Graphical abstract
Abstract
Attachment of viruses to cell-surface receptors is the initial step in infection. Many mammalian viruses have evolved to recognize receptors that are glycans on cell-surface glycoproteins or glycolipids. Although glycans are a ubiquitous component of mammalian cells, the types of terminal structures expressed vary among different cell-types and tissues, and even between comparable cells and tissues from different species, frequently leading to specific tissue and species tropisms as a direct consequence of glycan receptor recognition. Covering the majority of known virus families, this review provides an overview of mammalian viruses that use glycans as receptors, and their roles in determining in host recognition and tropism.
Current Opinion in Virology 2019, 34:117–129
This review comes from a themed issue on Viral immunology
Edited by Juan C de la Torre and John Teijaro
For a complete overview see the Issue and the Editorial
Available online 5th March 2019
https://doi.org/10.1016/j.coviro.2019.01.004
1879-6257/© 2019 Elsevier B.V. All rights reserved.
Introduction
For attachment to host cells, mammalian viruses recognize receptors that are conserved molecular features that mediate binding, and the subsequent internalization and replication of the virus. Since all mammalian cells display a dense network of glycans on glycoproteins and glycolipids, it is not surprising that many mammalian viruses have evolved to use glycans as host cell receptors [1,2••,3]. Indeed, over half of all mammalian virus families recognize glycans as receptors, including families with both protein capsids or membrane envelopes, and RNA-encoded and DNA-encoded genomes. In this review, we survey the major families of mammalian viruses that recognize host cell receptors that are glycans on cell-surface glycoproteins (N-linked and O-linked) and on glycolipids (Figure 1 ). For each virus family we cite recent literature documenting the specificity of virus recognition of glycan receptors and the role of receptor recognition in the biology of the virus. Although there is also clear evidence that some viruses recognize proteoglycans with extended glycan chains of up 200 sugar units (e.g. heparin sulfate, chondroitin sulfate) as receptors or co-receptors [3,4], the detailed specificity of virus–glycan recognition in these cases is typically less well understood, and thus we have considered this area outside the scope of our review. Also omitted from consideration here are examples of viruses with membrane-envelope glycoproteins whose own glycans are recognized by host glycan-binding proteins, including those on macrophages and/or dendritic cells that play an important role in virus tropism (e.g. for HIV) [5, 6, 7]. Finally, since the size of the review is limited, we also cite other excellent reviews with more in depth coverage for virus families with extensive literature on glycan receptors (e.g. orthomyxoviruses) [1,2••,8].
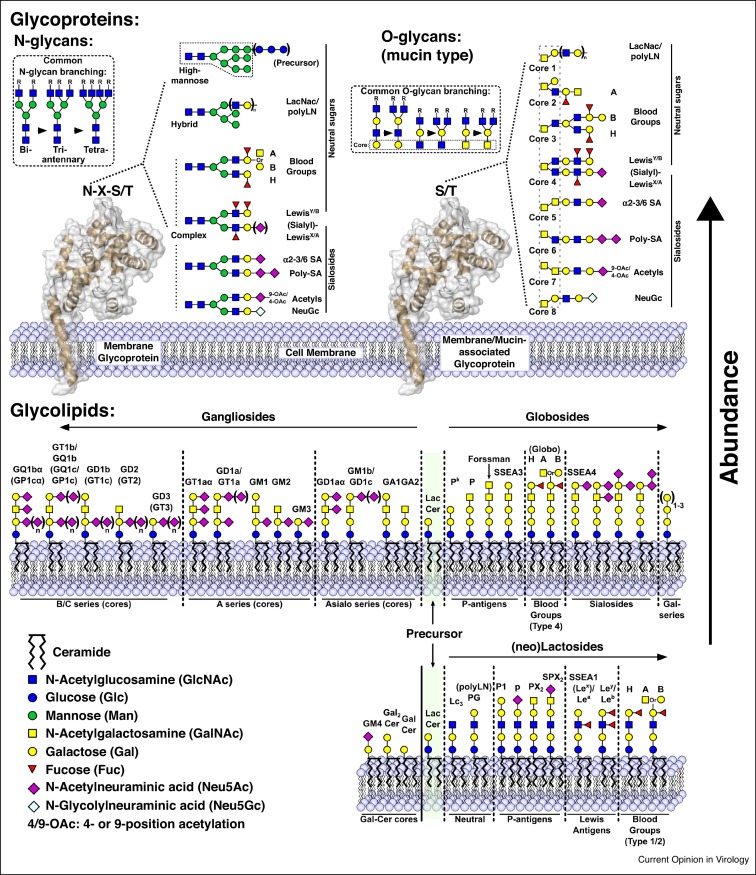
Figure 1.
Common glycan receptors for viruses found on mammalian host cells, including: protein N-linked glycans (upper left panel), protein O-linked glycans (upper right panel), and glycolipids (lower panel). N-glycans and O-glycans are assembled by combinations of specific glycosidases and glycosyl transferases from one or several shared cores. Combinations of branching (inset) and various terminal groups leads to huge variation and near-infinite possible receptor structures. Conversely, glycolipids maintain defined, and thus far fewer, individual structures, many of which are shown in the lower panel. For simplicity, linkage information has been omitted; however, common scaffolds include lactose (Galβ1-4Glc) and LacNAc (Galβ1-3/4GlcNAc; type 1/2), while terminal sialic acids are typically found in α2-3 (NeuAcα2-3Gal), α2-6 (NeuAcα2-6Gal), or α2-8 (NeuAcα2-8NeuAc) configurations.
Families of mammalian viruses that recognize cell-surface glycan receptors
Representative symbol structures of glycans of glycoproteins and glycolipids that are candidate receptors of mammalian viruses are illustrated in Figure 1 [9]. Glycans on cell-surface glycoproteins are N-linked to asparagine in the sequon Asn-X-Thr/Ser, or O-linked to surface Thr/Ser residues, while glycolipid glycans are attached to ceramide, a lipid that is directly embedded in the cell membrane. Although each glycan class differs in the core structures underlying their attachment to protein or lipid, all three classes of glycans often carry related or even identical terminal sequences. This is relevant since viruses typically recognize and coordinate only the terminal sugar(s) of glycan chains, typically within shallow pockets, as illustrated in Figure 2 . The most common terminal sequences recognized by mammalian viruses are listed in Table 1 , which compiles the glycan receptor specificities documented for each of the mammalian virus families discussed.
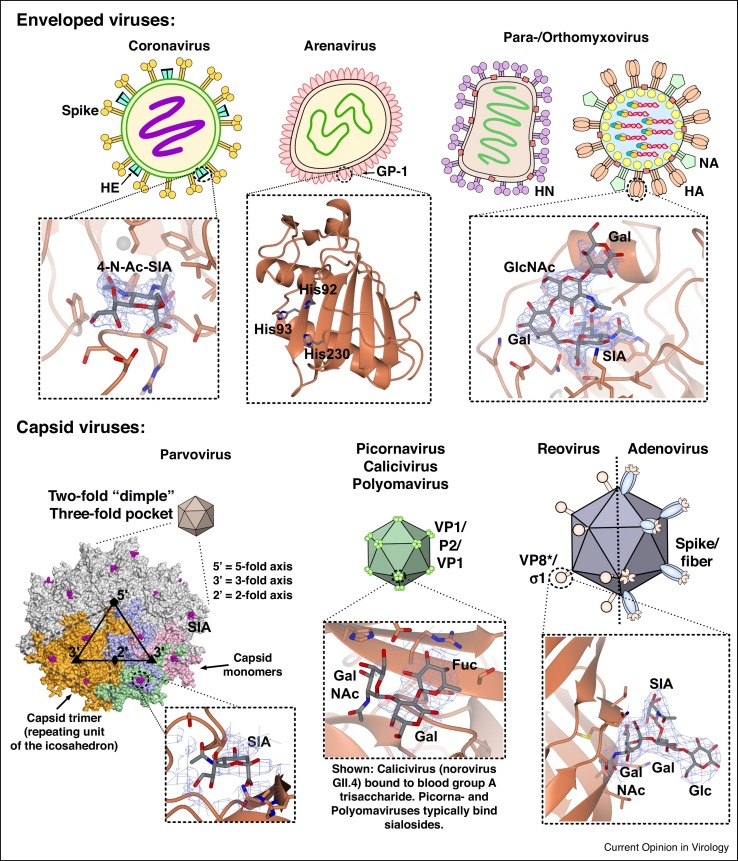
Figure 2.
Surface structures and glycan-binding profiles of mammalian viruses that use glycan receptors. Membrane-enveloped viruses (upper panels) typically coordinate glycans via a viral-surface glycoprotein, with examples shown for rat coronavirus (New Jersey strain) [133] bound to a non-hydrolysable 4-O-Ac-NeuAc analogue (PDB ID: 5JIF; left panel); the predicted αDG/LAMP1 binding domain of murine LFV [134] (PDB ID: 4ZJF; center panel); and LSTc bound to the 2009 pandemic H1N1 A/California/04/2009 [135] (PDB ID: 3UBE; right). Capsid viruses (lower panels) coordinate glycans either in shallow pockets directly on the outer shell, or within evolved glycan-binding domains that protrude from the surface. Examples are shown for an icosahedral pentamer of AAV1 [80•] which binds SA in pockets around the threefold axis (PDB ID: 5EGC; left panel); human norovirus (strain GII.4) [136] bound to blood group A trisaccharide (PDB ID: 3SLD; center); and the terminal σ1 domain of a type 1 (Lang) reovirus [100] bound to GM2 (PDB ID: 4GU3; right). For all panels, viral proteins are shown in coral; bound carbohydrates are shown as cylinders with carbons in grey, while electron density for bound ligands is depicted in blue mesh.
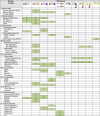
Table 1.
