Abstract
Background:
Improved understanding of the relationship between patient age and acute respiratory distress syndrome (ARDS) development and mortality following traumatic injury may help facilitate generation of new hypotheses about ARDS pathophysiology and the role of novel treatments to improve outcomes across the age spectrum.
Methods:
We conducted a retrospective cohort study of trauma patients included in the National Trauma Data Bank who were admitted to an intensive care unit from 2007–2016. We determined ARDS incidence and mortality across eight age groups for the entire 10-year study period and by year. We used generalized linear Poisson regression models adjusted for underlying mortality risk (injury mechanism, Injury Severity Score, admission Glasgow Coma Scale score, admission heart rate, and admission hypotension).
Results:
ARDS occurred in 3.1% of 1,297,190 trauma encounters. ARDS incidence was lowest among pediatric patients and highest among adults ages 35–64. ARDS mortality was highest among patients ≥80 years (43.9%) followed by 65–79 years (30.6%) and ≤4 years (25.3%). The relative risk of mortality associated with ARDS was highest among the pediatric age groups, with an adjusted relative risk (aRR) of 2.06 (95% CI 1.72–2.70) among patients ≤4 years old compared to an aRR of 1.51 (95% CI 1.42–1.62) for the entire cohort. ARDS mortality increased over the ten-year study period (aRR 1.03/year, 95% CI 1.02–1.05), while all-cause mortality decreased (aRR 0.98/year, 95% CI 0.98–0.99).
Conclusions:
While ARDS development following traumatic injury was most common in middle-aged adults, patients ≤4 years and ≥65 years with ARDS experienced the highest burden of mortality. Children ≤4 years were disproportionately affected by ARDS relative to their low underlying mortality following trauma that was not complicated by ARDS. ARDS-associated mortality following trauma has worsened over the past decade, emphasizing the need for new prevention and treatment strategies.
Keywords: Acute respiratory distress syndrome, hospital mortality, intensive care units, child
Background
Acute respiratory distress syndrome (ARDS) is a life-threatening complication of many pulmonary and extra-pulmonary insults that impacts patients across the age spectrum. Severe traumatic injury is a well-described trigger for ARDS, with 5–10% of adult trauma patients1–4 and up to 19% of adult trauma patients requiring intensive care unit (ICU) care5 developing ARDS. Most studies of injured adults with ARDS have found a mortality rate of 16–24%,2,3,6–9 with estimates as high as 35–45% in severely ill cohorts.1,10 The epidemiology of ARDS in the pediatric trauma population is not well described; the only study to date found an incidence of 1.8% with 20.0% mortality.11 Direct comparisons of ARDS epidemiology among trauma patients across the age spectrum have not been performed, and may facilitate improved understanding of ARDS pathophysiology following traumatic injury.
In general, pediatric ARDS of all etiologies is associated with lower incidence and mortality than ARDS in adults.12,13 Population-based ARDS-associated mortality rates are lowest in young children and rise with each sequentially older age group.13 The trauma population, however, has different demographic characteristics and underlying risk factors compared to patients with ARDS due to other etiologies such as sepsis or pneumonia. Adult trauma patients tend to be younger than adults hospitalized with medical conditions14,15 and have lower rates of immunosuppression and chronic comorbidities,15,16 which are associated with higher ARDS mortality.17,18 In contrast, pediatric trauma patients tend to be older than pediatric medical ICU patients.19,20 It is therefore unclear whether the trends across the age spectrum seen in ARDS as a whole are true for the trauma population as well.
It is also unknown whether trends in ARDS mortality over time are similar for the trauma population and for patients of different ages. A meta-analysis of adult ARDS of all etiologies found a linear reduction in mortality from 1994–2006,21 and population-based ARDS mortality rates decreased from 1999–2013.13 In contrast, a meta-analysis found no decrease in pediatric ARDS mortality from 1994–2014.22 In the trauma population, several adult single-center studies found declining mortality rates in the 1980s-1990s,23–25 but more recent data have not been published.
The aims of this study were to evaluate how the incidence and all-cause mortality associated with post-traumatic ARDS differ across the age spectrum, and how ARDS-associated mortality has changed over the past decade among different age groups. We hypothesized that pediatric patients would have a lower incidence of ARDS compared to adults, but that ARDS-associated mortality would be highest among both the youngest and oldest patients. Improved understanding of how the risk for ARDS development and subsequent mortality varies across the trauma population and over time is essential to be able to target improved prevention and treatment strategies for the highest risk groups and monitor progress over time.
Methods
Study design and participants:
We conducted a retrospective analysis of the National Trauma Data Bank (NTDB)26 research datasets from 2007–2016 to identify all trauma patients with ≥1 ICU day at a Level I or II adult or pediatric trauma center, based on either state or American College of Surgeons designation. We identified encounters with and without ARDS recorded in the NTDB as a hospital complication. The NTDB defined ARDS by American-European Consensus Conference (AECC) criteria27 through 2011, modified Berlin criteria28 from 2012–2014, and full Berlin criteria from 2015–2016 (Supplemental Digital Content 1). NTDB registrars record ARDS for patients who meet the designated criteria during the initial post-injury hospitalization based on review of physician notes, radiology reports, respiratory notes, lab reports, nursing charting, and discharge summaries.
We excluded patients who sustained burn- or drowning-related injuries given a different physiologic mechanism and risk for ARDS development than patients with traumatic injury. We also excluded patients from 14 facilities (n=22,737, 1.7%) that did not routinely report hospital complications to the NTDB. Finally, we excluded patients with no age recorded (n=37,631, 3.0%). The NTDB has not identified age of patients >90 years since 2011 and <1 year since 2016 due to privacy concerns.
Outcomes:
We assessed the incidence and in-hospital case-fatality of ARDS among eight age categories: ≤4 years, 5–12 years, 13–17 years, 18–34 years, 35–49 years, 50–64 years, 65–79 years, and ≥80 years. Age categories were initially developed a priori based on developmental stage and published literature on pediatric age-based vital signs,29,30 and combined post-hoc at natural cut-points for ARDS incidence and mortality. We evaluated whether ARDS mortality changed over the ten-year study period for the entire population and among the different age groups. We also determined the frequency of post-discharge care (home care, inpatient rehabilitation, skilled nursing facilities, long-term care facilities, and other inpatient care) among survivors to hospital discharge.
