Abstract
Objective:
To estimate the prevalence of major depressive episode (MDE) in patients with presumptive pulmonary tuberculosis (pre-PTB, defined by cough lasting ≥ 3 weeks) and compare it between patients with pulmonary tuberculosis (PTB) and without PTB.
Methods:
Patients with pre-PTB (n=260) were screened for depression using the Patient Health Questionnaire (PHQ-9). Those individuals with scores ≥ 10 were subsequently assessed with the depression module of the Mini International Neuropsychiatric Interview (MINI-Plus) to confirm diagnosis. Associations of categorical variables with PTB and MDE were calculated using the chi-square test and OR.
Results:
PTB was confirmed in 98 patients (37.7%). A high proportion of both groups (active PTB and no PTB) screened positive for depression (60.2 vs. 62.1%, respectively). Among 159 patients who screened positive for depression, a subset of 97 (61.0%) were further evaluated with the MINI-Plus; current MDE was confirmed in 54.6% (53/97). On univariate and multivariate analysis, female sex was the only factor associated with the diagnosis of current MDE (p = 0.04).
Conclusion:
The prevalence of MDE was high among individuals with prolonged respiratory symptoms, independent of PTB diagnosis. This is consistent with other studies of depression in primary care in Brazil.
Keywords: Depression, tuberculosis, prevalence
Introduction
Tuberculosis (TB) is one of the 10 leading causes of death worldwide and the leading cause of mortality among infectious diseases, surpassing HIV infection. In 2016, there were 10.4 million new cases of TB worldwide, 1 million of which occurred in people living with HIV.1 In addition, large outbreaks of multidrug-resistant tuberculosis (MDR-TB) have been observed in urban centers among vulnerable populations (HIV-positive, persons deprived of liberty or otherwise institutionalized, illicit drug users, refugees) and those with comorbid mental disorders, diabetes mellitus, and/or smoking.2
In 2016, 66,796 new cases and 12,809 incident and retreatment cases of TB were reported in Brazil.3 From 2007 to 2016, the incidence of TB in the country declined 1.7% per annum on average, from 37.9 per 100,000 population in 2007 to 32.4 per 100,000 in 2016. Reis-Santos et al.,4 in an analysis of the treatment outcomes of TB cases reported from 2001 to 2011, noted that comorbidities such as alcoholism, HIV infection, depression, and diabetes mellitus were highly prevalent (16.1, 8.1,4 5, and 3.7% of cases, respectively).
Major depressive episode (MDE) is a common mental disorder affecting more than 300 million people of all ages globally.5 It is the leading cause of disability worldwide, and is a major contributor to the global burden of disease. In the worst-case scenario, depression can lead to suicide. Globally, about 800,000 people die from suicide each year; it is the second leading cause of death in people aged 15 to 29.5
In Brazil, the prevalence of depression is estimated at 12.6%, and there is a high rate of recurrence; 46% of patients with MDE will experience a second episode, with a median of four episodes throughout life.6 The prevalence of MDE is unequally distributed in the population. It is more common among women,7 among the younger,8 the economically disadvantaged,9 and those who live without a partner.7 People with MDE may have biological changes that increase the risk of developing chronic diseases. On the other hand, the limitations in daily life experienced by patients with chronic diseases increases their likelihood of developing depression.10
MDE is common in patients with other medical conditions, such as arthritis, asthma, and COPD.11 However, there have been few studies on the association of MDE with TB.12 In a systematic review of 31 studies conducted in 11 countries, most of which relied on MDE screening instruments, the mean weighted prevalence of depression among individuals with TB was 48.9%.13 The high prevalence of MDE in people with TB may be attributed to a combination of biological, social, and behavioral factors.14 MDE and TB are both associated with social vulnerability, inadequate living conditions, and socioeconomic inequities.14 Subjects with MDE tend to seek less medical assistance to investigate respiratory symptoms and have a lower adherence to drug treatment15 -17; these behaviors increase the risk of developing resistant TB. Failure to treat often leads to death, making TB control even more difficult.18 -20
For these reasons, it is crucial to innovate and seek new indicators and interventions to address TB and other comorbidities, especially in large urban centers. In the present study, we used a screening instrument and a diagnostic confirmation tool to evaluate the prevalence of MDE in patients with presumptive pulmonary TB (pre-PTB) treated at a Municipal Health Center, seeking to explore the association between sociodemographic characteristics of these patients and treatment outcomes.
Methods
Study design
This was a cross-sectional, descriptive, survey-based study. Patients were screened at the Municipal Health Center (Centro Municipal de Saúde, CMSDC) of Duque de Caxias, Rio de Janeiro. The municipality of Duque de Caxias is among the five municipalities in the state of Rio de Janeiro with the highest incidences of TB (i.e., > 75.6 cases/100,000 population). In 2016, a total of 902 cases of TB were reported in Duque de Caxias, of which 75% were new cases of PTB. Of this total, 41% were diagnosed and treated at CMSDC that same year.21
Description of the sample
From July 2015 and December 2016, patients aged 18 and over who presented to CMSDC with a complaint of cough lasting 3 weeks or longer were invited by a staff nurse to participate in the study. Those who provided written informed consent then answered a sociodemographic and clinical questionnaire on sex, age, ethnicity, educational attainment, family income, signs and symptoms of TB, smoking, alcohol and drug abuse, HIV infection, and other comorbid conditions. Regardless of agreement to participate in the study, all eligible patients were screened for PTB and treated at the facility as needed.