Diverse glycan specificities of mammalian viruses. Virus families are organized by the Baltimore classification, which is based on single/double stranded, RNA/DNA, positive/negative nucleic acid genome. Glycan epitopes shown at the top are found as terminal sequences on N-linked and O-linked glycans of glycoproteins and on glycans of glycolipids. For each virus family, boxes are highlighted for glycan epitopes recognized as potential receptor determinants. Additional specificity information and class of glycan recognized as receptors for individual viruses are found in the text. For symbol nomenclature see Figure 1
 |
Membrane-enveloped viruses
Orthomyxoviridae
Orthomyxoviruses are membrane-enveloped, segmented negative-sense RNA viruses divided in seven genera known as influenza virus A, B, C, and D, isavirus, quaranjavirus, and thoratovirus. Influenza viruses are the most thoroughly studied of all viruses that recognize glycans as cell-surface receptors, with extensive literature and reviews covering the roles of receptor specificity in host cell and species tropism [2••,8,10]. Humans are infected by influenza A, B, and C viruses that cause respiratory disease, the ‘flu’, which in severe cases can be fatal. Influenza A virus (IAV) utilizes sialic acid (SA)-containing glycans as host cell receptors, mediated by two surface glycoproteins, hemagglutinin (H/HA) that attaches the virus to the cell, and neuraminidase (N/NA) that cleaves SA and releases budding virus from the infected cell. IAVs circulate in aquatic and domestic birds and other zoonotic species (e.g. horses, pigs, dogs, cats, seals, bats), and are characterized by 18 serologically different hemagglutinins and 11 different neuraminidases. Although avian viruses are considered to be the progenitors of all human viruses, only three serotypes, H1N1, H2N2, and H3N2, have caused pandemics and became established as seasonal viruses in humans. Despite their avian origin, human viruses exhibit a preference for glycan receptors with terminal NeuAcα2-6Gal linkages (Figure 2), while avian viruses recognize receptors with the NeuAcα2-3Gal linkage, commonly referred to as ‘avian-type’ and ‘human-type’ receptor specificity [2••,8]. These receptor differences result from only two amino acid mutations in the receptor binding pocket of the hemagglutinin, and are widely believed to mediate species specificity and tissue tropism [11,12••]. Human viruses are believed to acquire human-type receptor specificity due to the abundant expression of α2-6-linked SAs on glycans of epithelial cells in the upper airway, a phenotype also exhibited in ferrets which are used as a model for respiratory droplet transmission of human influenza [13,14,15•]. With the advent of glycan microarrays a wealth of detailed information on influenza receptor specificity has emerged, providing insights into how receptor specificity has evolved during passage in humans, including bi-dentate binding to extended, branched N-glycan structures (Figure 3 ) [16,17•,18,19••,20], and how avian influenza strains that cause zoonotic infections in humans (e.g. H5N1, H7N9) might acquire human type receptor specificity that enables transmission in humans [21, 22, 23, 24, 25]. Human influenza B viruses bind SAs to infect cells and exhibit preference for ‘human type’ receptors, but have been shown to drift to avian type receptor specificity when passaged in eggs [26•]. In contrast to influenza A and B viruses, influenza C virus recognizes 9-O-Ac-NeuAc containing glycans as receptors. Attachment is mediated by a dual function hemagglutinin/esterase glycoprotein that both binds 9-O-Ac-NeuAc and can hydrolyze the O-acetyl group that destroys receptor binding and releases virus from the infected cell [27]. More recently, influenza D which infects bovine species has also been demonstrated to have a hemagglutinin/esterase protein that recognizes 9-O-Ac-NeuAc as receptors [28]. Finally, Isavirus is a salmon-infecting virus with a hemagglutinin/esterase that has specificity for 4-O-Ac-NeuAc [29], similar to some coronaviruses that are presumed to share a common ancestor [30••].
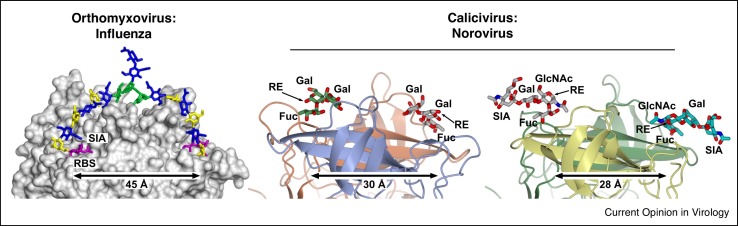
Figure 3.
Several virus species show potential for multivalent interactions with glycan receptors. Adaptation to human receptor specificity by influenza viruses (left panel) alters the receptor binding to mode to one where incoming glycans have potential to bivalently engage two monomeric receptor binding sites (RBSs) within a single HA trimer (figure adapted from glycan docking MD simulations reported in Peng, de Vries et al. [19••]). Similarly, the P2 glycan-binding domain of many caliciviruses typically present as dimers, likely sufficient to permit similar bivalent interactions. Structures depict P2 from human noroviruses VA387 bound to HBGA B trisaccharide [106•] (PDB ID: 2OBT; center panel) and VA207 bound to sialyl-Lewis X [118] (PDB ID: 3PVD). For influenza, the location of sialic acid and the RBS are marked, other sugar residues are colored according to CFG nomenclature. For calicivirus, all sugar residues are labelled, together with the location of respective sugar reducing ends (RE). Interestingly, the two different binding modes for HBGAs and Lewis antigens in caliciviruses lead to reducing-end sugars pointing in opposite directions within the receptor binding site.
Paramyxoviridae
Paramyxoviruses are membrane-enveloped viruses with a continuous, negative-sense single-stranded RNA genome. These viruses primarily infect airway epithelial cells and cause respiratory disease. The paramyxoviruses have a hemagglutinin-neuraminidase (HN) glycoprotein that both binds to SA-containing receptors on host cells, and can hydrolyze SAs to release budding virus from the infected cell ( Figure 2) [31]. Several viruses in this family have been studied for their specificity for glycan receptors, including Newcastle Disease (NDV), Sendai, mumps, and parainfluenza viruses 1,3,5 (hPIV-1, 3, 5) [31, 32, 33, 34, 35]. In general, paramyxoviruses recognize glycans with the terminal NeuAcα2-3Gal linkage, and in contrast to human influenza viruses do not exhibit binding to glycans with the terminal NeuAcα2-6Gal linkage (Table 1). The hPIV viruses show preference for the sequence NeuAcα2-3Galβ1-4GlcNAc sequence that often terminates N-linked glycans [32,33], but can also occur on O-glycans and glycolipids [36]. hPIV-1 and hPIV-3 show tolerance for substitutions of 6-SO3 and Fucα1-3 on the Gal and GlcNAc moieties, respectively, but only hPIV-1 binds to glycans with a terminal sequence Neu5Acα2-3(GalNAcβ1-4)Gal [32,33]. Sendai virus shows preferred binding to the terminal sequence Neu5Acα2-3Galβ1-3GalNAc on gangliosides and O-linked glycans [34, 35, 36, 37], and shows high-affinity binding to gangliosides terminating with NeuAcα2-8NeuAcα2-3Galβ1-3GalNAc [35]. For hPMV-1 there is evidence for a second SA-binding site that is exposed upon occupancy of the primary receptor site, and has similar, but not identical, binding specificity [32]. With regards to cell and tissue tropism, while epithelial cells of the upper airway have mainly α2-6 linked sialic acids, the α2-3-linked receptors of paramyxoviruses are also found to a lesser extent, and are enriched on epithelial cells deeper in the lung [14,18].
Coronaviridae
Coronaviruses (CoVs) are membrane-enveloped, single-stranded positive-sense RNA viruses that are divided into four genera (α, β, γ and δ). Coronaviruses cause respiratory or gastrointestinal infections, and many are known to use glycans as receptors [1,38,39]. CoVs typically contain two surface glycoproteins: the spike protein that is primarily responsible for attachment and membrane-fusion, and a hemagglutinin-esterase that can also participate in attachment [40]. While CoV spike proteins generally bind protein receptors, either of the two surface glycoproteins can bind glycans as primary or co-receptors. Among α-coronaviruses that cause gastrointestinal disease in cats (FCoV) and pigs (TGEV, PED), the spike protein binds to an aminopeptidase N, but uses SAs (NeuAc or NeuGc) as secondary receptors [41]. Some human β-coronaviruses (OC43, HKU1) contain a spike recognizing 9-O-Ac-NeuAc as a primary receptor on epithelial cells of the respiratory tract [42]. Other β-coronaviruses, and the torovirinea subfamily, both recognize and cleave 9-O-Ac-NeuAc, 4-O-Ac-NeuAc, or other O-Ac-SAs via hemagglutinin-esterase (Figure 2) [30••,43,44•]. The spike protein of the zoonotic MERS strain recognizes dipeptidyl peptidase 4 (DPP4) as a primary receptor, and NeuAc2-3Gal-containing glycans as co-receptors [45,46], similar to α-coronaviruses. γ-coronaviruses that infect the avian respiratory tract have spike proteins that bind Neu5Ac2-3Gal structures [47], while some gastrointestinal counterparts specifically bind to non-sialylated complex N-glycans with extended LacNAc (Galβ1-4GlcNAcβ3) repeats [48]. Finally, while there is limited information on δ-coronaviruses, porcine δ-coronavirus has been reported to bind a yet unidentified glycan receptor [49]. Clearly, coronaviruses have adapted to diverse modes of using glycans as receptors or co-receptors for interactions with their hosts.