Comorbidities:
The NTDB records the presence of 28 pre-existing conditions, from which we calculated a modified Charlson Comorbidity Index (mCCI)31 based on algorithms previously published using the NTDB.32,33 We did not include age in the mCCI to allow comparison of comorbidity status across the age spectrum. We grouped mCCI comorbidities into seven categories for analysis (respiratory, cardiovascular, renal, hepatic, neurologic, diabetes, and cancer). Our algorithm is detailed in Supplemental Digital Content 2.
Confounders:
We assessed crude mortality rates and mortality risk adjusted for known confounders. The choice of covariates to use for risk adjustment was based on Haider et al.’s description of the minimum set of confounders needed to adequately adjust for underlying risk of mortality in the NTDB.34 Those authors found that the best risk adjustment model, with an area under the receiver operating characteristics curve of 0.9578 for mortality prediction, included six covariates: age, Injury Severity Score (ISS), admission heart rate, admission hypotension, total Glasgow Coma Scale (GCS) score at admission, and need for ventilator use. Injury mechanism was added to a model for patients with an ISS >15. Given that the median ISS of our cohort was 15, we included injury mechanism in our risk adjustment model; we also adjusted for comorbidity burden using mCCI. We excluded mechanical ventilation as a covariate given collinearity with ARDS. We also adjusted all analyses for admission year and transfer status. For analyses in which age was a stratifying variable, we thus adjusted for mCCI, total ISS, admission heart rate, admission hypotension, total admission GCS, injury mechanism, year, and transfer status; analyses with year as a stratifying variable included linear splines of age as covariates. Hypotension and heart rate were categorized using age-adjusted normative values for patients ≤17 years old,29,35 and per Haider et al.’s categorization for adults.34
Statistical analysis:
We determined the distribution of demographic, injury, and clinical characteristics for patients with and without ARDS for the entire cohort and by age group. We assessed the frequency of any comorbidity (mCCI ≥1) among patients with and without ARDS and by age group, and the type of comorbidity by mCCI category. We estimated associations between the presence of any comorbidity and each comorbidity category with ARDS development and death using bivariate generalized linear Poisson regression models.
We calculated the incidence of ARDS for each of the age groups and compared the incidence across age groups using a generalized linear Poisson regression model. We determined frequency of mortality and post-discharge care for patients with and without ARDS for each age group and estimated associations between ARDS and risk of death and post-discharge care for each age group using bivariate generalized linear Poisson regression models. We then estimated adjusted associations between age group and ARDS development, death, and post-discharge care by including the pre-determined covariates in multivariable Poisson regression models. To evaluate for potential survivor bias as the date of onset of ARDS is not included in the NTDB, we conducted sensitivity analyses of our primary models including only patients with a length of stay (LOS) of ≥3 days.
We then calculated the annual all-cause mortality rate and mortality rate for patients with ARDS from 2007–2016. We compared the risk of death by year and determined the annual linear trend for mortality for each of the age groups using bivariate and multivariable Poisson regression. We clustered all bivariate and multivariable regressions by facility. We conducted all analyses using Stata/SE 14.2 statistical software (StataCorp LP, College Station, TX).
This study was exempt from review by the University of Washington Institutional Review Board as data were de-identified and not considered to involve human subjects.
Results
Characteristics of the patient population
Over the ten years of NTDB data evaluated, 1,297,190 trauma encounters from 474 facilities met inclusion criteria, including encounters with 148,749 pediatric patients ≤17 years old (11.5%), 808,834 patients 18–64 years old (62.4%), and 339,607 patients ≥65 years old (26.2%). The overall incidence of ARDS among all encounters was 3.1% (n=39,732). Patients with ARDS were more likely to be male (73.6% vs 68.9%) and non-Hispanic white (70.1% vs 65.3%), and more commonly had an mCCI ≥1 (27.5% vs 21.6%). The most common mechanism of injury among ARDS patients was motor vehicle crashes (41.9%), while falls were the most common injury mechanism among non-ARDS patients (35.4%). The rate of serious head injury (Abbreviated Injury Scale severity score ≥3) was similar (47.6% vs 44.1%), but ARDS patients had a higher occurrence of serious chest injury (48.3% vs 27.0%). ARDS patients had a higher median ISS (22 vs 14) and were more likely to have an abnormal heartrate and hypotension on admission (Table 1).