Individuals who had received anti-TB therapy for over 7 days, or who had taken a fluoroquinolone for more than 7 days in the preceding 30 days (thus reducing the sensitivity of cultures for Mycobacterium tuberculosis), as well as pregnant or lactating women and individuals for whom no final diagnosis of presence or absence of TB was established, were excluded from the study.
PTB was diagnosed following the recommendations of the Brazilian national TB program for evaluation of patients with respiratory symptoms. Patients with a positive sputum smear for acid-fast bacilli (AFB) and/or a positive rapid molecular test for M. tuberculosis (Xpert MTB/RIF) were considered to have active PTB cases.20 Patients with negative smears who followed routine procedures for clinical and laboratory evaluation at CMSDC (including chest radiograph) and ultimately received another diagnosis constituted the non-TB group.
Major depressive episode (MDE)
The Patient Health Questionnaire (PHQ-9)22 is a commonly used brief screening tool for current MDE that assesses nine symptoms (depressed mood, anhedonia, sleep problems, tiredness or lack of energy, change in appetite or weight, guilt or uselessness, trouble concentrating, feeling slow or restless, and thoughts of suicide) and the frequency of their occurrence in the previous 2 weeks (no days, several days, more than half of the days, almost every day). The maximum possible score is 27 points. The cutoff score considered suggestive of moderate and severe depression, validated in Brazil, is ≥ 10 points (sensitivity of 77.5% and specificity of 86.7%).23
The Mini International Neuropsychiatric Interview (MINI-Plus) is a brief, standardized diagnostic interview based on DSM-IV24 and ICD-1025 criteria that explores Axis I psychiatric disorders. The MINI-Plus can be used by trained mental health specialists, while non-specialist health professionals (e.g., nurses) can be taught to administer the instrument with more intensive training. Major depressive disorder is one of the modules the MINI-Plus, and was used to confirm diagnosis.26 Given the focus of the PHQ-9 on the preceding 2 weeks, we restricted assessment to current MDE.
A nurse and two medical students were trained to administer the PHQ-9 and confirm diagnosis using the MINI-Plus. Although the PHQ-9 was originally designed as a self-report instrument, given the low educational level in our sample, it was administered by interview instead. Although both tools explore the same depressive symptoms, their format is different; whereas the PHQ-9 is highly structured and questions and answers are repeated as written to avoid interviewer bias, the MINI-Plus diagnostic interview allows further questioning and exploration of symptoms followed by “clinical judgment” by the trained interviewer to determine whether symptoms surpass clinical thresholds and the associated social or occupational impairment is consistent with a clinical diagnosis of MDE.
Statistical analysis