Picornaviridae
Picornaviruses are a large family of non-enveloped viruses containing a single positive strand RNA genome (∼7.5 kb) within a 30 nm icosahedral capsid. Picornaviruses primarily infect enterocytes and the respiratory tract in mammals and birds, and comprise 34 genera, of which at least four have members that interact with SA-containing glycans [1,50]. A number of human (e.g. Coxsackie A24) enteroviruses have been reported to bind glycan receptors with terminal NeuAcα2-6Gal and/or NeuAcα2-3Gal linkages [51,52•]. In the case of human enterovirus 68, there is good evidence for the role of NeuAcα2-6Gal receptors on N-linked glycans as the functional receptor [52•,53]. In contrast, infection by the porcine sapelovirus, also an enterovirus, is believed to be mediated by ganglioside receptors such as GD1a [54]. Murine encephalomyocarditis virus binds to NeuAcα2-3Gal receptors on N-glycans, while high virulency viruses have adapted to a proteinacious receptor [55].
Arenaviridae
Arenaviruses are bi-segmented negative-sense RNA viruses that encode just four proteins. The family is subdivided into three genera, and of these, only Mammarenaviruses use glycans as receptors [56,57]. This genus is further subdivided into new-world and old-world viruses, the latter notably including Lassa fever virus (LFV) and lymphocytic choriomeningitis virus (LCMV) that cause hemorrhagic fever and febrile illness in humans, respectively [58]. Both of these viruses bind cells via envelope GP-1 (Figure 2), and rely on the highly glycosylated cell-surface glycoprotein α-dystroglycan as a receptor [57,59]. α-Dystroglycan contains an unusual O-mannose linked glycan structure that has long polymers comprising xylose (Xyl) and glucuronic acid (GlcU) in a repeating disaccharide sequence (-3-Xylα1-3-GlcUβ1-) produced by a bifunctional glycosyltransferase called Large [60, 61, 62, 63]. LFV and LCMV bind specifically to this repeat sequence, a shared property with laminin that also binds α-dystroglycan to link the surface of host cells to the extracellular matrix [60,62]. Upon endocytosis, LFV engages a secondary α2-3-terminal sialoglycan receptor (synthesized specifically by the sialyltransferase ST3Gal4 [64••]) on the lysosomal protein LAMP, which is essential for functional infection. This remarkable two-step process, involving interactions with two different glycans on both cell-surface and intracellular receptors, reflects a long evolutionary relationship of virus-host interaction mediated by glycan recognition.
Protein capsid viruses
Polyomaviridae
Polyomaviruses comprise a comparatively small family of non-enveloped icosahedral viruses with double stranded DNA genomes. Over 70 species of Polyomaviridae are known, divided into four genera: α-polyomavirus, β-polyomavirus, γ-polyomavirus, and δ-polyomavirus, with 14 human types present in all except γ-polyomavirus. Although systemic polyomavirus infections in humans are common, they are most often asymptomatic, except in immuno-compromised patients where symptoms can be severe, potentially leading to neoplasms and cancer [65,66]. The polyomavirus outer capsid is composed of pentamers of the VP1 coat protein, which houses the glycan-binding domain [65,67] where most species bind preferentially to sialoglycans [66,67]. Mouse polyomavirus (MPyV) recognizes the NeuAcα2-3Galβ1-3GalNAc sequence found in both gangliosides (e.g. GD1a) and glycoproteins (O-linked), although the precise contribution of these receptors in natural infections are still under investigation [68••,69•,70]. Some strains carrying mutations at position 91 in VP1 bind receptors with an additional NeuAc in the sequence NeuAcα2-3Galβ1-3(NeuAcα2-6)GalNAc, which is associated with a decreased tumorigenic phenotype [67]. Both human BK and JC polyomaviruses (HPyV-1 and HPyV-2, respectively) bind NeuAcα2-3Gal and NeuAcα2-6Gal sequences present on various ganglioside and glycoprotein glycans present on oligodendrocytes, astrocytes, urogenital tissue, lymphocytes and renal cells infected by these viruses [66,67,71•]. In addition, JC requires the proteinacious serotonin receptor 5HT2A for full infectivity [72]. Relative to the HPyVs, SV40 is highly specific for the GM1 ganglioside [73,74•], with NeuGc instead of NeuAc, consistent with its non-human host tropism [73].
Parvoviridae
Parvoviruses are a large family of compact, non-enveloped icosahedral viruses with single-stranded DNA genomes, subdivided into Densovirinae (invertebrates) and Parvovirinae (mammalian hosts). The parvovirinae comprise eight genera, from which three well-studied representatives bind glycan receptors: Protoparvovirus (canine parvovirus (CPV), feline panleukopenia virus (FPV), and minute virus of mice (MVM)), Erythroparvovirus (parvovirus B19 (B19V)), and Dependoparvovirus (adeno-associated virus (AAVs 1–11)). Primary receptors for CPV and FPV are NeuGcα2-3Gal terminated sialosides [75••,76] on GI epithelial cells, while transferrin is required as a specific co-receptor [77]. MVM infects connective tissues and exhibits specificity for glycan receptors with the terminal sequences NeuAcα2-3Galβ1-4(±Fucα1-3)GlcNAc and NeuAcα2-8α2-8NeuAcα2-3(8)Gal(NeuAc) [78]. These viruses bind SA in a conserved ‘dimple’ close to the capsid twofold symmetry axis (Figure 2) [76,79]. AAVs show wider receptor diversity, serotypes AAV1 and AAV4-6 all bind SA on a range of cell types [76,80•,81]; AAV9 binds terminal galactose on an unknown N-glycan [82]; and AAVs 2, 3, 6, and 13 are all specific for heparan sulfate proteoglycans [76]. Essential co-receptors for AAVs and B19V include: Ku80 autoantigen, α5β1 integrin, αvβ1 integrin and growth receptors [83,84]. Finally, human B19V binds globoside glycolipids of the P blood-group antigen series, particularly globotetraose (Gb4) [84,85••], on erythrocytes and hematopoietic progenitors. Gb4 interaction is thought to induce a conformational change in the B19V capsid, enabling binding to a co-receptor for viral entry [86]. In contrast to the protoparvoviruses, the AAVs and B19V bind glycan receptors in a pocket surrounding the icosahedral threefold axes [76,80•] (Figure 2), and have distinct host/tissue tropisms [81].
Reoviridae
The Reoviridae are a large family of double-stranded RNA viruses, with intricate, multilayered icosahedral capsids, divided into two subfamilies (Sedoreovirinae and Spinareovirinae) with over 15 genera. All viruses share a similar 120-subunit inner shell, with variable outer layers, giving distinct surface structures, antigenicities, and even naming conventions. The two most studied genera from respective subfamiles, Rotavirus and Orthoreovirus (referred to as ‘reovirus’), feature large spike proteins projecting from the icosahedral fivefolds [87,88•], whose terminal domains (VP8* in rotavirus [89] and δ1 in reovirus [88•,90]) bind glycans (Figure 2). Reoviridae also rely on specific protein cofactors for full entry/infectivity, including: α2β1, αxβ2, and α4β1 integrins (rotaviruses [84]); JAM-A/JAM-1 and Nogo receptor 1 (reoviruses [91,92]). Most animal rotaviruses bind terminal NeuAcα2-3Gal/GalNAc sequences as found on GM3 or GD1a gangliosides [93••,94]; however, some human strains, originally thought to be ‘SA-independent’, have specificity for internal SA such as those on gangliosides GM2 or GM1 (Figure 1, Figure 2) [93••]. Recent studies on a subset of human rotaviruses have revealed glycan specificities without SA such as human blood groups [95••,96•,97,98] and fucosylated human milk oligosaccharides that are proposed to be decoy receptors to prevent GI infections in infants [98,99]. While rotaviruses cause gastrointestinal disease, reoviruses cause neurological diseases [1]. Type 1 reoviruses (RV1) that infect ependymal cells and cause hydrocephalus bind GM2 [100,101•], whereas RV3s that infect neurons and cause encephalitis bind GM3 [88•]. These are potentially relevant to natural infections since gangliosides are abundant in brain tissues. Importantly, mutants of these viruses that have reduced binding to glycan receptors exhibit dramatically reduced disease severity in vivo [102,103].
Caliciviridae
Caliciviruses are a family of small, non-enveloped icosahedral viruses with single-stranded positive-sense RNA genomes, subdivided into five major genera: Norovirus (infecting humans, pigs and mice), Sapovirus (human and swine), Vesivirus (predominantly feline calicivirus (FCV)), Lagovirus (rabbit hemorrhagic disease virus (RHDV)), and Nebovirus (bovine Newbury-1). Caliciviruses are broadly glycan-dependent for attachment and cell entry [84], binding via the highly antigenic region (P2) of the viral coat P-domain [104,105]. Three major groups of glycoconjugates are targeted as receptors; human [106•], bovine [107], and canine [108] noroviruses, which all infect gastrointestinal epithelia, as well as RHDV [109•], which target hepatocytes all utilize human blood group associated antigens (HBGAs) as cell-surface receptors (Figure 2); while GI-specific murine norovirus (MNV) and porcine sapovirus bind sialic acids on O-glycans and some gangliosides [110•,111•]; and FCV which causes respiratory disease in cats, binds SA present on N-glycans on unknown glycoprotein receptor(s) [112]. There is strong evidence for that O-glycans are functional receptors for sapovirus [110•]. Certain animal viruses require protein coreceptors, includeing CD300lf/ld on murine enteric epithelia (MNV [113,114]), and respiratory JAM-1 (FCV [115] and newly identified Hom-1 calicivirus [116]). Underlying these glycan specificities in many cases, is a core requirement for recognition of fucose, with activity of FUT genes involved in HBGA synthesis conclusively shown to be a risk-factor for norovirus infection [117•]. Landmark structural [105,106•,118,119] and STD-NMR [109•,120] studies have revealed both strong fucose-dependent binding, and alternate binding modes for HBGAs (secretor-type Fucα1-2Gal modifications) and Lewis antigens (Fucα1-3/4GlcNAc), dependent on different mutations/genotypes within the P2 domain. It is interesting to note that close localization of P2 dimers could allow bivalent interactions with branched glycans similar to that for the influenza hemagglutinin trimer (see Figure 3). The ubiquity of these carbohydrate epitopes among mammalian species, and rapidity with which novel P2 variants appear to arise has led to concern for potential transmission of zoonotic caliciviruses to humans, and this area remains a major research focus [121,122•].
