Abstract
Objectives
To evaluate perceptions towards pharmacogenetic testing of patients undergoing percutaneous coronary intervention (PCI) who are prescribed dual anti-platelet therapy (DAPT) and whether geographical differences in these perceptions exist.
Methods
TAILOR-PCI is the largest genotype-based cardiovascular clinical trial randomizing participants to conventional DAPT or prospective genotyping guided DAPT. Enrolled patients completed surveys prior to and six-months after randomization.
Results
A total of 1,327 patients completed baseline surveys of whom 28%, 29%, and 43% were from Korea, Canada and the United States (US), respectively. Majority of patients (77%) valued identifying pharmacogenetic variants however, fewer Koreans (44%) as compared to Canadians (91%), and US (89%) patients identified pharmacogenetics as being important (P<0.001). After adjusting for age, sex, and country, those who were confident in their ability to understand genetic information were significantly more likely to value identifying pharmacogenetic variants (OR 30.0, 95% CI 20.5, 43.8). Only 21% of Koreans, as opposed to 86% and 77% of patients in Canada and US, respectively, were confident in their ability to understand genetic information (P<0.001).
Conclusions
Although genetically mediated clopidogrel resistance is more prevalent amongst Asians, Koreans undergoing PCI identified pharmacogenetic variants as less important to their healthcare, likely related to their lack of confidence in their ability to understand genetic information. To enable successful implementation of pharmacogenetic testing on a global scale, the possibility of international population differences in perceptions should be considered.
Keywords: Genetics, coronary artery disease, percutaneous coronary intervention, stent, treatment
Introduction
Although pharmacogenetic testing is widely available especially for clopidogrel [1], perceptions of patients with cardiovascular disease towards genetic testing remain uncertain, and whether geographical differences in these perceptions exist is unknown.
TAILOR-PCI (ClinicalTrials.gov Identifier: NCT01742117) is an international, prospective randomized clinical trial (RCT) enrolling 5,270 subjects [2] to investigate whether a strategy of point of care genotyping to guide antiplatelet therapy reduces major adverse cardiovascular events (MACE) after percutaneous coronary intervention (PCI) in CYP2C19 reduced or loss of function allele (LOF) carriers. Prior studies suggest that CYP2C19 *2 or *3 carriers have a reduced ability to metabolize the prodrug clopidogrel, have decreased clopidogrel active metabolite levels and therefore are at risk for treatment failure [3–6]. TAILOR-PCI is the largest genotype-based RCT testing the utility of a personalized anti-platelet therapy approach after PCI.
As a sub-study of TAILOR-PCI, a survey was administered prior to and 6 months after randomization, to assess patient perception of genetic testing, whether these perceptions change during continued participation in the study, and whether geographical differences exist. Conducting such surveys is important to enable effective bedside implementation of pharmacogenetic testing. A majority of participants in prior such survey studies have been of European descent and without cardiovascular disease. However, since the minor allele frequencies of CYP2C19 LOF alleles are higher in East Asians and most relevant to patients with cardiovascular disease [7], it is important to include these patients. TAILOR-PCI patients were from the United States, Canada, and Korea and therefore the trial was designed to detect potential international differences in patient perception of pharmacogenetic testing.
Methods
Participants and recruitment
The survey was approved by respective institutional research ethics boards. Patients with acute coronary syndromes (ACS) or stable coronary artery disease (CAD) undergoing PCI and requiring at least 12 months of dual anti-platelet therapy (DAPT) were enrolled. After obtaining informed written consent, patients are randomized to the conventional therapy or the prospective genotyping arm. Subjects in the conventional therapy arm receive clopidogrel with aspirin without prospective genotyping. Those in the prospective genotyping arm had buccal swabs performed and underwent rapid genotyping with the Spartan Bioscience genotyping platform and based on their genotype status receive aspirin with either clopidogrel (wild type (WT) CYP2C19) or ticagrelor (CYP2C19*2 or *3). A priori decision was made to limit the survey sub-study to the first approximately 1,300 of the 5,270 subjects enrolled.
Surveys
The survey was developed by the investigators in collaboration with the Mayo Clinic Survey Research Center and non-physician members of the Mayo Clinic Center for Individualized Medicine. Survey questions were based on past published literature and clinical experience. The surveys, study protocol, case report forms and manual of operations were comprehensively translated by a certified translation service. Bilingual Korean investigators, study coordinators and members of the Korean Clinical Research Organization tested the reliability of the survey questions with the other study documents prior to site IRB submission and at the site training and initiation visits. The survey was administered twice during the study. The first survey was administered after informed consent and prior to randomization, and the second survey was conducted 6 months (±28 days) after PCI. The six-month follow-up survey was conducted over the telephone by a study coordinator. The survey questions and responses offered are outlined in Supplementary Table S1. The surveys were designed to characterize the subject’s previous experience with genotyping, their general attitudes towards genotyping, and their perceived comfort level with genetic information. The first part of the survey gathered information on previous direct patient exposure to genetic testing and were only asked in the baseline survey. Patients were then asked whether they had considered undergoing any genetic testing. For these questions, subjects could respond “yes,” “no,” or “refuse to answer.”