Table 1:
Characteristics of intensive care unit patients with and without ARDS included in the National Trauma Data Bank, 2007–2016
| Patient or injury characteristic | Patients with ARDS No. (%) (n=39,732, 3.1%) | Patients without ARDS No. (%) (n=1,257,458, 96.9%) |
|---|---|---|
| Age (years), mean (± SD) | 47.4 (+22.0) | 46.0 (+24.0) |
| Male gender | 29,225 (73.6) | 865,322 (68.9) |
| Race/ethnicity | ||
| Non-Hispanic White | 27,828 (70.1) | 819,496 (65.3) |
| Non-Hispanic African American | 5,620 (14.2) | 156,814 (12.5) |
| Hispanic | 3,339 (8.4) | 149,237 (11.9) |
| Asian/Pacific Islander | 808 (2.0) | 29,115 (2.3) |
| Other/Unknown/Multiracial | 2,086 (5.3) | 99,835 (8.0) |
| Modified Charlson Comorbidity Index ≥1 | 10,907 (27.5) | 271,769 (21.6) |
| Mechanism of injury | ||
| Motor vehicle crash | 16,491 (41.9) | 378,718 (30.4) |
| Fall | 10,534 (26.8) | 441,127 (35.4) |
| Pedestrian/cyclist | 3,213 (8.2) | 99,946 (8.0) |
| Firearm | 2,920 (7.4) | 78,617 (6.3) |
| Struck by/against | 1,530 (3.9) | 82,394 (6.6) |
| Other | 4,650 (11.8) | 164,693 (13.2) |
| Injury type | ||
| Blunt | 33,791 (86.1) | 1059,970 (85.7) |
| Penetrating | 3,730 (9.5) | 119,694 (9.7) |
| Other | 1,719 (4.4) | 57,884 (4.7) |
| Injury Severity Score, median (IQR) | 22 (14–30) | 14 (9–22) |
| Head injury with AIS ≥3 | 18,448 (47.6) | 542,839 (44.1) |
| Chest injury with AIS ≥3 | 18,730 (48.3) | 332,200 (27.0) |
| Hypotensive on admissiona | 4,030 (10.4) | 60,760 (5.0) |
| Heart rate on admissiona | ||
| Pulseless | 194 (0.5) | 3,017 (0.3) |
| Bradycardic | 2,025 (5.2) | 65,347 (5.3) |
| Normal | 18,599 (48.1) | 732,345 (59.6) |
| Tachycardic | 17,869 (46.2) | 427,769 (34.8) |
| Glasgow Coma Scale score on admission | ||
| 3 | 12,351 (32.3) | 164,136 (13.7) |
| 4–5 | 1,065 (2.8) | 16,356 (1.4) |
| 6–8 | 2,850 (7.5) | 52,785 (4.4) |
| 9–12 | 2,937 (7.7) | 69,163 (5.8) |
| 13–15 | 19,018 (49.8) | 899,675 (74.8) |
The distribution of demographic and injury characteristics differed by age group (Supplemental Digital Content 3). Patients ≤17 years were less commonly non-Hispanic white, had fewer comorbidities, and were more commonly involved in pedestrian/cyclist injuries compared to adult patients. Adults ≥65 years were more evenly split by gender compared to the younger age groups, and nearly 50% had an mCCI ≥1. Over 70% of injuries in this age group were due to falls. Adults ≥65 years had a higher GCS and lower frequency of abnormal hemodynamics on presentation compared to the younger age groups. Adults 18–64 years had the highest median ISS, highest frequency of serious chest injury, lowest presenting GCS, and highest frequency of hypotension compared to younger and older patients.
Association of comorbidities with ARDS incidence and mortality:
The presence of an mCCI ≥1 was associated with a 36% higher risk of developing ARDS relative to having an mCCI of 0 (95% CI 1.23–1.49). Of the seven mCCI categories evaluated, respiratory disease, cardiovascular disease, hepatic disease, and diabetes were all associated with higher risk of ARDS relative to not having those conditions, but there was no association between chronic renal failure, neurologic conditions, or cancer with ARDS development. Among patients with ARDS, the risk of mortality was 36% higher among patients with an mCCI ≥1 relative to those with an mCCI of 0 (95% CI 1.29–1.44). The risk of death associated with ARDS was highest among patients with renal disease, cancer, and hepatic disease (Table 2).
Table 2:
Association between comorbid conditions and ARDS development and mortality
| Comorbidity | ARDS (%) | No ARDS (%) | ARDS Development | ARDS Mortality | ||
|---|---|---|---|---|---|---|
| RRa | 95% CI | RRb | 95% CI | |||
| mCCI ≥1 | 10,907 (27.5) | 271,769 (21.6) | 1.36 | 1.23 – 1.49 | 1.36 | 1.29 – 1.44 |
| Respiratory | 3,564 (9.0) | 78,962 (6.3) | 1.45 | 1.31 – 1.61 | 1.08 | 1.01 – 1.17 |
| Cardiovascular | 2,807 (7.1) | 52,451 (4.2) | 1.71 | 1.52 – 1.92 | 1.51 | 1.42 – 1.62 |
| Renal | 393 (1.0) | 12,019 (1.0) | 1.03 | 0.86 – 1.25 | 1.90 | 1.69 – 2.14 |
| Hepatic | 625 (1.6) | 10,409 (0.8) | 1.86 | 1.61 – 2.16 | 1.78 | 1.60 – 1.98 |
| Neurologic | 2,157 (5.4) | 62,246 (5.0) | 1.10 | 0.95 – 1.27 | 1.57 | 1.45 – 1.69 |
| Diabetes/PVD | 5,240 (13.2) | 136,399 (10.9) | 1.24 | 1.14 – 1.35 | 1.22 | 1.16 – 1.29 |
| Cancer | 289 (0.7) | 9,719 (0.8) | 0.94 | 0.77 – 1.15 | 1.83 | 1.57 – 2.13 |
ARDS, acute respiratory distress syndrome; RR, relative risk; CI, confidence interval; mCCI, modified Charlson Comorbidity Index; PVD, peripheral vascular disease
Risk of ARDS relative to patients without that comorbidity
Risk of death among patients with ARDS relative to not having that comorbidity
ARDS incidence across ages:
The incidence of an ARDS diagnosis was lowest among 5–12-year-olds (1.4%) and ≤4-year-olds (1.6%), and highest among 35–49-year-olds (3.5%) and 50–64-year-olds (3.5%). After adjusting for the predetermined covariates, all pediatric age groups were less likely to receive an ARDS diagnosis relative to patients ages 18–34, and all older age groups were more likely (Supplemental Digital Content 4).
ARDS mortality across ages:
The overall in-hospital all-cause mortality rate among ARDS patients was 22.8% compared to a mortality rate of 8.0% among non-ARDS patients. The unadjusted RR for mortality among ARDS patients compared to patients without ARDS was 2.84 (95% CI 2.66–3.03). After adjusting for underlying risk of mortality, the adjusted RR for death associated with ARDS was 1.51 (95% CI 1.42–1.62). Median time to death was 8 days (IQR 3–15) among ARDS patients and 3 days (IQR 1–8) among patients without ARDS; time to death was shortest among patients ≤4 years for both ARDS (3 days, IQR 2–5) and non-ARDS cohorts (2 days, IQR 1–4) and longer with each advancing age group until age 65–79 (ARDS 10 days [4–17]; non-ARDS 4 days [2–10]).
ARDS mortality was highest at the extremes of the age spectrum (Figure 1). Mortality was 25.3% among patients ≤4 years with ARDS, fell to <20% for all age groups between 5 and 49 years, and then rose again to 21.5% for 50–64-year-olds, 30.6% for 65–79-year-olds, and 43.9% among patients ≥80 years. In contrast, mortality among patients without ARDS was low across the pediatric age spectrum at 5.3% among patients ≤4 years, dropping to 3.0% among 5–12-year-olds, then rising steadily with each sequentially older age group to a peak of 15.8% among those ≥80 years.