Adenoviridae
Adenoviruses are a smaller family of non-enveloped icosahedral viruses with double-stranded DNA genomes, spread across five genera: Atadenovirus, Aviadenovirus, Ichtadenovirus, Mastadenovirus, and Siadenovirus. Human and mammalian adenoviruses are all within the Mastadenovirus genus, and principally infect mucosal tissue and epithelials cells, including the respiratory and GI tracts. The 57 human adenoviruses (HAdV1-57) are subdivided into 7 species (A–G). HAdVs bind host receptors via a large spike or ‘fiber protein’ extending from the protein shell at the icosahedral fivefold axes (Figure 2). Most, including HAdVs-A, B, C–F bind protein receptors and are glycan independent [123, 124, 125]. However, select members of HAdV-D (HAdV-8, 19a, and 37) that have tropism for the human eye and cause severe epidemic keratoconjunctivitis [126], are dependent on NeuAcα2-3Gal terminated glycans for binding and infectivity [127,128]. While HAdV-37 exhibits specifically for the glycan portion of GD1a, evidence suggests that the virus binds to glycoprotein glycans [129•]. Recently, a prototypical member of the HAdV-G group that causes human gastroenteritis, HAdV-52, has been shown to preferentially recognize poly sialic acid (NeuAcα2-8NeuAcα2-8) sequences [130••]. Finally, glycan-binding specificity linked to tropisms of animal AdVs that target the GI and respiratory tissues include bovine BAd3 (NeuAcα2-3- and NeuAcα2-6-specific [131]) and porcine PAd4 (lactose/LacNAc) [132].
Summary and future directions
It is remarkable that most families of mammalian viruses either predominantly recognize glycans as receptors, or have subfamilies or representative species that recognize them. Within these contexts, glycans can function either directly as sole, primary determinants of infection (tropism), likely via highly specific and higher avidity interactions with viral surface proteins; or, potentially more commonly, as primary co-receptors, functioning in tandem with host membrane proteins to accumulate virus particles on the cell surface via high-valency, but low-specificity interactions, where tropism is determined by presence of both glycan and proteinaceous receptors together. These similar, yet quite distinct models, form the basis for a system where apparently overlapping receptor specificities (e.g. for terminal SAs or HBGAs) still result in highly individualized interactions, leading to distinct cell, tissue, and host tropisms, symptoms, and disease progression. Nonetheless, while our understanding of these interactions has advanced significantly over recent decades, there is still much to be learned about how virus specificity for glycans matches the host cell expression of glycan receptors, and the degree to which this impacts virus tropism. Much of this accumulating knowledge is still yet to progress into treatments or therapeutics capable of preventing, rather than simply treating viral infections. With continually improving tools to look at the specificity of virus–glycan interactions, it is likely that an even greater understanding of the roles of glycan receptors in virus tropism and species specificity, potentially translating to future advances in the clinic, will soon be forthcoming.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
A.J.T. is a recipient of an EMBO Long-term Fellowship (EMBO ALTF 963-2014). R.P.dV. is a recipient of a VENI grant from the Netherlands Organization for Scientific Research (NWO). This work was funded in part by National Institutes of Health grant R01 AI114730 and the Kwang Hua Educational Foundation to J.C.P.
References
- 1.Stroh L.J., Stehle T. Glycan engagement by viruses: receptor switches and specificity. Annu Rev Virol. 2014;1:285–306. doi: 10.1146/annurev-virology-031413-085417. [DOI] [PubMed] [Google Scholar]
- 2••.de Graaf M., Fouchier R.A. Role of receptor binding specificity in influenza A virus transmission and pathogenesis. EMBO J. 2014;33:823–841. doi: 10.1002/embj.201387442. [DOI] [PMC free article] [PubMed] [Google Scholar]; Comprehensive review on roles of glycan specificity in tissue and species tropism of influenza virus.
- 3.Olofsson S., Bergstrom T. Glycoconjugate glycans as viral receptors. Ann Med. 2005;37:154–172. doi: 10.1080/07853890510007340. [DOI] [PubMed] [Google Scholar]
- 4.Horvath C.A., Boulet G.A., Renoux V.M., Delvenne P.O., Bogers J.P. Mechanisms of cell entry by human papillomaviruses: an overview. Virol J. 2010;7:11. doi: 10.1186/1743-422X-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akiyama H., Ramirez N.G., Gudheti M.V., Gummuluru S. CD169-mediated trafficking of HIV to plasma membrane invaginations in dendritic cells attenuates efficacy of anti-gp120 broadly neutralizing antibodies. PLoS Pathog. 2015;11 doi: 10.1371/journal.ppat.1004751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erikson E., Wratil P.R., Frank M., Ambiel I., Pahnke K., Pino M., Azadi P., Izquierdo-Useros N., Martinez-Picado J., Meier C., et al. Mouse siglec-1 mediates trans-infection of surface-bound murine leukemia virus in a sialic acid N-acyl side chain-dependent manner. J Biol Chem. 2015;290:27345–27359. doi: 10.1074/jbc.M115.681338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geijtenbeek T.B., van Kooyk Y. DC-SIGN: a novel HIV receptor on DCs that mediates HIV-1 transmission. Curr Top Microbiol Immunol. 2003;276:31–54. doi: 10.1007/978-3-662-06508-2_2. [DOI] [PubMed] [Google Scholar]
- 8.Paulson J.C., de Vries R.P. H5N1 receptor specificity as a factor in pandemic risk. Virus Res. 2013;178:99–113. doi: 10.1016/j.virusres.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varki A., Kornfeld S., et al. In: Essentials of Glycobiology. Varki A., Cummings R.D., Esko J.D., Stanley P., Hart G.W., Aebi M., Darvill A.G., Kinoshita T., Packer N.H., editors. 2015. Historical background and overview; pp. 1–18. [Google Scholar]
- 10.Byrd-Leotis L., Cummings R.D., Steinhauer D.A. The interplay between the host receptor and influenza virus hemagglutinin and neuraminidase. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18071541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gambaryan A.S., Matrosovich T.Y., Boravleva E.Y., Lomakina N.F., Yamnikova S.S., Tuzikov A.B., Pazynina G.V., Bovin N.V., Fouchier R.A.M., Klenk H.D., et al. Receptor-binding properties of influenza viruses isolated from gulls. Virology. 2018;522:37–45. doi: 10.1016/j.virol.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 12••.Tumpey T.M., Maines T.R., Van Hoeven N., Glaser L., Solorzano A., Pappas C., Cox N.J., Swayne D.E., Palese P., Katz J.M., et al. A two-amino acid change in the hemagglutinin of the 1918 influenza virus abolishes transmission. Science. 2007;315:655–659. doi: 10.1126/science.1136212. [DOI] [PubMed] [Google Scholar]; Elegant demonstration that human-type receptor specificity is critical for transmission of influenza virus in a ferret model.
- 13.Jia N., Barclay W.S., Roberts K., Yen H.L., Chan R.W., Lam A.K., Air G., Peiris J.S., Dell A., Nicholls J.M., et al. Glycomic characterization of respiratory tract tissues of ferrets: implications for its use in influenza virus infection studies. J Biol Chem. 2014;289:28489–28504. doi: 10.1074/jbc.M114.588541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shinya K., Ebina M., Yamada S., Ono M., Kasai N., Kawaoka Y. Avian flu: influenza virus receptors in the human airway. Nature. 2006;440:435–436. doi: 10.1038/440435a. [DOI] [PubMed] [Google Scholar]
- 15•.Walther T., Karamanska R., Chan R.W., Chan M.C., Jia N., Air G., Hopton C., Wong M.P., Dell A., Malik Peiris J.S., et al. Glycomic analysis of human respiratory tract tissues and correlation with influenza virus infection. PLoS Pathog. 2013;9 doi: 10.1371/journal.ppat.1003223. [DOI] [PMC free article] [PubMed] [Google Scholar]; Demonstration of extended N-linked glycans in human respiratory tissues.
- 16.Childs R.A., Palma A.S., Wharton S., Matrosovich T., Liu Y., Chai W., Campanero-Rhodes M.A., Zhang Y., Eickmann M., Kiso M., et al. Receptor-binding specificity of pandemic influenza A (H1N1) 2009 virus determined by carbohydrate microarray. Nat Biotechnol. 2009;27:797–799. doi: 10.1038/nbt0909-797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17•.Ji Y., White Y.J., Hadden J.A., Grant O.C., Woods R.J. New insights into influenza A specificity: an evolution of paradigms. Curr Opin Struct Biol. 2017;44:219–231. doi: 10.1016/j.sbi.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]; Overview of glycan orientation in the receptor-binding site of influenza hemagglutinins and how it relates to specificity.
- 18.Nicholls J.M., Bourne A.J., Chen H., Guan Y., Peiris J.S. Sialic acid receptor detection in the human respiratory tract: evidence for widespread distribution of potential binding sites for human and avian influenza viruses. Respir Res. 2007;8:73. doi: 10.1186/1465-9921-8-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19••.Peng W., de Vries R.P., Grant O.C., Thompson A.J., McBride R., Tsogtbaatar B., Lee P.S., Razi N., Wilson I.A., Woods R.J., et al. Recent H3N2 viruses have evolved specificity for extended, branched human-type receptors, conferring potential for increased avidity. Cell Host Microbe. 2017;21:23–34. doi: 10.1016/j.chom.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]; Demonstration of the evolution of the specificity of H3N2 influenza for human type receptor specificity, and potential for bi-dentate binding of N-linked glycans to the same HA trimer.
