Abstract
Background and Purpose-
Cellular fibronectin containing extra domain A (Fn-EDA) is expressed in activated endothelial cells and elevated in circulation in patients with cardiovascular diseases. While global deficiency of Fn-EDA in mice improves stroke outcome, the specific contribution of plasma versus endothelium Fn-EDA in stroke outcome is currently unknown. We investigated the role of plasma versus endothelial Fn-EDA in stroke exacerbation in the comorbid condition of hyperlipidemia.
Methods-
We generated novel plasma Fn-EDA−/− (Fn-EDAfl/flAlbCre) and endothelial Fn-EDA−/− (Fn-EDAfl/flTie2Cre) strains on hyperlipidemic apolipoprotein E-deficient (Apoe−/−) background. By following the STAIR guidelines, we evaluated stroke outcome in male and female mice. Susceptibility to ischemia/reperfusion injury was evaluated in two different models of stroke: intraluminal monofilament and embolic model, on day 1, 3 and 7. Quantitative assessment of stroke outcome was evaluated by measuring infarct volume (by MRI), cerebral blood flow (CBF, by laser speckle imaging), neurological and sensory-motor outcome, and post-ischemic thrombo-inflammation (platelet thrombi, fibrin, neutrophil, phospho-NFκB, TNFα, and IL-1β).
Results-
Stroke outcome was comparable in Apoe−/−Fn-EDAfl/flTie2Cre and control Apoe−/−Fn-EDAfl/fl mice suggesting endothelial Fn-EDA does not contribute to stroke. Apoe−/−Fn-EDAfl/flAlbCre mice exhibited significantly smaller infarcts and improved neurological and sensory-motor outcome at days 1, 3 and 7 in monofilament and embolic models of stroke. Improved stroke outcome was concomitant with enhanced survival, and decreased post-ischemic thrombo-inflammatory response (P<0.05 versus Apoe−/−Fn-EDAfl/fl). No sex-based differences were observed. Laser speckle imaging revealed significantly improved regional CBF at 1 hour in Apoe−/−Fn-EDAfl/flAlbCre mice suggesting plasma Fn-EDA promotes post-ischemic secondary thrombosis. Co-infusion of anti-Fn-EDA antibody with recombinant tissue plasminogen activator (rtPA) in Apoe−/− mice, 1 hour after embolization, improved stroke outcome with enhanced survival and improved neurological outcome (P<0.05 versus rtPA).
Conclusion:
Genetic evidence suggests that plasma Fn-EDA exacerbates stroke outcome by promoting post-ischemic thrombo-inflammation. Interventions targeting plasma Fn-EDA may reduce brain damage following reperfusion.
Keywords: Cellular fibronectin, Thrombosis, Inflammation, Stroke
Introduction
Reperfusion therapy with recombinant tissue plasminogen activator (rtPA) or mechanical thrombectomy (MT) of large vessels is the standard of care for patients with acute ischemic stroke. Although reperfusion of ischemic region has shown promising clinical outcomes, evidence from several patients and animal models suggest that cerebral reperfusion promotes oxidative stress, secondary thrombosis, and vascular inflammation, which aggravate neuronal death in the ischemic penumbra.1, 2 There is currently no effective intervention to reduce brain damage following reperfusion. Both thrombosis and inflammation contribute to the pathophysiology of acute ischemic stroke.3 A better understanding of molecular mechanisms that promote thrombo-inflammatory brain injury may result in novel therapeutic interventions to reduce brain damage following an acute ischemic stroke.
The extracellular matrix protein fibronectin (Fn) is known to contribute to inflammation and thrombosis.4–6 Fn exists in multiple isoforms that are generated by alternative processing of a single primary transcript at three sites: Extra Domain A (EDA), Extra Domain B (EDB), and the Type III Homologies Connecting Segment (IIICS). Two primary forms of FN isoforms exist in human and mice: 1) plasma Fn, which does not contain EDA and EDB. 2) cellular Fn, which contains EDA or EDB or both. Plasma level of Fn-EDA is significantly elevated in patients with cardiovascular disorders (CVD).7–9 Recently, we have reported that non-hematopoietic cell-derived Fn-EDA mediates ischemia/reperfusion brain injury.5 Patients with acute ischemic stroke have severe endothelial dysfunction10, 11 and reports suggest that endothelial injury results in an increased expression of Fn-EDA.12, 13 Furthermore, the influx of polymorphonuclear leukocytes at the ischemic site, following reperfusion, elicits a robust inflammatory response and was associated with increased Fn-EDA synthesis by endothelial cells.14 These observations prompted us to investigate the relative contribution of plasma versus endothelial Fn-EDA in stroke exacerbation.
Current Stroke Therapy Academic Industry Roundtable (STAIR) criterion for preclinical stroke evolution recommends determining the mechanisms in animal models with comorbidities including hyperlipidemia that adequately mimics the human scenario.15 Therefore, we generated novel plasma Fn-EDA−/− (Fn-EDAfl/flAlbCre) and endothelial Fn-EDA−/− (Fn-EDAfl/flTie2Cre) mutant strains on hyperlipidemic apolipoprotein E-deficient (Apoe−/−) background. We provide definitive evidence for the first time that it is Fn-EDA in the plasma and not in the endothelial cells that exacerbates stroke outcome.
Methods
Because of the word limit, a detailed description of the Materials and Methods is present in the Online Data Supplement. The data that supports the findings of this study are available from the corresponding author upon reasonable request.