Absolute and relative frequencies were calculated for categorical variables. Continuous variables are presented as mean and standard deviation (SD). Associations of categorical variables with TB and MDE were evaluated using the chi-square test (or Fisher’s exact test, when indicated); ORs were derived and 95% confidence intervals (95%CI) calculated. Student’s t-test was used to compare continuous variables. Logistic regression was used to control for variables independently associated with MDE. Statistical significance was accepted when p ≤ 0.05 (two-tailed). Results were generated in SPSS version 23.0.
Ethical considerations
The study was approved by the research ethics committee of Hospital Universitário Clementino Fraga Filho, Universidade Federal do Rio de Janeiro (CAAE 45637715.5.0000.5257).
Results
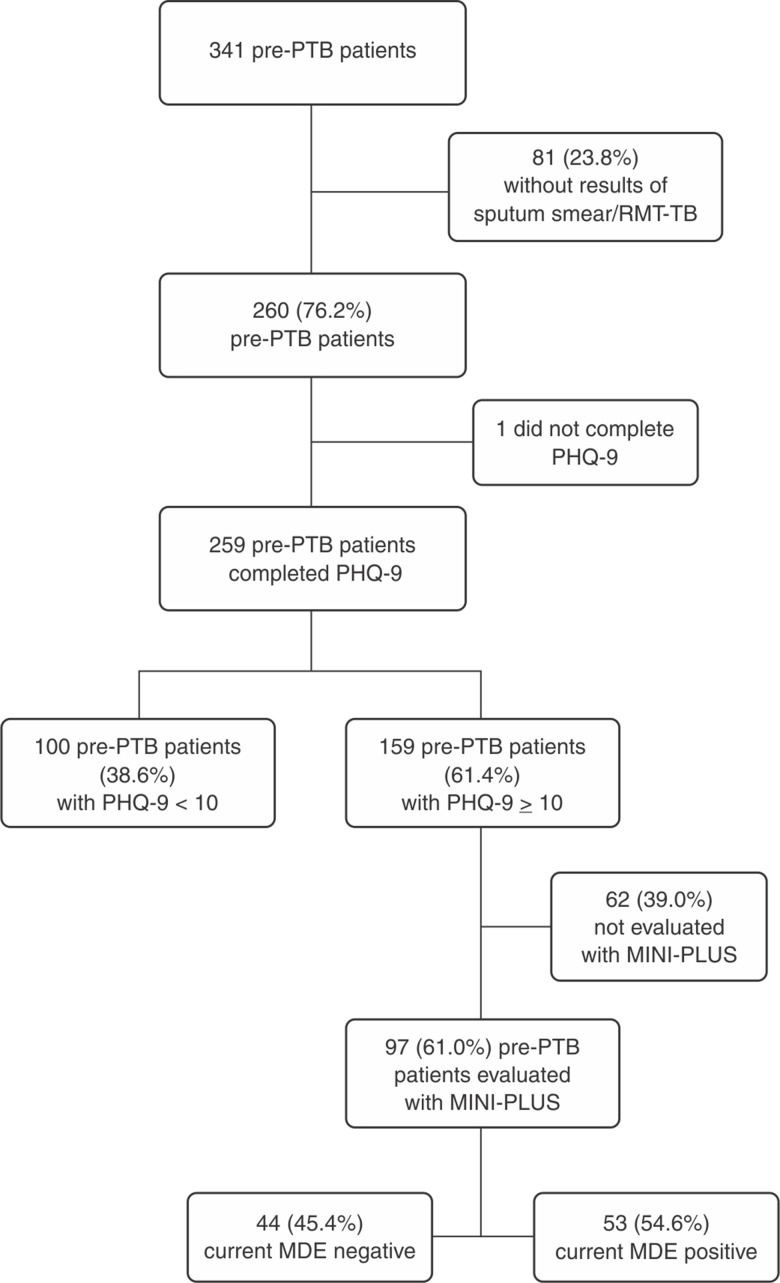
During the study period, 3,251 pre-PTB patients were assessed at CMSDC, of whom 341 (10.5%) were recruited. Of these, 81 (24%) were later excluded because they did not return with the results of sputum cultures. Among the 260 remaining subjects, 259 (99.6%) were screened for depression using the PHQ-9; 159 (61.4%) screened positive (score ≥ 10, suggestive of current MDE). Of these 159 participants who screened positive, 97 (61.0%) were subsequently evaluated with the MINI-Plus, and current MDE was confirmed in 54.6% (53/97) (Figure 1). Among the 260 pre-PTB participants, 37.7% (98/260) were diagnosed with active PTB (37.7%). The diagnosis of PTB was associated with a lower mean age (40.7 vs. 46.9, p = 0.003), body mass index (BMI) < 18.5 kg/m2 (35.1 vs. 20%; p = 0.05), cough duration > 8 weeks (24.1 vs. 10.4; p = 0.01), and a lower frequency of hypertension (3.0 vs. 11.3; p = 0.01) (Table 1).
Figure 1. Flow diagram of participants with pre-PTB evaluated for depression by PHQ-9 and MINI-Plus. MDE = major depressive episode; MINI-Plus = Mini International Neuropsychiatric Interview; PHQ-9 = Patient Health Questionnaire; pre-PTB = presumptive pulmonary tuberculosis; RMT-TB = rapid molecular test (Xpert MTB/RIF).
Table 1. Univariate analysis of factors associated with diagnosis of tuberculosis among patients with presumptive pulmonary tuberculosis.
| TB diagnosis, n=260 | ||||
|---|---|---|---|---|
| No, n=161 | Yes, n=99 | OR (95%CI) | p-value | |
| Sex | ||||
| Male | 98 (60.9) | 62 (62.6) | 0.92 (0.55-1.6) | 0.79 |
| Female | 63 (39.1) | 37 (37.4) | ||
| Mean age (SD), n=255 | 46.9 (16.0) | 40.67 (15.7) | N/A | 0.003 |
| Ethnicity, n=210 | ||||
| White | 26 (20.0) | 13 (16.3) | 1 | |
| Black | 39 (30.0) | 29 (36.3) | 11.49 (0.65-3.38) | 0.34 |
| Asian | 12 (9.2) | 4 (5.0) | 0.67 (0.18-2.48) | 0.54 |
| Mixed race (White/Black) | 52 (40.0) | 32 (40.0) | 1.23 (0.55-2.73) | 0.61 |
| Indigenous | 1 (0.8) | 2 (2.5) | 4.0 (0.33-48.3) | 0.27 |
| Marital status | ||||
| Married | 92 (57.1) | 51 (51.5) | 1.2 (0.75-2.0) | 0.44 |
| Single/widowed | 69 (42.9) | 48 (48.5) | ||
| Education, n=209 | ||||
| < 8 years | 89 (69.5) | 64 (79) | 0.6 (0.31-1.16) | 0.15 |
| ≥ 8 years | 39 (30.5) | 17 (21.0) | ||
| Body mass index, n=146 | ||||
| < 18.5 kg/m2 | 18 (20.2) | 20 (35.1) | 0.4 (0.2-0.9) | 0.05 |
| ≥ 18.5 kg/m2 | 71 (79.8) | 37 (64.9) | ||
| Cough duration, n=204 | ||||
| ≤ 8 weeks | 112 (89.6) | 60 (75.9) | 2.7 (1.2-5.9) | 0.01 |
| > 8 weeks | 13 (10.4) | 19 (24.1) | ||