The latter half of the survey assessed patient’s opinions on the importance of genetic variants that may be influential to their health and their general attitudes towards physician recommended genetic testing to guide healthcare. Other questions evaluated patient comfort in the sharing of their genetic results, confidence in ability to understand genetic information, and if participants would feel comfortable carrying their results on a smartphone or card. Respondents answered these questions by ranking whether they agree or disagree on a Likert scale.
Statistical methods
Continuous variables are summarized as mean (standard deviation) and compared by one-way analysis of variance. Discrete variables are presented as frequency (percentage) and compared using Pearson’s chi-squared test for independent group comparisons. For comparisons of index to follow-up responses, McNemar’s test was used. Questions with Likert scale responses were dichotomized to a “somewhat or strongly agree” result for analysis. These responses were analyzed with logistic regression to assess age, sex, and geographic location effects in a multivariable model. Two-way interactions for these factors were tested using a likelihood ratio test. Group-specific effects were estimated for interactions found to be significant at the 0.05 level. The association between a subject’s response to confidence in understanding genetic material and their responses to other questions regarding evaluation and comfort with genetic testing were also assessed with logistic regression. An interaction between confidence and country was assessed for all responses. Age was analyzed as a 4-level categorical variable for all models to simplify presentation of results and allow for non-linearity. SAS version 9 software (SAS Institute, Cary, North Carolina, USA) was used for all analyses.
Results
Patient population
A total of 1,397 patients were surveyed, of which 44 did not complete either of the surveys. Demographics of the remaining 1,353 patients are outlined in Table 1 of which 77% were males, 23% were females, the average age was 63 years, 29% were from Canada, 28% Korea, and 43% from the U.S. The age, sex, and ethnic composition of survey participants by country are outlined in Supplementary Table S2.
Table 1.
Baseline Demographics Of TAILOR PCI Participants Who Completed At Least One Of The Two Surveys Administered
| Summary of Demographics and Risk Factors | ||
|---|---|---|
| Variable | Overall (N=1353) | |
| Age, years | 62.8 | (11.1) |
| Age. years | ||
| ≤55 | 364 | (27%) |
| 56–65 | 429 | (32%) |
| 66–75 | 370 | (27%) |
| >75 | 190 | (14%) |
| Gender, N (%) | ||
| Male | 1041 | (77%) |
| Female | 312 | (23%) |
| Presentation, N (%) | ||
| ACS/STEMI | 1086 | (80%) |
| Stable | 267 | (20%) |
| Country, N (%) | ||
| Canada | 399 | (29%) |
| Korea | 374 | (28%) |
| USA | 580 | (43%) |
| Ethnicity, N (%) | ||
| White/Caucasian | 878 | (65%) |
| East Asian | 313 | (23%) |
| South Asian | 95 | (7%) |
| African-American / Black | 12 | (1%) |
| Hispanic or Latino | 6 | (0%) |
| Other/Unknown | 46 | (3%) |
| BMI, kg/m2 | 28.8 | (6.1) |
| Diabetes, N (%) | 345 | (26%) |
| Hypertension, N (%) | 829 | (61%) |
| Dyslipidemia, N (%) | 728 | (54%) |
| Peripheral Artery Disease, N (%) | 32 | (2%) |
| Current Smoker, N (%) | 325 | (24%) |
| Stroke/TIA, N (%) | 39 | (3%) |
| Heart failure ≤ 2 weeks, N (%) | 30 | (2%) |
| Family History of CAD, N (%) | 557 | (41%) |
| Chronic lung disease, N (%) | 50 | (4%) |
| Pre-PCI LV Ejection Fraction, % | 57.7 | (11.2) |
Baseline survey
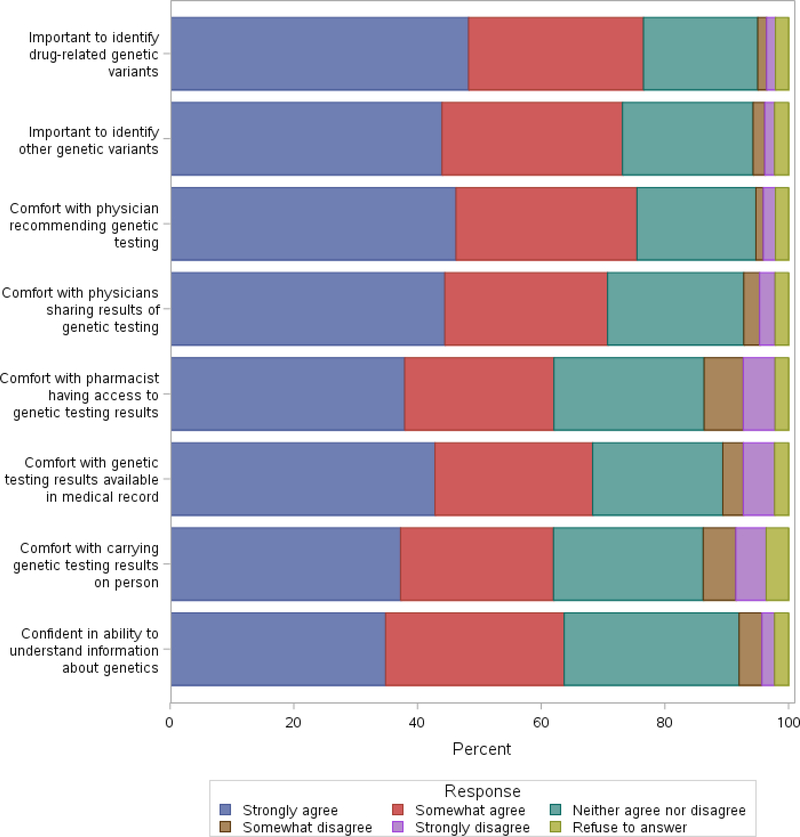
A vast majority (97% and up) of the patients who responded to the baseline survey did not have prior experience with genetic testing including pharmacogenetics. Approximately one-third of subjects (31%) had previously considered one of these genetic tests. A majority of patients (77%) were interested in finding out if they had pharmacogenetic variants or other genetic variants that were related to their health (73%). Similarly, 75% of patients felt comfortable if their physician recommended genetic testing to guide their healthcare, and over 70% felt comfortable if their physician shared these results with other physicians to guide use of medications. A vast majority of patients were comfortable if these genetic test results were made part of their medical record. Fewer patients (62%) felt comfortable if their pharmacist had access to genetic testing results to guide use of medications and were comfortable carrying the results of their genetic testing in the form of a card or on their smartphone. A substantial number of patients (64%) were confident of their ability to understand genetic information (Fig. 1).
