Abstract
False memories are elicited from exposure to misleading information. It is possible that self-provided misinformation, or lying, has similar effects. We hypothesized that lying impairs memory for younger adults, as increased cognitive control, necessary to inhibit a truthful response, comes at the expense of retaining veridical information in memory. Because older adults show deficits in cognitive control, we hypothesized their memory is unaffected by lying. In the present study, participants made truthful and deceptive responses on a computer while EEG data were recorded. We investigated medial frontal negativity (MFN), an ERP component associated with deception and cognitive control, which may be differentially generated across age groups due to differences in cognitive control. Unexpectedly, results revealed that older adults showed reduced accurate memory for items to which they previously lied compared to younger adults. There were no age differences in correct memory for truth items. We did not find the expected MFN effect, however results revealed long-lasting negative slow waves (NSW) to lie items across age in the pre-response period and following the response cue, suggesting the role of working memory processes in deception. These findings demonstrate that lying is another source of misinformation and influences memory differently across the lifespan.
Keywords: Lying, Cognitive Control, Aging, Memory, Working Memory
1. Introduction
1.1. False Information and Memory
Memory is malleable, which can lead to the occurrence of false memories. Research on false memories, remembering events that never happened or differently from the way they actually occurred, has largely considered how external factors such as information in our surroundings and interactions with others shape one’s memory (Meade & Roediger, 2009; Roediger & McDermott, 1995). False memories can occur in a variety of ways, but one way to elicit them is through the misinformation effect, or changes to memory as a result of exposure to misleading information from social (e.g., disguised research assistant) and nonsocial (e.g., suggestive questionnaire) sources (for a review see Loftus, 2005). Misinformation impacts memory because it can fill holes in personal recollections (Schacter, 2002).
Although there is extensive research investigating the effects of suggestive misinformation from an outside source on individual memory, there is less research on the role of endogenous influences. Unlike in the misinformation effect, where misleading information is provided post-event, in this situation people internally create information that is deliberately inconsistent with the original memory (Zuckerman, DePaulo, & Rosenthal, 1981). This creates a potentially stressful situation, not only because lying requires confidence in one’s delivery and the ability to be convincing, but also because lying requires rapid engagement of cognitive processes necessary to produce a response. Indeed, psychophysiological reactivity measured during deception is consistent with a stress response (Podlesny & Raskin, 1977). Research in emotion and memory has argued that stress can influence long-term memory processes (for a review see Dominique, Aerni, Schelling, & Roozendaal, 2009), but these effects are modulated by many factors including the stage of memory in which stress occurs or the degree of arousal induced by the stimuli (e.g., Buchanan & Lovallo, 2001; Cahill, Gorski, & Le, 2003). Previous work has shown that when stressed prior to learning false information, memory is resistant to the misinformation later presented (Hoscheidt, LaBar, Ryan, Jacobs, & Nadel, 2014). However, it is possible that when stressed while learning false information, there may be too great of a cognitive or physical burden for memory to maintain that same resistance to misinformation at later test. Ultimately, lying is important to study because it could potentially cause the deceiver to later actually believe the false information, a phenomenon known as self-deception (Von Hippel & Trivers, 2011).
Because exogenous misinformation robustly increases later false recognition (Loftus, 2005), self-generated false information may have a similar effect. Previous work has found that feigning amnesia, or simulating obliviousness about the knowledge of an event, leads to deficits in true memory for the event, leading to both errors of omission (Christianson & Bylin, 1999; Oorsouw & Merckelbach, 2004) and errors of commission (Oorsouw & Giesbrecht, 2008; Oorsouw & Merckelbach, 2004, 2006). Similarly, imagination inflation, or imagining the occurrence of events, increases confidence the event actually occurred and influences judgements of perceived happiness in or causality of a past event (Goff & Roediger, 1998; Wells & Gavanski, 1989). Analogously, lying has been shown to critically impair true memory for an event, leading to fewer correct details and more incorrect details remembered (for a review see Otgaar & Baker, 2018; Pickel, 2004). Several mechanisms have been suggested for these deceptive memory effects, including source monitoring, whereby fabricating a new story could lead to confusion with the original event (Johnson, Hashtroudi, & Lindsay, 1993), and lack of rehearsal of true information, whereby memory does not receive the beneficial effects from rehearsing information (Christianson & Bylin, 1999). Of particular interest for this line of work is the suggestion that retrieval-induced forgetting may be responsible for such memory effects: deceptive responding requires successful inhibition of truthful information, which could lead to later retrieval difficulties when trying to remember the correct information (Anderson, Bjork, & Bjork, 2000). The present study will investigate the contribution of lying on memory effects.
1.2. Neural Correlates of Lying
In contrast to the slim literature investigating the effects of lying on memory, there has been growing interest over the past few decades in implementing neural measures as a form of lie detection. Prior work has suggested that truth telling is our default brain setting (Christ, Van Essen, Watson, Brubaker, & McDermott, 2009) and lying requires successful suppression of truthful information (Verschuere, Spruyt, Meijer, & Otgaar, 2011). Although neuroimaging research has not specifically investigated the underlying neural processes involved in the effects of lying on memory, it is possible that processes involved in the act of lying also contribute to whether false information is later remembered. Specifically, cognitive control and memory processes may work in concert with one another to modulate the effects of lying on memory.
Extant work has shown that lying increases demands on cognitive control, which is necessary for allocating mental resources, inhibiting predominant responses, and resolving response conflict (e.g., Botvinick, Braver, Barch, Carter, & Cohen, 2001). Using functional magnetic resonance imaging (fMRI), research has shown that lying is associated with increased activity in prefrontal regions such as the anterior cingulate cortex (ACC), dorsolateral prefrontal cortex (DLPFC), and inferior frontal cortex (IFC) that are crucially involved in cognitive control (Christ et al., 2009; Ganis, Kosslyn, Stose, Thompson, & Yurgelun-Todd, 2003; Langleben et al., 2002).
These frontally based control processes have also been implicated in research employing event-related potentials (ERP), wherein electrical activity of the brain is measured through electrodes on the scalp. One component of interest for this line of work is medial frontal negativity (MFN). In a non-deceptive setting, this component is also known as the N450 or Error Related Negativity (ERN) and has an amplitude (change from peak to trough) that peaks around 450 ms after stimulus onset (e.g., West, Jakubek, Wymbs, Perry, & Moore, 2005) or 70 ms after an error response (e.g., Falkenstein, Hoormann, Christ, & Hohnsbein, 2000; Gehring, Goss, Coles, Meyer, & Donchin, 1993; Scheffers & Coles, 2000). This component has also been implicated in prior research with deception where its generation reflects cognitive control and source/conflict monitoring (Johnson Jr, Barnhardt, & Zhu, 2004; Johnson Jr, Henkell, Simon, & Zhu, 2008). Although ERP cannot directly associate the locations of scalp electrodes with underlying brain structures, research suggests that the MFN is generated in or near a region associated with cognitive control- the ACC (Johnson Jr et al., 2004; Johnson Jr et al., 2008; West et al., 2005). The ACC has been shown to monitor and detect cognitive conflict between sources (Botvinick, Nystrom, Fissell, Carter, & Cohen, 1999) and increases in activation during deceptive responses, suggesting a greater need for control when there is conflict between sources of information (Abe et al., 2006). This insinuates that lie statements would elicit a larger MFN response, as there is an increased need for frontally based control processes when separating true from false information in memory.