Animals
10–12 weeks old male and female mice weighing around 20–26 g were used in the present study. Fn-EDAfl/fl mice have been described previously.16 To generate endothelial-specific Fn-EDA deficient mice (Apoe−/−Fn-EDAfl/flTie2Cre), Apoe−/−Fn-EDAfl/fl mouse was crossed to the Apoe−/−Tie2Cre mouse. Similarly, to generate plasma-specific Fn-EDA deficient mice (Apoe−/−Fn-EDAfl/flAlbCre), Apoe−/−Fn-EDAfl/fl mouse was crossed to Apoe−/−AlbCre mouse. Controls were littermates. All mice used in the present study were on the C57BL/6J background. The University of Iowa Animal Care and Use Committee approved all the procedures, and studies were performed according to the current Animal Research: Reporting of In Vivo Experiment guidelines (https://www.nc3rs.org.uk/arrive-guidelines).
Transient middle cerebral artery occlusion (tMCAO) by intraluminal monofilament
Focal cerebral ischemia was achieved by transiently occluding the right middle cerebral artery for 60 minutes as described.5 Mice were anesthetized with 1–1.5% isoflurane mixed with medical air. After a midline incision, the right common carotid artery was temporarily clamped, and a filament (6.0 siliconized) was inserted via the external carotid artery into the internal carotid artery up to the origin of the middle cerebral artery. Reperfusion was achieved by removing the filament after 60 minutes and opening the common carotid artery. Laser Doppler flowmetry (Perimed instruments, Sweden) was used for each mouse to confirm the successful induction of ischemia and reperfusion. Animals having more than 70% reduction in the regional cerebral blood flow (rCBF) were included in the study.
Embolic middle cerebral artery occlusion
50 μl arterial blood from the experimental mice was supplemented with human fibrinogen (2 mg/ml) and clotted in a PE-10 tube for 4 hours at room temperature, followed by storage at 4°C overnight. The PE-10 catheter containing homologous washed fibrin-rich clots (~20 mm) was then introduced into the internal carotid artery. Recombinant tissue plasminogen activator (rtPA; 10 mg/kg, 10% volume by bolus and remaining slow infusion for 30 minutes) was infused via a jugular vein 60 minutes post-embolization. Laser Doppler flowmetry was performed to confirm the ischemia and reperfusion.
MRI imaging and Infarct volume quantification
MRI was performed on day-1 post reperfusion as per previously described protocol.17 Briefly, animals were anesthetized with isoflurane (2.5% induction, 1.2% maintenance) and placed in the bore of the 7.0 Tesla MRI (Agilent Technologies Inc., Santa Clara, CA, USA) with a two-channel receive-only surface coil. Following scout scans to orient the imaging planes; high-resolution images were acquired with a 9-minute T2-weighted 2D fast spin-echo pulse sequence oriented coronally. Imaging parameters included TR/TE = 6380 ms/83 ms, echo train length of 12, voxel resolution 0.10 mm × 0.10 mm × 0.50 mm, field of view covering 25.0 mm × 18.8 mm × 14.0 mm with no gaps, and 7 signal averages. Total imaging time for each animal was approximately 20 minutes. The area of infarction was quantified using NIH Image J software by outlining the zone with abnormally hyperintense regions in each brain slice, and the total infarct volume was obtained by summation of the infarcted areas multiplied by the slice thickness. To correct for brain swelling due to edema after ischemia, the corrected total infarct volume (%) was calculated. Corrected infarct volume (%) = [volume of contralateral hemisphere- (volume of ipsilateral hemisphere-volume of infarct)]/ Volume of contralateral hemisphere X 100.
Statistical analysis
Results are reported as mean ± SEM except for the neurological scores (Bederson score and mNSS), where the median ± range was used. The number of experimental animals in each group was based on power calculations for the primary parameter (infarct volume) with mean differences and standard deviations taken from pilot data at power 80% with an alpha of 0.05. For statistical analysis, GraphPad Prism software, version 7.04 was used. Shapiro-Wilk test was used to check normality and Bartlett’s test was used to check equal variance. The statistical significance was assessed using either unpaired t-test or one-way ANOVA followed by Holm-Sidak’s multiple comparisons test (for normally distributed data) and Mann Whitney test or Kruskal-Wallis test followed by Dunn’s multiple comparisons test (for not normally distributed data). P<0.05 was considered to be statistically significant.
Results
Generation and characterization of plasma Fn-EDA−/− and endothelial Fn-EDA−/− mice
To determine the relative contribution of plasma versus endothelial Fn-EDA in the pathophysiology of acute ischemic stroke, we generated two novel mouse strains (Apoe−/−Fn-EDAfl/flAlbCre and Apoe−/−Fn-EDAfl/flTie2Cre) in which Fn-EDA expression was inhibited specifically in hepatocytes (AlbCre) and endothelial cells (Tie2Cre) by Cre-Lox recombination (Online Supplementary Figure IAB). The presence of Tie2 and AlbCre were confirmed using genomic PCR (Online Supplementary Figure IC). RT-PCR confirmed the absence of the EDA exon in the FN mRNA in endothelial cells of Apoe−/−Fn-EDAfl/flTie2Cre mice and the hepatocytes of Apoe−/−Fn-EDAfl/flAlbCre mice (Online Supplementary Figure IIA). Next, we analyzed plasma levels of Fn-EDA in these mice by using ELISA. Control was littermate Apoe−/−Fn-EDAfl/fl strain that constitutively expresses Fn-EDA in the plasma and tissues. Plasma Fn-EDA levels were not altered in Apoe−/−Fn-EDAfl/flTie2Cre (2.87±0.5 μg/mL) but markedly reduced in Apoe−/−Fn-EDAfl/flAlbCre (0.05±0.01 μg/mL) when compared to control Apoe−/−Fn-EDAfl/fl (3.37±1.0 μg/mL) (Online Supplementary Figure II2B). The successful deletion of Fn-EDA from endothelial cells in the brain vasculature and hepatocytes were further confirmed by immunohistochemistry (Online Supplementary Figure III). Previously, we have found that Fn-EDAfl/fl strain, which constitutively expresses Fn-EDA, have a significant decrease in total Fn levels in the plasma and tissues when compared to WT.16 Notably, hepatocyte-specific deletion of EDA exon in Fn-EDAfl/fl strain normalizes total Fn levels both in the plasma and tissues.18 In agreement with these studies, we found that hepatocyte-specific deletion of EDA exon in Apoe−/−Fn-EDAfl/fl, but not endothelial-specific deletion, normalize total Fn levels both in the plasma and tissues when compared to Apoe−/− mice (Online Supplementary Figure IV).
