Abstract
Background:
Increasing number of methods are being used to map atrial fibrillation (AF), yet the sensitivity of identifying potential localized AF sources of these novel methods are unclear. Here we report a comparison of two approaches to map AF based upon (a) electrographic flow mapping and (b) phase mapping in a multicenter registry of patients in whom ablation terminated persistent AF.
Methods:
53 consecutive patients with persistent AF in whom ablation terminated AF in an international multicenter registry were enrolled. Electrographic flow mapping (EGF) and phase mapping were applied to the multipolar simultaneous electrograms recorded from a 64-pole basket catheter in the chamber (left vs right atrium) where AF termination occurred. We analyzed if the mapping methods were able to detect localized sources at the AF termination site. We also analyzed global results of mapping AF for each method, patterns of activation of localized sources.
Results:
Patients were 64.3±9.4 years old and 69.8% were male. EGF and phase mapping identified localized sources at AF termination sites in 81% and 83% of the patients respectively. Methods were complementary and in only n=2 (3.7%) neither method identified a source. Globally, EGF identified more localized sources than phase mapping (5.3 ± 2.8 vs 1.8 ± 0.5, p<0.001), with a higher prevalence of focal (compared to rotational) activation pattern (49% vs 2%, p<0.01).
Conclusions:
EGF is a novel vectorial-based AF mapping method, that can detect sites of AF termination, agreeing with, and complementary to an alternative AF mapping method using phase analysis.
Keywords: Atrial fibrillation, Electrophysiology, Mapping, Phase, Electrographic Flow
Introduction
Atrial fibrillation (AF) is the most common sustained atrial tachyarrhythmia worldwide and is associated with a significant burden. Pulmonary vein isolation (PVI) is the cornerstone of AF ablation therapy, but with suboptimal long-term success rates1 that may not increase by ablation of lines or complex fractionated electrograms.2 An increasing number of methods are being used to map AF and reveal localized drivers, whose ablation produces success ranging from excellent to poor in meta-analyses.3,4 Heterogeneity may reflect current limitations of AF mapping, and combining methods to map persistent AF might help to improve outcomes.
Phase mapping is used for mapping of fibrillatory conduction with endocardial5–7 and body surface electrograms.8 Phase mapping-based algorithms have been shown to demonstrate localized sources and/or improve clinical outcomes9,10,11 but also have been criticized for false positive reports.12,13 It has been suggested by Rudy et al. that combining phase with activation mapping may improve AF mapping.12
Electrographic flow (EGF) mapping is a non-phase based mapping technique which uses spatial and temporal reconstruction of panoramic, simultaneous endocardial electrograms,14 which may address certain limitations of mapping algorithms using only phase analysis, such as false positive detection of localized sources or over-emphasis of rotational activity.
In this study, we hypothesized that EGF mapping could detect localized AF sources at atrial sites where ablation terminates persistent AF. We also hypothesized that EGF mapping would have different sensitivity and specificity in determining total number of localized sources compared to phase mapping, given intrinsic technical differences of the mapping methods.
Methods
Study population
We identified 53 consecutive patients with persistent AF that was refractory to ≥1 anti-arrhythmic medication enrolled as part of the international registry (COMPARE-AF: COMParison of Algorithms for Rotational Evaluation in Atrial Fibrillation, NCT02997254) at 5 centers (Stanford University Hospital, Palo Alto, CA; University of California, San Diego, CA; University of Colorado, Denver, CO; Indiana University, Indianapolis, IN; and Klinikum Coburg, Germany) in whom ablation in a documented region terminated persistent AF to sinus rhythm or atrial tachycardia prior to PVI. We excluded patients in whom AF terminated during PVI, linear lesions, or ablation of complex fractionated electrograms. The study was approved by each local IRB.
Electrophysiology study and ablation
Patients were studied in the post-absorptive state. Class I and III anti-arrhythmic medications were discontinued for > 5 half-lives (>30 days for amiodarone). Catheters were advanced to the right atrium (RA), coronary sinus and transseptally to left atrium (LA). Contact basket catheters (64 poles, FIRMap, Abbott) were placed at multiple positions in the RA then LA for AF mapping, registered to 3-dimensional anatomic shells (NavX, St Jude Medical, Sylvar, CA; or Carto, Biosense-Webster, Diamond Bar, CA). All patients had prospective ablation at regions of interest identified by a clinical system (RhythmView™, Abbott, Inc) which uses activation and phase-based analysis and may reveal localized rotational or focal sources during AF. This mapping method has previously been compared to phase mapping11 and activation mapping15 at sites of termination but, other than identifying sites where ablation terminated AF, was not the focus of this study. Radiofrequency energy was delivered via an irrigated radiofrequency ablation catheter (Thermocool, Biosense-Webster; or Safire BLU, St Jude Medical) at these sites,16 followed by wide area circumferential pulmonary vein isolation. Each case in this series had termination of persistent AF, at sites marked prospectively on the electroanatomic shell relative to basket catheter electrodes.
