, 2011. Exercise, stress resistance, and central serotonergic systems. Exerc. Sport Sci. Rev 39, 140–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, 2011. The Utility of Animal Models in Understanding Links between Psychosocial Processes and Cardiovascular Health. Soc. Personal. Psychol. Compass 5, 164–179. doi: 10.1111/j.1751-9004.2011.00342.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Cushing BS, Carter CS, 2007. Depression-like behavior and stressor-induced neuroendocrine activation in female prairie voles exposed to chronic social isolation. Psychosom. Med 69, 149–57. doi: 10.1097/PSY.0b013e31802f054b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Gerena D, Huang J, Kumar N, Shah M, Ughreja R, Sue Carter C, 2007. Social isolation induces behavioral and neuroendocrine disturbances relevant to depression in female and male prairie voles. Psychoneuroendocrinology 32, 966–980. doi: 10.1016/J.PSYNEUEN.2007.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Ihm E, Wardwell J, McNeal N, Scotti M-AL, Moenk DA, Chandler DL, LaRocca MA, Preihs K, 2014. The effects of environmental enrichment on depressive and anxiety-relevant behaviors in socially isolated prairie voles. Psychosom. Med 76, 277–84. doi: 10.1097/PSY.0000000000000052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Wu KD, Hassan I, Carter CS, 2008. Social isolation in prairie voles induces behaviors relevant to negative affect: toward the development of a rodent model focused on co-occurring depression and anxiety. Depress. Anxiety 25, E17–E26. doi: 10.1002/da.20375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller J, Fuchs E, Halász J, Makara GB, 1999. Defeat is a major stressor in males while social instability is stressful mainly in females: towards the development of a social stress model in female rats. Brain Res. Bull 50, 33–39. doi: 10.1016/S0361-9230(99)00087-8 [DOI] [PubMed] [Google Scholar]
- Hare BD, Beierle JA, Toufexis DJ, Hammack SE, Falls WA, 2014a. Exercise-associated changes in the corticosterone response to acute restraint stress: Evidence for increased adrenal sensitivity and reduced corticosterone response duration. Neuropsychopharmacology 39, 1262–1269. doi: 10.1038/npp.2013.329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare BD, Beierle JA, Toufexis DJ, Hammack SE, Falls WA, 2014b. Exercise-Associated Changes in the Corticosterone Response to Acute Restraint Stress: Evidence for Increased Adrenal Sensitivity and Reduced Corticosterone Response Duration. Neuropsychopharmacology 39, 1262–1269. doi: 10.1038/npp.2013.329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkley LC, Cole SW, Capitanio JP, Norman GJ, Cacioppo JT, 2012. Effects of social isolation on glucocorticoid regulation in social mammals. Horm. Behav 62, 314–23. doi: 10.1016/j.yhbeh.2012.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head D, Singh T, Bugg JM, 2012. The moderating role of exercise on stress-related effects on the hippocampus and memory in later adulthood. Neuropsychology 26, 133–143. doi: 10.1037/a0027108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaney JLJ, Carroll D, Phillips AC, 2014. Physical Activity, Life Events Stress, Cortisol, and DHEA: Preliminary Findings That Physical Activity May Buffer Against the Negative Effects of Stress. J. Aging Phys. Act 22, 465–473. doi: 10.1123/JAPA.2012-0082 [DOI] [PubMed] [Google Scholar]
- Heiderstadt KM, McLaughlin RM, Wrighe DC, Walker SE, Gomez-Sanchez CE, 2000. The effect of chronic food and water restriction on open-field behaviour and serum corticosterone levels in rats. Lab. Anim 34, 20–28. doi: 10.1258/002367700780578028 [DOI] [PubMed] [Google Scholar]
- Herman JP, McKlveen JM, Ghosal S, Kopp B, Wulsin A, Makinson R, Scheimann J, Myers B, Herman JP, McKlveen JM, Ghosal S, Kopp B, Wulsin A, Makinson R, Scheimann J, Myers B, 2016. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response, in: Comprehensive Physiology. John Wiley & Sons, Inc., Hoboken, NJ, USA, pp. 603–621. doi: 10.1002/cphy.c150015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog CJ, Czéh B, Corbach S, Wuttke W, Schulte-Herbrüggen O, Hellweg R, Flügge G, Fuchs E, 2009. Chronic social instability stress in female rats: A potential animal model for female depression Neuroscience 159, 982–992. doi: 10.1016/j.neuroscience.2009.01.059 [DOI] [PubMed] [Google Scholar]