Figure 1.
Responses to baseline survey of attitudes towards genetic testing.
Follow-up survey
A follow-up genotyping survey was completed by 860 subjects, approximately 6-months after randomization. The 6-month response rate was significantly better in the U.S. (82%) versus Canada (51%) and Korea (47%). The purpose of the follow-up genotyping survey was to assess any change in subjects’ attitudes after undergoing the procedures involved with the clinical trial. Overall, there did not appear to be a statistically significant difference between patients’ attitudes towards undergoing genetic testing in the baseline and the follow-up survey. There was a trend (80% vs 77%, P=0.06) towards fewer patients feeling comfortable regarding physicians recommending genetic testing to help guide their healthcare (Supplementary Table S3). Subject comfort remained the same regarding the access of genetic results by physicians or pharmacists or having it available in the medical record. Subjects’ confidence in their ability to understand genetic information did not change.
Country of origin
There were a significantly greater proportion of subjects in Canada (59%) and Korea (30%) who had considered obtaining health related genetic tests as compared to the U.S. (11%) (Supplementary Table S4). In contrast, the perceived importance of pharmacogenetic and other health related genetic variants was far greater in patients in both U.S. (89%) and Canada (91%) than in Korea (44%) (Table 2). There were also a larger proportion of patients in Canada and the U.S. who were comfortable with physicians ordering genetic testing, sharing genetic results with other physicians and pharmacists, incorporating these results in the medical record, or carrying these results on their person.
Table 2.
Summary Of Responses From The Baseline Genotyping Survey Stratified Based On National Origin Of TAILOR PCI Participants
| Somewhat or Strongly Agree Response | |||||||
|---|---|---|---|---|---|---|---|
| Variable | Canada (N=381) | Korea (N=374) | USA (N=572) | p Value | |||
| It is important to me to find out if I have prescription drug-related genetic variants that might be important to my health, N (%) | 345 | (91%) | 164 | (44%) | 506 | (89%) | <.001 |
| It is important to me to find out if I have other genetic variants (not related to how my body uses prescription drugs) that might be important to my health, N (%) | 339 | (89%) | 160 | (43%) | 471 | (82%) | <.001 |
| In the future, I would feel comfortable if my physician recommended genetic testing to help guide my health care (if cost were not a factor), N (%) | 344 | (90%) | 160 | (43%) | 497 | (87%) | <.001 |
| Not related to this trial, I would feel comfortable if my physician shared results of my genetic testing with other physicians to guide use of medications, N (%) | 341 | (90%) | 107 | (29%) | 490 | (86%) | <.001 |
| Not related to this trial, I would feel comfortable if my pharmacist had access to results of my genetic testing to guide use of medications, N (%) | 332 | (87%) | 105 | (28%) | 386 | (68%) | <.001 |
| Not related to this trial, I would feel comfortable if genetic testing results were available as part of my medical record, N (%) | 336 | (88%) | 126 | (34%) | 444 | (78%) | <.001 |
| Not related to this trial, I would feel comfortable carrying results of my genetic testing to guide use of medications in the form of a card or on my smart phone, N (%) | 322 | (85%) | 95 | (25%) | 405 | (71%) | <.001 |
| I am confident in my ability to understand information about genetics, N (%) | 329 | (86%) | 79 | (21%) | 437 | (77%) | <.001 |
This perception could be related to a lack of confidence, as only 21% of Koreans as opposed to 86% and 77% (P<0.001) of patients in Canada and the U.S., respectively, were confident in their ability to understand genetic information. The differences between the Koreans as compared to Canada and the U.S. persisted in the six-month follow-up genotyping survey despite continued participation in the trial. However, during follow up, Korean subjects felt more comfortable than previously reported in the baseline survey with a physician sharing their genetic results (33% vs 23%, P=0.004) or if their pharmacists had access to genetic test results (33% vs 25%, P=0.028). Also, in the six-month survey, an increased proportion of Koreans were comfortable carrying results of their genetic testing (32% vs 24%, P=0.033). The comparisons of the 6 month follow-up surveys based on country of origin need to be interpreted with caution due to the geographic differences in response rates as discussed above.