| Presence of one or more comorbidities | ||||
| No | 129 (80.1) | 75 (75.8) | 1.2 (0.7-2.4) | 0.43 |
| Yes | 32 (19.9) | 24 (24.2) | ||
| Diabetes | ||||
| No | 154 (95.7) | 92 (92.9) | 1.6 (0.56-5.0) | 0.4 |
| Yes | 7 (4.3) | 7 (7.1) | ||
| Drug use, n=258 | ||||
| No | 137 (85.6) | 81 (82.7) | 1.2 (0.6-2.5) | 0.5 |
| Yes | 23 (14.4) | 17 (17.3) | ||
| Hypertension, n=259 | ||||
| No | 142 (88.8) | 96 (97.0) | 0.2 (0.7-0.8) | 0.01 |
| Yes | 18 (11.3) | 3 (3.0) | ||
| HIV status, n=138 | ||||
| Negative | 65 (89.0) | 61(93.8) | 0.5 (0.1-1.8) | 0.3 |
| Positive | 8 (11.0) | 4 (6.2) | ||
| Smoking, n=204 | ||||
| No | 68 (54.0) | 34 (43.6) | 1.5 (0.8-2.6) | 0.2 |
| Yes | 58(46.0) | 44 (56.4) | ||
| Alcoholism (CAGE), n=178 | ||||
| Positive | 27 (25.2) | 27(38.0) | 1.8 (0.9-3.5) | 0.09 |
| Negative | 80 (74.8) | 44 (62.0) | ||
| Homeless | ||||
| No | 158 (98.1) | 97 (98.0) | 1.0 (0.1-6.6) | 1.0 |
| Yes | 3 (1.9) | 2 (2.0) | ||
| Contact with resistant TB, n=157 | ||||
| No | 90 (93.8) | 55 (90.2) | 1.6 (0.5-5.3) | 0.5 |
| Yes | 6 (6.3) | 6 (9.8) | ||
| Incarcerated, n=207 | ||||
| No | 120 (94.5) | 75 (93.8) | 1.1 (0.3-3.7) | 1.0 |
| Yes | 7 (5.5) | 5 (6.3) | ||
| Contact with TB, n=203 | ||||
| No | 86 (68.3) | 52 (67.5) | 1.0 (0.5-1.9) | 1.0 |
| Yes | 40 (31.7) | 25 (32.5) | ||
| Living in shelter, n=208 | ||||
| No | 120 (93.8) | 74 (92.5) | 1.2 (0.4-3.6) | 0.7 |
| Yes | 8 (6.3) | 6 (7.5) | ||
Data presented as n (%), unless otherwise specified.
95%CI = 95% confidence interval; CAGE = “Cutting down, Annoyance by criticism, Guilty feeling, and Eye-openers” screening instrument for alcohol dependence; N/A = not applicable; OR = odds ratio; SD = standard deviation; TB = tuberculosis.
In the screening test for depression, the proportion of TB and non-TB patients with a PHQ-9 score ≥ 10 was 60.2 vs. 62.1% (OR = 0.92, 95%CI 0.55-1.54, p = 0.79). Among 97 patients with a PHQ-9 score ≥ 10 who were subsequently assessed with the MINI-Plus, current MDE was identified in 59.5% of TB and 50.9% of non-TB patients, with no statistically significant difference (OR = 1.42, 95%CI 0.63-3.19, p = 0.42) (Table 2). All individuals with depression were referred for outpatient mental health treatment within CMSDC. In both groups, the most frequently reported symptoms were depressed mood, sleep disturbance, fatigue, and appetite change. Individuals with PTB were more likely than those without PTB to endorse reduced appetite (87 vs. 73%, p = 0.009) (Table 3).
Table 2. Prevalence of MDE assessed by PHQ-9 and MINI-Plus in TB and non-TB patients.
| Non-TB | TB | OR (95%CI) | p-value | |
|---|---|---|---|---|
| PHQ-9 | (n=161) | (n=98) | ||
| < 10 | 61 (37.9) | 39 (39.8) | 0.92 | 0.79 |
| ≥ 10 | 100 (62.1) | 59 (60.2) | (0.55-1.54) | |
| MINI-Plus | (n=55) | (n=42) | ||
| Previous or absent MDE | 27 (49.1) | 17 (40.5) | 1.42 (0.63-3.19) | 0.42 |
| Current MDE | 28 (50.9) | 25 (59.5) |
Data presented as n (%).
95%CI = 95% confidence interval; MDE = major depressive episode; MINI-Plus = Mini International Neuropsychiatric Interview; OR = odds ratio; PHQ-9 = Patient Health Questionnaire; TB = tuberculosis.
Table 3. Differences in responses to PHQ-9 items* by TB status.