- Hill EE, Zack E, Battaglini C, Viru M, Viru A, Hackney AC, 2008. Exercise and circulating Cortisol levels: The intensity threshold effect. J. Endocrinol. Invest 31, 587–591. doi: 10.1007/BF03345606 [DOI] [PubMed] [Google Scholar]
- Hills AP, Street SJ, Byrne NM, 2015. Physical Activity and Health. pp. 77–95. doi: 10.1016/bs.afnr.2015.06.001 [DOI] [PubMed] [Google Scholar]
- Hoffman BM, Babyak MA, Craighead WE, Sherwood A, Doraiswamy PM, Coons MJ, Blumenthal JA, 2011. Exercise and pharmacotherapy in patients with major depression: one-year follow-up of the SMILE study. Psychosom. Med 73, 127–33. doi: 10.1097/PSY.0b013e31820433a5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iñiguez SD, Flores-Ramirez FJ, Riggs LM, Alipio JB, Garcia-Carachure I, Hernandez MA, Sanchez DO, Lobo MK, Serrano PA, Braren SH, Castillo SA, 2018. Vicarious Social Defeat Stress Induces Depression-Related Outcomes in Female Mice. Biol. Psychiatry 83, 9–17. doi: 10.1016/J.BIOPSYCH.2017.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarcho MR, Massner KJ, Eggert AR, Wichelt EL, 2016. Behavioral and physiological response to onset and termination of social instability in female mice. Horm. Behav 78, 135–140. doi: 10.1016/j.yhbeh.2015.11.004 [DOI] [PubMed] [Google Scholar]
- Kenkel WM, Carter CS, 2016. Voluntary exercise facilitates pair-bonding in male prairie voles. Behav. Brain Res 296, 326–330. doi: 10.1016/j.bbr.2015.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein SL, Schiebinger L, Stefanick ML, Cahill L, Danska J, de Vries GJ, Kibbe MR, McCarthy MM, Mogil JS, Woodruff TK, Zucker I, 2015. Opinion: Sex inclusion in basic research drives discovery. Proc. Natl. Acad. Sci. U. S. A. 112, 5257–8. doi: 10.1073/pnas.1502843112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvam S, Klepp CL, Nordhus IH, Hovland A, 2016. Exercise as a treatment for depression: A meta-analysis. J. Affect. Disord 202, 67–86. doi: 10.1016/J.JAD.2016.03.063 [DOI] [PubMed] [Google Scholar]
- Lawlor DA, Hopker SW, 2001. The effectiveness of exercise as an intervention in the management of depression: systematic review and meta-regression analysis of randomised controlled trials. BMJ 322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberwirth C, Liu Y, Jia X, Wang Z, 2012. Social isolation impairs adult neurogenesis in the limbic system and alters behaviors in female prairie voles. Horm. Behav 62, 357–366. doi: 10.1016/J.YHBEH.2012.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesenjohann M, Liesenjohann T, Palme R, Eccard J, 2013. Differential behavioural and endocrine responses of common voles (Microtus arvalis) to nest predators and resource competitors. BMC Ecol. 13, 33. doi: 10.1186/1472-6785-13-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon EM, Corcoran P, O’Regan G, Keeley H, Cannon M, Carli V, Wasserman C, Hadlaczky G, Sarchiapone M, Apter A, Balazs J, Balint M, Bobes J, Brunner R, Cozman D, Haring C, Iosue M, Kaess M, Kahn J-P, Nemes B, Podlogar T, Poštuvan V, Sáiz P, Sisask M, Tubiana A, Värnik P, Hoven CW, Wasserman D, 2017. Physical activity in European adolescents and associations with anxiety, depression and well-being. Eur. Child Adolesc. Psychiatry 26, 111–122. doi: 10.1007/s00787-016-0875-9 [DOI] [PubMed] [Google Scholar]
- McNeal N, Appleton KM, Johnson AK, Scotti M-AL, Wardwell J, Murphy R, Bishop C, Knecht A, Grippo AJ, 2017. The protective effects of social bonding on behavioral and pituitary-adrenal axis reactivity to chronic mild stress in prairie voles. Stress 20, 175–182. doi: 10.1080/10253890.2017.1295444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeal N, Scotti M-AL, Wardwell J, Chandler DL, Bates SL, Larocca M, Trahanas DM, Grippo AJ, 2014. Disruption of social bonds induces behavioral and physiological dysregulation in male and female prairie voles. Auton. Neurosci 180, 9–16. doi: 10.1016/j.autneu.2013.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JS, Novak MA, 2012. Minireview: Hair Cortisol: A Novel Biomarker of Hypothalamic-Pituitary-Adrenocortical Activity. Endocrinology 153, 4120–4127. doi: 10.1210/en.2012-1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelini LC, Stern JE, 2009. Exercise-induced neuronal plasticity in central autonomic networks: role in cardiovascular control. Exp. Physiol 94, 947–960. doi: 10.1113/expphysiol.2009.047449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mota-Pereira J, Silverio J, Carvalho S, Ribeiro JC, Fonte D, Ramos J, 2011. Moderate exercise improves depression parameters in treatment-resistant patients with major depressive disorder. J. Psychiatr. Res 45, 1005–1011. doi: 10.1016/J.JPSYCHIRES.2011.02.005 [DOI] [PubMed] [Google Scholar]