Figure 1:

Frequency of mortality among patients with and without ARDS by age
The risk of mortality among patients with ARDS relative to patients without ARDS was highest among the three pediatric age groups and lower with each subsequent age group to a nadir among patients aged 65–79 years followed by a slight rise among patients ≥80 years. After adjustment for underlying mortality risk, patients ≤4 years experienced the highest risk of mortality associated with ARDS (aRR 2.06, 95% CI 1.72–2.70) (Table 3).
Table 3:
Risk of death associated with ARDS, stratified by age group
| Age Group | ARDS deaths (%) | Non-ARDS deaths (%) | Unadjusted RR | 95% CI | Adjusted RRa | 95% CI |
|---|---|---|---|---|---|---|
| Entire Cohort | 9045 (22.8) | 100699 (8.0) | 2.84 | 2.66 – 3.03 | 1.51 | 1.42 – 1.62 |
| ≤4 years | 169 (25.3) | 2209 (5.3) | 4.78 | 3.86 – 5.93 | 2.06 | 1.72 – 2.70 |
| 5–12 years | 89 (15.8) | 1193 (3.0) | 5.34 | 4.20 – 6.79 | 1.92 | 1.44 – 2.57 |
| 13–17 years | 265 (19.3) | 2913 (4.6) | 4.21 | 3.74 – 4.74 | 1.30 | 1.15 – 1.47 |
| 18–34 years | 1809 (17.1) | 18536 (5.7) | 2.97 | 2.70 – 3.27 | 1.30 | 1.20 – 1.41 |
| 35–49 years | 1316 (17.3) | 12668 (6.0) | 2.91 | 2.64 – 3.19 | 1.35 | 1.22 – 1.50 |
| 50–64 years | 1907 (21.5) | 19284 (8.0) | 2.70 | 2.49 – 2.93 | 1.47 | 1.36 – 1.60 |
| 65–79 years | 1973 (30.6) | 22353 (11.7) | 2.62 | 2.47 – 2.79 | 1.67 | 1.56 – 1.80 |
| ≥80 years | 1517 (43.9) | 21543 (15.8) | 2.78 | 2.62 – 2.96 | 1.87 | 1.73 – 2.03 |
ARDS, acute respiratory distress syndrome; RR, relative risk; CI, confidence interval
Adjusted relative risks are adjusted for modified Charlson Comorbidity Index, injury mechanism, Injury Severity Score, admission heart rate, admission hypotension, admission total Glasgow Coma Scale score, year, and transfer status
Among patients with ARDS, the risk of ARDS-associated mortality was highest among patients ≥80 years relative to those 18–34 years both before and after adjustment for underlying risk of mortality, followed by patients 65–79 years and patients ≤4 years (Table 4). Exclusion of patients with a LOS <3 days did not change the trends in ARDS-associated mortality across age groups (Supplemental Digital Content 5).
Table 4:
Risk of in-hospital mortality among patients with ARDS by age group
| Age Group | Number of deaths (%) | Unadjusted RR | 95% CI | Adjusted RRa | 95% CI |
|---|---|---|---|---|---|
| ≤4 years | 169 (25.3) | 1.48 | 1.21 – 1.82 | 1.53 | 1.26 – 1.86 |
| 5–12 years | 89 (15.8) | 0.93 | 0.75 – 1.15 | 0.96 | 0.77 – 1.19 |
| 13–17 years | 265 (19.3) | 1.13 | 1.01 – 1.26 | 1.11 | 1.00 – 1.23 |
| 18–34 years | 1809 (17.1) | Ref | Ref | ||
| 35–49 years | 1316 (17.3) | 1.01 | 0.95 – 1.08 | 1.09 | 1.02 – 1.17 |
| 50–64 years | 1907 (21.5) | 1.26 | 1.18 – 1.34 | 1.44 | 1.35 – 1.53 |
| 65–79 years | 1973 (30.6) | 1.79 | 1.66 – 1.93 | 2.19 | 2.03 – 2.36 |
| ≥80 years | 1517 (43.9) | 2.57 | 2.34 – 2.83 | 3.36 | 3.08 – 3.67 |
ARDS, acute respiratory distress syndrome; RR, relative risk; CI, confidence interval
Adjusted relative risks are adjusted for modified Charlson Comorbidity Index, injury mechanism, Injury Severity Score, admission heart rate, admission hypotension, admission total Glasgow Coma Scale score, year, and transfer status
Trends over time:
The annual mortality rate for ARDS gradually rose over the course of the ten years evaluated, from 20.5–21.3% in 2007–2009 up to 26.6–27.4% in 2014–2016 (Figure 2). The risk of mortality among ARDS patients increased by 3% per year (95% CI 1.02–1.05) both before and after risk adjustment. All-cause mortality ranged from 8.2–9.7% annually, with a slight negative linear trend before (RR 0.98/year, 95% CI 0.97–0.98) and after (aRR 0.98/year, 95% CI 0.98–0.99) risk adjustment. All age groups between 18 and 79 years had a rising annual ARDS mortality rate, while the remaining age groups did not demonstrate any linear trend in mortality over the course of the study period.