Because lying increases demands on cognitive control, it is important to consider how engagement of these processes works in concert with memory. Prior work with feigning amnesia has suggested that retrieval-induced forgetting may be one potential mechanism responsible for reported memory effects (Christianson & Bylin, 1999), whereby cognitive control is necessary to inhibit correct information. The think/no-think paradigm by Anderson and Green (2001) is an example of this. In the task, participants learn a series of cue-response word pairs (e.g., “Tape-Radio”) and are later presented with these same cues (e.g., “Tape”) across two types of trials. In the “think” trials, participants are presented with the cue word and asked to think of the response word (e.g., “Radio”). In the “no-think” trials, participants are presented with the cue word and asked to suppress recall of the response word (e.g., do not think of “Radio”). The authors found that “no-think” trials led to increased cognitive control, likely because of increased conflict or competition between multiple pieces of information. Further, increased executive control in order to suppress a dominant response (e.g., suppressing the word “Radio” when presented with the cue “Tape”) leads to later difficulty in recalling the original information (e.g., “Radio”) (Anderson & Green, 2001; Anderson et al., 2004; Bergström, de Fockert, & Richardson-Klavehn, 2009). This finding provides evidence that people can successfully inhibit retrieval of information, but that in doing so memory for that information is impaired at later test (Levy & Anderson, 2008). The more the information is suppressed, the more memory is impaired (Anderson & Green, 2001; Anderson & Huddleston, 2012). In regards to the present line of work, this suggests that suppressing a habitual truthful response in order to lie requires increased cognitive control, which could facilitate that information being misremembered at later test. Further, extant work has shown that increased stress leads to greater inhibitory control, whereby there is better inhibition of truthful information but lower recall of this information at later test (Gillie, Vasey, & Thayer, 2014). Taken together, this suggests that suppressing information via cognitive control in a potentially stressful situation, such as while lying, can directly influence memory processes.
1.3. Influence of Age on the Effects of Lying
Because cognitive control and memory processes are critically involved in lying, it is important to consider whether aging influences the effects of lying on memory, as these neural processes are impacted throughout the lifespan. It is well established that older adults are more vulnerable to memory errors relative to younger adults (Balota, Dolan, & Duchek, 2000) and neurological changes affecting both cognitive control and memory processes may be another way to modulate that vulnerability. It is not known what age-related effects lying could have on memory nor is there an understanding for how the influence lying has on memory may change across the lifespan.
Prior work has substantiated deficits in cognitive control with age that lead to difficulty in mental flexibility and efficiency (e.g., Hasher, Stoltzfus, Zacks, & Rypma, 1991). One such region that exhibits dysfunction with healthy aging is the ACC (e.g., Martin, Friston, Colebatch, & Frackowiak, 1991; Pardo et al., 2007; Schultz et al., 1999). The ACC exhibits changes such as decline in metabolism rate (Pardo et al., 2007) and cerebral blow flow (Martin et al., 1991; Schultz et al., 1999), among others, all of which coincide with cognitive decline in aging. As a result, older adults show deficits in the ability to ignore interfering stimuli compared to younger adults, particularly as executive functioning demands increase (Friedman, Nessler, Johnson Jr, Ritter, & Bersick, 2007). These age-related changes have also been shown with ERP, as older adults fail to generate larger MFN responses to interfering stimuli (Tays, Dywan, Mathewson, & Segalowitz, 2008), reflecting an inability to ignore distracting items. In relation to the present line of work, this suggests that older adults might show little change in MFN response when lying compared to younger adults, as it may be more difficult for them to suppress truthful information when providing a deceptive response.
Because older adults have difficulty engaging cognitive control processes, this leads to age-related differences in memory for information that was supposed to be ignored or suppressed. Older adults show structural and functional decline in the DLPFC that is accompanied by impairments in down-regulating activation in other areas that represent distracting or irrelevant information and occurs concomitantly with impaired memory performance (Gazzaley, Cooney, Rissman, & D’Esposito, 2005). Engagement of the DLPFC has been linked to memory inhibition in younger adults (Anderson et al., 2004), but declines in DLPFC functionality for older adults would cause information that should be suppressed to be more distracting and less easily regulated in long-term memory (Anderson, Reinholz, Kuhl, & Mayr, 2011). As a result, in tasks like the think/no think paradigm older adults exhibit less forgetting of to-be-suppressed items on a later memory test compared to younger adults (Anderson et al., 2011). Further, older adults show reduced functional connectivity within the frontoparietal network concomitant with recognition of items that were to be ignored relative to younger adults (Campbell, Grady, Ng, & Hasher, 2012).
Due to deficits in cognitive control and an increased vulnerability to memory errors in older adults, age-related differences in the effects of lying on memory are possible. If older adults have difficulty engaging cognitive control processes, they may not have the necessary resources to suppress a truthful response in order to lie. Because prior work has shown that older adults have a tendency to remember more of the to-be-suppressed information, this could mean their memory is relatively unaffected by lying. In other words, older adults may be unable to adequately suppress the truthful response when instructed to lie, thereby preserving this information for later test relative to younger adults.
The present study is novel in its consideration of the influence cognitive control has on memory in a deceptive setting that is potentially stressful, and whether this effect changes with age. Because lying requires increased cognitive control, we predicted there would be a greater MFN response for lies relative to the truth. Because lying increases conflict and competition between sources of information, we predicted that deceptive information would be misremembered at later test. Because older adults have deficits in cognitive control, this should prevent them from detecting as much conflict between truth and lie information. As a consequence, we predicted that older adults would produce a smaller MFN and be less affected by deceptive information in memory. Because processes like cognitive control and memory are impacted across the lifespan, it is important to consider that age may be a significant moderator in the effects lying has on memory.
2. Methods
2.1. Participants
Participants included 22 younger adults (M age = 19.53, SD = 1.68; age range = 18–24; 7 male) and 20 older adults (M age = 74.95, SD = 8.76; age range = 60–92; 8 male), recruited from Brandeis University and the greater Boston area (for a summary of demographics, see Table 1). An additional 3 younger adults and 5 older adults were excluded due to failure to follow instructions (3 older adults), correct memory performance for truth items more than 2.5 standard deviations below the mean (1 younger adult), EEG data that was collected using the incorrect sampling rate (1 younger adult), and having more than 33% of trials rejected because of artifacts in the EEG data (1 younger adult, 2 older adults). All participants were right-handed, with no usage of medications known to affect the central nervous system, and no neurological, psychological, or physical conditions that were problematic for EEG recording. Education levels were similar for younger (M = 14.43, SD = 1.66) and older adults (M = 14.18, SD = 4.02), t(36)=.26, p=.80. Younger adults (M = 40.95, SD = 6.69) had significantly greater speed of processing scores than older adults (M = 18.55, SD = 5.44), as measured by Pattern Matching (Salthouse & Babcock, 1991), t(40)=11.83, p<.001. Younger adults (M = 9.50, SD = 5.02) had significantly greater working memory capacity than older adults (M = 4.30, SD = 3.76), as measured by Letter Number Sequencing (WAIS-III, Wechsler, 1997), t(34)=3.56, p=.001. Older adults completed the Mini-Mental State Examination (Folstein, Folstein, & McHugh, 1975) and all older adults included in the analyses scored above a 24 (M = 28.05, SD = 1.79), which is the suggested cutoff for dementia. To ensure that participants with lower MMSE scores were not driving the results, we re-ran the memory analyses (see section 3.1.1) excluding an additional two participants who scored between 24–26 on the MMSE, as 26 is often used as a more stringent cutoff. Results did not change. Participants gave their written consent and the study was performed with approval from the Brandeis University Institutional Review Board.