Gender and age
There were important gender differences in perceptions of genotyping at baseline. Overall, a statistically higher proportion of males felt that pharmacogenetic variants (78% vs. 72%, P=0.026) and genetic variants (75% vs. 66%, P=0.002) associated with health were important. The proportion of females who felt comfortable allowing their pharmacists access to genetic test results to guide use of medications was also statistically significantly lower than that of males (64% vs. 55%, P=0.004). There were a greater proportion of males than females who felt comfortable if physicians used genetic testing to guide their healthcare (77% vs. 71%, P=0.045). Comparing these demographics by gender and country revealed that these statistically significant differences occurred more frequently in Koreans (Supplementary Table S5); these differences were less evident during the six-month follow-up survey (Supplementary Table S6). There were no significant associations between age and perceptions of genotyping at baseline. At the follow-up survey, the only age-related difference meeting significance (P=0.039) was the importance of finding out if there was a prescription drug-related genetic variant that might be important to the patient’s health.
Conventional arm vs. prospective genotyping arm
There were no significant differences in the conventional or the prospective genotyping arm in confidence regarding genetic testing, perception regarding the importance of pharmacogenetic testing, and the ability of physicians or pharmacists to use genetic information. This lack of differences between the two arms persisted in the follow-up surveys.
Knowledge of CYP2C19 genotype
At baseline, among subjects assigned to prospective genotyping, differences in attitudes between those identified as WT and those with a CYP2C19 LOF allele were adjusted for country, due to the significantly higher prevalence of LOF alleles in Korean subjects versus North American subjects. With regards to attitudes and comfort with genetic testing between these 2 groups, there were no significant differences after accounting for country of origin. Amongst the subjects who were identified to possess the CYP2C19 WT allele by prospective genotyping, there was a greater proportion who felt comfortable if their physicians shared genetic test results with other physicians to guide use of medications in the six-month follow-up survey (82% vs 76%, P=0.032). Amongst the patients who had the CYP2C19 LOF allele by prospective genotyping there were no significant differences between baseline and six-month follow-up surveys.
Multivariable models
Multivariable models were constructed to estimate the age and gender associations with baseline survey responses, accounting for country as well (Table 3). The differences observed in the unadjusted analysis between Canadians, Koreans and Americans with regards to genetic-based guidance and sharing of test results persisted after adjusting for age and gender. Additionally, after adjusting for country, age was significantly associated with a patient’s comfort level with having their genetic testing results available to the pharmacist. Also, after adjustment, men remained significantly more likely than women to agree on the importance of knowing about genetic variants, as well as to be comfortable with using genetic testing to guide their health decisions and to be comfortable with their pharmacist having access to their genetic testing results. Nine interactions between country and age or gender were found to be statistically significant and are outlined in Supplementary Fig. S1.
Table 3.