Figure 2:
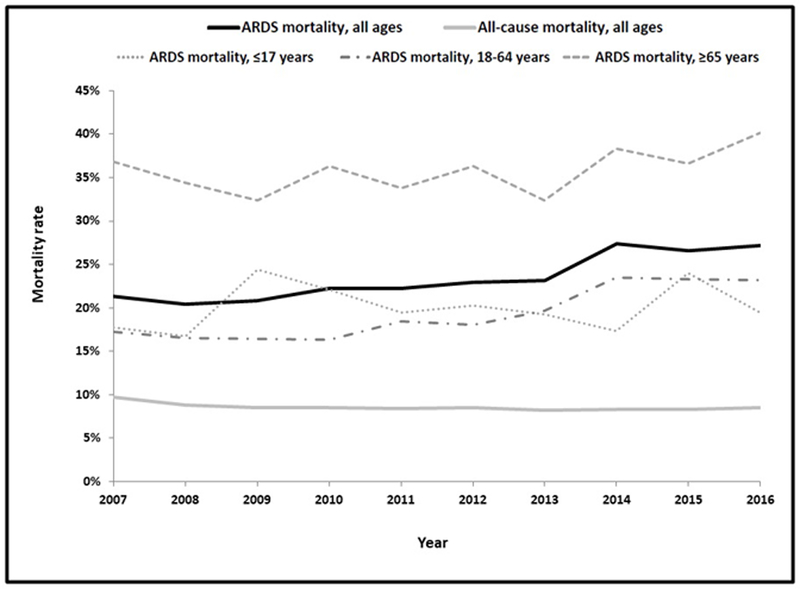
Trends in mortality among patients with ARDS from 2007–2016, stratified by age group
Outcomes among survivors:
Among survivors to hospital discharge, median LOS was 20 days (IQR 12–32) for patients with ARDS and 6 days (IRQ 3–12) for patients without ARDS; LOS was shortest in the youngest and oldest age groups. LOS increased slightly over the course of the study duration for ARDS survivors (0.30 days/year, 95% CI 0.20–0.40) and decreased for non-ARDS patients (−0.08 days/year, −0.09 – −0.07). Of patients who survived ARDS, 71.1% required ongoing care after discharge, compared to 42.7% of survivors without ARDS. The frequency of post-discharge care was lowest among patients ≤4 years both with ARDS (41.5%) and without ARDS (10.5%), and rose with sequentially older age groups to a peak among patients ≥80 years, with 92.8% of ARDS survivors and 77.7% of survivors without ARDS requiring post-discharge care. Patients of all ages with ARDS were significantly more likely than patients without ARDS to require post-discharge care after risk adjustment (Supplemental Digital Content 6).
Discussion
This ten-year evaluation of nearly 1.3 million encounters with critically injured trauma patients demonstrates that the risk of ARDS development and ARDS-associated all-cause mortality following traumatic injury varied considerably across the age spectrum. While middle-aged adults represented the majority of the trauma population included in the NTDB and were the most likely to be diagnosed with ARDS, patients at the extremes of age experienced the highest burden of mortality associated with ARDS. The risk of death among patients ≤4 years and those ≥65 years remained disproportionately elevated even after adjustment for comorbidities, injury mechanism and severity, hemodynamic abnormalities, and GCS.
The lower incidence of ARDS among older adults appears to be largely related to their lower injury severity, higher GCS, and less hemodynamic instability compared to younger patients. Before adjustment for these covariates, the unadjusted risk of ARDS was highest among 35–64-year-olds and sequentially lower in patients aged 65–79 years and ≥80 years, consistent with prior literature in trauma patients.36 After risk adjustment, however, patients age 65 and older had the highest risk of ARDS development of any of the age groups. In contrast, pediatric patients had relatively similar injury severity, GCS, and hemodynamics compared to adults, suggesting that the lower incidence of ARDS in the pediatric population may be more attributable to differences in underlying biologic mechanisms of ARDS development or differences in diagnosis patterns given the use of adult ARDS criteria.
While patients at the extremes of age were the least likely to be diagnosed with ARDS, they were the most likely to die if they did develop it. Previous studies of adults with ARDS of all etiologies have consistently established a higher risk of mortality with advancing age,13,37,38 and our data demonstrate that this remains true in the trauma population despite less severe illness at admission among patients over age 65 compared to younger adults. Because older patients experienced a high risk for mortality both before and after adjustment for confounders, many of the factors contributing to such high mortality may be unrelated to the injury itself or the clinical status at the time of hospital presentation. The role of underlying comorbidities in ARDS development and mortality is well-described;38–40 the fact that nearly 50% of adults ≥65 had an mCCI ≥1 compared to only 14% of younger adults and 5% of pediatric patients likely contributed greatly to their susceptibility to ARDS despite their lower injury severity. This is consistent with previous findings demonstrating that the presence of pre-existing medical conditions in trauma patients has a substantial impact on mortality among patients with minor and moderate injuries, while comorbidities do not further increase the risk for mortality among patients with severe injuries.16 Older adults may also have been more likely than younger adults and children to have had limitations of life-sustaining therapy following an ARDS diagnosis given the known high risk of mortality associated with ARDS in these age groups.13,37,38
While the risk of mortality among patients with ARDS was highest in those ages 65 and older, patients in this age group also experienced the highest non-ARDS mortality. In contrast, pediatric patients experienced low non-ARDS mortality, but a much higher mortality risk associated with ARDS, with 4.2–5.3 times higher mortality among patients who developed ARDS compared to those who did not. This suggests that the variety of factors that provide relative protection against mortality for pediatric trauma patients compared to adults, including differences in injury mechanism, pre-existing health status, biomechanics, and the immunologic and inflammatory response to injury, may not be as protective in the setting of ARDS.
The risk of mortality associated with ARDS was particularly striking among patients age four and younger, whose absolute mortality rate of 25.3% was higher than any other age group until age 65 and older and was nearly five times their non-ARDS mortality rate. This was not explainable by factors present on hospital arrival; compared to older pediatric patients, those ≤4 years had lower rates of comorbidities, lower median ISS, lower frequency of hemodynamic abnormalities, and similar GCS. They did have a markedly higher frequency of serious head injuries compared to patients aged 5–17 (63% versus 44%), but the risk of mortality associated with head injury in this age group was in fact higher among those without ARDS than with ARDS. Previous studies of all-cause ARDS in pediatric patients have not found an association with younger age and higher risk of mortality,17,41 and one evaluation of the Berlin definition in children <18 months with a variety of ARDS etiologies found a mortality rate of 17.2%,42 much lower than the mortality rate in our cohort for this age group. Further evaluation of in-hospital and quality of care factors is needed to better understand why the youngest trauma patients experience such a substantially higher risk of mortality after developing ARDS compared to older trauma patients and young children with medical triggers for ARDS.