Table 1.
Summary of demographic information (means; standard deviations in parentheses).
| Younger adults | Older adults | |
|---|---|---|
| N | 22 | 20 |
| Age | 19.53 (1.68) | 74.95 (8.76) |
| Years Education | 14.43 (1.66) | 14.18 (4.02) |
| Mini-Mental Exam | n/a | 28.05 (1.79) |
| Pattern Matching* | 40.95 (6.69) | 18.55 (5.44) |
| Letter Number Sequencing* | 9.50 (5.02) | 4.30 (3.76) |
| PANAS Positive Pre-task* | 23.36 (5.73) | 37.80 (8.15) |
| PANAS Positive Post-task* | 20.23 (5.55) | 36.75 (7.99) |
| PANAS Negative Pre-task | 12.14 (2.10) | 11.90 (4.84) |
| PANAS Negative Post-task | 12.00 (2.29) | 11.65 (2.46) |
| EPI ‘lie’* | 2.73 (1.80) | 4.20 (2.42) |
| EPI ‘neurotic’* | 12.41 (3.83) | 8.10 (4.40) |
| Social Desirability* | 15.00 (4.74) | 21.05 (7.02) |
Note: Table provides information from participants included in analyses only
=significant age differences at p<.05).
2.2. Stimuli
Stimuli for this study included 102 items that comprised a questionnaire asking participants whether they completed any of these actions in the course of the day yesterday (e.g., have a conversation with someone you hadn’t met before, press “snooze” on your alarm clock, use a fork to eat your lunch). This design was similar to prior experiments from Verschuere et al. (2011) and Spence et al. (2001) that also assessed deception. These items were selected from a larger list of 121 items. Seventy-five of the items were the same as those from Verschuere et al. (2011), but updated if needed (e.g., changed from British English to American English), and an additional 46 items were generated following the same construction. All 121 items were rated by 8 pilot participants on a scale of 1 (low likelihood) to 5 (high likelihood) as to how 1) likely an item would occur on any given day and 2) likely a participant would remember doing that activity on any given day. Items were excluded if, on average, they were considered to be unlikely to occur on any given day and easily memorable/difficult to forget. The 102 items selected to be included in the questionnaire also had additional details added to the root phrase (e.g., “pay for food” was changed to “pay for food with cash,” “drank water” was changed to “drank water from a water bottle”), such that the items were more specific and detailed allowing for a greater burden of information on participants. This was to ensure the task was not too easy and to prevent correct memory performance from being at ceiling. In other words, it might be easier to remember whether the participant took a nap yesterday, even after lying, but perhaps less so when the participant has to remember whether they took a nap on the couch yesterday (as opposed to on the bed, in a chair, etc.).
2.3. Procedure
2.3.1. Encoding task.
In this study, participants completed the 102-item questionnaire twice over the course of the experiment. Participants completed the first questionnaire on the computer while EEG data were collected (Figure 1). During the questionnaire, participants were instructed to tell the truth or lie to each question. The order of items and instruction type were randomized for every trial, with 51 “truth” items and 51 “lie” items in total. The questionnaire began with the prompt, “Yesterday, did you do any of the following?” and participants responded with either “yes” or “no” via button press. Response type (truth vs. lie) was cued with each item. Participants were instructed that when cued for “truth” they should provide a truthful response and when cued for “lie” they should lie (questions were counterbalanced). Items/instructions were presented for 4000 ms, followed by a 3000 ms window during which participants made their button response. A jittered fixation cross ranging from 900–1100 ms occurred between trials. For each of the items, participants were prompted to confirm their response a second time, as prior work has shown that elaboration can increase the occurrence of false memories (e.g., Drivdahl, Zaragoza, & Learned, 2009; Zaragoza, Belli, & Payment, 2007). Following completion of the questionnaire, EEG equipment was removed and participants completed some basic behavioral pen and paper tasks for approximately 45 minutes. Participants completed Pattern Matching (Salthouse & Babcock, 1991), which assesses speed of processing, as well as Letter Number Sequencing (Wechsler, 1997), which measures working memory capacity. We administered the Positive and Negativity Affect Scale (PANAS; Watson, Clark, & Tellegen, 1988), a self-reported assessment of affect and mood. This was administered before and after the first 102-item questionnaire in which participants were asked to lie as a way to assess whether the task of lying influences subjective mood or affect. Participants also completed a series of personality measures. The Eysenck Personality Inventory (EPI; Eysenck & Eysenck, 1964), including the ‘lie’ and ‘neurotic’ subscales, and the Social Desirability Scale (Crowne & Marlowe, 1960) both provide measures of the participants’ propensity to present socially desirable images. Participants who score high on measures like ‘lie’ or ‘social desirability’ may have an easier time providing deceptive responses in the task than those who score lower on those measures.
Figure 1.
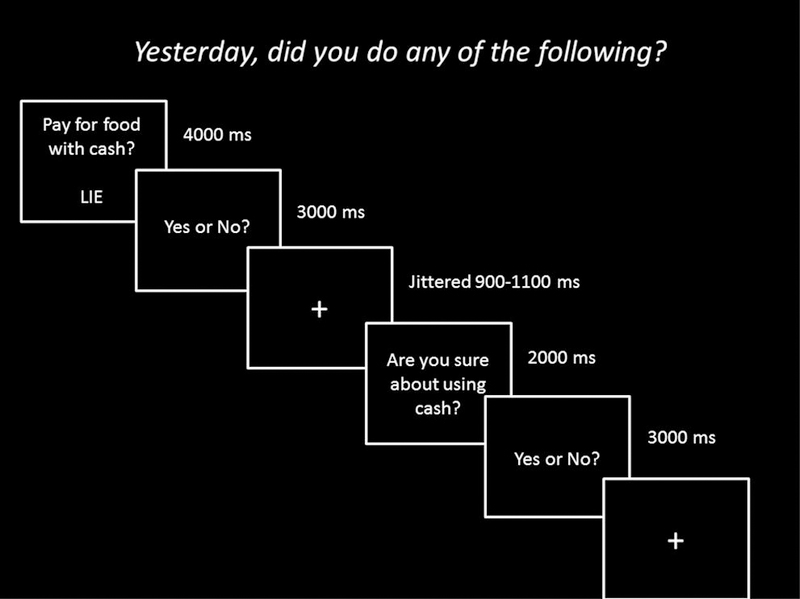
Design of Questionnaire 1 with example stimuli. Participants completed the 102-item questionnaire on the computer where there were asked whether they completed certain actions in the course of the day yesterday. For some of the questions, participants were instructed to lie and for other questions, participants were instructed to tell the truth (randomized). Questions required a “yes” or “no” response via button press.
2.3.2. Retrieval task.
After an approximately 45 minute delay, participants completed the second questionnaire on the computer, which served as a recognition test to assess the influence of lying on memory (Figure 2). The questionnaire began with the prompt, “Yesterday, did you do any of the following?” Participants responded with “yes” or “no” via button press. The items included in the questionnaire were the same items participants previously saw in the first questionnaire. However, this time participants were instructed that all items should be answered truthfully. Additionally, on this questionnaire participants only responded to each item once, as there was no elaboration component. Items were presented for 5000 ms, during which time participants made their button response. A 250 ms fixation cross was displayed between trials.