AF mapping methods and quantification of localized AF sources
Two novel AF mapping methods were applied to the same electrogram data: A novel electrographic flow mapping method (Ablacon Inc., Wheat Ridge, CO) which was compared to a novel endocardial phase mapping approach that we have made freely available.17,18
Data acquisition:
Unipolar electrograms were recorded at 0.05 to 500 Hz bandpass, at 1 kHz sampling with electroanatomic location turned off to reduce electromagnetic interference. Recording duration of 4 seconds during atrial fibrillation (the same segment that was used clinically to guide ablation that terminated persistent AF) was used for this analysis of localized sources at the sites of AF termination, as well as for analysis of global AF mapping. Raw electrograms comprising 64 electrode basket and other intracardiac channels (e.g. coronary sinus), and 12-lead ECG were exported from Bard (LabSystem Pro), Prucka (GE Cardiolab) or Siemens recorders for analysis.
Mapping method 1:
Electrographic Flow (EGF) designates the matrix of velocity vectors describing the average propagation of action potentials in the physiological or pathological electrical activity of the myocardium.14 Ablamap is proprietary software that processes endocardial electrogram data with electrographic flow mapping to visualize AF drivers.14 The system analyzes the first two-seconds of electrograms for training and the remaining two-seconds for finalization of the maps. An EGF map represents a spatial and temporal reconstruction of electrographic potentials and their flow derived from endocardial unipolar electrogram data collected with a 64-pole basket catheter. The results for a 4 second segment include a prevalence map of drivers, n=6 still electrographic flow images with color representing action potential wave directionality, and one movie with arbitrary colors representing action potential flow (Figure 1A, Movie 1 right panel). The still maps and videos are displayed on a square (8×8) grid reflecting each electrode of the 64-pole basket catheter. For the still EGF images, each color on the grid implicitly represents a flow direction and overlaid arrows explicitly inform the viewer. Localized AF sources are classified by type (rotational vs focal source), and rotational sites are further classified to have centrifugal or centripetal activation. The Ablamap algorithm automatically marks rotational and focal source locations on each still image. We included localized sources for analysis, only if their presence is spatially conserved in >2 out of the 6 still electrographic flow images. Supplemental Figure 1 shows an example of marking a rotational source on electrodes DE5 and a focal source on electrodes E12. The prevalence map and the movies provided visual guidance but did not affect marking localized source locations, to ensure objectivity in marking sources.
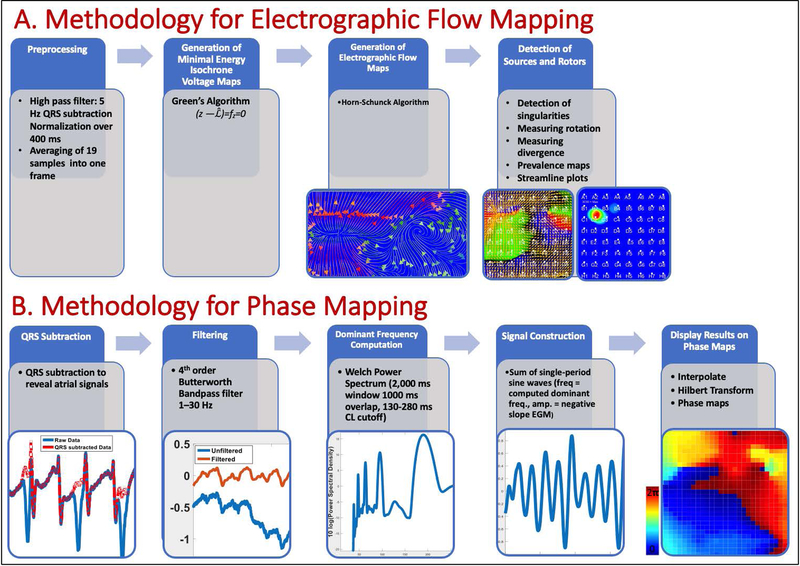
Figure 1: Critical Elements of AF Mapping: A. EGF mapping:
Core Algorithms of EGF mapping are (1) the Green’s Algorithm to generate high resolution isochrone voltage maps from normalized QRS-complex adjusted unipolar electrograms assuming minimal energy electrical field distribution and (2) the reconstruction of velocity vector flow fields from 2 seconds of consecutive voltage maps using the Horn-Schunck Algorithm. Location of sources are determined as singularities in the flow field and sources are classified according their divergence and rotation. Prevalence maps over one-minute show stability of the sources and centripetal Rotors over time. B. Phase mapping: QRS’s are subtracted using a series of methods. High frequency noise and baseline oscillations are removed via filtering, which is used to focus on the signal components that are most characteristic of AF electrograms. Rate of each atrial signal is calculated using dominant frequency and signals were then reconstructed as the sum of sine waves, each having a period corresponding to the dominant frequency. Finally, spatial maps were interpolated by the 64 electrode signals in a 29 by 29-point matrix. Then, the Hilbert transform was used to obtain the phase signal at each pixel to construct a phase map.