- 20.Stevens J., Blixt O., Paulson J.C., Wilson I.A. Glycan microarray technologies: tools to survey host specificity of influenza viruses. Nat Rev Microbiol. 2006;4:857–864. doi: 10.1038/nrmicro1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Vries R.P., Peng W., Grant O.C., Thompson A.J., Zhu X., Bouwman K.M., de la Pena A.T.T., van Breemen M.J., Ambepitiya Wickramasinghe I.N., de Haan C.A.M., et al. Three mutations switch H7N9 influenza to human-type receptor specificity. PLoS Pathog. 2017;13 doi: 10.1371/journal.ppat.1006390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herfst S., Schrauwen E.J., Linster M., Chutinimitkul S., de Wit E., Munster V.J., Sorrell E.M., Bestebroer T.M., Burke D.F., Smith D.J., et al. Airborne transmission of influenza A/H5N1 virus between ferrets. Science. 2012;336:1534–1541. doi: 10.1126/science.1213362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imai M., Watanabe T., Hatta M., Das S.C., Ozawa M., Shinya K., Zhong G., Hanson A., Katsura H., Watanabe S., et al. Experimental adaptation of an influenza H5 HA confers respiratory droplet transmission to a reassortant H5 HA/H1N1 virus in ferrets. Nature. 2012;486:420–428. doi: 10.1038/nature10831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watanabe T., Kiso M., Fukuyama S., Nakajima N., Imai M., Yamada S., Murakami S., Yamayoshi S., Iwatsuki-Horimoto K., Sakoda Y., et al. Characterization of H7N9 influenza A viruses isolated from humans. Nature. 2013;501:551–555. doi: 10.1038/nature12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu R., de Vries R.P., Zhu X., Nycholat C.M., McBride R., Yu W., Paulson J.C., Wilson I.A. Preferential recognition of avian-like receptors in human influenza A H7N9 viruses. Science. 2013;342:1230–1235. doi: 10.1126/science.1243761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26•.Velkov T. The specificity of the influenza B virus hemagglutinin receptor binding pocket: what does it bind to? J Mol Recognit. 2013;26:439–449. doi: 10.1002/jmr.2293. [DOI] [PubMed] [Google Scholar]; Excellent review of glycan receptors of influenza B viruses.
- 27.Rogers G.N., Herrler G., Paulson J.C., Klenk H.D. Influenza C virus uses 9-O-acetyl-N-acetylneuraminic acid as a high affinity receptor determinant for attachment to cells. J Biol Chem. 1986;261:5947–5951. [PubMed] [Google Scholar]
- 28.Song H., Qi J., Khedri Z., Diaz S., Yu H., Chen X., Varki A., Shi Y., Gao G.F. An open receptor-binding cavity of hemagglutinin-esterase-fusion glycoprotein from newly-identified influenza D virus: basis for its broad cell tropism. PLoS Pathog. 2016;12 doi: 10.1371/journal.ppat.1005411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hellebo A., Vilas U., Falk K., Vlasak R. Infectious salmon anemia virus specifically binds to and hydrolyzes 4-O-acetylated sialic acids. J Virol. 2004;78:3055–3062. doi: 10.1128/JVI.78.6.3055-3062.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30••.Zeng Q., Langereis M.A., van Vliet A.L., Huizinga E.G., de Groot R.J. Structure of coronavirus hemagglutinin-esterase offers insight into corona and influenza virus evolution. Proc Natl Acad Sci U S A. 2008;105:9065–9069. doi: 10.1073/pnas.0800502105. [DOI] [PMC free article] [PubMed] [Google Scholar]; An important paper on the structural recognition of sialic acid analogs by both corona and influenza virus.
- 31.Bowden T.A., Crispin M., Jones E.Y., Stuart D.I. Shared paramyxoviral glycoprotein architecture is adapted for diverse attachment strategies. Biochem Soc Trans. 2010;38:1349–1355. doi: 10.1042/BST0381349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alymova I.V., Portner A., Mishin V.P., McCullers J.A., Freiden P., Taylor G.L. Receptor-binding specificity of the human parainfluenza virus type 1 hemagglutinin-neuraminidase glycoprotein. Glycobiology. 2012;22:174–180. doi: 10.1093/glycob/cwr112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amonsen M., Smith D.F., Cummings R.D., Air G.M. Human parainfluenza viruses hPIV1 and hPIV3 bind oligosaccharides with α2-3-linked sialic acids that are distinct from those bound by H5 avian influenza virus hemagglutinin. J Virol. 2007;81:8341–8345. doi: 10.1128/JVI.00718-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferreira L., Villar E., Munoz-Barroso I. Gangliosides and N-glycoproteins function as newcastle disease virus receptors. Int J Biochem Cell Biol. 2004;36:2344–2356. doi: 10.1016/j.biocel.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 35.Markwell M.A., Svennerholm L., Paulson J.C. Specific gangliosides function as host cell receptors for sendai virus. Proc Natl Acad Sci U S A. 1981;78:5406–5410. doi: 10.1073/pnas.78.9.5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suzuki Y., Suzuki T., Matsunaga M., Matsumoto M. Gangliosides as paramyxovirus receptor. Structural requirement of sialo-oligosaccharides in receptors for hemagglutinating virus of Japan (Sendai virus) and Newcastle disease virus. J Biochem. 1985;97:1189–1199. doi: 10.1093/oxfordjournals.jbchem.a135164. [DOI] [PubMed] [Google Scholar]
- 37.Markwell M.A., Paulson J.C. Sendai virus utilizes specific sialyloligosaccharides as host cell receptor determinants. Proc Natl Acad Sci U S A. 1980;77:5693–5697. doi: 10.1073/pnas.77.10.5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwegmann-Wessels C., Herrler G. Sialic acids as receptor determinants for coronaviruses. Glycoconj J. 2006;23:51–58. doi: 10.1007/s10719-006-5437-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hulswit R.J., de Haan C.A., Bosch B.J. Coronavirus spike protein and tropism changes. Adv Virus Res. 2016;96:29–57. doi: 10.1016/bs.aivir.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Groot R.J. Structure, function and evolution of the hemagglutinin-esterase proteins of corona- and toroviruses. Glycoconj J. 2006;23:59–72. doi: 10.1007/s10719-006-5438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu C., Tang J., Ma Y., Liang X., Yang Y., Peng G., Qi Q., Jiang S., Li J., Du L., et al. Receptor usage and cell entry of porcine epidemic diarrhea coronavirus. J Virol. 2015;89:6121–6125. doi: 10.1128/JVI.00430-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang X., Dong W., Milewska A., Golda A., Qi Y., Zhu Q.K., Marasco W.A., Baric R.S., Sims A.C., Pyrc K., et al. Human coronavirus HKU1 spike protein uses O-Acetylated sialic acid as an attachment receptor determinant and employs hemagglutinin-esterase protein as a receptor-destroying enzyme. J Virol. 2015;89:7202–7213. doi: 10.1128/JVI.00854-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Langereis M.A., Zeng Q., Gerwig G.J., Frey B., von Itzstein M., Kamerling J.P., de Groot R.J., Huizinga E.G. Structural basis for ligand and substrate recognition by torovirus hemagglutinin esterases. Proc Natl Acad Sci U S A. 2009;106:15897–15902. doi: 10.1073/pnas.0904266106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44•.Langereis M.A., Bakkers M.J., Deng L., Padler-Karavani V., Vervoort S.J., Hulswit R.J., van Vliet A.L., Gerwig G.J., de Poot S.A., Boot W., et al. Complexity and diversity of the mammalian sialome revealed by nidovirus virolectins. Cell Rep. 2015;11:1966–1978. doi: 10.1016/j.celrep.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]; A demonstration of strict glycan specificity of viral glycan proteins and their possible use as lectin tools in glycobiology.
- 45.Li W., Hulswit R.J.G., Widjaja I., Raj V.S., McBride R., Peng W., Widagdo W., Tortorici M.A., van Dieren B., Lang Y., et al. Identification of sialic acid-binding function for the Middle East respiratory syndrome coronavirus spike glycoprotein. Proc Natl Acad Sci U S A. 2017;114:E8508–E8517. doi: 10.1073/pnas.1712592114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raj V.S., Mou H., Smits S.L., Dekkers D.H., Muller M.A., Dijkman R., Muth D., Demmers J.A., Zaki A., Fouchier R.A., et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature. 2013;495:251–254. doi: 10.1038/nature12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schultze B., Enjuanes L., Cavanagh D., Herrler G. N-acetylneuraminic acid plays a critical role for the haemagglutinating activity of avian infectious bronchitis virus and porcine transmissible gastroenteritis virus. Adv Exp Med Biol. 1993;342:305–310. doi: 10.1007/978-1-4615-2996-5_47. [DOI] [PubMed] [Google Scholar]
- 48.Ambepitiya Wickramasinghe I.N., de Vries R.P., Weerts E.A., van Beurden S.J., Peng W., McBride R., Ducatez M., Guy J., Brown P., Eterradossi N., et al. Novel receptor specificity of avian gammacoronaviruses that cause enteritis. J Virol. 2015;89:8783–8792. doi: 10.1128/JVI.00745-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shang J., Zheng Y., Yang Y., Liu C., Geng Q., Tai W., Du L., Zhou Y., Zhang W., Li F. Cryo-electron microscopy structure of porcine deltacoronavirus spike protein in the prefusion state. J Virol. 2018;92 doi: 10.1128/JVI.01556-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.ICTV . 2017. Picornaviridea. [Google Scholar]
- 51.Zocher G., Mistry N., Frank M., Hahnlein-Schick I., Ekstrom J.O., Arnberg N., Stehle T. A sialic acid binding site in a human picornavirus. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52•.Imamura T., Okamoto M., Nakakita S., Suzuki A., Saito M., Tamaki R., Lupisan S., Roy C.N., Hiramatsu H., Sugawara K.E., et al. Antigenic and receptor binding properties of enterovirus 68. J Virol. 2014;88:2374–2384. doi: 10.1128/JVI.03070-13. [DOI] [PMC free article] [PubMed] [Google Scholar]; Excellent example of using glycan arrays to identify-specific receptors for a picornavirus.