Figure 2.
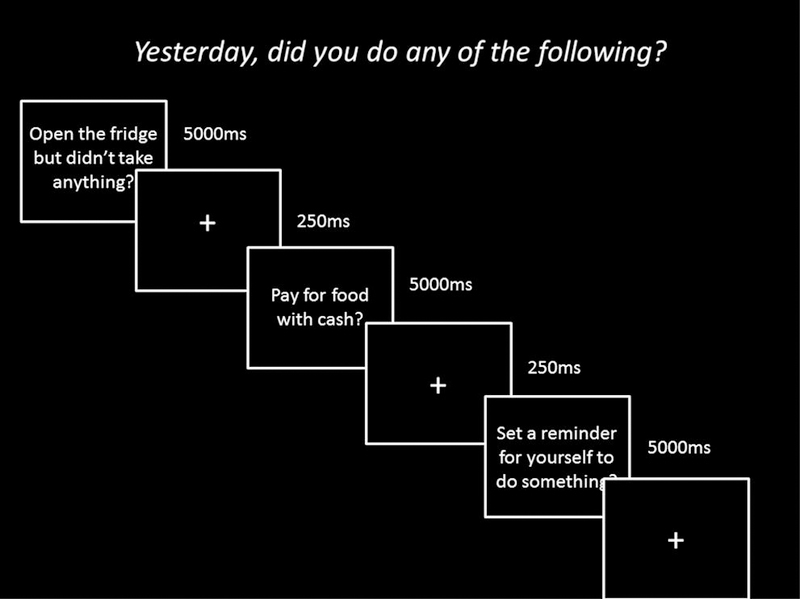
Design of Questionnaire 2 with example stimuli. Participants completed the 102-item questionnaire on the computer where they were asked whether they completed certain actions in the course of the day yesterday. Participants were instructed to answer truthfully to all questions. Questions required a “yes” or “no” response via button press.
2.3.3. Behavioral analysis.
One concern with having participants lie about the actions they completed yesterday is that it is possible some participants do not remember whether they completed an action, and therefore guess. It is also possible participants may accidentally make a mistake during the task. To control for this, participants completed a post task error-rating questionnaire. In this questionnaire, participants were asked to review the items again to determine if there were any items for which their response was in error. Participants were asked to denote whether their responses fell under one of two types of errors. First, participants were asked to mark any items to which they accidentally gave the incorrect response (e.g., during the first questionnaire, if the participant was supposed to lie to a question but accidentally gave a truthful response or if during the recognition test, the participant pressed the wrong button). Second, participants were asked to denote any items for which they were not sure whether they actually did or did not do something yesterday. If an item was marked as either “mistake” or “unsure,” the item was thrown out of the analyses. Out of the 102 items shown at either encoding or retrieval, the proportion of items to which participants made a mistake was .03 (SD = .04) and the proportion of items to which participants were unsure whether they actually did/did not do an action yesterday was .03 (SD = .03). Looking across younger and older adults, there were no significant differences in either proportion of mistakes, t(40)=.10, p=.31, or unsure items, t(40)=1.25, p=.10 (Mistakes: [YA: M = .03, SD = .02; OA: M = .03, SD = .05]; Unsure: [YA: M = .03, SD = .02; OA: M = .04, SD = .04]).
For the behavioral results, we predicted impaired correct memory performance (i.e., correctly remembering whether the participant did or did not do something yesterday) on the second questionnaire for items to which participants previously lied. To assess this, we examined how responses at recognition on the second questionnaire compared to previous responses on the first questionnaire. For example, responses at recognition that were identical as those made to truthful statements on the first questionnaire were considered “correct,” whereas responses at recognition that were identical as those made to lie statements on the first questionnaire were considered “incorrect.” For a summary of memory performance, see Table 2. Because participants were asked to confirm their response to each item a second time at encoding, we had hoped to be able to compare memory performance across responses from both the first prompt and second prompt. However, confusion with the instructions of the second prompt rendered it no longer possible for us to include those responses in the analyses. Therefore, we only included participants’ first response for each trial at encoding in the memory analyses.
Table 2.
Mean (standard deviation) correct memory performance for each of the instruction types by age.
| Younger adults | Older Adults | |
|---|---|---|
| Truth items | .92 (.07) | .91 (.06) |
| Lie items* | .89 (.08) | .80 (.10) |
Note: Values are presented as the proportion of items correctly remembered
(=significant age differences at p<.05).
2.3.4. EEG recording and analysis.
EEG signals were recorded using Ag/AgCl electrodes from thirty-two channels according to the International 10–20 system. Two additional electrodes were used for support in signal processing: one below the left eye and one at the outer right canthus. Signals were recorded using a BioSemi ActiveTwo amplifier (Cortech Solutions, Wilmington, NC) with a 512 Hz sampling rate. A 5th order sinc response anti-aliasing filter with half-power cutoff at 102.4 Hz was applied prior to digitization.
Preprocessing and analyses were performed offline in EEGLAB (Delorme & Makeig, 2004) and ERPLAB, an open-source toolbox for analyzing event-related potentials (Lopez-Calderon & Luck, 2014). EEG signals were re-referenced to the left and right mastoids and filtered using a 2nd order Butterworth high pass filter with a half-amplitude cutoff at 0.1 Hz to remove drift. ICA was run to correct for ocular artifacts using the extended infomax algorithm (Lee, Girolami, & Sejnowski, 1999), as implemented in EEGLAB. ERP waveforms were epoched according to the instruction type (i.e. truth or lie) for the first response in each trial, again because we sought to analyze the first participant response only. The epochs were created from 200 ms before the participant response to 800 ms after. Epochs were baseline corrected by subtracting the mean amplitude from the −200 ms to 0 ms pre-response time window. ICA components were manually inspected and rejected for eye (e.g., blink, saccade) or EMG (muscular) artifacts and the resulting EEG data were semi-automatically inspected for remaining eye movements, EMG noise, or other abnormalities. Thresholds were adjusted on a subject-by-subject basis. Participants with more than 33% of trials rejected due to artifacts were excluded from the dataset. EEG signals were then averaged per participant and across age group (young, old), generating averaged ERPs. The ERP signals were filtered with a 2nd order Butterworth low pass filter with a half amplitude cutoff of 20 Hz.
Statistical analysis of ERP data was conducted via the mass univariate approach. This method involves conducting a statistical test at each electrode and time point and applying specialized multiple comparison corrections (Groppe, Urbach, & Kutas, 2011). The mass univariate approach allows for a more data-driven approach to identifying when and where effects occur, but maintains the same or better power than traditional mean amplitude approaches when spatial and temporal assumptions are matched (Fields & Kuperberg, 2018). All statistical analyses were performed using the Mass Univariate Toolbox (Groppe et al., 2011) and the Factorial Mass Univariate Toolbox (Fields, 2017).