Mapping method 2:
The comparator method to map AF in this study was a phase mapping method validated in animal models, where it did not produce false rotations (<1%),12,19 and in human persistent AF where we have shown that it correlates with commercial clinical mapping.7,20 Briefly, we first compute an average QRS complex for each electrogram channel that is subtracted from its parent channel. Next, we applied a 1.5–25 Hz fourth-order Butterworth band pass filter and computed the dominant cycle length for each channel from the Welch Power Spectrum Density estimate. The recomposed signal was constructed as a sum of single-period sinusoidal waves, then the Hilbert Transform was used to compute phase maps, which are then displayed as a video on a square (8×8) grid reflecting each electrode of the 64-pole basket catheter. A focal impulse was defined as an electrical origin of centrifugal activation during AF, either uniform or anisotropic, and present for at least 50% of the segment (2 seconds). Rotational activation was defined as at least one full rotation (360o) and present for at least 50% of the segment (2 seconds). All videos were analyzed by 3 reviewers and agreement between at least 2 out of 3 reviewers was required to mark a localized source. Figure 1B and Movie 1 left panel shows an example of a rotational source at GH23 produced in this way. Phase mapping software and raw basket recordings of AF from the registry are available online at http://narayanlab.stanford.edu.
Analysis of overlap between mapping methods
Each localized source determined by both mapping methods was marked with their corresponding electrodes on the 8×8 grid (i.e. rotational source on DE5, focal source on E2, Supplemental Figure 1), for later use in correlation analyses. Because such sites show limited spatial meander, patterns repeating within ≤1 electrode were considered to represent precession (‘wobble’)21 of the same organized AF pattern, we considered localized sources with different methods that were marked at neighboring electrodes as the same localized source (i.e., phase mapping determining a localized source at electrode G2 and electrographic flow mapping determining a localized source at electrode G3 on the 64 electrode grid (Figure 1D and Movie 1 (left panel)).
Statistical analysis
Continuous data are represented as mean ± standard deviation (SD) or median and interquartile range (IQR) as appropriate. Normality was evaluated using the Kolmogorov-Smirnov test. Comparisons between two groups were made with independent samples and paired samples t-tests and summarized with means and standard deviations for independent samples if normally distributed; or if not normally distributed, with the Mann-Whitney U test and summarized with medians and quartiles. Nominal values were expressed as n (%) and compared with chi-square tests, the Fisher exact test when expected cell frequency was < 5. Pearson`s and point biserial correlation was applied for clinical demographic correlations. A probability of < 0.05 was considered statistically significant.
Results
Patient demographics
Table 1 shows demographics of the patients. All patients had persistent AF. Median history of AF was 3.9 years [2.2–8.8] and median CHADS2Vasc score was 2 [1–3]. Twenty-seven patients (50.9%) had a history of prior ablation. Termination sites lay in the left atrium in 48 (90.6%), and in the right atrium in 5 (9.4%) patients. AF termination with ablation was guided by clinical mapping of localized sources, and occurred before pulmonary vein isolation (PVI) in 49 patients (92.5%) and after the PVs were isolated by PVI in the remaining 4 (7.5%) patients.
Table 1.
Demographics
| Clinical characteristics | |
|---|---|
| Age (years) | 64.3±9.4 |
| Male gender (n, %) | 37 (69.8%) |
| Persistent AF (n, %) | 53 (100%) |
| Left atrial diameter (mm) | 47.2 ± 6.2 |
| LV ejection fraction (%) | 55.7±9.8 |
| CHADS2VAS2c score | 2.3±1.4 |
| Body mass index (BMI, kg/m2) | 30.7±5.8 |
| Comorbidities | |
| Diabetes mellitus | 23.9% |
| Hypertension | 71.7% |
| Hyperlipidemia | 43.4% |
| Congestive heart failure | 21.9% |
| Chronic kidney disease | 9.4% |
AF mapping at sites of termination
Sites at which ablation terminated AF demonstrated localized sources with phase mapping in n=44 (83%) and with EGF mapping in n=43 (81%) of patients. In 2 patients, neither method showed any localized sources at AF termination site (Table 2). Both mapping methods agreed at the AF terminating site in 38/53 patients (71.7%), of which n=36 (67.9%) represented agreement on the presence of a source and n=2 (3.8%) represented agreement on the absence of a source.
Table 2.
Determination and type of localized sources by phase and EGF mapping at sites of AF termination.
| Phase Mapping | |||||
|---|---|---|---|---|---|
| Rotational activation | Focal activation | No sources detected | Total | ||
| Electrographic Flow Mapping | Rotational activation | 22 | 0 | 3 | 25 |
| Focal activation | 14 | 0 | 4 | 18 | |
| No sources detected | 8 | 0 | 2 | 10 | |
| Total | 44 | 0 | 9 | 53 | |
At AF termination sites, phase mapping identified localized AF sources to be 100% rotational in pattern; whereas EGF identified terminations sites to be 51.2% rotational (7.0% centrifugal, 44.2% centripetal activation) and 48.8% focal activation (p<0.01) sources.