Multivariable Models For Somewhat/Strongly Agree Response
| Endpoint | Variable | OR (95% CI) | p Value |
|---|---|---|---|
| Importance of drug-related genetic variants | Age group (overall) | 0.43 | |
| Age 56–65 v <=55 | 1.14 (0.77, 1.68) | 0.51 | |
| Age 66–75 v <=55 | 0.91 (0.61, 1.35) | 0.64 | |
| Age >75 v <=55 | 1.31 (0.81, 2.12) | 0.28 | |
| Men v Women | 1.48 (1.05, 2.09) | 0.024 | |
| Canada v USA | 1.21 (0.78, 1.86) | 0.39 | |
| Korea v USA | 0.10 (0.07, 0.14) | <0.001 | |
| Importance of other genetic variants | Age group (overall) | 0.51 | |
| Age 56–65 v <=55 | 1.29 (0.90, 1.85) | 0.17 | |
| Age 66–75 v <=55 | 1.03 (0.72, 1.50) | 0.86 | |
| Age >75 v <=55 | 1.16 (0.74, 1.80) | 0.51 | |
| Men v Women | 1.62 (1.18, 2.22) | 0.003 | |
| Canada v USA | 1.66 (1.13, 2.45) | 0.011 | |
| Korea v USA | 0.15 (0.11, 0.21) | <0.001 | |
| Comfort with genetic testing to guide decisions | Age group (overall) | 0.75 | |
| Age 56–65 v <=55 | 1.20 (0.82, 1.75) | 0.35 | |
| Age 66–75 v <=55 | 1.11 (0.75, 1.64) | 0.60 | |
| Age >75 v <=55 | 1.24 (0.78, 1.99) | 0.36 | |
| Men v Women | 1.42 (1.01, 1.98) | 0.041 | |
| Canada v USA | 1.37 (0.90, 2.09) | 0.14 | |
| Korea v USA | 0.11 (0.08, 0.15) | <0.001 | |
| Comfort sharing results with other physicians | Age group (overall) | 0.39 | |
| Age 56–65 v <=55 | 1.10 (0.75, 1.62) | 0.62 | |
| Age 66–75 v <=55 | 1.10 (0.74, 1.64) | 0.63 | |
| Age >75 v <=55 | 1.53 (0.94, 2.49) | 0.088 | |
| Men v Women | 1.31 (0.93, 1.85) | 0.12 | |
| Canada v USA | 1.43 (0.95, 2.14) | 0.087 | |
| Korea v USA | 0.06 (0.05, 0.09) | <0.001 | |
| Comfort with pharmacist having test results | Age group (overall) | 0.013 | |
| Age 56–65 v <=55 | 1.40 (1.00, 1.97) | 0.048 | |
| Age 66–75 v <=55 | 1.06 (0.75, 1.49) | 0.74 | |
| Age >75 v <=55 | 1.83 (1.20, 2.80) | 0.005 | |
| Men v Women | 1.53 (1.13, 2.06) | 0.006 | |
| Canada v USA | 3.31 (2.33, 4.70) | <0.001 | |
| Korea v USA | 0.18 (0.14, 0.24) | <0.001 | |
| Comfort with genetic tests in medical record | Age group (overall) | 0.11 | |
| Age 56–65 v <=55 | 1.36 (0.96, 1.92) | 0.085 | |
| Age 66–75 v <=55 | 1.23 (0.86, 1.76) | 0.26 | |
| Age >75 v <=55 | 1.69 (1.09, 2.62) | 0.020 | |
| Men v Women | 1.23 (0.90, 1.68) | 0.20 | |
| Canada v USA | 2.20 (1.52, 3.19) | <0.001 | |
| Korea v USA | 0.14 (0.11, 0.19) | <0.001 | |
| Comfort carrying test results on person | Age group (overall) | 0.078 | |
| Age 56–65 v <=55 | 1.16 (0.83, 1.63) | 0.38 | |
| Age 66–75 v <=55 | 1.12 (0.79, 1.58) | 0.53 | |
| Age >75 v <=55 | 1.76 (1.14, 2.70) | 0.010 | |
| Men v Women | 1.31 (0.97, 1.77) | 0.078 | |
| Canada v USA | 2.29 (1.64, 3.21) | <0.001 | |
| Korea v USA | 0.14 (0.10, 0.18) | <0.001 | |
| Confident in ability to understand genetics | Age group (overall) | 0.89 | |
| Age 56–65 v <=55 | 0.94 (0.65, 1.35) | 0.73 | |
| Age 66–75 v <=55 | 0.87 (0.60, 1.27) | 0.48 | |
| Age >75 v <=55 | 0.87 (0.55, 1.36) | 0.54 | |
| Men v Women | 1.21 (0.87, 1.66) | 0.25 | |
| Canada v USA | 1.88 (1.32, 2.68) | <0.001 | |
| Korea v USA | 0.08 (0.06, 0.11) | <0.001 |
Confidence in understanding genetic information
To assess whether differences in confidence in understanding genetic information might be a potential explanation for the geographic differences in attitudes observed towards the importance of pharmacogenetics, we looked at the association between confidence in understanding and other baseline responses. Subjects who were confident in understanding genetic information were significantly more likely to agree that it was important to identify drug-related genetic variants (OR 30.0, 95% CI (20.5, 43.8)) and similarly strong associations were seen with the other survey responses as well (Supplementary Table S7). These associations were attenuated, but remained highly significant after adjusting for age, sex, and country of origin. Additionally, significant interactions were found between subject’s confidence and their country of origin. Confidence in understanding genetic information was significantly less associated with other attitudes in the US, as compared to Canada and Korea. These associations remained statistically significant in the US, but adjusted odds ratios were frequently 3 to 5 times larger in Canada and Korea. In particular, with regards to the importance of identifying drug-related variants, the odds ratio was 8.94 (95% CI 5.09, 15.7) in the USA, compared to 34.0 (14.7, 78.6) in Canada and 42.8 (15.2, 121) in Korea (interaction P-value=0.005).