3. Results
3.1. Behavioral
3.1.1. Memory performance.
For the behavioral memory results, we predicted that correct memory (i.e., correctly remembering whether the participant did something yesterday) for items to which the participants had lied would be impaired for younger adults relative to older adults, with older adults’ memory remaining relatively consistent across lie and truth items. Because older adults show deficits in cognitive control, they may be unable to reconcile veridical and deceptive information and, therefore, may be relatively unaffected by false information. To assess this, we performed a 2 (Between-subjects factor: Age Group − younger adults, older adults) × 2 (Within-subjects factor: Instruction type - Truth, Lie) mixed ANOVA looking at correct memory performance (see Figure 3). Results revealed there was a significant Age Group by Instruction type interaction, F(1, 40)=10.58, p=.002, partial ɳ2=.21. Younger adults (M = .92, SD = .07) and older adults (M = .91, SD = .06) did not differ in correct memory for truth items, t(40)=.50, p=.62. However, counter to predictions, older adults (M = .80, SD = .10) showed reduced accurate memory for lie items relative to younger adults (M = .89, SD = .08), t(40)=3.1, p=.004. Additionally, there was a main effect of Age Group, F(1, 40)=5.01, p=.03, partial ɳ2=.11, as younger adults (M = .90, SD = .07) had higher levels of correct memory than older adults (M = .86, SD = .07). There was a main effect of Instruction type, F(1, 40)=36.40, p<.001, partial ɳ2=.48. Correct memory performance was greater for truth items (M = .91, SD = .07) than lie items (M = .85, SD = .10), t(41)=5.30, p<.001.
Figure 3.
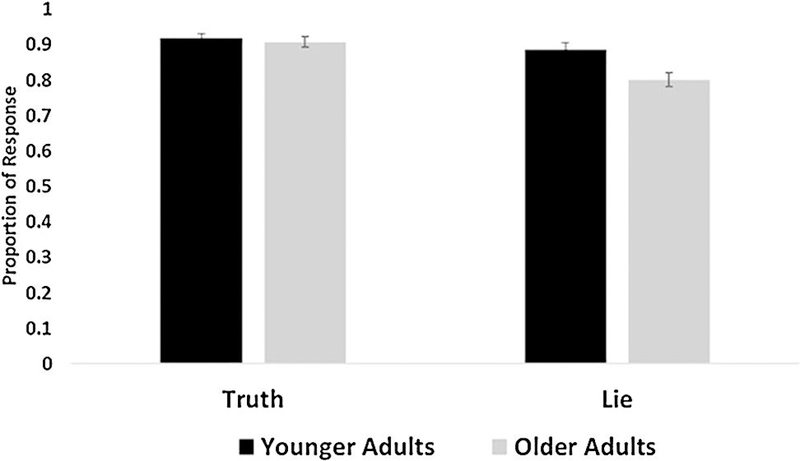
Correct memory performance by instructions and age. The graph depicts the proportion of correctly remembered items, separated by age group, based on whether participants were instructed to tell the truth or lie. Younger and older adults did not differ in correct memory for truth items. However, older adults had reduced correct memory for items to which they previously lied compared to younger adults. Correct memory was greater for truth items than lie items.
3.1.2. Response time.
In addition, we assessed reaction times for correctly remembered items in a 2 (Between-subjects factor: Age Group − younger adults, older adults) × 2 (Within-subjects factor: Instruction type Truth, Lie) mixed ANOVA (see Figure 4). Previous work has suggested that response time is negatively correlated with decision accuracy, as well as confidence, when testing memory (Rotello & Zeng, 2008). If during Questionnaire 1 participants confuse events they actually completed yesterday and events to which they lied, longer response times during retrieval on Questionnaire 2 would reflect that difficulty and the heightened cognitive control demands during encoding. Results revealed there was no significant Age Group by Instruction type interaction, F(1, 40)=.002, p=.97, partial ɳ2=.00. There was a significant main effect of Age Group, F(1, 40)=5.91, p=.02, partial ɳ2=.13. Younger adults had significantly faster response times than older adults for both truth items (Younger: M = 1630.76, SD = 301.98; Older: M = 1864.75, SD = 366.76), t(40)=2.27, p=.03, and lie items (Younger: M = 1664.28, SD = 281.47; Older: M = 1899.98, SD = 331.56), t(40)=2.49, p=.02. There was no main effect of Instruction type, F(1, 40)=2.51, p=.12, partial ɳ2=.06.
Figure 4.
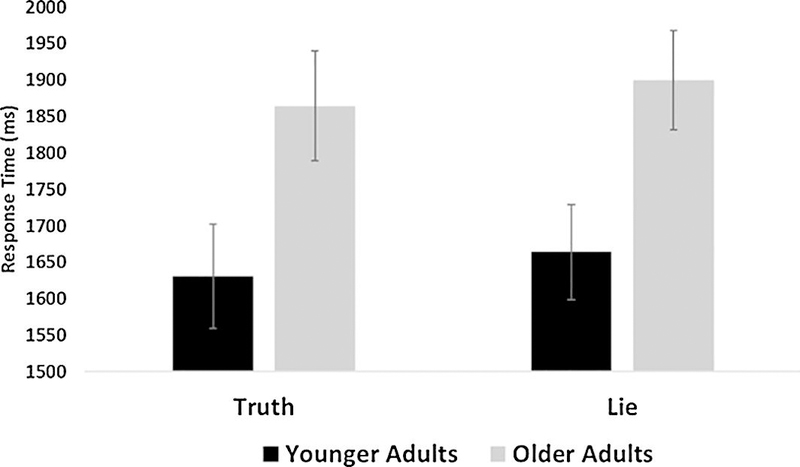
Response time for correctly remembered items by instructions and age. The graph depicts the average response time, separated by age group, for items to which participants told the truth and lied. Younger adults had a faster response time than older adults for both truth items and lie items.
3.1.3. Measures of emotion and personality.
To assess whether the task differently impacted the groups’ arousal levels, we assessed scores on the PANAS (Watson et al., 1988) in a 2 (Between subjects: Age Group − younger adults, older adults) × 2 (Within-subjects factor: Administration – pre-, post-task) mixed ANOVA (see Table 1). For positive affect, there was a marginal Age Group by Administration interaction, F=3.16, p=.08, partial ɳ2=.07. There was a main effect of Administration, F=12.72, p=.001, partial ɳ2=.24, where positive affect scores were greater pre- (M = 30.24, SD =10.04) than post-task (M = 28.10, SD = 10.73), t=3.56, p=.001. There was a main effect of Age Group, F=56.97, p<.001, partial ɳ2=.59, where older adults had significantly greater positive affect on the PANAS pre-task (M = 37.80, SD = 8.15), t(40)=6.69, p<.001, and post-task (M = 36.75, SD = 7.99), t(40)=7.84, p<.001, relative to younger adults (Pre: M = 23.36, SD = 5.73; Post: M = 20.23, SD = 5.55). For negative affect scores, there was no Age Group by Administration interaction, F=.01, p=.92, partial ɳ2=0. There was no main effect in Administration, F=.12, p=.73, partial ɳ2=0, as scores did not differ between between pre- (M= 12.02, SD=3.63) and post-task (M=11.83, 2.35), t=.35, p=.73. There was no main effect of Age Group, F=.14, p=.71, partial ɳ2=0. Younger and older adults did not differ in negative affect scores on the PANAS pre-task, t(40)=.21, p=.84, or post-task, t(40)=.48, p=.64 (Pre: [YA: M = 12.14, SD = 2.10; OA: M = 11.90, SD = 4.84]; Post: [YA: M = 12.00, SD = 2.29; OA: M = 11.65, SD = 2.46]). Older adults (M = 4.20, SD = 2.42) had a significantly greater ‘lie’ score, as measured by the Eysenck Personality Inventory (EPI; Eysenck & Eysenck, 1964), relative to younger adults (M = 2.73, SD = 1.80), t(40)=2.25, p=.03. Younger adults (M = 12.41, SD = 3.83) had a significantly greater ‘neurotic’ score relative to older adults (M = 8.10, SD = 4.40), as measured by the EPI (Eysenck & Eysenck, 1964), t(40)=3.40, p=.002. Older adults (M = 21.05, SD = 7.02) scored higher on social desirability than younger adults (M = 15.00, SD = 4.74), as measured by the Social Desirability Scale (Crowne & Marlowe, 1960), t(40)=3.30, p=.002.