| Non-TB (n=161) | TB (n=98) | OR (95%CI) | p-value | |
|---|---|---|---|---|
| During the last 2 weeks, how often have you been bothered by any of the problems below? | ||||
| 1. Little interest or little pleasure in doing things? | 105 (65.2) | 65 (66.3) | 1.01 (0.62-1.80) | 0.9 |
| 2. Feeling “down,” depressed or out of perspective? | 107 (66.5) | 71 (72.4) | 1.33 (0.77-2.3) | 0.34 |
| 3. Difficulty getting to sleep or staying asleep, or sleeping more than usual? | 125 (77.6) | 76 (77.6) | 1.0 (0.55-1.9) | 1.0 |
| 4. Feeling tired or low energy? | 143 (88.8) | 80 (81.6) | 0.56 (0.27-1.2) | 0.14 |
| 5. Lack of appetite or overeating? | 117 (72.7) | 85 (86.7) | 2.5 (1.3-4.9) | 0.009 |
| 6. Do you feel bad about yourself – or do you feel that you are a failure or that you have disappointed your family or yourself? | 91 (56.5) | 55 (56.1) | 0.99 (0.6-1.7) | 1.0 |
| 7. Difficulty concentrating on things, such as reading the newspaper or watching TV? | 77 (47.8) | 42(42.9) | 0.81(0.5-1.4) | 0.44 |
| 8. Slow to move or talk, to the point that other people perceive? Or the opposite – being so agitated or restless that you walk around much more than usual? | 99 (61.5) | 54 (55.5) | 0.77(0.4-1.3) | 0.36 |
| 9. Think about hurting yourself in some way or it would be better to be dead? | 34 (21.1) | 21 (21.4) | 1.1(0.5-1.9) | 1.0 |
Data presented as n (%).
95%CI = 95% confidence interval; OR = odds ratio; PHQ-9 = Patient Health Questionnaire; TB = tuberculosis.
Symptoms were considered present if endorsed at least some of the time.
Table 4 presents the univariate analysis of sociodemographic and clinical factors associated with depression. Based on this analysis, women were two and a half times more likely to present with current MDE than men were (OR = 2.52, 95%CI, 1.10-5.76; p = 0.04). The effect of the association of female sex with MDE was controlled by age, education, and smoking in logistic regression; being female remained the only variable independently associated with MDE (OR = 2.72, 95%CI 1.39-5.30; p = 0.003). TB treatment outcomes were available for 23 patients. Loss to follow-up was higher among individuals with MDE (8.7%) compared to those without MDE (4.3%); however, this difference was not statistically significant. There were also no statistically significant associations of alcohol, drug abuse, or lower education with current MDE.
Table 4. Univariate analysis of the sociodemographic factors associated with current MDE among individuals with presumptive TB with PHQ-9 ≥ 10 and diagnosis of depression confirmed using the MINI-Plus (n=97).
| Current MDE | ||||
|---|---|---|---|---|
| Variables | No | Yes | OR (95%CI) | p-value |
| Sex | ||||
| Male | 29 (55.8) | 23 (44.2) | 2.52 (1.10-5.76) | 0.04 |
| Female | 15 (33.3) | 30 (66.7) | ||
| Ethnicity, n=93 | ||||
| White | 5 (33.3) | 10 (66.7) | 1 | |
| Black | 19 (61.3) | 12 (38.7) | 0.32 (0.09-1.15) | 0.08 |
| Asian | 4 (50.0) | 4 (50.0) | 0.50 (0.09-2.89) | 0.44 |
| Mixed race (White/Black) | 14 (36.8) | 24 (63.2) | 0.86 (0.24-3.02) | 0.81 |
| Indigenous | 0 (0.0) | 1 (100.0) | N/A | N/A |
| Education, n=94 | ||||
| ≤ 8 years | 30 (42.9) | 40 (57.1) | 0.63 (0.25-1.61) | 0.35 |
| ≥ 8 years | 13 (54.2) | 11 (45.8) | ||
| Marital status | ||||
| Married | 20 (46.5) | 23 (53.5) | 1.09 (0.49-2.42) | 1.00 |
| Single | 24 (44.4) | 30 (55.6) | ||
| Body mass index, n=75 | ||||
| ≥ 18.5 kg/m2 | 11 (44.0) | 14 (56.0) | 1.28 (0.48-3.40) | 0.63 |
| < 18.5 kg/m2 | 19 (38.0) | 31(62.0) | ||
| Cough duration, n=92 | ||||
| ≤ 8 weeks | 33 (47.8) | 36 (52.2) | 1.43 (0.54-3.73) | 0.63 |
| ≥ 8 weeks | 9 (39.1) | 14 (60.9) | ||
| Presence of comorbidities | ||||
| Yes | 16 (42.1) | 22 (57.9) | 1.24 (0.55-2.83) | 0.68 |
| No | 28 (47.5) | 31 (52.5) | ||
| Diabetes | ||||
| Yes | 6 (54.5) | 5 (45.5) | 0.66 (0.19-2.33) | 0.54 |
| No | 38 (44.2) | 48(55.8) | ||
| Hypertension, n=96 | ||||
| Yes | 8 (53.3) | 7 (46.7) | 0.70 (0.23-2.11) | 0.58 |
| No | 36 (44.4) | 45 (55.6) | ||
| HIV status, n=61 | ||||
| Positive | 5 (71.4) | 2 (28.6) | 0.30 (0.05-1.67) | 0.23 |
| Negative | 23 (42.6) | 31 (57.4) | ||
| Smoking, n=92 | ||||
| Yes | 20 (47.6) | 22 (52.4) | 086 (0.38-1.97) | 0.83 |
| No | 22 (44.0) | 28 (56.0) | ||
| Drug abuse | ||||
| Yes | 5 (31.3) | 11 (68.8) | 2.04 (0.65-6.41) | 0.28 |
| No | 39 (48.1) | 42 (51.9) | ||
| Alcoholism (CAGE), n=85 | ||||
| Yes | 13 (50.0) | 13 (50.0) | 0.73 (0.29-1.86) | 0.64 |
| No | 25 (42.4) | 34 (57.6) | ||
| Homeless | ||||
| Yes | 0 (0) | 2 (100.0) | N/A | 0.50 |
| No | 44 (46.3) | 51 (53.7) | ||
| Contact with resistant TB, n=69 | ||||
| Yes | 2 (33.3) | 4 (66.7) | 1.94 (0.33-11.3) | 0.67 |
| No | 31 (49.2) | 32 (50.8) | ||
| Incarcerated, n=94 | ||||
| Yes | 1 (33.3) | 2 (66.7) | 1.71 (0.15-19.6) | 1.00 |
| No | 42 (46.2) | 49 (53.8) | ||
| Living in shelter, n=94 | ||||
| Yes | 4 (57.1) | 3 (42.9) | 0.61 (0.13-2.89) | 0.70 |
| No | 39 (44.8) | 48 (55.2) | ||
| Contact with TB, n=90 | ||||
| Yes | 9 (36.0) | 16 (64.0) | 1.72 (0.67-4.46) | 0.35 |
| No | 32 (49.2) | 33 (50.8) | ||
| TB treatment outcome, n=23 | ||||
| Treatment success | 11 (47.8) | 9 (39.1) | 2.44 (0.19-31.53) | 0.59 |
| Loss to follow-up | 1 (4.3) | 2 (8.7) | ||
Data presented as n (%).