Discussion
The field of pharmacogenomics is rapidly progressing with increasing availability of low cost pharmacogenomic panels, efforts at introducing preemptive pharmacogenomics in patient populations and recent clinical trials demonstrating benefit of such testing [2,8,9]. Studies must therefore identify possible barriers to adopting and performing these tests and determine the best way to disseminate the practice of precision medicine to the bedside. The survey sub-study of the TAILOR-PCI trial is one of the first and largest studies to explore attitudes of post-PCI patients enrolled in a CYP2C19 genotype based antiplatelet therapy clinical trial. The design of TAILOR-PCI enabled assessment of whether attitudes changed with time and knowledge of genotype status, and whether geographical differences exist. The study demonstrates the following key findings: 1) Most patients do not have experience with genetic testing irrespective of country of origin; 2) Patients are interested in finding out if they have genetic variants that are related to their treatment or health; 3) Patients are comfortable with their physicians using these results to manage their healthcare and having these results incorporated in their medical record; 4) US and Canadian participants are more comfortable with the use of pharmacogenetics than Koreans, likely related to a confidence gap; 5) These attitudes do not change significantly after participation in a pharmacogenetic-based clinical trial; 6) Knowledge of genetic results does not appear to change these attitudes; and, 7) Females are initially less likely to be comfortable with the use and dissemination of pharmacogenetics but these differences resolve with time.
Surveying public reactions towards pharmacogenetic testing to assess attitudes in subjects from diverse backgrounds is critical. Due to a difference in allele frequencies amongst different populations [10] particularly in the case of the clopidogrel-CYP2C19 drug-gene pair, in which approximately 50% in Asians and 30% in Caucasians have CYP2C19 LOF alleles [7] it becomes especially important to assess patient perceptions in Asia and compare those to subjects from other countries. Patients from Korea, despite undergoing the informed consent process for enrollment in TAILOR-PCI were not comfortable with the routine clinical use of pharmacogenetics in their care. The lack of comfort was likely related to their lack of confidence in their ability to understand genetic information. While more than 3 out of 4 North American patients were confident in their ability to understand genetic information, this was only true for 1 in 5 from Korea. In the 1,200 Patients Project, a non-randomized observational study, subjects who received an explanation of pharmacogenomics during study enrollment and experienced pharmacogenomic testing were more receptive towards the implementation of pharmacogenomics in their care compared to those who received conventional care [11]. However, differences were observed in the Korean participants of our study despite all subjects, irrespective of national origin and the arm that they were randomized to, received information regarding pharmacogenetic testing during the informed consent process. The reasons for the lack of confidence in understanding pharmacogenetic testing are not clear and cannot be directly linked to information provided in the consent form especially since the information provided was uniform across all 3 countries. The lack of confidence could be linked to the differential exposure to and poor knowledge of pharmacogenomics of the Korean patient prior to study participation. Supporting this possible explanation, in a separate study of 703 Korean subjects revealed that only 28% had prior knowledge that genetics could affect drug response [12]. A large Finnish study in the general population has demonstrated a significant association between knowledge and attitudes towards genetic testing [13]. However, our study did not directly assess knowledge of pharmacogenetic testing prior to enrollment.
There may also be social barriers towards genetic testing that may need to be taken into account as Asian patients may be apprehensive about the stigmas that are possibly associated with possessing a certain “defective” gene [14]. In our study, consistent with prior studies, female patients had less favorable attitudes towards pharmacogenetic testing and were more concerned about the negative impacts of precision medicine [14–19]. Subgroup analysis revealed that these statistically significant differences occurred more frequently in Korean females. In addition to geographical differences, a study based in an outpatient Baltimore clinic highlights the importance of addressing perceptions of minorities by demonstrating that minorities express a reduced intention to adhere to medical therapy which is prescribed on the basis of genetic information [20].
Patients appear to be fearful that precision medicine may result in an increase in costs and thereby promoting a greater inequality in the access of such healthcare [18,21]. However patients appear to be more willing to pay out-of-pocket if the disease associated with pharmacogenetic testing is high risk and the risk of recurrent acute coronary or cerebrovascular events associated with clopidogrel failures would certainly fall in this category [21]. Our finding that there is a high acceptance rate for the use of pharmacogenetics to guide treatment in patients with cardiovascular disease is consistent with studies conducted in other populations [10,15,19,22,23]. The utilization of precision medicine may improve drug adherence and result in a decrease in the overall cost of healthcare for patients by decreasing the occurrence of clinical events [24,25]. The effect of ongoing participation and engagement in the TAILOR-PCI clinical trial may have had an effect on the Korean population as the number of participants who felt comfortable if physicians shared genetic results with other physicians to guide use of medications (23% to 33%), comfort if pharmacist had access to genetic results (25% to 33%), and comfort in carrying genetic results on a card or smartphone (24% to 32%) increased during follow up at 6 months. The gender differences in perception towards genetic testing in the subset of Korean patients also resolved at six-month follow-up.