3.2. ERP
3.2.1. MFN in post-response window across younger and older adults.
For the ERP results, we predicted older adults would have a reduced MFN response relative to younger adults. Older adults have deficits in engaging cognitive control processes and should, therefore, exhibit a reduced MFN response compared to younger adults when providing a deceptive response.
Based on the prior literature (Johnson Jr et al., 2004; Johnson Jr, Barnhardt, & Zhu, 2005), statistical analyses were conducted in a priori time window of 0–150 ms at four frontocentral electrodes (FC2, FC2, Fz, and Cz). Correction for multiple comparisons was achieved via the Fmax correction. This correction uses a permutation approach to estimate the null distribution of the maximum F-value across time points and electrodes (Blair & Karniski, 1993; Groppe et al., 2011).
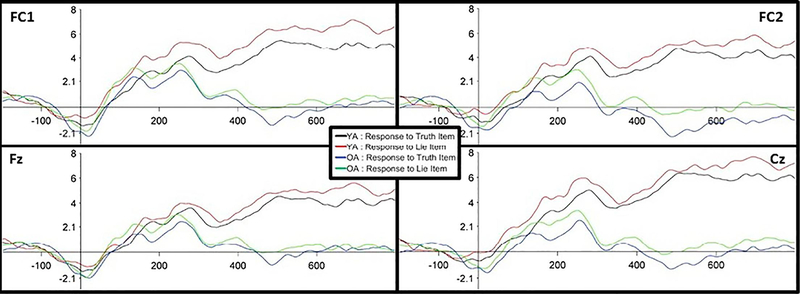
Across just the four electrodes of interest, there was no Age Group by Instruction type interaction. However, there was a significant main effect of Instruction type at FC1 (p=.037), FC2 (p=.018), and Cz (p=.038), and a trend at Fz (p=.102) (see Figure 5), in which there was a greater negative amplitude for truth responses than lie responses (see Figure 6). This occurred at the very end of the time window (~150 ms). Because the MFN typically occurs around 70 ms post-response, this finding may reflect the beginning of a later effect rather than the MFN. There was no main effect of Age Group. Full results are reported in the supplementary materials.
Figure 5.
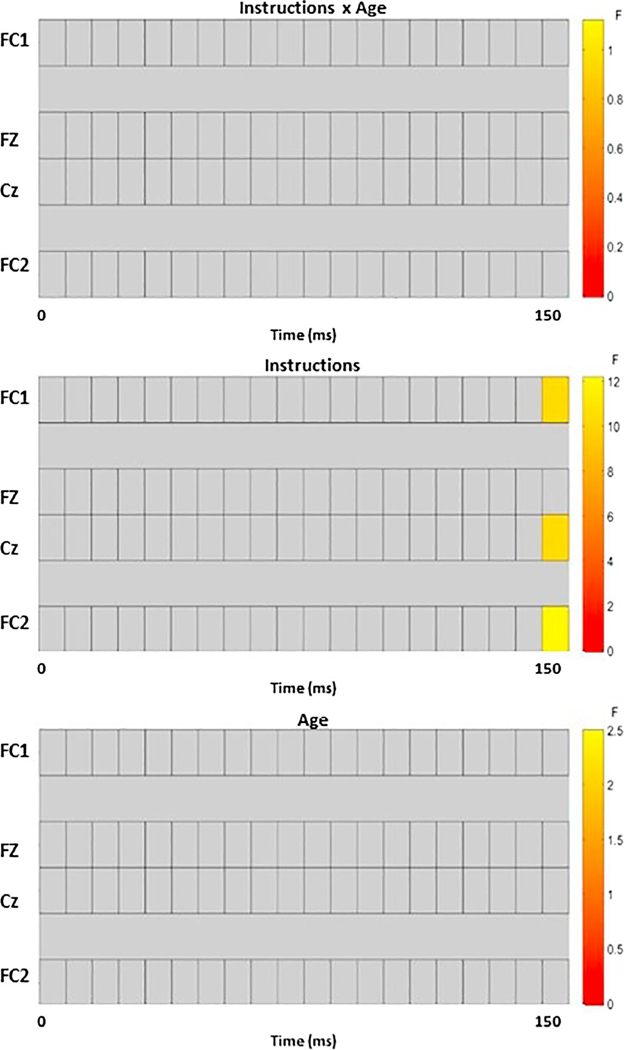
ERP results using mass univariate analysis to look at the four electrodes of interest. The raster plots presented here show a graphic representation of results, where the warmer the colors, the more significant a given time point in the electrode is. Within the 0–150 ms time window post response, response to truth items was more negative than response to lie items at FC1, FC2, and Cz towards the end of the selected time window (~150 ms). There were no differences across age.
Figure 6.
Activity at each of the four electrodes of interest. Activity is shown at FC1, FC2, Fz, and Cz. The black (response to truth item) and red (response to lie item) lines depict activity for younger adults. The blue (response to truth item) and green (response to lie item) lines depict activity for older adults. ERP data is time-locked to the response (e.g., 0 point on the x-axis) and epoched from 200 ms prior to response to 800 ms after a response is made. The y-axis reflects magnitude in μV. Analysis was conducted within the 0–150 ms time window post response. Results show a greater negative amplitude for truth responses than lie responses, for both younger and older adults.
3.2.2. Baseline differences in younger and older adults
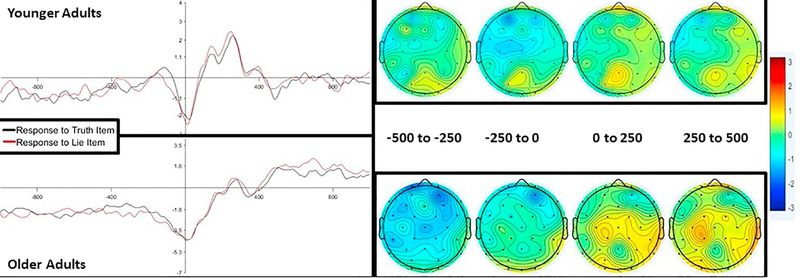
One concern with time-locking the ERP data to the response, as opposed to the presentation of a stimulus, is that there may be differences between conditions in the baseline period. Because baseline correction computes the average of the points from the baseline period and subtracts this average from each point in the waveform, if there are differences at baseline, this could be carried over to the rest of the data. In other words, baseline correction could be driving the effects or potentially masking other effects.