95%CI = 95% confidence interval; CAGE = Cutting down, Annoyance by criticism, Guilty feeling, and Eye-openers, screening instrument for alcohol dependence; N/A = not applicable; MDE = major depressive episode; MINI-Plus = Mini International Neuropsychiatric Interview; OR = odds ratios; SD = standard deviation; TB = tuberculosis.
Discussion
The objective of the present study was to evaluate the prevalence of current MDE among patients with presumptive pulmonary TB evaluated at a municipal health center. The prevalence of probable depression, based on the PHQ-9, among individuals with confirmed PTB (60.2%) was comparable to that described in two studies in Pakistan and Ethiopia (56 and 54%, respectively) using the same instrument.27,28 On the other hand, it was considerably higher than the prevalence found in Nigeria, Cameroon, the Philippines, and India, where studies reported moderate to severe depression rates of 6.2 to 41.5% among TB patients, using the PHQ-9 and other depression screening instruments.29,30,31,32,33-34 In our sample, there was no significant difference in the prevalence of depression among patients with and without active PTB with any of the instruments used (PHQ-9 and MINI-Plus). Using the MINI-Plus to confirm diagnosis of MDE in a subset of individuals with PHQ ≥ 10 was a significant strength of our study.
Depression and TB are both associated with a combination of biological, social and behavioral factors.14 In a population-based cohort study in Korea, Oh et al.35 observed that individuals with depression at baseline had a 2.63-fold higher risk of active TB over a 10-year period. The association of TB and depression is associated with all negative outcomes, including morbidity, mortality, drug resistance, loss to follow-up, treatment failure, and death.14 Treating depression is therefore critical for global TB control.13
Importantly, while extremely high, the prevalence found in our sample is consistent with those from a previous study conducted in four cities of Brazil, including Rio de Janeiro, in which the prevalence of common mental disorders in individuals seeking treatment in primary care ranged from 51.9 to 64.3%.36 In another longitudinal study of adult health in Brazil, the prevalence of common mental disorders in a sample of more than 15,000 civil servants from five universities was 26.8%; these disorders were especially prevalent among women, the unemployed, those with low educational attainment, and those with low income.37
Our study has several limitations. Most can be attributed to the fact that it was performed under routine conditions in a municipal basic health unit, in an area of high social vulnerability. Nearly one-quarter (24%) of subjects eligible for the study did not return for sputum culture, making it impossible to diagnose TB and include them in the analysis. The small sample size included in the study precluded proper evaluation of the effect associated with MDE and TB. Another limitation is related to the study design, which precluded evaluation of the causality or effect of some variables dependent on time (for instance, BMI could be a causal factor or a consequence of TB).
Moreover, confirmation of the diagnosis of MDE using the MINI-Plus was only possible in a subset of individuals with PHQ-9 scores ≥ 10 (97/159; 61%), and no individuals with scores < 10 were assessed with the MINI-Plus, making it impossible to assess the sensitivity and specificity of the PHQ-9 in this population of pre-PTB patients. This is a critical need that should be addressed in future studies.
In summary, the prevalence of MDE (detected by the PHQ-9) among individuals with pre-PTB was high, and there was little difference between individuals with confirmed PTB and those with other respiratory illnesses (60.2 vs. 62.1%, respectively). Notably, a previous study of common mental disorders in Brazilian primary care found comparably high rates (51.9 to 64.3%).36 Among the sociodemographic variables evaluated, only female sex conferred a greater risk for MDE. Further studies are needed to better understand the magnitude of comorbid TB and depression and improve treatment of MDE and TB, as well as to elucidate the sensitivity and specificity of the PHQ-9 among individuals affected by TB.
Disclosure
The authors report no conflicts of interest.
Acknowledgements
The project was funded in part by the National Science and Technology Institute for Tuberculosis (CNPq/INCT 465318/2014-2), the Graduate Program in Clinical Medicine of the Faculty of Medicine, Universidade Federal do Rio de Janeiro, and the National Institute of Mental Health (USA) (K01MH104514).