The morbidity associated with ARDS among survivors to discharge was also substantial, with prolonged hospitalizations and high rates of post-discharge care across the age spectrum. ARDS survivors were nearly twice as likely to require ongoing care after discharge than non-ARDS patients even after adjusting for factors such as injury severity, serious neurologic injury, and chronic comorbidities. In contrast to the trends observed with mortality, however, the frequency of post-discharge care was lowest among the younger age groups and rose with advancing age; this is most likely attributable to lack of care facilities for young patients, greater ease of administering care in the home, and lower rates of chronic comorbidities.
Finally, we found that ARDS-associated mortality in the trauma population increased steadily over the past decade, in contrast to a general downtrend in mortality for all-cause ARDS from the mid-1990s through 2006–2013.13,21 This does not appear to be due to changes in injury factors or patient demographics, as all-cause mortality in this cohort declined steadily over the same time period. Although changes in fluid and transfusion practices and ventilator strategy may have contributed to improved management of post-traumatic ARDS in the early 2000s,43 our findings suggest that this progress has not been sustained over the most recent decade. It has been demonstrated that adherence to standard treatment protocols is associated with reduction in organ failure and mortality after trauma,44 suggesting that further efforts to develop processes of care across the broader range of trauma centers included in the NTDB may help improve outcomes among critically injured patients.
Limitations:
This study had several limitations. The NTDB expanded considerable over the study period, and thus while it is now felt to be representative of all US trauma centers, trends over the 10-year period may not be nationally representative given changes in the characteristics of the facilities included. Additionally, we cannot verify the accuracy of the ARDS diagnoses as recorded by NTDB registrars. While registrars are given clear guidelines for chart abstraction for ARDS with well-defined and standardized criteria across facilities, there remains a possibility of misclassification bias. The ARDS criteria used by the NTDB changed over the course of the 10-year time period, and thus annual incidence and mortality may have been affected by these definitional changes. Previous studies have found that while ARDS incidence varies slightly between the AECC and Berlin definitions, mortality is very similar in both adult45,46 and pediatric47 patients. We thus elected not to evaluate ARDS incidence by year but believe that annual mortality estimates are likely comparable across the study period. Analyses in which year was not the primary exposure variable were adjusted by year to mitigate any effects of definition changes.
The NTDB has not yet adopted pediatric ARDS consensus criteria (PALICC) published in 2015,48 and thus pediatric patients were diagnosed with ARDS based on adult consensus definitions, which likely underestimate ARDS incidence in children.49 Based on recent studies demonstrating that children with more severe disease are identified by both PALICC criteria and AECC/Berlin criteria,47,50 our cohort likely represents a more severely ill subpopulation of the patients who would have been identified by PALICC criteria.
While we based our risk adjustment strategy on previously published recommendations for evaluating trauma mortality in the NTDB, there may have been additional confounders of the association between ARDS and mortality that we did not include. We also used the same covariates identified for mortality risk adjustment to adjust for ARDS incidence and risk of post-discharge care across age groups, which have not been validated but likely approximate the actual confounders of the association between age and ARDS development and morbidity.
Conclusions:
While post-traumatic ARDS most commonly occurs in middle-aged adults, patients at the extremes of age experience the highest burden of mortality associated with ARDS. Over one-quarter of patients ≤4 years and ≥65 years who develop ARDS after trauma die despite admission with lower injury severity, less hemodynamic instability, and higher GCS compared to the remainder of the trauma population. This is most likely attributable to comorbid medical conditions and frailty in the older population, but pediatric patients experience ARDS mortality after trauma out of proportion to their underlying post-trauma mortality risk and what has been previously reported for pediatric mortality due to other ARDS etiologies. Mortality associated with post-traumatic ARDS has risen steadily over the past decade, underscoring the need for new prevention and treatment strategies to improve outcomes for patients following trauma.
Supplementary Material
Acknowledgments
Sources of Funding: Supported by NICHD grant T32 HD057822-08
Footnotes
Conflicts of Interest: The authors have no conflicts of interest relevant to this article to disclose
References
- 1.Navarrete-Navarro P, Rivera-Fernández R, Rincón-Ferrari MD, García-Delgado M, Muñoz A, Jiménez HM, Ortega FJ, García DM, GITAN multicenter project. Early markers of acute respiratory distress syndrome development in severe trauma patients. J Crit Care 2006. September;21(3):253–8. [DOI] [PubMed] [Google Scholar]
- 2.Chaiwat O, Lang JD, Vavilala MS, Wang J, MacKenzie EJ, Jurkovich GJ, Rivara FP. Early packed red blood cell transfusion and acute respiratory distress syndrome after trauma. Anesthesiology 2009. February;110(2):351–60. [DOI] [PubMed] [Google Scholar]