Fn-EDA in the plasma, but not in the endothelial cells, mediates stroke exacerbation in hyperlipidemic Apoe−/− mice
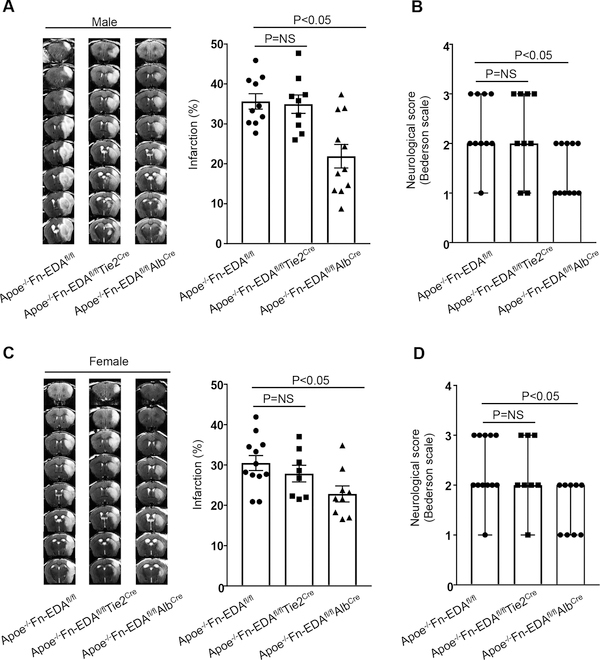
First, we determined stroke outcome in Apoe−/− and Apoe−/−Fn-EDAfl/fl mice subjected to 60 minutes of tMCAO/ 23 hours of reperfusion. We found that infarct volume and neurological outcome were comparable between Apoe−/− and Apoe−/−Fn-EDAfl/fl (Online Supplementary Figure V). To minimize the potential confounding effect of the floxed background, in the subsequent experiments, we utilized Apoe−/−Fn-EDAfl/fl littermates as controls to evaluate stroke outcome in the Apoe−/−Fn-EDAfl/flAlbCre and Apoe−/−Fn-EDAfl/flTie2Cre mice. To minimize the potential confounding effect of advanced atherosclerotic lesions in Apoe−/− mice, which can impair collateral flow and may exacerbate the stroke outcome, all mice were fed a normal chow diet until 8–12 weeks of age, an age at which no significant vascular lesions are found (not shown). Body weight, plasma cholesterol, triglycerides, and complete blood counts were comparable (Online Supplementary Table I & II). Infarct volume and neurological outcome were comparable between male Apoe−/−Fn-EDAfl/flTie2Cre (34.9±2.3 %) and Apoe−/−Fn-EDAfl/fl (35.6±1.9 %, Figure 1AB). On the other hand, we found significantly reduced infarct volume and improved neurological outcome in male Apoe−/−Fn-EDAfl/flAlbCre mice (21.9±2.9%, P<0.05, Figure 1AB). Laser Doppler flow measurements (Online Supplementary Table III) were similar among groups before, during and after ischemia. Female mice were subjected to 60 minutes of tMCAO/ 23 hours of reperfusion to determine any potential sex-specific effects. Similar to males, we found significantly reduced infarct volume and improved neurological outcome in the Apoe−/−Fn-EDAfl/flAlbCre mice (22.8±1.9 %, P<0.05, Figure 1CD), but not in Apoe−/−Fn-EDAfl/flTie2Cre mice, when compared with littermate control Apoe−/−Fn-EDAfl/fl mice (30.5±1.9%). To determine whether Fn-EDA in the plasma contributes to stroke outcome not only in hyperlipidemic Apoe−/− mice but also in wild-type mice, Fn-EDAfl/flAlbCre and Fn-EDAfl/fl mice subjected to 60 minutes of tMCAO/ 23 hours of reperfusion. We found significantly reduced infarct volume and improved neurological outcome in Fn-EDAfl/flAlbCre mice (Online Supplementary Figure VI).
Figure 1. Plasma Fn-EDA−/−, but not endothelial Fn-EDA−/−, mice exhibit improved stroke outcome.
A and C. The left panels show representative MRI from one mouse (male and female) of each genotype on day 1. White is the infarct area. The right panels show corrected mean infarct volumes of each genotype (N=8–12/group). Data are mean ± SEM. Statistical analysis: One-way ANOVA followed by Holm-Sidak’s multiple comparisons test. B and D. Neurological outcome of male and female mice from each genotype as assessed prior to sacrifice on day 1 (depicted as scatter plots including median). Analysis of variance on ranks was applied to test for significant differences in the neurological score.