Determinants of detecting localized sources at sites of AF termination
Phase mapping was more likely or trended, respectively, to detect localized sources at sites of AF termination in patients who had a lower prevalence of hypertension (p=0.045) and have a lower prevalence of hyperlipidemia (p=0.065). EGF was also more likely to detect localized sources at sites of AF termination in patients who had a lower prevalence of hyperlipidemia (p=0.025), and in patients who did not have a history of TIA or CVA (p=0.028) or a history of MI (p=0.048).
Clinical examples of concordant and discordant results at AF termination sites
We illustrate four case examples of comparison of the mapping methods. Figure 2 shows two similar examples in which electrographic flow and phase mapping showed concordant results. The top panel is a 56-year-old man in whom ablation in the anterior left atrium (Figure 2A), led to termination of atrial fibrillation (Figure 2B). Phase mapping and electrographic flow mapping identified clockwise rotational activation localized at the AF termination site, over electrodes GH23 on the grid (Figure 2C–D). A similar agreement is shown in the bottom panel, which illustrates a 53-year-old man with persistent AF in whom ablation in the left atrial roof, proximal to the antrum of the right upper pulmonary vein (Figure 1E), terminated persistent AF to sinus rhythm (Figure 2F). Phase mapping and electrographic flow mapping identified counterclockwise rotational activation localized to the AF termination site, over electrodes BC45 on the grid (Figure 2G–H).
Figure 2.
Two patients in whom electrographic flow and phase mapping showed concordant results. The top panel shows AF in a 56-year-old man and where ablation in the anterior left atrium (A), led to termination of atrial fibrillation (B). Phase mapping and electrographic flow mapping identified a clockwise rotational activation localized at the AF termination site, over electrodes GH23 on the grid (C-D). Movie 1 demonstrates phase and EGF videos of this case. Bottom panel shows AF in a 53-year-old man in whom ablation in the left atrial roof, proximal to the right upper pulmonary vein antrum (E), terminated persistent AF to sinus rhythm (F). Phase mapping and electrographic flow mapping identified clockwise rotational activation localized to the AF termination site, over electrodes BC45 on the grid (G-H).
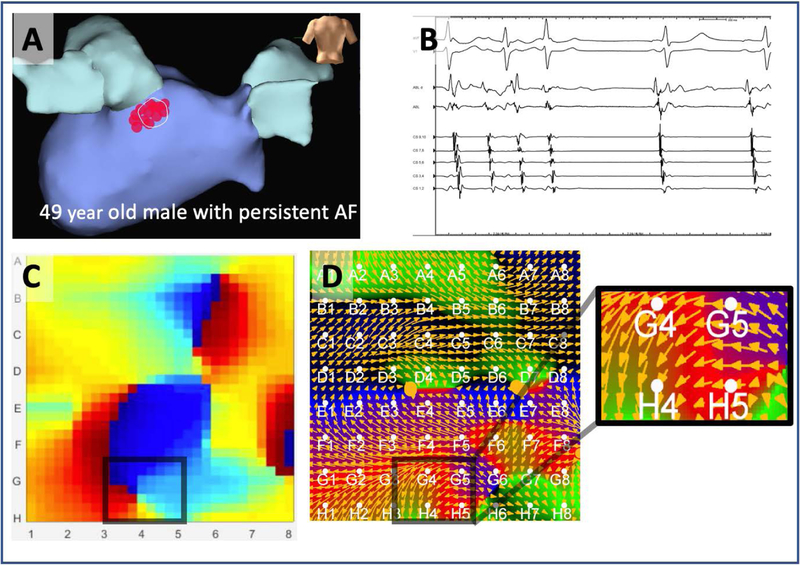
Figure 3 shows a case of disagreement between methods. Here, figure 3A shows the atrial electroanatomic shell of a 49-year-old man in whom ablation in the posterior left atrium, inferior to the LIPV terminated persistent AF to sinus rhythm (Figure 3A,B). Mapping at this site identified a rotational source at termination site GH45 by Phase (Figure 3C) but EGF flow showed no clear rotational or focal activation (Figure 3D).
Figure 3. Disagreement on rotational activity at AF termination site, which revealed rotational source by phase mapping, but not by EGF mapping in a.
49 year old man in whom ablation in the left atrium, inferior to the left inferior pulmonary vein (A) terminated persistent AF to sinus rhythm (B) where phase mapping identified a rotational source (electrodes GH45) (C) but electrographic flow mapping showed no clear rotational or focal activation (D).