- 3.Afshar M, Smith GS, Cooper RS, Murthi S, Netzer G. Trauma indices for prediction of acute respiratory distress syndrome. J Surg Res 2016. April;201(2):394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pfeifer R, Neussen N, Michalewicz E, Hilgers RD, Pape HC. Incidence of adult respiratory distress syndrome in trauma patients: A systematic review and meta-analysis over a period of three decades. J Trauma Acute Care Surg 2017. September;83(3):496–506. [DOI] [PubMed] [Google Scholar]
- 5.O’Leary MP, Keeley JA, Yule A, Suruki C, Plurad DS, Moazzez A, Neville AL, Putnam BA, Kim DY. Clinical predictors of early acute respiratory distress syndrome in trauma patients. Am J Surg 2016. December;212(6):1096–100. [DOI] [PubMed] [Google Scholar]
- 6.Ryb GE, Cooper C. Race/ethnicity and acute respiratory distress syndrome: A National Trauma Data Bank study. J Natl Med Assoc 2010. October;102(10):865–9. [DOI] [PubMed] [Google Scholar]
- 7.Ingraham AM, Xiong W, Hemmila MR, Shafi S, Goble S, Neal ML, Nathens AB. The attributable mortality and length of stay of trauma-related complications: A matched cohort study. Ann Surg 2010. August;252(2):358–62. [DOI] [PubMed] [Google Scholar]
- 8.Treggiari MM, Hudson LD, Martin DP, Weiss NS, Caldwell E, Rubenfeld G. Effect of acute lung injury and acute respiratory distress syndrome on outcome in critically ill trauma patients. Crit Care Med 2004. February;32(2):327–31. [DOI] [PubMed] [Google Scholar]
- 9.Shah CV, Localio AR, Lanken PN, Kahn JM, Bellamy S, Gallop R, Finkel B, Gracias VH, Fuchs BD, Christie JD. The impact of development of acute lung injury on hospital mortality in critically ill trauma patients. Crit Care Med 2008. August;36(8):2309–15. [DOI] [PubMed] [Google Scholar]
- 10.Howard BM, Kornblish LZ, Hendrickson CM, Redick BJ, Conroy AS, Nelson MF, Callcut RA, Calfee CS, Cohen MJ. Differences in degree, differences in kind: Characterizing lung injury in trauma. J Trauma Acute Care Surg 2015. April;78(4):735–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Killien EY, Mills B, Watson RS, Vavilala MS, Rivara FP. Morbidity and Mortality Among Critically Injured Children With Acute Respiratory Distress Syndrome. Crit Care Med 2018. October 30 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 12.Zimmerman JJ, Akhtar SR, Caldwell E, Rubenfeld GD. Incidence and outcomes of pediatric acute lung injury. Pediatrics 2009. July;124(1):87–95. [DOI] [PubMed] [Google Scholar]
- 13.Cochi SE, Kempker JA, Annangi S, Kramer MR, Martin GS. Mortality trends of acute respiratory distress syndrome in the United States from 1999–2013. Ann Am Thorac Soc 2016. October;13(10):1742–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minei JP, Schmicker RH, Kerby JD, Stiell IG, Schreiber MA, Bulger E, Tisherman S, Hoyt DB, Nichol G, Resuscitation Outcome Consortium Investigators. Severe traumatic injury: regional variation in incidence and outcome. Ann Surg 2010. July;252(1):149–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wunsch H, Angus DC, Harrison DA, Linde-Zwirble WT, Rowan KM. Comparison of medical admissions to intensive care units in the United States and United Kingdom. Am J Respir Crit Care Med 2011. June;183(12):1666–73. [DOI] [PubMed] [Google Scholar]
- 16.Hollis S, Lecky F, Yates DW, Woodford M. The effect of pre-existing medical conditions and age on mortality after injury. J Trauma 2006. November;61(5):1255–60. [DOI] [PubMed] [Google Scholar]
- 17.Erickson S, Schibler A, Numa A, Nuthall G, Yung M, Pascoe E, Wilkins B, Paediatric Study Group, Australian and New Zealand Intensive Care Society. Acute lung injury in pediatric intensive care in Australia and New Zealand: A prospective, multicenter, observational study. Pediatr Crit Care Med 2007. July;8(4):317–23. [DOI] [PubMed] [Google Scholar]
- 18.Calfee CS, Eisner MD, Ware LB, Thompson BT, Parsons PE, Wheeler AP, Korpak A, Matthay MA, Acute Respiratory Distress Syndrome Network, National Heart, Lung, and Blood Institute. Trauma-associated lung injury differs clinically and biologically from acute lung injury due to other clinical disorders. Crit Care Med 2007. October;35(10):2243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tracy ET, Englum BR, Barbas AS, Foley C, Rice HE, Shapiro ML. Pediatric injury patterns by year of age. J Pediatr Surg 2013. June;48(6):1384–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edwards JD, Houtrow AJ, Vasilevskis EE, Rehm RS, Markovitz BP, Graham RJ, Dudley RA. Chronic conditions among children admitted to U.S. pediatric intensive care units: their prevalence and impact on risk for mortality and prolonged length of stay. Crit Care Med 2012. July;40(7):2196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zambon M, Vincent J. Mortality rates for patients with acute lung injury/ARDS have decreased over time. Chest 2008. May;133(5):1120–7. [DOI] [PubMed] [Google Scholar]
- 22.Schouten LR, Veltkamp F, Bos AP, van Woensel JB, Serpa Neto A, Schultz MJ, Wosten-van Asperen RM. Incidence and mortality of acute respiratory distress syndrome in children: A systematic review and meta-analysis. Crit Care Med 2016. April;44(4):819–29. [DOI] [PubMed] [Google Scholar]
- 23.Navarrete-Navarro P, Rodriguez A, Reynolds N, West R, Habashi N, Rivera R, Chiu WC, Scalea T. Acute respiratory distress syndrome among trauma patients: trends in ICU mortality, risk factors, complications and resource utilization. Intensive Care Med 2001. July;27(7):1133–40. [DOI] [PubMed] [Google Scholar]
- 24.Rocco TR Jr, Reinert SE, Cioffi W, Harrington D, Buczko G, Simms HH. A 9-year single-institution, retrospective review of death rate and prognostic factors in adult respiratory distress syndrome. Ann Surg 2001. March;233(3):414–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milberg JA, Davis DR, Steinberg KP, Hudson LD. Improved survival of patients with acute respiratory distress syndrome (ARDS): 1983–1993. JAMA 1995. January;273(4):306–9. [PubMed] [Google Scholar]
- 26.National Trauma Data Bank. American College of Surgeons https://www.facs.org/quality-programs/trauma/ntdb. Accessed 15 August 2017.