Lack of Fn-EDA in the plasma improves post-stroke survival and long-term sensorimotor recovery
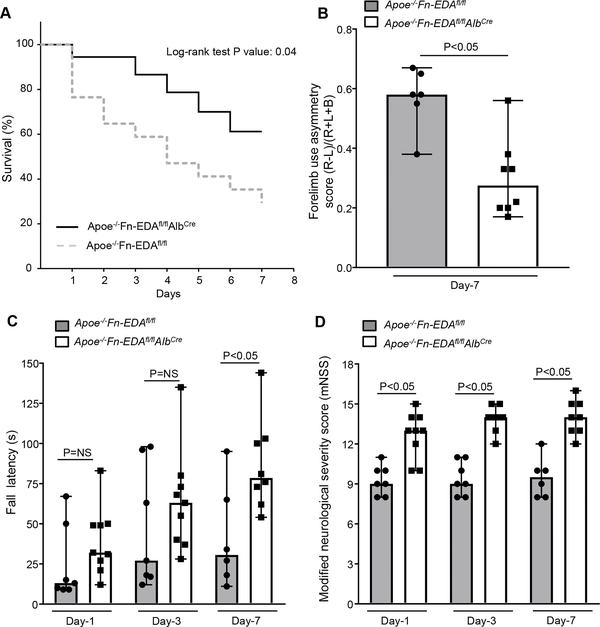
Because both male and female endothelial Fn-EDA−/− (Fn-EDAfl/flTie2Cre) mice did not exhibit improved stroke outcome on day-1 following the filament model, in the subsequent experiments, we utilized plasma Fn-EDA−/− (Fn-EDAfl/flAlbCre) mice to dissect the mechanism. To determine whether Apoe−/−Fn-EDAfl/flAlbCre mice have improved stroke outcome not only at day 1 but also at later time points, we followed male mice for survival up to 7 days following 60 minutes of transient ischemia. We found that on day 7, Apoe−/−Fn-EDAfl/flAlbCre mice had a better survival rate (~60%) when compared with Apoe−/−Fn-EDAfl/fl mice (~30%, P=0.04; Figure 2A). Post-stroke sensorimotor recovery was evaluated using a cylinder test and accelerated rotarod test. Cylinder test is a sensorimotor test to assess asymmetry in forelimb use during vertical exploratory behavior inside a glass cylinder, while accelerated rotarod test evaluates motor coordination and balance. We found that Apoe−/−Fn-EDAfl/flAlbCre mice had improved post-stroke sensorimotor behavioral recovery at day 3 and 7 compared with Apoe−/−Fn-EDAfl/fl mice (Figure 2BC). Next, we evaluated the modified Neurological Severity Score (mNSS) which rates neurological functioning based on spontaneous activity, symmetry in the movement of four limbs, forepaw outstretching, climbing, body proprioception and responses to vibrissae touch (on the scale of 3 to 18; higher score indicates a better outcome).19 We found that Apoe−/−Fn-EDAfl/flAlbCre mice had significantly improved neurological outcome on day-1, day-3 and day-7 (P<0.05 versus Apoe−/−Fn-EDAfl/fl mice; Figure 2D).
Figure 2. Plasma Fn-EDA−/− mice exhibit enhanced long-term survival and improved post-stroke sensorimotor behavioral recovery.
A. Mortality rate between day 0 and day 7 after 60 minutes transient ischemia (N = 10 −12/ group). B. Post-stroke sensorimotor behavioral recovery as analyzed by cylinder test on day 7. Data were analyzed by unpaired t-test. C. Post-stroke motor function as analyzed by accelerated rotarod on day-1, 3 and 7. Data were analyzed by Kruskal-Wallis test followed by Dunn’s multiple comparisons test. D. Modified Neurological Severity Score (mNSS) at day 1, 3 and 7 based on spontaneous activity, symmetry in the movement of four limbs, forepaw outstretching, climbing, body proprioception and responses to vibrissae touch (higher score indicates a better outcome). Data were analyzed by Kruskal-Wallis test followed by Dunn’s multiple comparisons test.
Lack of Fn-EDA in the plasma improves regional cerebral blood flow and limits post-ischemic thrombo-inflammatory response
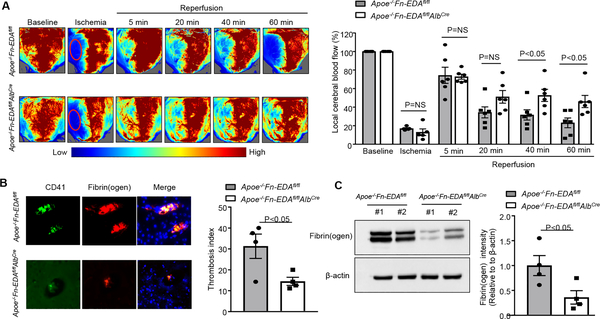
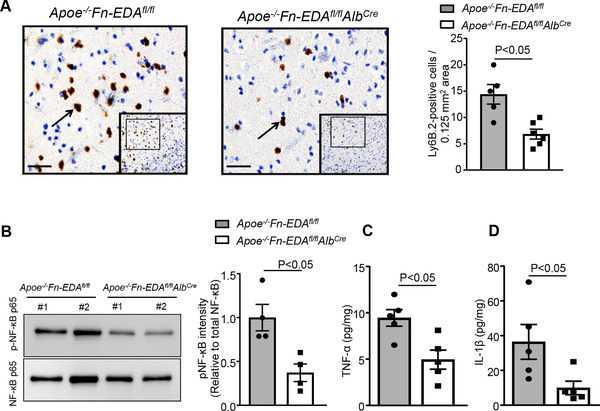
To determine whether improved stroke outcome in Apoe−/−Fn-EDAfl/flAlbCre mice is associated with improved regional cerebral blood flow (CBF), laser speckle imaging (LSI) was performed at early time points (5–60 minutes). We found that CBF was significantly improved at 40 and 60 minutes after reperfusion in Apoe−/−Fn-EDAfl/flAlbCre mice (P<0.05 vs. Apoe−/−Fn-EDAfl/flAlbCre mice, Figure 3A). Furthermore, we observed that intracerebral platelet (CD41-positive) and fibrin(ogen) deposition as determined by Western blot were markedly reduced in Apoe−/−Fn-EDAfl/flAlbCre mice (P<0.05 vs. Apoe−/−Fn-EDAfl/fl mice, Figure 3BC) suggesting that Fn-EDA present in the plasma may promote secondary intracerebral thrombosis, and thereby worsens stroke outcome. Following ischemia-reperfusion injury, neutrophils are the first immune cells to respond and also significantly contribute to ischemic brain damage by promoting inflammation.20, 21 To determine whether improved stroke outcome in Apoe−/−Fn-EDAfl/flAlbCre mice was associated with reduced postischemic inflammation, we evaluated neutrophil influx in infarct and peri-infarct region of the perfused brain after 60 minutes of tMCAO followed by 23 hours of reperfusion. Immunohistochemistry