- 53.Liu Y., Sheng J., Baggen J., Meng G., Xiao C., Thibaut H.J., van Kuppeveld F.J., Rossmann M.G. Sialic acid-dependent cell entry of human enterovirus D68. Nat Commun. 2015;6 doi: 10.1038/ncomms9865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim D.S., Son K.Y., Koo K.M., Kim J.Y., Alfajaro M.M., Park J.G., Hosmillo M., Soliman M., Baek Y.B., Cho E.H., et al. Porcine sapelovirus uses α2,3-linked sialic acid on GD1a ganglioside as a receptor. J Virol. 2016;90:4067–4077. doi: 10.1128/JVI.02449-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lipton H.L., Kumar A.S., Hertzler S., Reddi H.V. Differential usage of carbohydrate co-receptors influences cellular tropism of Theiler’s murine encephalomyelitis virus infection of the central nervous system. Glycoconj J. 2006;23:39–49. doi: 10.1007/s10719-006-5436-x. [DOI] [PubMed] [Google Scholar]
- 56.Raaben M., Jae L.T., Herbert A.S., Kuehne A.I., Stubbs S.H., Chou Y.Y., Blomen V.A., Kirchhausen T., Dye J.M., Brummelkamp T.R., et al. NRP2 and CD63 are host factors for lujo virus cell entry. Cell Host Microbe. 2017;22:688–696. doi: 10.1016/j.chom.2017.10.002. e685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cao W., Henry M.D., Borrow P., Yamada H., Elder J.H., Ravkov E.V., Nichol S.T., Compans R.W., Campbell K.P., Oldstone M.B. Identification of α-dystroglycan as a receptor for lymphocytic choriomeningitis virus and lassa fever virus. Science. 1998;282:2079–2081. doi: 10.1126/science.282.5396.2079. [DOI] [PubMed] [Google Scholar]
- 58.Hallam S.J., Koma T., Maruyama J., Paessler S. Review of mammarenavirus biology and replication. Front Microbiol. 2018;9:1751. doi: 10.3389/fmicb.2018.01751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spiropoulou C.F., Kunz S., Rollin P.E., Campbell K.P., Oldstone M.B. New world arenavirus clade C, but not clade A and B viruses, utilizes α-dystroglycan as its major receptor. J Virol. 2002;76:5140–5146. doi: 10.1128/JVI.76.10.5140-5146.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rojek J.M., Campbell K.P., Oldstone M.B., Kunz S. Old world arenavirus infection interferes with the expression of functional α-dystroglycan in the host cell. Mol Biol Cell. 2007;18:4493–4507. doi: 10.1091/mbc.E07-04-0374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kunz S., Rojek J.M., Kanagawa M., Spiropoulou C.F., Barresi R., Campbell K.P., Oldstone M.B. Posttranslational modification of α-dystroglycan, the cellular receptor for arenaviruses, by the glycosyltransferase LARGE is critical for virus binding. J Virol. 2005;79:14282–14296. doi: 10.1128/JVI.79.22.14282-14296.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Briggs D.C., Yoshida-Moriguchi T., Zheng T., Venzke D., Anderson M.E., Strazzulli A., Moracci M., Yu L., Hohenester E., Campbell K.P. Structural basis of laminin binding to the LARGE glycans on dystroglycan. Nat Chem Biol. 2016;12:810–814. doi: 10.1038/nchembio.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kunz S. Receptor binding and cell entry of Old World arenaviruses reveal novel aspects of virus-host interaction. Virology. 2009;387:245–249. doi: 10.1016/j.virol.2009.02.042. [DOI] [PubMed] [Google Scholar]
- 64••.Jae L.T., Raaben M., Herbert A.S., Kuehne A.I., Wirchnianski A.S., Soh T.K., Stubbs S.H., Janssen H., Damme M., Saftig P., et al. Virus entry. Lassa virus entry requires a trigger-induced receptor switch. Science. 2014;344:1506–1510. doi: 10.1126/science.1252480. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using state-of-the-art haploid screening techniques this study identified the extreme glycan specificity of Lass virus. The use of a specific N-glycan that must be sialylated by ST3G4 is fascinating.
- 65.Neu U., Stehle T., Atwood W.J. The polyomaviridae: contributions of virus structure to our understanding of virus receptors and infectious entry. Virology. 2009;384:389–399. doi: 10.1016/j.virol.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maginnis M.S., Nelson C.D., Atwood W.J. JC polyomavirus attachment, entry, and trafficking: unlocking the keys to a fatal infection. J Neurovirol. 2015;21:601–613. doi: 10.1007/s13365-014-0272-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.O’Hara S.D., Stehle T., Garcea R. Glycan receptors of the polyomaviridae: structure, function, and pathogenesis. Curr Opin Virol. 2014;7:73–78. doi: 10.1016/j.coviro.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 68••.Tsai B., Gilbert J.M., Stehle T., Lencer W., Benjamin T.L., Rapoport T.A. Gangliosides are receptors for murine polyoma virus and SV40. EMBO J. 2003;22:4346–4355. doi: 10.1093/emboj/cdg439. [DOI] [PMC free article] [PubMed] [Google Scholar]; Elegant study and one of the first to identify gangliosides as potential glycan receptors for polyomaviruses.
- 69•.Qian M., Tsai B. Lipids and proteins act in opposing manners to regulate polyomavirus infection. J Virol. 2010;84:9840–9852. doi: 10.1128/JVI.01093-10. [DOI] [PMC free article] [PubMed] [Google Scholar]; An excellent study utilizing ganglioside-deficient cell lines; establishes dual roles for glycolipids and glycoproteins in polyomavirus infection.
- 70.Chen M.H., Benjamin T. Roles of N-glycans with alpha2,6 as well as alpha2,3 linked sialic acid in infection by polyoma virus. Virology. 1997;233:440–63442. doi: 10.1006/viro.1997.8596. [DOI] [PubMed] [Google Scholar]
- 71•.Stroh L.J., Maginnis M.S., Blaum B.S., Nelson C.D., Neu U., Gee G.V., O’Hara B.A., Motamedi N., DiMaio D., Atwood W.J., et al. The greater affinity of JC polyomavirus capsid for α2,6-linked lactoseries tetrasaccharide than for other sialylated glycans is a major determinant of infectivity. J Virol. 2015;89:6364–6375. doi: 10.1128/JVI.00489-15. [DOI] [PMC free article] [PubMed] [Google Scholar]; Important study showing that JC polyomavirus binds with high specificity to a terminal α2-6 epitope likely found on glycoproteins. Secreted LSTc milk oligosaccharide may act as a decoy in vivo.
- 72.Elphick G.F., Querbes W., Jordan J.A., Gee G.V., Eash S., Manley K., Dugan A., Stanifer M., Bhatnagar A., Kroeze W.K., et al. The human polyomavirus, JCV, uses serotonin receptors to infect cells. Science. 2004;306:1380–1383. doi: 10.1126/science.1103492. [DOI] [PubMed] [Google Scholar]
- 73.Campanero-Rhodes M.A., Smith A., Chai W., Sonnino S., Mauri L., Childs R.A., Zhang Y., Ewers H., Helenius A., Imberty A., et al. N-glycolyl GM1 ganglioside as a receptor for simian virus 40. J Virol. 2007;81:12846–12858. doi: 10.1128/JVI.01311-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74•.Neu U., Woellner K., Gauglitz G., Stehle T. Structural basis of GM1 ganglioside recognition by simian virus 40. Proc Natl Acad Sci U S A. 2008;105:5219–5224. doi: 10.1073/pnas.0710301105. [DOI] [PMC free article] [PubMed] [Google Scholar]; Important structural study revealing the first complex of a capsid virus with a ganglioside glycan.
- 75••.Lofling J., Lyi S.M., Parrish C.R., Varki A. Canine and feline parvoviruses preferentially recognize the non-human cell surface sialic acid N-glycolylneuraminic acid. Virology. 2013;440:89–96. doi: 10.1016/j.virol.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]; Important work linking-specific binding of a carbohydrate absent in humans to select species tropism.
- 76.Huang L.Y., Halder S., Agbandje-McKenna M. Parvovirus glycan interactions. Curr Opin Virol. 2014;7:108–118. doi: 10.1016/j.coviro.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Parker J.S., Parrish C.R. Cellular uptake and infection by canine parvovirus involves rapid dynamin-regulated clathrin-mediated endocytosis, followed by slower intracellular trafficking. J Virol. 2000;74:1919–1930. doi: 10.1128/jvi.74.4.1919-1930.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nam H.J., Gurda-Whitaker B., Gan W.Y., Ilaria S., McKenna R., Mehta P., Alvarez R.A., Agbandje-McKenna M. Identification of the sialic acid structures recognized by minute virus of mice and the role of binding affinity in virulence adaptation. J Biol Chem. 2006;281:25670–25677. doi: 10.1074/jbc.M604421200. [DOI] [PubMed] [Google Scholar]
- 79.Lopez-Bueno A., Rubio M.P., Bryant N., McKenna R., Agbandje-McKenna M., Almendral J.M. Host-selected amino acid changes at the sialic acid binding pocket of the parvovirus capsid modulate cell binding affinity and determine virulence. J Virol. 2006;80:1563–1573. doi: 10.1128/JVI.80.3.1563-1573.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80•.Huang L.Y., Patel A., Ng R., Miller E.B., Halder S., McKenna R., Asokan A., Agbandje-McKenna M. Characterization of the adeno-associated virus 1 and 6 sialic acid binding site. J Virol. 2016;90:5219–5230. doi: 10.1128/JVI.00161-16. [DOI] [PMC free article] [PubMed] [Google Scholar]; First structural analysis confirming alternate location of SA binding sites in AAVs, around the threefold symmetry axis of the virus capsid.