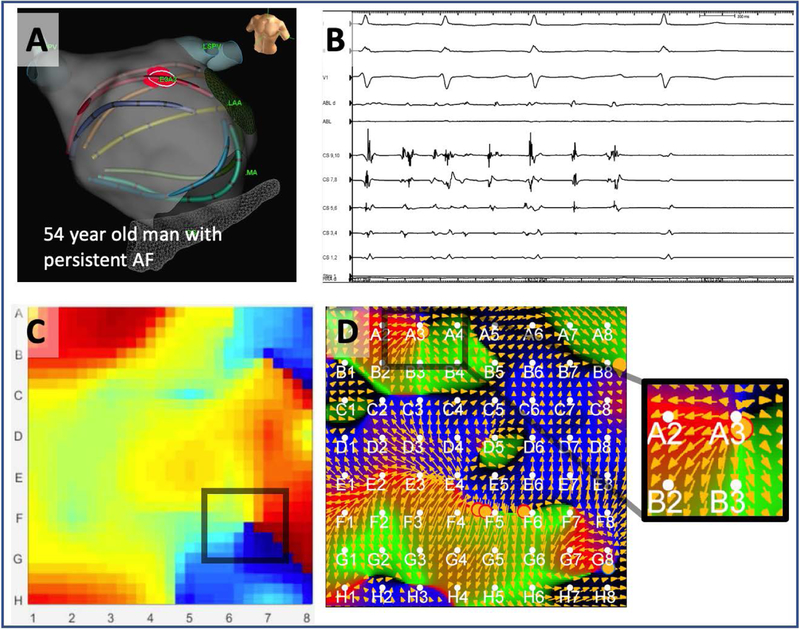
Figure 4 illustrates another case of disagreement between methods, in a 54-year-old man in whom ablation anterior to left atrial appendage terminated persistent AF (Figure 4A–B). Phase mapping at this site did not identify a rotational source (Figure 4C), but electrographic flow shows a clear focal source at the A3 electrode overlying the termination site (Figure 4D).
Figure 4. Disagreement on rotational activity at AF termination site, which revealed rotational source by EGF mapping but missed by phase mapping in.
54 year old man in whom ablation anterior to left atrial appendage terminated persistent AF (A-B). Mapping at this site did not identify a rotational source by phase (C), but electrographic flow shows a clear rotational source at the A3 electrode overlying the termination site (D). Both methods agree on a non-terminating AF source on this map, at electrode F6.
Global AF mapping with phase and electrographic flow
Examining all potential localized sources, i.e. where AF termination was observed as well as non-AF-terminating sites, phase mapping determined a total of 96 localized AF sources in 53 patients, with an average of 1.8 ± 0.5 total sources per patient. Ninety-eight percent of these localized sources had rotational and two percent focal activation patterns.
EGF determined a total of 276 localized AF sources with an average of 5.3 ± 2.8 total sources per patient, which was significantly higher compared to phase mapping (p<0.01). We observed that 51% of sources identified by EGF mapping had rotational activation, significantly fewer than the proportion identified as rotational by phase mapping (p<0.01), although the absolute number of rotational sites was higher by EGF than phase (139 vs 95). Of sources with rotational activation by EGF, 21% were determined to have centrifugal activation, 79% to have centripetal activation. 49% of the localized sources determined with EGF had a focal activation pattern.
Agreement on localization of all sources during left atrial mapping between phase mapping and EGF was 31%, reflecting the higher number of sources detected by EGF. If analyzing the agreement of EGF to phase mapping, i.e. how often EGF determined the same sites determined by phase mapping, the agreement was 63%.
Discussion
In this study, we demonstrate that electrographic flow mapping, which is a novel way to identify propagation patterns in AF that does not use phase analysis, is able to detect localized sources at sites where persistent AF terminated with ablation. We show that this mapping approach correlates with localized AF sources identified by an open access phase mapping algorithm, at anatomic sites which could potentially play a role in the mechanism of the rhythm disorder.
There was agreement among the two methods in this multicenter cohort, and a combination of phase and electrographic flow mapping appeared to be complimentary.
Regarding the activation patterns of localized AF sources (focal vs rotational activation) where AF termination occurred, there was disagreement between methods. Although phase mapping is able to identify both types of activation patterns of localized sources, Vijayakumar et al. reported that phase mapping is more likely to demonstrate rotational than focal activation.12 Sites determined to have rotational activation by phase mapping may be marked as focal by EGF, which does not use Hilbert transform or sum of single-period sinusoidal wave reconstruction. On the other hand, optical mapping of human AF shows that repetitive focal activity is most likely breakthrough from sites of rotational activity on the other side of the atrial wall 20, and so higher resolution mapping or bisurface mapping may be needed to resolve this issue. Also, with EGF mapping, we observed that a focal activation was often accompanied by a nearby centripetal rotational source, which may represent passive activation driven by the focal activation (Supplemental Figure 2). Future studies should reconcile the nature of these activation patterns across methods, as well as within electrographic flow mapping itself (centrifugal vs centripetal activation). Using mapping methods as complementary tools in mapping AF may have promise, and may include the combination of activation plus phase mapping (as in our clinical system11), contact recordings plus body surface mapping, or electrical plus structural mapping.