and flow cytometry analysis revealed decreased neutrophil infiltration in Apoe−/−Fn-EDAfl/flAlbCre mice when compared with Apoe−/−Fn-EDAfl/fl mice as (P<0.05, Figure 4A and online Supplementary Figure VII). Previously, we have shown that Fn-EDA promotes thrombo-inflammatory brain injury via TLR4 expressed on hematopoietic cells.5, 22 Therefore, we measured levels of phospho-NFκB p65, and inflammatory cytokine (TNFα and IL1β) levels, which are components of the canonical signaling pathway downstream of TLR4, in brain homogenates prepared from the infarcted and surrounding areas following tMCAO. We found markedly reduced phospho-NFκB p65, TNFα and IL1β levels in Apoe−/−Fn-EDAfl/flAlbCre mice when compared to Apoe−/−Fn-EDAfl/fl mice (P<0.05, Figure 4BCD). To determine whether Fn-EDA in the immune cells contributes to stroke exacerbation, we transplanted lethally irradiated Apoe−/−Fn-EDAfl/fl Tie2Cre mice with bone marrow cells from either Apoe−/−Fn-EDAfl/flTie2Cre or Apoe−/−Fn-EDAfl/fl mice. This bone marrow transplantation protocol resulted in chimeric mice (Apoe−/−Fn-EDAfl/fl BM→Apoe−/−Fn-EDAfl/flTie2Cre) that express Fn-EDA in the bone-marrow cells but lack Fn-EDA expression in the endothelial cells. Complete blood counts were comparable (not shown). Infarct volume and neurological score were comparable between Apoe−/−Fn-EDAfl/fl Tie2Cre-BM→Apoe−/−Fn-EDAfl/flTie2Cre) and (Apoe−/−Fn-EDAfl/fl-BM→Apoe−/−Fn-EDAfl/fl-Tie2Cre), suggesting that Fn-EDA in immune cells is not involved in stroke severity or post-stroke recovery (Supplementary Figure VIII). These results are in agreement with previously published studies.5
Figure 3. Plasma Fn-EDA−/− mice have improved regional cerebral blood flow and reduced cerebral thrombosis.
A. The left panel shows regional cerebral blood flow (CBF) as analyzed by laser speckle imaging (LSI). The right panel shows quantification at different time points (5–60 minutes). N=6 mice/group. B. The left panel shows representative immunostaining for platelet CD41 (green) and fibrin(ogen) (red). The right panel shows the thrombotic index (CD41 positive area/ total area of the section) X 100. C. Quantification of fibrin(ogen) in brain homogenates as determined by immunoblotting and densitometry (N=4 mice/group). Actin was used as loading control. Data were analyzed by Kruskal-Wallis test followed by Dunn’s multiple comparisons test (CBF) and unpaired t-test (thrombosis index and fibrin).
Figure 4. Plasma Fn-EDA−/− mice displayed reduced cerebral inflammation.
A. The left panel shows representative low-magnification (boxed region) and higher-magnification (insert in the boxed region) stained for neutrophils (brown Ly6B.2-positive cells). The right panel shows quantification. B. The left panels show representative immunoblots and quantification (densitometry) of NFκB p65 in brain homogenates prepared from the infarcted and surrounding areas. C and D. Quantification of TNFα and IL-1β levels by ELISA in brain homogenates. Data are presented as mean ± SEM. N = 5 mice/group. Data were analyzed by unpaired t-test (neutrophils, pNFκB p65, TNF-α, and IL-1β).
Targeting Fn-EDA in the plasma improves stroke outcome in the embolic stroke model
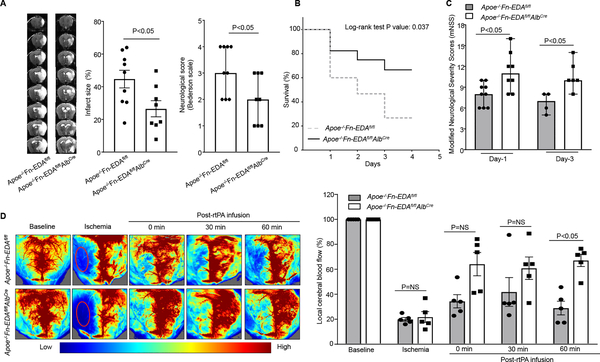
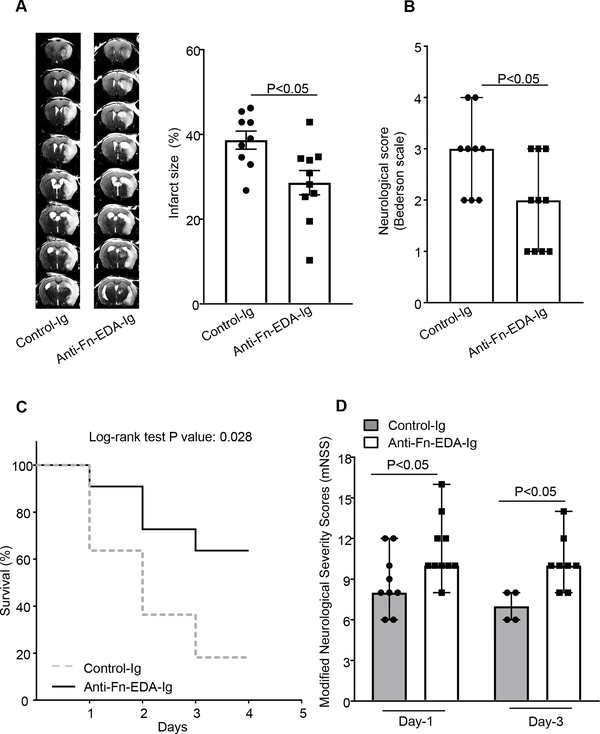
To determine whether the observed effect is not limited to one model, but generalizable to a broader context, we evaluated the role of plasma Fn-EDA in the embolic model of stroke. In this model, homologous washed fibrin-rich clots (~20 mm) were infused through the internal carotid artery. Laser Doppler flowmetry and LSI were performed to confirm ischemia and reperfusion. After 60 minutes of ischemia, rtPA (10 mg/kg, 10% volume by bolus and remaining slow infusion for 30 minutes) was infused through the jugular vein. We found significantly reduced infarct volume and improved neurological outcome in male Apoe−/−Fn-EDAfl/flAlbCre mice when compared to Apoe−/−Fn-EDAfl/fl mice (Figure 5A). Furthermore, Apoe−/−Fn-EDAfl/flAlbCre mice had significantly reduced mortality and