- 81.Srivastava A. In vivo tissue-tropism of adeno-associated viral vectors. Curr Opin Virol. 2016;21:75–80. doi: 10.1016/j.coviro.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shen S., Bryant K.D., Brown S.M., Randell S.H., Asokan A. Terminal N-linked galactose is the primary receptor for adeno-associated virus 9. J Biol Chem. 2011;286:13532–13540. doi: 10.1074/jbc.M110.210922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Di Pasquale G., Davidson B.L., Stein C.S., Martins I., Scudiero D., Monks A., Chiorini J.A. Identification of PDGFR as a receptor for AAV-5 transduction. Nat Med. 2003;9:1306–1312. doi: 10.1038/nm929. [DOI] [PubMed] [Google Scholar]
- 84.Taube S., Jiang M., Wobus C.E. Glycosphingolipids as receptors for non-enveloped viruses. Viruses. 2010;2:1011–1049. doi: 10.3390/v2041011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85••.Brown K.E., Anderson S.M., Young N.S. Erythrocyte P antigen: cellular receptor for B19 parvovirus. Science. 1993;262:114–117. doi: 10.1126/science.8211117. [DOI] [PubMed] [Google Scholar]; Seminal study linking a virus with strong tropism for human blood to a rare carbohydrate antigen present on erythrocytes.
- 86.Bonsch C., Zuercher C., Lieby P., Kempf C., Ros C. The globoside receptor triggers structural changes in the B19 virus capsid that facilitate virus internalization. J Virol. 2010;84:11737–11746. doi: 10.1128/JVI.01143-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Settembre E.C., Chen J.Z., Dormitzer P.R., Grigorieff N., Harrison S.C. Atomic model of an infectious rotavirus particle. EMBO J. 2011;30:408–416. doi: 10.1038/emboj.2010.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88•.Reiter D.M., Frierson J.M., Halvorson E.E., Kobayashi T., Dermody T.S., Stehle T. Crystal structure of reovirus attachment protein sigma1 in complex with sialylated oligosaccharides. PLoS Pathog. 2011;7 doi: 10.1371/journal.ppat.1002166. [DOI] [PMC free article] [PubMed] [Google Scholar]; First crystal structures of a complex with a complete receptor glycan for this family. Previous structures had only been solved with an SA monosaccharide.
- 89.Dormitzer P.R., Sun Z.Y., Wagner G., Harrison S.C. The rhesus rotavirus VP4 sialic acid binding domain has a galectin fold with a novel carbohydrate binding site. EMBO J. 2002;21:885–897. doi: 10.1093/emboj/21.5.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dermody T.S., Nibert M.L., Bassel-Duby R., Fields B.N. A sigma 1 region important for hemagglutination by serotype 3 reovirus strains. J Virol. 1990;64:5173–5176. doi: 10.1128/jvi.64.10.5173-5176.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Guglielmi K.M., Johnson E.M., Stehle T., Dermody T.S. Attachment and cell entry of mammalian orthoreovirus. Curr Top Microbiol Immunol. 2006;309:1–38. doi: 10.1007/3-540-30773-7_1. [DOI] [PubMed] [Google Scholar]
- 92.Konopka-Anstadt J.L., Mainou B.A., Sutherland D.M., Sekine Y., Strittmatter S.M., Dermody T.S. The Nogo receptor NgR1 mediates infection by mammalian reovirus. Cell Host Microbe. 2014;15:681–691. doi: 10.1016/j.chom.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93••.Haselhorst T., Fleming F.E., Dyason J.C., Hartnell R.D., Yu X., Holloway G., Santegoets K., Kiefel M.J., Blanchard H., Coulson B.S., et al. Sialic acid dependence in rotavirus host cell invasion. Nat Chem Biol. 2009;5:91–93. doi: 10.1038/nchembio.134. [DOI] [PubMed] [Google Scholar]; Excellent paper utilizing STD-NMR to establish specificity of various rotaviruses for internal SAs present on certain gangliosides. This work provided crucial evidence towards settling a long-standing debate over the binding specificity of so-called ‘SA-dependent’ and ‘SA-independent’ viruses.
- 94.Coulson B.S. Expanding diversity of glycan receptor usage by rotaviruses. Curr Opin Virol. 2015;15:90–96. doi: 10.1016/j.coviro.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 95••.Hu L., Crawford S.E., Czako R., Cortes-Penfield N.W., Smith D.F., Le Pendu J., Estes M.K., Prasad B.V. Cell attachment protein VP8* of a human rotavirus specifically interacts with A-type histo-blood group antigen. Nature. 2012;485:256–259. doi: 10.1038/nature10996. [DOI] [PMC free article] [PubMed] [Google Scholar]; First description of rotavirus binding to HBGAs, this work also highlighted a further route towards infection independent of sialoglycans.
- 96•.Huang P., Xia M., Tan M., Zhong W., Wei C., Wang L., Morrow A., Jiang X. Spike protein VP8* of human rotavirus recognizes histo-blood group antigens in a type-specific manner. J Virol. 2012;86:4833–4843. doi: 10.1128/JVI.05507-11. [DOI] [PMC free article] [PubMed] [Google Scholar]; Significant study showing that different strains of human rotavirus are highly specific for subtly different HBGA structures. The study also highlights that many well-recognized epitopes are present in secreted oligosaccharides in saliva and human milk, highlighting a potential natural defense.
- 97.Sun X., Wang L., Qi J., Li D., Wang M., Cong X., Peng R., Chai W., Zhang Q., Wang H., et al. Human group C rotavirus VP8*s recognize type A histo-blood group antigens as ligands. J Virol. 2018;92 doi: 10.1128/JVI.00442-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yu Y., Lasanajak Y., Song X., Hu L., Ramani S., Mickum M.L., Ashline D.J., Prasad B.V., Estes M.K., Reinhold V.N., et al. Human milk contains novel glycans that are potential decoy receptors for neonatal rotaviruses. Mol Cell Proteomics. 2014;13:2944–2960. doi: 10.1074/mcp.M114.039875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu Y., Huang P., Jiang B., Tan M., Morrow A.L., Jiang X. Poly-LacNAc as an age-specific ligand for rotavirus P[11] in neonates and infants. PLoS One. 2013;8 doi: 10.1371/journal.pone.0078113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Reiss K., Stencel J.E., Liu Y., Blaum B.S., Reiter D.M., Feizi T., Dermody T.S., Stehle T. The GM2 glycan serves as a functional coreceptor for serotype 1 reovirus. PLoS Pathog. 2012;8 doi: 10.1371/journal.ppat.1003078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101•.Stencel-Baerenwald J., Reiss K., Blaum B.S., Colvin D., Li X.N., Abel T., Boyd K., Stehle T., Dermody T.S. Glycan engagement dictates hydrocephalus induction by serotype 1 reovirus. mBio. 2015;6 doi: 10.1128/mBio.02356-14. [DOI] [PMC free article] [PubMed] [Google Scholar]; Important paper connecting binding of a specific receptor, GM2, to tropism for ependymal cells, leading to hydrocephalus in mice.
- 102.Barton E.S., Youree B.E., Ebert D.H., Forrest J.C., Connolly J.L., Valyi-Nagy T., Washington K., Wetzel J.D., Dermody T.S. Utilization of sialic acid as a coreceptor is required for reovirus-induced biliary disease. J Clin Invest. 2003;111:1823–1833. doi: 10.1172/JCI16303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Frierson J.M., Pruijssers A.J., Konopka J.L., Reiter D.M., Abel T.W., Stehle T., Dermody T.S. Utilization of sialylated glycans as coreceptors enhances the neurovirulence of serotype 3 reovirus. J Virol. 2012;86:13164–13173. doi: 10.1128/JVI.01822-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Prasad B.V., Hardy M.E., Dokland T., Bella J., Rossmann M.G., Estes M.K. X-ray crystallographic structure of the Norwalk virus capsid. Science. 1999;286:287–290. doi: 10.1126/science.286.5438.287. [DOI] [PubMed] [Google Scholar]
- 105.Tan M., Huang P., Meller J., Zhong W., Farkas T., Jiang X. Mutations within the P2 domain of norovirus capsid affect binding to human histo-blood group antigens: evidence for a binding pocket. J Virol. 2003;77:12562–12571. doi: 10.1128/JVI.77.23.12562-12571.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106•.Cao S., Lou Z., Tan M., Chen Y., Liu Y., Zhang Z., Zhang X.C., Jiang X., Li X., Rao Z. Structural basis for the recognition of blood group trisaccharides by norovirus. J Virol. 2007;81:5949–5957. doi: 10.1128/JVI.00219-07. [DOI] [PMC free article] [PubMed] [Google Scholar]; Excellent study and one of the first to highlight a structural basis for HBGA recognition by caliciviruses.
- 107.Zakhour M., Ruvoen-Clouet N., Charpilienne A., Langpap B., Poncet D., Peters T., Bovin N., Le Pendu J. The αGal epitope of the histo-blood group antigen family is a ligand for bovine norovirus Newbury2 expected to prevent cross-species transmission. PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Caddy S., Breiman A., le Pendu J., Goodfellow I. Genogroup IV and VI canine noroviruses interact with histo-blood group antigens. J Virol. 2014;88:10377–10391. doi: 10.1128/JVI.01008-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109•.Rademacher C., Krishna N.R., Palcic M., Parra F., Peters T. NMR experiments reveal the molecular basis of receptor recognition by a calicivirus. J Am Chem Soc. 2008;130:3669–3675. doi: 10.1021/ja710854r. [DOI] [PubMed] [Google Scholar]; Exceptional work underlining the importance of fucose recognition by caliciviruses, and one of the first to demonstrate the power of STD-NMR in dissection of carbohydrate binding epitopes.