The ability of the patient to trust in the physician is critical when drugs are prescribed based on genetic information especially in minority communities and therefore a strong physician-patient relationship is important to enable effective implementation of pharmacogenomics [20]. In addition to patients’ perspectives, physicians’ perceptions and their willingness to utilize pharmacogenetic testing in their clinical practice play an important role. A study on physicians’ perspectives of warfarin pharmacogenetic testing demonstrated that major barriers to adoption were inadequate literature evidence, testing’s impracticality and unproven applicability [26]. TAILOR-PCI trial addresses these concerns by not only assessing the effectiveness of a genetic based anti-platelet therapy approach using meaningful clinical endpoints but by also demonstrating the use of a “point of care” genotyping platform that can provide results accurately within an hour.
There are limitations to our study. The patient population surveyed had already consented to be involved in a genetic study and were perhaps predisposed to having a favorable attitude towards genetic testing. Despite this limitation the study demonstrated significant differences in perceptions in Koreans and that positive attitudes of North Americans towards genetic testing are comparable to other North American random population based surveys. We also are unaware whether education levels played a role in the lower level of understanding and lack of comfort towards genetic testing expressed by participants of TAILOR PCI since this data was not collected. However, we do not believe lack of literacy in Koreans compared to North Americans played a major role in the wide disparity of perceptions between the 2 populations because the literacy rate in Korea is 98% (99% males, 97% females) and is similar to that of a developed country with average school life expectancy being 17 years and 83% of high school graduates advancing to tertiary education (http://sites.miis.edu/southkoreaeducation/diversity-and-access/).
Conclusion
In conclusion, the majority of patients enrolled in a CYP2C19 pharmacogenetic based study recognized the importance of prescription drug-related genetic variants, although important geographical differences exist between Korea and North America. The reason for these differences may be related to lack of confidence regarding genetic testing. To enable successful implementation of pharmacogenomic testing on a global scale, international population differences in perceptions should be considered.
Supplementary Material
Acknowledgements
We are grateful for the outstanding effort of these TAILOR PCI clinical trial sites: Dr. Christopher Overgaard, UHN-Toronto General Hospital; Dr. Charles Cagin, Mayo Clinic Health System-Franciscan Healthcare; Dr. Gary Lane, Mayo Clinic in Florida; Dr. Adam Frank, Naples Community Hospital Incorporated; Dr. Wilson Ginete, Essentia Health Saint Mary’s Medical Center; Dr. John Sweeney, Mayo Clinic in Arizona; Dr. Jacqueline Saw, Vancouver General Hospital-Gordon and Leslie Diamond Health Care Centre; Dr. Jorge Saucedo, NorthShore University Health System-Evanston Hospital; Dr. Hong-seok Lim, Ajou University Hospital; Dr. D.P. Suresh, Saint Elizabeth Medical Center South; and Dr. Mohammed El-Hajjar, Albany Medical Center.
Conflict of Interest and Source of Funding
The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services. Shaun G. Goodman reports research grant support and speaker/consulting honoraria from Spartan, AstraZeneca, Bristol-Myers Squibb, Daiichi-Sankyo, Eli Lilly, and Sanofi. This study was funded by NIH/NHLBI grant U01 HL128606.
Footnotes
Supplemental information is available for this article.