improved post-ischemic recovery (analyzed by mNSS) on day 1 and 3 compared with Apoe−/−Fn-EDAfl/fl mice (Figure 5BC). LSI revealed improved regional CBF in Apoe−/− Fn-EDAfl/flAlbCre mice when compared with Apoe−/−Fn-EDAfl/fl mice (Figure 5D) suggesting that plasma Fn-EDA promotes post-ischemic secondary thrombosis and may thereby worsen stroke outcome in an embolic model of stroke. Importantly, co-infusion of specific anti-Fn EDA antibody with rtPA, 1 hr post embolization, improved stroke outcome and enhanced post-stroke survival and improved neurological recovery in the embolic stroke model (P<0.05 vs. Apoe−/− mice infused with tPA; Figure 6). Due to higher mortality, we could not evaluate long-term functional outcome after rtPA infusion.
Figure 5. Plasma Fn-EDA−/− mice exhibit significantly improved stroke outcome in the embolic stroke model.
A. The left panels show representative MRI from one mouse of each genotype on day 1. White is the infarct area. The middle panel shows corrected mean infarct size in the ipsilateral hemisphere (%) of each genotype (N=8–9/group). Data are mean ± SEM. The right panel shows neurological outcome as assessed before sacrifice on day 1 (depicted as scatter plots including median). B. Mortality rate C. modified Neurological Severity Score (mNSS). D. The left panel shows regional cerebral blood flow (CBF) as analyzed by laser speckle imaging. The right panel shows quantification at different time points (0–60 minutes). N=5 mice/group). Data were analyzed by unpaired student’s t-test (% infarct), Mann-Whitney test (neurological score) and Kruskal-Wallis test followed by Dunn’s multiple comparisons test (CBF and mNSS).
Figure 6. Targeting plasma Fn-EDA in hyperlipidemic Apoe−/− mice significantly improves stroke outcome in the embolic stroke model.
A. The left panel shows representative MRI from one Apoe−/− mouse of each group on day 1 infused with anti-Fn-EDA Ig or control Ig. The right panels show corrected mean infarct size (%). B. Neurological outcome (N=9–10 mice/group). C. Mortality rate between day 0 and day 4 after 60 minutes of transient ischemia and reperfusion with rtPA (N = 8–9/ group). D. mNSS at day 1 and 3 (N = 8–9/ group). Data were analyzed by unpaired student’s t-test (% infarct), Mann-Whitney test (neurological score) and Kruskal-Wallis test followed by Dunn’s multiple comparisons test (mNSS).
Discussion
Although several interventions have shown efficacy in preclinical stroke models, they have been unsuccessful in the clinics. It is now believed that a critical factor for the lack of translation from “bench to bedside” is that pre-clinical evaluation of mechanisms of ischemic stroke progression and therapeutic studies are typically done in healthy animals, whereas human stroke usually occurs during the pathophysiological progression of underlying diseases or risk factors. Patients at risk for stroke are those with the comorbid conditions including hyperlipidemia, obesity, diabetes, and hypertension. Therefore, STAIR criterion for preclinical stroke evolution recommends determining the mechanisms and response to treatment in animal models with comorbid conditions such as hyperlipidemia, diet-induced obesity, hypertension that adequately mimics the human scenario.15 In the present study, we have implemented several STAIR-RIGOR guidelines including a) utilizing two different models for experimental stroke, with preexisting comorbidity (hyperlipidemia), b) predefined inclusion-exclusion criteria, c) conducting experiments in cohorts and blind fashion, d) using both males and females, e) assessment of neurological outcome measures up to one week on the same mouse. Utilizing novel mutant strains, we provide genetic evidence that cellular Fn-EDA in the plasma, but not in the endothelial cells, contributes to the stroke exacerbation in the setting of comorbid hyperlipidemia. We found that lack of cellular Fn-EDA in the plasma significantly reduced infarct volume, improves post-stroke survival and sensorimotor recovery by reducing thrombo-inflammatory responses.