Footnotes
How to cite this article: de Castro-Silva KM, Carvalho AC, Cavalcanti MT, Martins PS, França JR, Oquendo M, et al. Prevalence of depression among patients with presumptive pulmonary tuberculosis in Rio de Janeiro, Brazil. Braz J Psychiatry. 2019;41:316-323. http://dx.doi.org/10.1590/1516-4446-2018-0076
References
- 1.World Health Organization (WHO) Global tuberculosis report 2017 [Internet] 2017. http:/www.who.int/tb/publications/global_report/en/ [cited 2017 Nov 7] [Google Scholar]
- 2.World Health Organization (WHO) Global tuberculosis report 2016 [Internet] 2016. [cited 2017 Nov 7]. http:/apps.who.int/medicinedocs/documents/s23098en/s23098en.pdf. [Google Scholar]
- 3.Brasil, Ministério da Saúde, Secretaria de Vigilância em Saúde . Indicadores prioritários para o monitoramento do Plano Nacional pelo Fim da Tuberculose como Problema de Saúde Pública no Brasil [Internet] 2017. [cited 2017 Nov 20] http:/portalarquivos2.saude.gov.br/images/pdf/2017/marco/23/2017-V-48-N-8-Indicadores-priorit--rios-para-o-monitoramento-do-Plano-Nacional-pelo-Fim-da-Tuberculose-como-Problema-de-Sa--de-P--blica-no-Brasil.pdf. [Google Scholar]
- 4.Reis-Santos B, Gomes T, Locatelli R, de Oliveira ER, Sanchez MN, Horta BL, et al. Treatment outcomes in tuberculosis patients with diabetes: a polytomous analysis using Brazilian surveillance system. PLoS One. 2014;9:e100082. doi: 10.1371/journal.pone.0100082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization (WHO) Depression (fact sheet) [Internet] 2018. Mar 22 [cited 2018 Aug 13]. http:/www.who.int/mediacentre/factsheets/fs369/en/ [Google Scholar]
- 6.Andrade L, Caraveo-Anduaga JJ, Berglund P, Bijl RV, De Graaf R, Vollebergh W, et al. The epidemiology of major depressive episodes: results from the international consortium of psychiatric epidemiology (ICPE) surveys. Int J Methods Psychiatr Res. 2003;12:3–21. doi: 10.1002/mpr.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van de Velde S, Bracke P, Levecque K. Gender differences in depression in 23 European countries. Cross sectional variation in the gender gap in depression. Soc Sci Med. 2010;71:305–13. doi: 10.1016/j.socscimed.2010.03.035. [DOI] [PubMed] [Google Scholar]
- 8.Blazer DG, Kessler RC, McGonaqle KA, Swartz MS. The prevalence and distribution of major depression in a national community sample: the national comorbidity survey. Am J Psychiatry. 1994;151:979–86. doi: 10.1176/ajp.151.7.979. [DOI] [PubMed] [Google Scholar]
- 9.Everson SA, Maty SC, Lynch JW, Kaplan GA. Epidemiologic evidence for the relation between socioeconomic status and depression, obesity and diabetes. J Psychosom Res. 2002;53:891–5. doi: 10.1016/s0022-3999(02)00303-3. [DOI] [PubMed] [Google Scholar]
- 10.Katon WJ. Epidemiology and treatment of depression in patients with chronic medical illness. Dialogues Clin Neurosci. 2011;13:7–23. doi: 10.31887/DCNS.2011.13.1/wkaton. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patten SB, Beck CA, Kassam A, Williams JV, Barbui C, Metz LM. Long-term medical conditions and major depression: strength of association for specific conditions in the general population. Can J Psychiatry. 2005;50:195–202. doi: 10.1177/070674370505000402. [DOI] [PubMed] [Google Scholar]
- 12.Araújo GS, Pereira SM, Santos DN. Revisão sobre tuberculose e transtornos mentais comuns. Rev Eletr Gestao Saude. 2014;5:716–26. [Google Scholar]
- 13.Sweetland A, Oquendo M, Wickramaratne P, Weissman M, Wainberg M. Depression: a silent driver of the global tuberculosis epidemic. World Psychiatry. 2014;13:325–6. doi: 10.1002/wps.20134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sweetland AC, Kritski A, Oquendo MA, Sublette ME, Norcini Pala A, Silva LR, et al. Addressing the tuberculosis-depression syndemic to end the tuberculosis epidemic. Int J Tuberc Lung Dis. 2017;21:852–61. doi: 10.5588/ijtld.16.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pachi A, Bratis D, Moussas G, Tselebis A. Psychiatric morbidity and other factors affecting treatment adherence in pulmonary tuberculosis patients. Tuberc Res Treat. 2013;2013:489865. doi: 10.1155/2013/489865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castro-Costa E, Dewey M, Stewart R, Banerjee S, Huppert F, Mendonca-Lima C, et al. Prevalence of depressive symptoms and syndromes in later life in ten European countries: the SHARE study. Br J Psychiatry. 2007;191:393–401. doi: 10.1192/bjp.bp.107.036772. [DOI] [PubMed] [Google Scholar]