- 110•.Kim D.S., Hosmillo M., Alfajaro M.M., Kim J.Y., Park J.G., Son K.Y., Ryu E.H., Sorgeloos F., Kwon H.J., Park S.J., et al. Both α2,3-and α2,6-linked sialic acids on O-linked glycoproteins act as functional receptors for porcine Sapovirus. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004172. [DOI] [PMC free article] [PubMed] [Google Scholar]; Excellent example of using a variety of techniques methods to establish the role of O-linked glycans as functional receptors for sapovirus.
- 111•.Taube S., Perry J.W., Yetming K., Patel S.P., Auble H., Shu L., Nawar H.F., Lee C.H., Connell T.D., Shayman J.A., et al. Ganglioside-linked terminal sialic acid moieties on murine macrophages function as attachment receptors for murine noroviruses. J Virol. 2009;83:4092–4101. doi: 10.1128/JVI.02245-08. [DOI] [PMC free article] [PubMed] [Google Scholar]; Clear demonstration of gangliosides as functional receptors for norovirus.
- 112.Stuart A.D., Brown T.D. α2,6-Linked sialic acid acts as a receptor for Feline calicivirus. J Gen Virol. 2007;88:177–186. doi: 10.1099/vir.0.82158-0. [DOI] [PubMed] [Google Scholar]
- 113.Orchard R.C., Wilen C.B., Doench J.G., Baldridge M.T., McCune B.T., Lee Y.C., Lee S., Pruett-Miller S.M., Nelson C.A., Fremont D.H., et al. Discovery of a proteinaceous cellular receptor for a norovirus. Science. 2016;353:933–936. doi: 10.1126/science.aaf1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Haga K., Fujimoto A., Takai-Todaka R., Miki M., Doan Y.H., Murakami K., Yokoyama M., Murata K., Nakanishi A., Katayama K. Functional receptor molecules CD300lf and CD300ld within the CD300 family enable murine noroviruses to infect cells. Proc Natl Acad Sci U S A. 2016;113:E6248–E6255. doi: 10.1073/pnas.1605575113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ossiboff R.J., Parker J.S. Identification of regions and residues in feline junctional adhesion molecule required for feline calicivirus binding and infection. J Virol. 2007;81:13608–13621. doi: 10.1128/JVI.01509-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sosnovtsev S.V., Sandoval-Jaime C., Parra G.I., Tin C.M., Jones R.W., Soden J., Barnes D., Freeth J., Smith A.W., Green K.Y. Identification of human junctional adhesion molecule 1 as a functional receptor for the hom-1 calicivirus on human cells. mBio. 2017;8 doi: 10.1128/mBio.00031-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117•.Lindesmith L., Moe C., Marionneau S., Ruvoen N., Jiang X., Lindblad L., Stewart P., LePendu J., Baric R. Human susceptibility and resistance to Norwalk virus infection. Nat Med. 2003;9:548–553. doi: 10.1038/nm860. [DOI] [PubMed] [Google Scholar]; Important early study in human subjects showing FUT genes associated with secretor phenotypes to be a strong risk factor for norovirus infection.
- 118.Chen Y., Tan M., Xia M., Hao N., Zhang X.C., Huang P., Jiang X., Li X., Rao Z. Crystallography of a Lewis-binding norovirus, elucidation of strain-specificity to the polymorphic human histo-blood group antigens. PLoS Pathog. 2011;7 doi: 10.1371/journal.ppat.1002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nasir W., Frank M., Koppisetty C.A., Larson G., Nyholm P.G. Lewis histo-blood group α1,3/α1,4 fucose residues may both mediate binding to GII.4 noroviruses. Glycobiology. 2012;22:1163–1172. doi: 10.1093/glycob/cws084. [DOI] [PubMed] [Google Scholar]
- 120.Fiege B., Rademacher C., Cartmell J., Kitov P.I., Parra F., Peters T. Molecular details of the recognition of blood group antigens by a human norovirus as determined by STD NMR spectroscopy. Angew Chem Int Ed Engl. 2012;51:928–932. doi: 10.1002/anie.201105719. [DOI] [PubMed] [Google Scholar]
- 121.Kocher J.F., Lindesmith L.C., Debbink K., Beall A., Mallory M.L., Yount B.L., Graham R.L., Huynh J., Gates J.E., Donaldson E.F., et al. Bat caliciviruses and human noroviruses are antigenically similar and have overlapping histo-blood group antigen binding profiles. mBio. 2018;9 doi: 10.1128/mBio.00869-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122•.Cho E.H., Soliman M., Alfajaro M.M., Kim J.Y., Seo J.Y., Park J.G., Kim D.S., Baek Y.B., Kang M.I., Park S.I., et al. Bovine nebovirus interacts with a wide spectrum of histo-blood group antigens. J Virol. 2018;92 doi: 10.1128/JVI.02160-17. [DOI] [PMC free article] [PubMed] [Google Scholar]; Recent paper highlighting specificity of a bovine calicivirus member for glycan epitopes also present in humans. Underscores potential concerns over zoonosis within this family.
- 123.Bergelson J.M., Cunningham J.A., Droguett G., Kurt-Jones E.A., Krithivas A., Hong J.S., Horwitz M.S., Crowell R.L., Finberg R.W. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 124.Gaggar A., Shayakhmetov D.M., Lieber A. CD46 is a cellular receptor for group B adenoviruses. Nat Med. 2003;9:1408–1412. doi: 10.1038/nm952. [DOI] [PubMed] [Google Scholar]
- 125.Wang H., Li Z.Y., Liu Y., Persson J., Beyer I., Moller T., Koyuncu D., Drescher M.R., Strauss R., Zhang X.B., et al. Desmoglein 2 is a receptor for adenovirus serotypes 3, 7, 11 and 14. Nat Med. 2011;17:96–104. doi: 10.1038/nm.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kemp M.C., Hierholzer J.C., Cabradilla C.P., Obijeski J.F. The changing etiology of epidemic keratoconjunctivitis: antigenic and restriction enzyme analyses of adenovirus types 19 and 37 isolated over a 10-year period. J Infect Dis. 1983;148:24–33. doi: 10.1093/infdis/148.1.24. [DOI] [PubMed] [Google Scholar]
- 127.Arnberg N., Edlund K., Kidd A.H., Wadell G. Adenovirus type 37 uses sialic acid as a cellular receptor. J Virol. 2000;74:42–48. [PMC free article] [PubMed] [Google Scholar]
- 128.Arnberg N., Pring-Akerblom P., Wadell G. Adenovirus type 37 uses sialic acid as a cellular receptor on Chang C cells. J Virol. 2002;76:8834–8841. doi: 10.1128/JVI.76.17.8834-8841.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129•.Nilsson E.C., Storm R.J., Bauer J., Johansson S.M., Lookene A., Angstrom J., Hedenstrom M., Eriksson T.L., Frangsmyr L., Rinaldi S., et al. The GD1a glycan is a cellular receptor for adenoviruses causing epidemic keratoconjunctivitis. Nat Med. 2011;17:105–109. doi: 10.1038/nm.2267. [DOI] [PubMed] [Google Scholar]; High-quality multidisciplinary study highlighting a potential role for a select ganglioside in adenovirus infection.
- 130••.Lenman A., Liaci A.M., Liu Y., Frangsmyr L., Frank M., Blaum B.S., Chai W., Podgorski I.I., Harrach B., Benko M., et al. Polysialic acid is a cellular receptor for human adenovirus 52. Proc Natl Acad Sci U S A. 2018;115:E4264–E4273. doi: 10.1073/pnas.1716900115. [DOI] [PMC free article] [PubMed] [Google Scholar]; Very recent paper demonstrating novel specificity of a glycan-binding human adenovirus for polysialic epitopes.
- 131.Li X., Bangari D.S., Sharma A., Mittal S.K. Bovine adenovirus serotype 3 utilizes sialic acid as a cellular receptor for virus entry. Virology. 2009;392:162–168. doi: 10.1016/j.virol.2009.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Guardado-Calvo P., Munoz E.M., Llamas-Saiz A.L., Fox G.C., Kahn R., Curiel D.T., Glasgow J.N., van Raaij M.J. Crystallographic structure of porcine adenovirus type 4 fiber head and galectin domains. J Virol. 2010;84:10558–10568. doi: 10.1128/JVI.00997-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bakkers M.J., Zeng Q., Feitsma L.J., Hulswit R.J., Li Z., Westerbeke A., van Kuppeveld F.J., Boons G.J., Langereis M.A., Huizinga E.G., et al. Coronavirus receptor switch explained from the stereochemistry of protein-carbohydrate interactions and a single mutation. Proc Natl Acad Sci U S A. 2016;113:E3111–E3119. doi: 10.1073/pnas.1519881113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cohen-Dvashi H., Cohen N., Israeli H., Diskin R. Molecular mechanism for LAMP1 recognition by lassa virus. J Virol. 2015;89:7584–7592. doi: 10.1128/JVI.00651-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Xu R., McBride R., Nycholat C.M., Paulson J.C., Wilson I.A. Structural characterization of the hemagglutinin receptor specificity from the 2009 H1N1 influenza pandemic. J Virol. 2012;86:982–990. doi: 10.1128/JVI.06322-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shanker S., Choi J.M., Sankaran B., Atmar R.L., Estes M.K., Prasad B.V. Structural analysis of histo-blood group antigen binding specificity in a norovirus GII.4 epidemic variant: implications for epochal evolution. J Virol. 2011;85:8635–8645. doi: 10.1128/JVI.00848-11. [DOI] [PMC free article] [PubMed] [Google Scholar]