- Nabkasorn C, Miyai N, Sootmongkol A, Junprasert S, Yamamoto H, Arita M, Miyashita K, 2005. Effects of physical exercise on depression, neuroendocrine stress hormones and physiological fitness in adolescent females with depressive symptoms. Eur. J. Public Health 16, 179–184. doi: 10.1093/eurpub/cki159 [DOI] [PubMed] [Google Scholar]
- Netz Y, 2017. Is the Comparison between Exercise and Pharmacologic Treatment of Depression in the Clinical Practice Guideline of the American College of Physicians Evidence-Based? Front. Pharmacol 8, 257. doi: 10.3389/fphar.2017.00257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishijima T, Kawakami M, Kita I, Steffen C, Ang E, 2013. Long-Term Exercise Is a Potent Trigger for ΔFosB Induction in the Hippocampus along the dorso–ventral Axis. PLoS One 8, e81245. doi: 10.1371/journal.pone.0081245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka T, Nishii A, Amemiya S, Kubota N, Nishijima T, Kita I, 2016. Effects of acute treadmill running at different intensities on activities of serotonin and corticotropin-releasing factor neurons, and anxiety- and depressive-like behaviors in rats. Behav. Brain Res 298, 44–51. doi: 10.1016/j.bbr.2015.10.055 [DOI] [PubMed] [Google Scholar]
- Page RM, Hammermeister J, 1995. Shyness and Loneliness: Relationship to the Exercise Frequency of College Students. Psychol. Rep 76, 395–398. doi: 10.2466/pr0.1995.76.2.395 [DOI] [PubMed] [Google Scholar]
- Peuler JD, Scotti M-AL, Phelps LE, McNeal N, Grippo AJ, 2012. Chronic social isolation in the prairie vole induces endothelial dysfunction: implications for depression and cardiovascular disease. Physiol. Behav 106, 476–484. doi: 10.1016/j.physbeh.2012.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pournajafi-Nazarloo H, Partoo L, Yee J, Stevenson J, Sanzenbacher L, Kenkel W, Mohsenpour SR, Hashimoto K, Carter CS, 2011. Effects of social isolation on mRNA expression for corticotrophin-releasing hormone receptors in prairie voles. Psychoneuroendocrinology 36, 780–9. doi: 10.1016/j.psyneuen.2010.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast BJ, Onishi KG, Zucker I, 2014. Female mice liberated for inclusion in neuroscience and biomedical research. Neurosci. Biobehav. Rev 40, 1–5. doi: 10.1016/J.NEUBIOREV.2014.01.001 [DOI] [PubMed] [Google Scholar]
- Prince M, Harwood R, Blizard R, Thomas A, 1997. Social support deficits, loneliness and life events as risk factors for depression in old age. The Gospel Oak Project VI. Psychol. Med. 27, 323–332. doi: 10.1017/S0033291796004485 [DOI] [PubMed] [Google Scholar]
- Rebar AL, Stanton R, Geard D, Short C, Duncan MJ, Vandelanotte C, 2015. A meta-meta-analysis of the effect of physical activity on depression and anxiety in non-clinical adult populations. Health Psychol. Rev 9, 366–378. doi: 10.1080/17437199.2015.1022901 [DOI] [PubMed] [Google Scholar]
- Rosenquist JN, Fowler JH, Christakis NA, 2011. Social network determinants of depression. Mol. Psychiatry 16, 273–281. doi: 10.1038/mp.2010.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell E, Koren G, Rieder M, Van Uum S, 2012. Hair cortisol as a biological marker of chronic stress: current status, future directions and unanswered questions. Psychoneuroendocrinology 37, 589–601. doi: 10.1016/j.psyneuen.2011.09.009 [DOI] [PubMed] [Google Scholar]
- Sandi C, Haller J, 2015. Stress and the social brain: behavioural effects and neurobiological mechanisms. Nat. Rev. Neurosci 16. doi: 10.1038/nrn3918 [DOI] [PubMed] [Google Scholar]