Mapping of Fibrillatory Conduction:
Fibrillatory propagation in the atrium has been mapped in detail during surgical procedures. The historical surgical maps during fibrillation showed complicated activation patterns with no localized sources.22 However, because they involved manual assignment of activation at multiple electrodes, few cycles of AF were analyzed (typically 2–4). Moreover, the rules of marking activation during fibrillatory conduction may occasionally be ambiguous i.e. how to mark electrograms with multiple deflections or low amplitude signals.15 Electrographic flow mapping is a new way to map propagation, which applies a consistent rule for multiple cycles. A potentially similar approach was published by Vidmar et al. in 2016,23 termed as ‘wave-front flow field’ in that study, analyzing synthetic data as well as two clinical cases with this methodology: a case of atrial tachycardia and another with ventricular fibrillation, where this method is able to map localized sources in either rhythm. The other study analyzing electrographic flow mapping is by Bellman et al.14 where the same method used in our analysis was applied. In that study, 25 patients with persistent AF were analyzed, and electrographic flow maps were compared to another mapping system, FIRM, which uses activation timings and phase analyses to analyze fibrillatory conduction. Our study adds to this data, by including a clinical endpoint of AF termination by ablation for validation, as well analyzing a much larger cohort of patients from multicenter cohort compared to earlier studies. Long-term implications of termination of AF with ablation have been debated with multiple positive and negative studies, but it still remains as the only intra-procedural end-point, especially in persistent AF, that may have a mechanistic value.
Localized sources and AF
Mechanisms of AF are debated, but a large number of techniques have shown sources in recent years.4 Notably, optical mapping for AF,20 often considered a gold standard, showed rotational AF sources in human atria, which correlate well with clinical mapping methods (FIRM)24 on simultaneous mapping of AF.25 Other AF mapping methods also reveal localized sources, including electrocardiographic imaging (ECGi),9 dipole density mapping or AcQMap,26 CartoFinder,27 and dominant frequency mapping.28 In general, the EGF method identifies more source regions than ECGi, FIRM or CartoFinder methods. This may be an advantage in identifying sites missed by other methods, or a disadvantage in identifying more ‘false positives’, but both have yet to be established. Whether these systems each identify the same atrial regions remains to be determined. In the present study, we have noted that mapping strategies using different algorithms could indeed be complimentary, i.e. if the AF termination site is not detected by one method, it may be detected by the other, only being unable to detect sources in 3.8% of cases when combined (Table 2).
Conclusions
Electrographic flow mapping is a novel AF mapping method, that can detect sites of AF termination, agreeing with phase mapping of AF. Combination of these mapping methods may provide a complimentary approach to AF, and studies should further examine differences in the activation patterns of localized sources between the two methods.
Limitations
Although phase mapping is non-proprietary and open-source code is available, Ablamap™, the mapping method analyzed in this study, and RhythmView™, the mapping method with which clinical sites were originally identified that resulted in AF termination are proprietary. However, identifying sites where ablation terminates persistent AF helps validate that the sites identified by each method in this study. Although comparison of mapping methods was done prospectively, the enrollment and creation of the registry is retrospective in nature in our relatively small cohort, and a prospective validation of these mapping methods in a larger study is needed. We realize there is a debate in AF mechanisms, but the goal of this paper was to compare a new method, EGF, to phase mapping, that has previously been described and used in clinical studies. We accept that no mapping system currently is optimal, and ideal studies should use multiple mapping methods in the same patients prospectively. Ablamap currently does not have FDA clearance to guide AF ablation therapy prospectively, and given the inconsistency of re-mapping following ablation among centers to assess complete elimination of all drivers, an on-treatment analysis of long term outcomes is not available at this point.
Supplementary Material
Supplemental Figure 1. Analysis of localized sources with EGF. A. Still EGF images: 4 second analysis during AF shows focal activation (red circle) from E12 is present in all six still images. A rotational source at D5 (green circle, centrifugal activation; orange circle, centripetal activation) is also present in at least two out of the six still images, therefore marked as a localized source for our analysis. B. Prevalence map of drivers with red denoting area of highest prevalence throughout the time segment. AF termination had occurred with ablation at electrodes DE2 in this 52-year-old male with persistent AF.
Supplemental Figure 2. Focal activation accompanied by a nearby localized source with centripetal activation, potentially driven by the focal activation. Mapping at this site of AF termination (A-B) identified a rotational source by phase mapping (C) and a rotational source with centripetal activation with electrographic flow mapping at the A3/A4 electrodes overlying the termination site (D). This centripetal rotor is accompanied by the flow of a very stable localized source with focal activation at electrode C2. This is demonstrated in a stream line plot displaying the focal source surrounded by a high velocity flow field connected with the rotor through a low velocity bridge (E) A similar pattern of a nearby focal activation accompanying a rotational centripetal activation at a termination site was observed in 18 of the 53 patients.
Movie 1. Phase (left panel) and electrographic flow (right panel) mapping videos of the same case in Figure 2 tope panel is shown, demonstrating a clockwise rotational activation localized at the AF termination site, over electrodes GH23 on the grid.