To check whether baseline differences could be inducing or accounting for effects, we re-processed the data without performing baseline correction. To better examine pre-response effects, we epoched the data from 1000 ms before the response was made to 1000 ms after the response was made. Visual examination of the waveforms suggested a frontally distributed negativity throughout the entire pre-baseline period, with responses to lie items showing a greater negative amplitude than responses to truth items (see Figure 7). When we replicated the a priori analyses described above (see section 3.2.1) on the data with no baseline correction, we did not observe effects of instruction type (all ps > .88), suggesting the difference observed above may have been due to differences in the baseline. Full results are reported in the supplementary materials.
Figure 7.
ERP waveforms that were not baseline corrected. Given the differences across age groups, data were re-processed without baseline correcting to check whether baseline activity was driving the effects or masking other effects. The graphs are from electrode Fz (left), which was representative of the other electrodes. A black line depicts response to truth items. A red line depicts response to lie items. ERP data is time-locked to the response (e.g., 0 point on the x-axis) and epoched from 1000 ms prior to the response to 1000 ms after the response. The y-axis reflects magnitude in μV. For all panels, younger adults are shown on the top, older adults are shown on the bottom. Older adults had greater negativity than younger adults. Visually inspecting the waveforms revealed that response to lie items exhibited a greater negativity than response to truth items, albeit this difference did not reach significance. The topographic maps (right) depict the distribution of effects across the scalp, where the warmer the colors, the more significant the effect in a given location is.
3.2.3. Exploratory analyses.
In the present task, the participants’ response was significantly delayed from the onset of the trial, when participants were informed whether they were to lie or tell the truth. This may have reduced neurocognitive effects at the time of the response. Given this and the baseline problems described above, we decided to examine the ERP response time-locked to the beginning of the trial, rather than the response.
We conducted two broad exploratory analyses using the 200 ms before the onset of the trial as a neutral period for baseline correction. Analyses were conducted across all electrodes to examine the ERP response to the initial instruction (0 – 4000 ms after trial onset) and the response cue (4000 – 7000 ms after trial onset). For these analyses we used the cluster mass correction, which provides better power for exploratory analyses when broadly distributed and/or long-lasting effects are expected (Fields & Kuperberg, 2018; Groppe et al., 2011). Briefly, this approach involves finding adjacent electrodes and/or time points with an F-value over a specified threshold to form clusters, summing the F-values across locations in each cluster to calculate a cluster mass statistic, and using a permutation approach to estimate the null distribution for this statistic (see Groppe et al., 2011; Maris & Oostenveld, 2007).
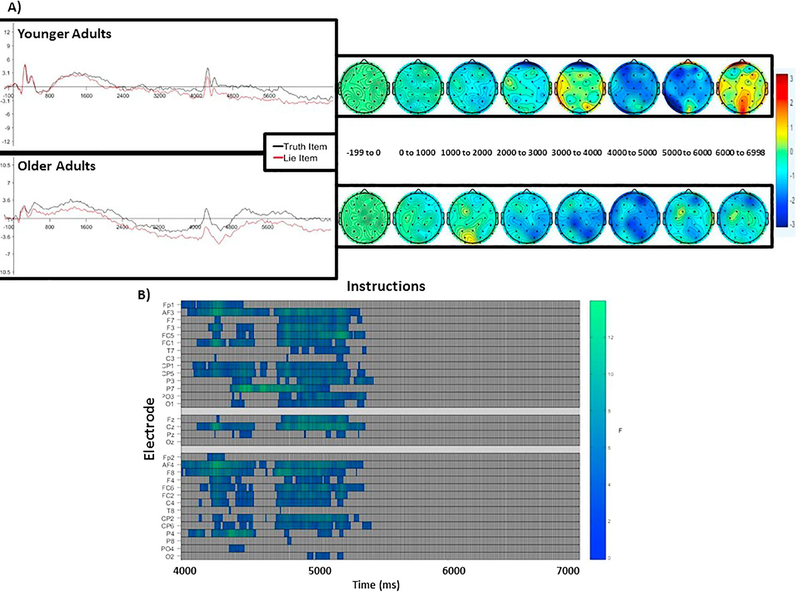
Visual examination revealed a frontally distributed negativity that was greater for lie items than truth items, starting around the time of the response cue and continuing for at least 1000 ms (see Figure 8A). This effect was apparent for both older and younger adults.
Figure 8.
ERP data with long epoch. Data were re-processed with a longer epoch from −200 to 7000 ms to better characterize the landscape against which effects emerged. ERP data is time-locked to the item/instruction cue (e.g., 0 point on the x-axis). The y-axis depicts magnitude in μV. A) The graphs are from electrode AF4 (left), which was representative of the other electrodes. A black line depicts truth items. A red line depicts lie items. For all panels, younger adults are shown on the top, older adults are shown on the bottom. Both younger and older adults show greater frontal negativity for lie items than truth items around 4000 ms. The topographic maps (right) depict the distribution of effects across the scalp during this time window, where the warmer the colors, the more significant the effect is in a given location. B) Differences in instruction emerged after the onset of the response cue, where lie items show greater frontal negativity than truth items.
No significant clusters emerged between the beginning of the trial and the response cue (all ps > 0.1). However, as shown in Figure 8B, the main effect of Instruction generated a significant cluster representing the frontal negativity described above after the onset of the response cue (p = 0.017). This effect did not interact with age (all ps > 0.7). Full results are reported in the supplementary materials.
3.3. Frontal negativity and correct memory performance.
Because a significant cluster emerged revealing a main effect of Instruction type prior to a response being made, where responses to lie items are more negative than truth items, it is possible this signal difference is indicative of cognitive processes that could predict later memory performance. One way to test this is to enter values of frontal negativity into a regression. To determine whether frontal negativity predicted correct memory performance, the mean activity of lie minus truth differences was extracted for each participant across all electrodes/time point locations in the significant cluster reported above (see section 3.2.3).
As previously mentioned, younger and older adults differed on the Social Desirability Scale (Crowne & Marlowe, 1960), whereby older adults exhibited a greater propensity to present a socially desirable image. Socially desirable responding has often been thought of as a response bias that leads to inaccuracy in self-reports or behaviors (Paulhus & Reid, 1991) and could play a large role in the ease with which participants complete the given task. This could then subsequently influence memory performance. To ensure that a variable like social desirability is not entirely responsible for any association between activity and correct memory performance, it was controlled for in the regression. Therefore, any observed effect of frontal negativity on correct memory performance would be independent of the exhibited age differences in social desirability.
Frontal negativity was treated as a continuous variable with age as a categorical predictor. Social desirability and frontal negativity were standardized into z-scores prior to inclusion in the regression to prevent multicollinearity. Two separate regressions were conducted: one for correct memory performance for truth items and one for correct memory performance for lie items (see Table 3, Figure 9).
Table 3.