Several studies suggest that activated endothelial cells synthesize and secrete Fn-EDA.23, 24 Moreover, PMNs, which are known to contribute to stroke progression, were implicated in the modulation of the endothelial Fn (most likely Fn-EDA) synthesis.14 These observations suggest that endothelial Fn-EDA may contribute to stroke evolution. Surprisingly, we found that Fn-EDA present in the endothelial cells was not essential for stroke exacerbation. The possibility that a threshold of endothelial-derived Fn-EDA is required in the plasma to exacerbate stroke outcome cannot be ruled out. Significantly increased plasma levels of Fn-EDA have been reported in patients with CVDs,7–9, and acute ischemic stroke.25 While global deficiency of Fn-EDA improves stroke outcome in hyperlipidemic Apoe−/− mice,5 the specific contribution of Fn-EDA in the plasma remains unclear. Herein, we provide definitive evidence that Fn-EDA in the plasma mediates stroke exacerbation. Previous studies have shown that lack of total Fn in the plasma results in stroke exacerbation, suggesting a beneficial effect of total Fn in the plasma.26 This raises the question of whether Apoe−/−Fn-EDAfl/fl mice have stroke severity because they have sub optimal levels of total Fn in the plasma and brain tissue when compared to Apoe−/−Fn-EDAfl/flAlbCre mice.16 The possibility that Apoe−/−Fn-EDAfl/fl have stroke severity when compared to Apoe−/−Fn-EDAfl/flAlbCre mice due to a decrease in total plasma Fn levels is minimal because of the following reasons. First, Fn-EDA heterozygous (Fn-EDA+/wt) mice, which have normal levels of FN but contain Fn-EDA,16 also exhibit exacerbated brain injury similar to Fn-EDAfl/fl mice.27 Second, Fn-EDA−/−Apoe−/− mice exhibited improved stroke outcome compared to Apoe−/− mice, though total plasma Fn levels in Fn-EDA−/−Apoe−/− and Apoe−/− mice were comparable.5
Next, using laser speckle imaging that measures real-time CBF with high spatial and temporal resolution, we found that lack of Fn-EDA in the plasma improves post-reperfusion regional CBF in the core region. Improved regional CBF was associated with significantly reduced platelet thrombi and fibrin deposition. These results are in agreement with previous studies that have suggested a pro-thrombotic role for plasma Fn-EDA, which was mediated by platelet TLR4.5, 28, 29 Platelets are known to have molecular machinery that is necessary for downstream signaling of TLR4, including components of the canonical NF-κB pathway.30 Notably, Fn-EDA was associated with increased clot density in type 2 diabetic patients favoring a prothrombotic state.31 This study also identified that Fn-EDA specifically in the plasma promotes post-ischemic inflammation. We found that lack of Fn-EDA in the plasma reduces neutrophil influx that was associated with decreased expression of phospho-NFκB and inflammatory cytokines TNFα, and IL-1β in the infarcted and surrounding region. Fn-EDA is an endogenous ligand for TLR432 and known to promote inflammation via TLR4 expressed on hematopoietic cells in experimental models.5, 6 Together our data suggests that Fn-EDA in the plasma exacerbates stroke outcome by promoting post-ischemic thrombosis and vascular inflammation.
There is currently no effective intervention to reduce brain damage following reperfusion with rtPA. A particular strength of this study is that targeting Fn-EDA specifically in the plasma improved stroke outcome followed by reperfusion with rtPA in the comorbid condition of hyperlipidemia. A limitation of this study is that we did not confirm these findings in another species or with other preexisting comorbidities including hypertension and aging. Extending these studies to large-animal models with preexisting comorbidities will be required to validate the translational feasibility of our current findings. In summary, our studies unequivocally support a causal connection between Fn-EDA present in the plasma and stroke exacerbation. These observations suggest that interventions targeting plasma Fn-EDA may reduce brain damage following reperfusion with rtPA in the humans.
Supplementary Material
Acknowledgments
Sources of funding
The A.K.C lab is supported by grants from the National Heart, Lung and Blood Institute (R35 HL139926), and National Institute of Neurological Disorders and Stroke (R01 NS109910) of the National Institutes of Health, and by Established Investigator Award 18EIA33900009 from American Heart Association. N.D. is supported by the postdoctoral award 17POST33650044 from American Heart Association.
Footnotes
Disclosures
None
References
- 1.Dietrich WD. Morphological manifestations of reperfusion injury in brain. Ann N Y Acad Sci. 1994;723:15–24 [PubMed] [Google Scholar]
- 2.Yang GY, Betz AL. Reperfusion-induced injury to the blood-brain barrier after middle cerebral artery occlusion in rats. Stroke. 1994;25:1658–1664; discussion 1664–1655 [DOI] [PubMed] [Google Scholar]
- 3.Nieswandt B, Kleinschnitz C, Stoll G. Ischaemic stroke: A thrombo-inflammatory disease? J Physiol. 2011;589:4115–4123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y, Gallant RC, Ni H. Extracellular matrix proteins in the regulation of thrombus formation. Curr Opin Hematol. 2016;23:280–287 [DOI] [PubMed] [Google Scholar]
- 5.Dhanesha N, Ahmad A, Prakash P, Doddapattar P, Lentz SR, Chauhan AK. Genetic ablation of extra domain a of fibronectin in hypercholesterolemic mice improves stroke outcome by reducing thrombo-inflammation. Circulation. 2015;132:2237–2247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chorawala MR, Prakash P, Doddapattar P, Jain M, Dhanesha N, Chauhan AK. Deletion of extra domain a of fibronectin reduces acute myocardial ischaemia/reperfusion injury in hyperlipidaemic mice by limiting thrombo-inflammation. Thromb Haemost. 2018;118:1450–1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanters SD, Banga JD, Algra A, Frijns RC, Beutler JJ, Fijnheer R. Plasma levels of cellular fibronectin in diabetes. Diabetes Care. 2001;24:323–327 [DOI] [PubMed] [Google Scholar]