- 27.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American-European Consensus Conference on ARDS: definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994. March;149(3 Pt 1):818–24. [DOI] [PubMed] [Google Scholar]
- 28.ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin definition. JAMA 2012. June;307(23):2526–33. [DOI] [PubMed] [Google Scholar]
- 29.Fleming S, Thompson M, Stevens R, Heneghan C, Pluddemann A, Maconochie I, Tarassenko L, Mant D. Normal ranges of heart rate and respiratory rate in children from birth to 18 years: a systematic review of observational studies. Lancet 2011. March;377(9770):1011–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldstein B, Giroir B, Randolph A; International Consensus Conference on Pediatric Sepsis. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med 2005;6:2–8. [DOI] [PubMed] [Google Scholar]
- 31.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40(5):373–83. [DOI] [PubMed] [Google Scholar]
- 32.Samuel AM, Grant RA, Bohl DD, Basques BA, Webb ML, Lukasiewicz AM, Diaz-Collado PJ, Grauer JN. Delayed surgery after acute traumatic central cord syndrome is associated with reduced mortality. Spine 2015. March;40(5):349–56. [DOI] [PubMed] [Google Scholar]
- 33.Anandasivam NS, Russo GS, Swallow MS, Basques BA, Samuel AM, Ondeck NT, Chung SH, Fischer JM, Bohl DD, Grauer JN. Tibial shaft fracture: A large-scale study defining the injured population and associated injuries. J Clin Orthop Trauma 2017. Jul-Sep;8(3):225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haider AH, Hashmi ZG, Zafar SN, Castillo R, Haut ER, Schneider EB, Cornwell EE, Mackenzie EJ, Efron DT. Developing best practices to study trauma outcomes in large databases: An evidence-based approach to determine the best mortality risk adjustment model. J Trauma Acute Care Surg 2014. April;76(4):1061–9. [DOI] [PubMed] [Google Scholar]
- 35.Kleinman ME, Chameides L, Schexnayder SM, Samson RA, Hazinski MF, Atkins DL, Berg MD, de Caen AR, Fink EL, Freid EB, et al. Part 14: Pediatric advanced life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2010. November;122(18 Suppl 3):S876–908. [DOI] [PubMed] [Google Scholar]
- 36.Johnston CJ, Rubenfeld GD, Hudson LD. Effect of age on the development of ARDS in trauma patients. Chest 2003. August;124(2):653–9. [DOI] [PubMed] [Google Scholar]
- 37.Eachempati SR, Hydo LJ, Shou J, Barie PS. Outcomes of acute respiratory distress syndrome (ARDS) in elderly patients. J Trauma 2007. August;63(2):344–50. [DOI] [PubMed] [Google Scholar]
- 38.Zilberberg MD, Epstein SK. Acute lung injury in the medical ICU: Comorbid conditions, age, etiology, and hospital outcome. Am J Respir Crit Care Med 1998. April;157(4 Pt 1):1159–64. [DOI] [PubMed] [Google Scholar]
- 39.Ando K, Doi T, Moody SY, Ohkuni Y, Sato S, Kaneko N. The effect of comorbidity on the prognosis of acute lung injury and acute respiratory distress syndrome. Intern Med 2012. July;51(14):1835–40. [DOI] [PubMed] [Google Scholar]
- 40.Azoulay E, Lemiale V, Mourvillier B, Garrouste-Orgeas M, Schwebel C, Ruckly S, Argaud L, Cohen Y, Souweine B, Papazian L, et al. ; OUTCOMEREA Study Group. Management and outcomes of acute respiratory distress syndrome patients with and without comorbid conditions. Intensive Care Med. Epub 2018. June 7. [DOI] [PMC free article] [PubMed]
- 41.Flori HR, Glidden DV, Rutherford GW, Matthay MA. Pediatric acute lung injury: Prospective evaluation of risk factors associated with mortality. Am J Respir Crit Care Med 2005. May;171(9):995–1001. [DOI] [PubMed] [Google Scholar]
- 42.De Luca D, Piastra M, Chidini P, Tissieres P, Calderini E, Essouri S, Medina Villanueva A, Vivanco Allende A, Pons-Odena M, Perez-Baena L, et al. ; Respiratory Section of the European Society for Pediatric Neonatal Intensive Care (ESPNIC). The use of the Berlin definition for acute respiratory distress syndrome during infancy and early childhood: multicenter evaluation and expert consensus. Intensive Care Med 2013. December;39(12):2083–91. [DOI] [PubMed] [Google Scholar]
- 43.Plurad D, Martin M, Green D, Salim A, Inaba K, Belzberg H, Demetriades D, Rhee P. The decreasing incidence of late posttraumatic acute respiratory distress syndrome: The potential role of lung protective ventilation and conservative transfusion practice. J Trauma 2007. July;63(1):1–7. [DOI] [PubMed] [Google Scholar]
- 44.Cuschieri J, Johnson JL, Sperry J,West MA, Moore EE, Minei JP, Bankey PE, Nathens AB, Cuenca AG, Efron PA, et al. , Inflammation and Host Response to Injury, Large Scale Collaborative Research Program. Benchmarking outcomes in the critically injured trauma patient and the effect of implementing standard operating procedures. Ann Surg 2012;255(5):993–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hernu R, Wallet F, Thiollière F, Martin O, Richard JC, Schmitt Z, Wallon G, Delannoy B, Rimmelé T, Démaret C, et al. An attempt to validate the modification of the American-European consensus definition of acute lung injury/acute respiratory distress syndrome by the Berlin definition in a university hospital. Intensive Care Med 2013. December;39(12):2161–70. [DOI] [PubMed] [Google Scholar]
- 46.Sine CR, Belenkiy SM, Buel AR, Waters JA, Lundy JB, Henderson JL, Stewart IJ, Aden JK, Liu NT, Batchinsky A, et al. Acute Respiratory Distress Syndrome in Burn Patients: A Comparison of the Berlin and American-European Definitions. J Burn Care Res 2016. Sep-Oct;37(5):e461–9. [DOI] [PubMed] [Google Scholar]
- 47.Parvathaneni K, Belani S, Leung D, Newth CJ, Khemani RG. Evaluating the Performance of the Pediatric Acute Lung Injury Consensus Conference Definition of Acute Respiratory Distress Syndrome. Pediatr Crit Care Med 2017. January;18(1):17–25. [DOI] [PubMed] [Google Scholar]
- 48.Pediatric Acute Lung Injury Consensus Conference Group. Pediatric acute respiratory distress syndrome: consensus recommendations from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med 2015. June;16(5):428–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khemani RG, Smith LS, Zimmerman JJ, Erickson S, Pediatric Acute Lung Injury Consensus Conference Group. Pediatric Acute Respiratory Distress Syndrome: Definition, Incidence, and Epidemiology: Proceedings From the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med 2015. June; 16(5 Suppl 1):S23–40. [DOI] [PubMed] [Google Scholar]
- 50.Gupta S, Sankar J, Lodha R, Kabra SK. Comparison of Prevalence and Outcomes of Pediatric Acute Respiratory Distress Syndrome Using Pediatric Acute Lung Injury Consensus Conference Criteria and Berlin Definition. Front Pediatr 2018. April;6:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