Footnotes
Author Disclosures: Swerdlow M, No disclosures; Tamboli M, No disclosures; Alhusseini MI, No disclosures; Moosvi N, No disclosures; Rogers AJ, NIH (F32HL144101): Fellowship support, Stanford SSPS: I1; Leef G, No disclosures; Wang PJ, Biosense Webster: Fellowship support, Boston Scientific: Fellowship support, Medtronic: Fellowship support, St. Jude Medical: Fellowship support; Rillig A, Biosense, Medtronic, EPSolutions, Abbott: Compensation for services, Abbott, Medtronic, and Boehringer Ingelheim: Speaker’s Bureau, Ablamap: Other, Medtronic: Other, Boston Scientific: Fellowship support; Brachmann J, Medtronic, Inc, SJM, Boehringer Ingelheim, Biotronik: Compensation for services, Medtronic, Abbott, SJM, Boehringer, Biotronik: Speaker’s Bureau; Sauer W, Abbott: Compensation for services; Ruppersberg P, Ablacon: Equity interest; Narayan SM, NIH: Fellowship support, Topera: Royalty income, Abbott, Medtronic, JACC: Compensation for services, UC Regents: Intellectual property rights, Uptodate: Royalty income; Baykaner T, No disclosures
References
- 1.Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: Executive summary. Europace 2018; 20:157–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verma A, Jiang C-Y, Betts TR, Chen J, Deisenhofer I, Mantovan R, Macle L, Morillo C a, Haverkamp W, Weerasooriya R, Albenque J-P, Nardi S, Menardi E, Novak P, Sanders P. Approaches to Catheter Ablation for Persistent Atrial Fibrillation. NEJM 2015; 372:1812–1822. [DOI] [PubMed] [Google Scholar]
- 3.Ramirez FD, Birnie DH, Nair GM, Szczotka A, Redpath CJ, Sadek MM, Nery PB. Efficacy and safety of driver-guided catheter ablation for atrial fibrillation: A systematic review and meta-analysis. JCE 2017; 28:1371–1378. [DOI] [PubMed] [Google Scholar]
- 4.Baykaner T, Rogers AJ, Meckler GL, Zaman J, Navara R, Rodrigo M, Alhusseini M, Kowalewski CAB, Viswanathan MN, Narayan SM, Clopton P, Wang PJ, Heidenreich PA. Clinical Implications of Ablation of Drivers for Atrial Fibrillation. Circ: AE 2018; 11:e006119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Umapathy K, Nair K, Masse S, Krishnan S, Rogers J, Nash MP, Nanthakumar K. Phase mapping of cardiac fibrillation. Circ: AE 2010; 3:105–114. [DOI] [PubMed] [Google Scholar]
- 6.Kuklik P, Zeemering S, Maesen B, Maessen J, Crijns HJ, Verheule S, Ganesan AN, Schotten U. Reconstruction of Instantaneous Phase of Unipolar Atrial Contact Electrogram Using a Concept of Sinusoidal Recomposition and Hilbert Transform. IEEE Trans Biomed Eng 2015; 62:296–302. [DOI] [PubMed] [Google Scholar]
- 7.Alhusseini M, Vidmar D, Meckler GL, Kowalewski CA, Shenasa F, Wang PJ, Narayan SM, Rappel WJ. Two Independent Mapping Techniques Identify Rotational Activity Patterns at Sites of Local Termination During Persistent Atrial Fibrillation. JCE 2017; 28:615–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramanathan C, Ghanem RN, Jia P, Ryu K, Rudy Y. Noninvasive electrocardiographic imaging for cardiac electrophysiology and arrhythmia. Nat Med 2004; 10:422–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haissaguerre M, Hocini M, Denis A, et al. Driver domains in persistent atrial fibrillation. Circulation 2014; 130:530–538. [DOI] [PubMed] [Google Scholar]
- 10.Kuklik P, Schaffer B, Hoffmann BA, Schreiber D, Moser J, Akbulak RO, Klatt N, Sultan A, Eickholt C, Luker J, Steven D, Meyer C, Willems S. Catheter ablation of persistent atrial fibrillation guided by electrogram dyssynchrony: acute and long term effect. Heart Rhythm Scientific Sess 2015; PO04.