- Sasse SK, Greenwood BN, Masini CV, Nyhuis TJ, Fleshner M, Day HEW, Campeau S, 2008. Chronic voluntary wheel running facilitates corticosterone response habituation to repeated audiogenic stress exposure in male rats. Stress 11, 425–437. doi: 10.1080/10253890801887453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scorrano F, Carrasco J, Pastor-Ciurana J, Belda X, Rami-Bastante A, Bacci ML, Armario A, 2015. Validation of the long-term assessment of hypothalamic-pituitary-adrenal activity in rats using hair corticosterone as a biomarker. FASEB J. 29, 859–67. doi: 10.1096/fj.14-254474 [DOI] [PubMed] [Google Scholar]
- Sipp TL, Blank SE, Lee EG, Meadows GG, 1993. Plasma corticosterone response to chronic ethanol consumption and exercise stress. Proc. Soc. Exp. Biol. Med 204, 184–90. [DOI] [PubMed] [Google Scholar]
- Skoluda N, Dettenborn L, Stalder T, Kirschbaum C, 2012. Elevated hair cortisol concentrations in endurance athletes. Psychoneuroendocrinology 37, 611–617. doi: 10.1016/J.PSYNEUEN.2011.09.001 [DOI] [PubMed] [Google Scholar]
- Slavich GM, Irwin MR, 2014. From stress to inflammation and major depressive disorder: A social signal transduction theory of depression. Psychol. Bull 140, 774–815. doi: 10.1037/a0035302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalder T, Kirschbaum C, 2012. Analysis of cortisol in hair – State of the art and future directions. Brain. Behav. Immun 26, 1019–1029. doi: 10.1016/J.BBI.2012.02.002 [DOI] [PubMed] [Google Scholar]
- Starzec JJ, Berger DF, Hesse R, 1983. Effects of stress and exercise on plasma corticosterone, plasma cholesterol, and aortic cholesterol levels in rats. Psychosom. Med 45, 219–26. [DOI] [PubMed] [Google Scholar]
- Stupnicki R, Obminski Z, 1992. Glucocorticoid response to exercise as measured by serum and salivary cortisol. Eur. J. Appl. Physiol. Occup. Physiol 65, 546–549. doi: 10.1007/BF00602363 [DOI] [PubMed] [Google Scholar]
- Sun P, Smith AS, Lei K, Liu Y, Wang Z, 2014. Breaking bonds in male prairie vole: Long-term effects on emotional and social behavior, physiology, and neurochemistry. Behav. Brain Res 265, 22–31. doi: 10.1016/J.BBR.2014.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tharp GD, 1975. The role of glucocorticoids in exercise. Med. Sci. Sports 7, 6–11. [PubMed] [Google Scholar]
- Tremblay MS, Copeland JL, Van Helder W, 2004. Effect of training status and exercise mode on endogenous steroid hormones in men. J. Appl. Physiol 96, 531–539. doi: 10.1152/japplphysiol.00656.2003 [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP, 2009. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci 10, 397–409. doi: 10.1038/nrn2647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanasriyakul WT, Wardwell J, McNeal N, Schultz R, Woodbury M, Dagner A, Cox M, Grippo AJ, 2018. Voluntary physical exercise protects against behavioral and endocrine reactivity to social and environmental stressors in the prairie vole. Soc. Neurosci 13, 602–615. doi: 10.1080/17470919.2017.1365761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner M, Helmich I, Machado S, Nardi A,E, Arias-Carrion O, Budde H, 2014. Effects of Exercise on Anxiety and Depression Disorders: Review of Meta- Analyses and Neurobiological Mechanisms. CNS Neurol. Disord. - Drug Targets 13, 1002–1014. [DOI] [PubMed] [Google Scholar]
- Weiss IC, Pryce CR, Jongen-Rêlo AL, Nanz-Bahr NI, Feldon J, 2004. Effect of social isolation on stress-related behavioural and neuroendocrine state in the rat. Behav. Brain Res 152, 279–295. doi: 10.1016/J.BBR.2003.10.015 [DOI] [PubMed] [Google Scholar]
- Young KA, Gobrogge KL, Liu Y, Wang Z, 2011. The neurobiology of pair bonding: Insights from a socially monogamous rodent. Front. Neuroendocrinol 32, 53–69. doi: 10.1016/J.YFRNE.2010.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T, Xu H, Wang W, Li S, Chen Z, Deng H, 2015. Determination of endogenous corticosterone in rodent’s blood, brain and hair with LC–APCI–MS/MS. J. Chromatogr. B 1002, 267–276. doi: 10.1016/J.JCHROMB.2015.08.035 [DOI] [PubMed] [Google Scholar]
- Zhai L, Zhang Y, Zhang D, 2015. Sedentary behaviour and the risk of depression: a meta-analysis. Br. J. Sports Med 49, 705–709. doi: 10.1136/bjsports-2014-093613 [DOI] [PubMed] [Google Scholar]
- Zschucke E, Renneberg B, Dimeo F, Wüstenberg T, Ströhle A, 2015. The stress-buffering effect of acute exercise: Evidence for HPA axis negative feedback. Psychoneuroendocrinology 51, 414–425. doi: 10.1016/j.psyneuen.2014.10.019 [DOI] [PubMed] [Google Scholar]