- 8.Lemanska-Perek A, Krzyzanowska-Golab D, Pupek M, Klimeczek P, Witkiewicz W, Katnik-Prastowska I. Analysis of soluble molecular fibronectin-fibrin complexes and eda-fibronectin concentration in plasma of patients with atherosclerosis. Inflammation. 2016;39:1059–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peters JH, Maunder RJ, Woolf AD, Cochrane CG, Ginsberg MH. Elevated plasma levels of ed1+ (“cellular”) fibronectin in patients with vascular injury. J Lab Clin Med. 1989;113:586–597 [PubMed] [Google Scholar]
- 10.Cherian P, Hankey GJ, Eikelboom JW, Thom J, Baker RI, McQuillan A, et al. Endothelial and platelet activation in acute ischemic stroke and its etiological subtypes. Stroke. 2003;34:2132–2137 [DOI] [PubMed] [Google Scholar]
- 11.Blum A, Vaispapir V, Keinan-Boker L, Soboh S, Yehuda H, Tamir S. Endothelial dysfunction and procoagulant activity in acute ischemic stroke. J Vasc Interv Neurol. 2012;5:33–39 [PMC free article] [PubMed] [Google Scholar]
- 12.Kang YH, Williams R. Endotoxin-induced endothelial injury and subendothelial accumulation of fibronectin in rat aorta. Anat Rec. 1991;229:86–102 [DOI] [PubMed] [Google Scholar]
- 13.Rizk T, Rebres R, Vincent P, Lewis E, McKeown-Longo P, Saba TM. Ed1-containing cellular fibronectin release into lung lymph during lung vascular injury with postoperative bacteremia. Am J Physiol. 1993;264:L66–73 [DOI] [PubMed] [Google Scholar]
- 14.Macarak EJ, Gorfien S, MacGregor RR. Modulation of endothelial fibronectin synthesis by polymorphonuclear granulocytes. J Cell Physiol. 1989;139:517–523 [DOI] [PubMed] [Google Scholar]
- 15.Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, et al. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40:2244–2250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muro AF, Chauhan AK, Gajovic S, Iaconcig A, Porro F, Stanta G, et al. Regulated splicing of the fibronectin eda exon is essential for proper skin wound healing and normal lifespan. The Journal of cell biology. 2003;162:149–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dhanesha N, Vazquez-Rosa E, Cintron-Perez CJ, Thedens D, Kort AJ, Chuong V, et al. Treatment with uric acid reduces infarct and improves neurologic function in female mice after transient cerebral ischemia. J Stroke Cerebrovasc Dis. 2018;27:1412–1416 [DOI] [PubMed] [Google Scholar]
- 18.Moretti FA, Chauhan AK, Iaconcig A, Porro F, Baralle FE, Muro AF. A major fraction of fibronectin present in the extracellular matrix of tissues is plasma-derived. J Biol Chem. 2007;282:28057–28062 [DOI] [PubMed] [Google Scholar]
- 19.Garcia JH, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke. 1995;26:627–634; discussion 635 [DOI] [PubMed] [Google Scholar]
- 20.Kumar AD, Boehme AK, Siegler JE, Gillette M, Albright KC, Martin-Schild S. Leukocytosis in patients with neurologic deterioration after acute ischemic stroke is associated with poor outcomes. J Stroke Cerebrovasc Dis. 2013;22:e111–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Segel GB, Halterman MW, Lichtman MA. The paradox of the neutrophil’s role in tissue injury. J Leukoc Biol. 2011;89:359–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhattacharyya S, Tamaki Z, Wang W, Hinchcliff M, Hoover P, Getsios S, et al. Fibronectineda promotes chronic cutaneous fibrosis through toll-like receptor signaling. Sci Transl Med. 2014;6:232ra250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peters JH, Sporn LA, Ginsberg MH, Wagner DD. Human endothelial cells synthesize, process, and secrete fibronectin molecules bearing an alternatively spliced type iii homology (ed1). Blood. 1990;75:1801–1808 [PubMed] [Google Scholar]
- 24.Tan MH, Sun Z, Opitz SL, Schmidt TE, Peters JH, George EL. Deletion of the alternatively spliced fibronectin eiiia domain in mice reduces atherosclerosis. Blood. 2004;104:11–18 [DOI] [PubMed] [Google Scholar]
- 25.Castellanos M, Leira R, Serena J, Blanco M, Pedraza S, Castillo J, et al. Plasma cellular-fibronectin concentration predicts hemorrhagic transformation after thrombolytic therapy in acute ischemic stroke. Stroke. 2004;35:1671–1676 [DOI] [PubMed] [Google Scholar]
- 26.Sakai T, Johnson KJ, Murozono M, Sakai K, Magnuson MA, Wieloch T, et al. Plasma fibronectin supports neuronal survival and reduces brain injury following transient focal cerebral ischemia but is not essential for skin-wound healing and hemostasis. Nat Med. 2001;7:324–330 [DOI] [PubMed] [Google Scholar]
- 27.Khan MM, Gandhi C, Chauhan N, Stevens JW, Motto DG, Lentz SR, et al. Alternatively-spliced extra domain a of fibronectin promotes acute inflammation and brain injury after cerebral ischemia in mice. Stroke. 2012;43:1376–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prakash P, Kulkarni PP, Lentz SR, Chauhan AK. Cellular fibronectin containing extra domain a promotes arterial thrombosis in mice through platelet toll-like receptor 4. Blood. 2015;125:3164–3172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chauhan AK, Kisucka J, Cozzi MR, Walsh MT, Moretti FA, Battiston M, et al. Prothrombotic effects of fibronectin isoforms containing the eda domain. Arterioscler Thromb Vasc Biol. 2008;28:296–301 [DOI] [PubMed] [Google Scholar]
- 30.Spinelli SL, Casey AE, Pollock SJ, Gertz JM, McMillan DH, Narasipura SD, et al. Platelets and megakaryocytes contain functional nuclear factor-kappab. Arterioscler Thromb Vasc Biol. 2010;30:591–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Konieczynska M, Bryk AH, Malinowski KP, Draga K, Undas A. Interplay between elevated cellular fibronectin and plasma fibrin clot properties in type 2 diabetes. Thromb Haemost. 2017;117:1671–1678 [DOI] [PubMed] [Google Scholar]
- 32.Okamura Y, Watari M, Jerud ES, Young DW, Ishizaka ST, Rose J, et al. The extra domain a of fibronectin activates toll-like receptor 4. J Biol Chem. 2001;276:10229–10233 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.