- 11.Kowalewski CA, Shenasa F, Rodrigo M, et al. Interaction of Localized Drivers and Disorganized Activation in Persistent Atrial Fibrillation. Circ: AE 2018; 11:e005846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vijayakumar R, Vasireddi SK, Cuculich PS, Faddis MN, Rudy Y. Methodology considerations in phase mapping of human cardiac arrhythmias. Circ: AE 2016; 9:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roney CH, Cantwell CD, Bayer JD, Qureshi NA, Lim PB, Tweedy JH, Kanagaratnam P, Peters NS, Vigmond EJ, Ng FS. Spatial Resolution Requirements for Accurate Identification of Drivers of Atrial Fibrillation. Circ: AE 2017; 10:e004899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bellmann B, Lin T, Ruppersberg P, Zettwitz M, Guttmann S, Tscholl V, Nagel P, Roser M, Landmesser U, Rillig A. Identification of active atrial fibrillation sources and their discrimination from passive rotors using electrographical flow mapping. Clin Res Cardiol 2018; 0:1–12. [DOI] [PubMed] [Google Scholar]
- 15.Zaman JABB, Sauer WH, Alhusseini MI, et al. Identification and Characterization of Sites Where Persistent Atrial Fibrillation Is Terminated by Localized Ablation. Circ: AE 2018; 11:e005258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller JM, Kalra V, Das MK, Jain R, Garlie JB, Brewster JA, Dandamudi G. Clinical Benefit of Ablating Localized Sources for Human Atrial Fibrillation: The Indiana University FIRM Registry. JACC 2017; 69:1247–1256. [DOI] [PubMed] [Google Scholar]
- 17.Narayan SM, Krummen DE, Shivkumar K, Clopton P, Rappel W-J, Miller JM. Treatment of Atrial Fibrillation by the Ablation of Localized Sources CONFIRM (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation) Trial. JACC 2012; 60:628–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Narayan SM, Krummen DE, Enyeart MW, Rappel W-J. Computational Mapping Identifies Localized Mechanisms for Ablation of Atrial Fibrillation. Bondarenko VE, ed. PLoS One 2012; 7:e46034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuklik P, Lau DH, Ganesan AN, Brooks AG, Sanders P. High-density mapping of atrial fibrillation in a chronic substrate: Evidence for distinct modes of repetitive wavefront propagation. Int J Cardiol 2015; 199:407–414. [DOI] [PubMed] [Google Scholar]
- 20.Hansen BJ, Zhao J, Csepe T a., et al. Atrial fibrillation driven by micro-anatomic intramural re-entry revealed by simultaneous sub-epicardial and sub-endocardial optical mapping in explanted human hearts. EHJ 2015; 36:2390–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pandit SV, Jalife J Rotors and the Dynamics of Cardiac Fibrillation. Circ Res 2013; 112:849–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Groot NMS De, Houben RPM, Smeets JL, Boersma E, Schotten U, Schalij MJ, Crijns H, Allessie M a. Electropathological substrate of longstanding persistent atrial fibrillation in patients with structural heart disease: epicardial breakthrough. Circulation 2010; 122:1674–1682. [DOI] [PubMed] [Google Scholar]
- 23.Vidmar D, Narayan SM, Krummen DE, Rappel W-J. Determining conduction patterns on a sparse electrode grid: Implications for the analysis of clinical arrhythmias. Phys Rev E 2016; 94:050401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Narayan SM, Baykaner T, Clopton P, Schricker A, Lalani GG, Krummen DE, Shivkumar K, Miller JM. Ablation of rotor and focal sources reduces late recurrence of atrial fibrillation compared with trigger ablation alone: Extended follow-up of the CONFIRM trial (conventional ablation for atrial fibrillation with or without focal impulse and rotor modulat. JACC 2014; 63:1761–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hansen BJ, Li N, Csepe TA, Pederson B, Kilic A, Janssen P, Mohler P, Hummel J, Fedorov VV. Intramural Drivers Of Human Atrial Fibrillation Visualized Differently From The Endocardium And Epicardium By Simultaneous Dual-Sided Electrode And Optical Mapping. Heart Rhythm Scientific Sess 2016; Featured P:S115.
- 26.Grace A, Verma A, Willems S. Dipole Density Mapping of Atrial Fibrillation. EHJ 2017; 38:5–9. [DOI] [PubMed] [Google Scholar]
- 27.Honarbakhsh S, Schilling RJ, Dhillon G, Ullah W, Keating E, Providencia R, Chow A, Earley MJ, Hunter RJ. A Novel Mapping System for Panoramic Mapping of the Left Atrium: Application to Detect and Characterize Localized Sources Maintaining Atrial Fibrillation. JACC: CE 2018; 4:124–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Atienza F, Almendral J, Ormaetxe JM, et al. Comparison of radiofrequency catheter ablation of drivers and circumferential pulmonary vein isolation in atrial fibrillation: A noninferiority randomized multicenter RADAR-AF trial. JACC 2014; 64:2455–2467. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Analysis of localized sources with EGF. A. Still EGF images: 4 second analysis during AF shows focal activation (red circle) from E12 is present in all six still images. A rotational source at D5 (green circle, centrifugal activation; orange circle, centripetal activation) is also present in at least two out of the six still images, therefore marked as a localized source for our analysis. B. Prevalence map of drivers with red denoting area of highest prevalence throughout the time segment. AF termination had occurred with ablation at electrodes DE2 in this 52-year-old male with persistent AF.
Supplemental Figure 2. Focal activation accompanied by a nearby localized source with centripetal activation, potentially driven by the focal activation. Mapping at this site of AF termination (A-B) identified a rotational source by phase mapping (C) and a rotational source with centripetal activation with electrographic flow mapping at the A3/A4 electrodes overlying the termination site (D). This centripetal rotor is accompanied by the flow of a very stable localized source with focal activation at electrode C2. This is demonstrated in a stream line plot displaying the focal source surrounded by a high velocity flow field connected with the rotor through a low velocity bridge (E) A similar pattern of a nearby focal activation accompanying a rotational centripetal activation at a termination site was observed in 18 of the 53 patients.
Movie 1. Phase (left panel) and electrographic flow (right panel) mapping videos of the same case in Figure 2 tope panel is shown, demonstrating a clockwise rotational activation localized at the AF termination site, over electrodes GH23 on the grid.