References
- 1.Pereira NL, Weinshilboum RM. Cardiovascular pharmacogenomics and individualized drug therapy. Nat Rev Cardiol. 2009; 6:632–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pereira NL, Sargent DJ, Farkouh ME, Rihal CS. Genotype-based clinical trials in cardiovascular disease. Nat Rev Cardiol. 2015; 12:475–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simon T, Verstuyft C, Mary-Krause M, Quteineh L, Drouet E, Meneveau N, et al. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med. 2009; 360:363–375. [DOI] [PubMed] [Google Scholar]
- 4.Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009; 361:1045–1057. [DOI] [PubMed] [Google Scholar]
- 5.Mega JL, Close SL, Wiviott SD, Shen L, Hockett RD, Brandt JT, et al. Cytochrome P-450 polymorphisms and response to clopidogrel. New Engl J Med. 2009; 360:354–362. [DOI] [PubMed] [Google Scholar]
- 6.Cavallari LH, Lee CR, Beitelshees AL, Cooper-DeHoff RM, Duarte JD, Voora D, et al. Multisite investigation of outcomes with implementation of CYP2C19 genotype-guided antiplatelet therapy after percutaneous coronary intervention. JACC Cardiovasc Interv. 2018; 11:181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oh IY, Park KW, Kang SH, Park JJ, Na SH, Kang HJ, et al. Association of cytochrome P450 2C19*2 polymorphism with clopidogrel response variability and cardiovascular events in Koreans treated with drug-eluting stents. Heart. 2012; 98:139–144. [DOI] [PubMed] [Google Scholar]
- 8.Gage BF, Bass AR, Lin H, Woller SC, Stevens SM, Al-Hammadi N, et al. Effect of genotype-guided warfarin dosing on clinical events and anticoagulation control among patients undergoing hip or knee arthroplasty: The gift randomized clinical trial. JAMA. 2017; 318:1115–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pereira NL, Stewart AK. Clinical implementation of cardiovascular pharmacogenomics. Mayo Clin Proc. 2015; 90:701–704. [DOI] [PubMed] [Google Scholar]
- 10.Haga SB, O’Daniel JM, Tindall GM, Lipkus IR, Agans R. Survey of US public attitudes toward pharmacogenetic testing. Pharmacogenomics J. 2012; 12:197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKillip RP, Borden BA, Galecki P, Ham SA, Patrick-Miller L, Hall JP, et al. Patient perceptions of care as influenced by a large institutional pharmacogenomic implementation program. Clin Pharmacol Ther. 2017; 102:106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee IH, Kang HY, Suh HS, Lee S, Oh ES, Jeong H. Awareness and attitude of the public toward personalized medicine in Korea. PLoS One. 2018; 13:e0192856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jallinoja P, Aro AR. Does knowledge make a difference? The association between knowledge about genes and attitudes toward gene tests. J Health Commun. 2000; 5:29–39. [DOI] [PubMed] [Google Scholar]
- 14.Glenn BA, Chawla N, Bastani R. Barriers to genetic testing for breast cancer risk among ethnic minority women: an exploratory study. Ethn Dis. 2012; 22:267–273. [PubMed] [Google Scholar]
- 15.Chan CY, Chua BY, Subramaniam M, Suen EL, Lee J. Clinicians’ perceptions of pharmacogenomics use in psychiatry. Pharmacogenomics. 2017; 18:531–538. [DOI] [PubMed] [Google Scholar]
- 16.Tan E-K, Lee J, Hunter C, Shinawi L, Fook-Chong S, Jankovic J. Comparing knowledge and attitudes towards genetic testing in Parkinson’s disease in an American and Asian population. J Neurol Sci. 2007; 252:113–120. [DOI] [PubMed] [Google Scholar]
- 17.Rogausch A, Prause D, Schallenberg A, Brockmoller J, Himmel W. Patients’ and physicians’ perspectives on pharmacogenetic testing. Pharmacogenomics. 2006; 7:49–59. [DOI] [PubMed] [Google Scholar]
- 18.Almarsdóttir AB, Björnsdóttir I, Traulsen JM. A lay prescription for tailor-made drugs—focus group reflections on pharmacogenomics. Health Policy. 2005; 71:233–241. [DOI] [PubMed] [Google Scholar]
- 19.Sanderson SC, Wardle J, Jarvis MJ, Humphries SE. Public interest in genetic testing for susceptibility to heart disease and cancer: a population-based survey in the UK. Prev Med. 2004; 39:458–464. [DOI] [PubMed] [Google Scholar]
- 20.Butrick M, Roter D, Kaphingst K, Erby LH, Haywood C Jr., Beach MC, et al. Patient reactions to personalized medicine vignettes: an experimental design. Genet Med. 2011; 13:421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Issa AM, Tufail W, Hutchinson J, Tenorio J, Baliga MP. Assessing patient readiness for the clinical adoption of personalized medicine. Public Health Genomics. 2009; 12:163–169. [DOI] [PubMed] [Google Scholar]
- 22.Olson JE, Rohrer Vitek CR, Bell EJ, McGree ME, Jacobson DJ, St. Sauver JL, et al. Participant-perceived understanding and perspectives on pharmacogenomics: the Mayo Clinic RIGHT protocol (Right Drug, Right Dose, Right Time). Genet Med. 2017; 19:819–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meisel SF, Rahman B, Side L, Fraser L, Gessler S, Lanceley A, et al. Genetic testing and personalized ovarian cancer screening: a survey of public attitudes. BMC Women’s Health. 2016; 16:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fagerness J, Fonseca E, Hess GP, Scott R, Gardner KR, Koffler M, et al. Pharmacogenetic-guided psychiatric intervention associated with increased adherence and cost savings. Am J Manag Care. 2014; 20:e146–156. [PubMed] [Google Scholar]
- 25.Iuga AO, McGuire MJ. Adherence and health care costs. Risk Manag Healthc Policy. 2014; 7:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kadafour M, Haugh R, Posin M, Kayser SR, Shin J. Survey on warfarin pharmacogenetic testing among anticoagulation providers. Pharmacogenomics. 2009; 10:1853–1860. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.