- 17.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160:2101–7. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- 18.Duarte PS, Miyazaki MC, Blay SL, Sesso R. Cognitive-behavioral group therapy is an effective treatment for major depression in hemodialysis patients. Kidney Int. 2009;76:414–21. doi: 10.1038/ki.2009.156. [DOI] [PubMed] [Google Scholar]
- 19.Johnson J, Kagal A, Bharadwaj R. Factors associated with drug resistance in pulmonary tuberculosis. Indian J Chest Dis Allied Sci. 2003;45:105–9. [PubMed] [Google Scholar]
- 20.Brasil, Ministério da Saúde, Secretaria de Vigilância em Saúde, Departamento de Vigilância Epidemiológica . Manual de recomendações para o controle da tuberculose no Brasil [Internet] 2011. [cited 2017 Nov 8]. http:/bvsms.saude.gov.br/bvs/publicacoes/manual_recomendacoes_controle_tuberculose_brasil.pdf. [Google Scholar]
- 21.Brasil, Ministério da Saúde, Departamento de Vigilância Epidemiológica . Sistema de Notificação de Agravos de Notificação (SINAN) [Internet] 2006. [cited 2018 Aug 13] http:/bvsms.saude.gov.br/bvs/publicacoes/sistema_informacao_agravos_notificacao_sinan.pdf. [Google Scholar]
- 22.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santos IS, Tavares BF, Munhoz TN, Almeida LS, Silva NT, Tams BD, et al. [Sensitivity and specificity of the patient health questionnaire-9 (PHQ-9) among adults from the general population] Cad Saude Publica. 2013;29:1533–43. doi: 10.1590/0102-311x00144612. [DOI] [PubMed] [Google Scholar]
- 24.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) Arlington: American Psychiatric Publishing; 1994. [Google Scholar]
- 25.World Health Organization (WHO) Schedules for clinical assessment in neuropsychiatry (SCAN) [Internet] 1999. [cited 2017 Nov 21]. http:/whoscan.org/wp-content/uploads/2014/10/xinterview.pdf. [Google Scholar]
- 26.Amorim P. Mini International Neuropsychiatric Interview (MINI): validação de entrevista breve para diagnóstico de transtornos mentais. Rev Bras Psiquiatr. 2000;22:106–15. [Google Scholar]
- 27.Amreen, Nadeem Rizvi. Frequency of depression and anxiety among tuberculosis patients. J Tuberc Res. 2016;4:183–90. [Google Scholar]
- 28.Ambaw F, Mayston R, Hanlon C, Alem A. Burden and presentation of depression among newly diagnosed individuals with TB in primary care settings in Ethiopia. BMC Psychiatry. 2017;17:57. doi: 10.1186/s12888-017-1231-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Issa BA, Yussuf AD, Kuranga SI. Depression comorbidity among patients with tuberculosis in a university teaching hospital outpatient clinic in Nigeria. Ment Health Fam Med. 2009;6:133–8. [PMC free article] [PubMed] [Google Scholar]
- 30.Kehbila J, Ekabe CJ, Aminde LN, Noubiap JJ, Fon PN, Monekosso GL. Prevalence and correlates of depressive symptoms in adult patients with pulmonary tuberculosis in the Southwest Region of Cameroon. Infect Dis Poverty. 2016;5:51. doi: 10.1186/s40249-016-0145-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masumoto S, Yamamoto T, Ohkado A, Yoshimatsu S, Querri AG, Kamiya Y. Prevalence and associated factors of depressive state among pulmonary tuberculosis patients in Manila, The Philippines. Int J Tuberc Lung Dis. 2014;18:174–9. doi: 10.5588/ijtld.13.0335. [DOI] [PubMed] [Google Scholar]
- 32.Singh L, Pardal PK, Prakash J. Psychiatric morbidity in patients of pulmonary tuberculosis-an observational study. Ind Psychiatry J. 2015;24:168–71. doi: 10.4103/0972-6748.181722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mandaknalli R, Giriraj B. Prevalence of depression in tuberculosis patients in a tertiary hospital care hospital. Sch J App Med Sci. 2015;3:2445–8. [Google Scholar]
- 34.Basu G, Chatterjee C, Sing R, Biswas S. Prevalence of depression in tuberculosis patients: an experience from a DOTS clinic. IJRRMS. 2012;2:14–7. [Google Scholar]
- 35.Oh KH, Choi H, Kim EJ, Kim HJ, Cho SI. Depression and risk of tuberculosis: a nationwide population-based cohort study. Int J Tuberc Lung Dis. 2017;21:804–9. doi: 10.5588/ijtld.17.0038. [DOI] [PubMed] [Google Scholar]
- 36.Goncalves DA, Mari Jde J, Bower P, Gask L, Dowrick C, Tofoli LF, et al. Brazilian multicentre study of common mental disorders in primary care: rates and related social and demographic factors. Cad Saude Publica. 2014;30:623–32. doi: 10.1590/0102-311x00158412. [DOI] [PubMed] [Google Scholar]
- 37.Nunes MA, Pinheiro AP, Bessel M, Brunoni AR, Kemp AH, Benseñor IM, et al. Common mental disorders and sociodemographic characteristics: baseline findings of the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil) Rev Bras Psiquiatr. 2016;38:91–7. doi: 10.1590/1516-4446-2015-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]