This table depicts the results (unstandardized coefficient B, standardized coefficient Beta, t and p values) of the regression analyses, examining the predictive effects of differences in frontal negativity on memory performance, including age as a categorical predictor, and controlling for social desirability. The regression was performed for both truth and lie items (*=significant at p<.05).
| Correct Memory for Truth Items | Correct Memory for Lie Items | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| B |
Beta (β) |
t | p | B |
Beta (β) |
t | p | ||
| Social Desirability | .02 | .24 | 1.34 | .19 | Social Desirability | .00 | .04 | .26 | .80 |
| Age | −.03 | −.21 | −1.17 | .25 | Age | −.09 | −.48 | −2.93 | .01* |
| Frontal Negativity | .02 | .28 | 1.35 | .19 | Frontal Negativity | .02 | .20 | 1.03 | .31 |
|
Age-by-Frontal Negativity |
−.01 | −.11 | −.52 | .60 |
Age-by-Frontal Negativity |
.00 | −.01 | −.04 | .97 |
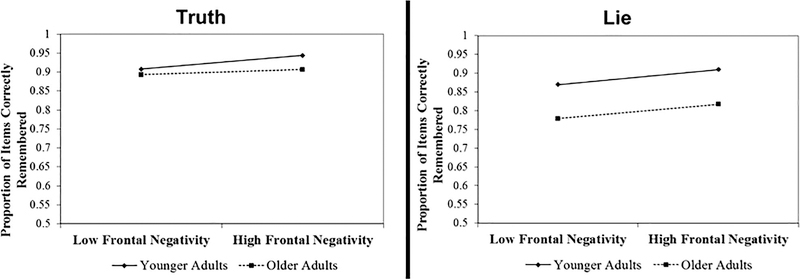
Figure 9.
Regression results showing predictive effects of frontal negativity on correct memory performance. To assess whether frontal negativity predicts correct memory performance, mean values from lie minus truth and memory performance were included in a linear regression. Frontal negativity was treated as a continuous variable with age as a categorical predictor, controlling for social desirability. Two separate regressions, one for correct memory for truth items and one for correct memory for lie items, were conducted. The graphs represent the two-way interaction depicting the relationship between correct memory performance (y axis) and frontal negativity (x axis) for each instruction type, moderated by age group. Neither age nor frontal negativity predicted correct memory for truth items. Age predicted correct memory for lie items, as younger adults had better correct memory for lie items relative to older adults.
For correct memory to truth items, neither the interaction term (β=−.11, t=−.52, p=.60), nor age (β=−.21, t=−1.17, p=.25), nor frontal negativity (β=.28, t=1.35, p=.19) predicted correct memory performance. For correct memory to lie items, age significantly predicted correct memory (β=−.48, t=−2.93, p=.01). Neither frontal negativity (β=.20, t=1.03, p=.31) nor the interaction term (β=−.01, t=−.04, p=.97) predicted correct memory performance.
4. Discussion
Although much of the prior work has investigated the physiological and neural means by which lies can be detected, more research has considered the influence deceptive behavior, like feigning amnesia (Christianson & Bylin, 1999; Oorsouw & Merckelbach, 2004) and deliberate fabrication (for a review see Otgaar & Baker, 2018; Pickel, 2004), can have on memory. In the present study, we extended this literature by considering the influence of lying on memory effects and assessed whether age is a potential moderator of the effects. Older adults fail to engage inhibitory processes when faced with distracting stimuli and, as a result, tend to remember information that was supposed to be suppressed at later test (Anderson et al., 2011). In relation to lying, we predicted that older adults would show less of the MFN response compared to younger adults because of these deficits in cognitive control and, consequently, would not misremember items to which they previously lied, compared to younger adults. The study produced two main findings. First, older adults exhibited reduced correct memory for lie items compared to younger adults. Second, both younger and older adults exhibited long-lasting frontal negativity to lie items versus truth items primarily between the response cue and the response. These findings will be discussed below.
Behavioral results revealed that older adults did not show enhanced memory relative to younger adults for the to-be-suppressed truthful information, as initially predicted. In contrast to our predictions, older adults had reduced correct memory for lie items at later test. Prior work has shown that older adults show deficits in DLPFC functioning that are associated with impairments in down-regulating activation in areas of the brain that represent distracting information (Gazzaley et al., 2005). The DLPFC has been linked to memory inhibition in younger adults (Anderson et al., 2004), but declines in functionality result in older adults exhibiting less forgetting of information that was supposed to be suppressed in tasks like think/no think (Anderson et al., 2011). In relation to the present study, this would suggest that older adults have difficulty inhibiting truthful information in order to provide a deceptive response and, in turn, remember the to-be-inhibited truthful information at later test. However, that is not the case here.
One possibility for our unexpected finding is the types of lies that are involved in this design. Prior work has investigated how the type of lie can influence the impact it has on later memory, as it is possible some lies require greater cognitive resources to produce than others (DePaulo et al., 2003; Vru & Heaven, 1999). Lying by falsely describing something that had not actually been seen relies heavily on executive processes so as to produce a realistic response consistent with the situation (e.g., Sporer & Schwandt, 2007; Walczyk, Roper, Seemann, & Humphrey, 2003). On the other hand, lying by denial, such as by feigning amnesia, may be less taxing, requiring less effort to produce, and likely does not create memory traces with distinct perceptual or contextual features (Vieira & Lane, 2013). Extant work has found that lying about something that was seen leads to a greater memory impairment than lying about something that was not seen (Vieira & Lane, 2013). In the present study, participants commit both types of lies within the same questionnaire. Given the findings from previous research, it is possible that for items to which participants remember completing an action (truthful “yes” responses), those memory traces could be different from the absence of a memory for an action to which participants did not complete (truthful “no” responses).1 In our data, there is a slight bias towards “yes” responses, or responses of having either truthfully completed an action or lying about the fact that the action was completed. In relation to our findings, if older adults showed a bias towards “yes” response compared to younger adults, this could explain their tendency to exhibit lower levels of correct memory as a result of lying. However, we found no significant age differences in response rates. Therefore, we do not have reason to believe that our behavioral results are driven by the type of response. Future work should scrutinize the nuances between the types of lies more closely when designing a deceptive task.
Instead, our pattern of behavioral results may be driven by the fact that the task requires greater source monitoring for lie items. Older adults have difficulty with source monitoring, or difficulty distinguishing between sources of information, particularly as sources share increasing similarities (Bayen & Murnane, 1996; Bayen, Murnane, & Erdfelder, 1996; Henkel, Johnson, & De Leonardis, 1998; Johnson, De Leonardis, Hashtroudi, & Ferguson, 1995). The more similar perceptual and contextual features of truth and lie items are for older adults, the more difficult it is to distinguish between truth and lie, and the easier it is for memory to be impaired (Vieira & Lane, 2013). In fact, prior work with feigning amnesia has suggested that source monitoring may be one potential mechanism underlying reported memory effects (e.g., Christianson & Bylin, 1999). In the present study, the information to which older adults lied is both autobiographical and commonplace, in that items were selected as likely to occur on any given day for the participants. Therefore, it is possible that older adults have a much harder time deciphering between truth versus lie at later test, leading to an overall reduced correct memory performance relative to younger adults.
For the ERP results, we predicted that older adults would show a reduced MFN response relative to younger adults, due to impairments in cognitive control and difficulty reconciling conflicting information in memory. However, we did not observe the expected MFN effect, and the results that were seen in the MFN time window seem to have been driven by pre-response differences. One reason for the lack of a MFN effect may be the delayed response window. In the present study, participants had to wait for the “yes or no?” screen to appear before responding. Given this design, it is possible that participants were ready to respond shortly after the item and instruction cue first appeared on the screen, but had to wait to make their response. This may have led the processes that would generally be engaged at the time of the response to occur earlier, before the response, or increase variability in the timing of these neural responses. A similar paradigm with a speeded response to the item/instruction cue may be more likely to show response-locked MFN or control effects.
This explanation would be consistent with the ERP effects we did find: a long-lasting frontal negativity to lie items that primarily appears between the response cue and the response. One explanation of this effect is that it represents the same neural processes and sources as the MFN, which were simply shifted earlier and spread out in time due to the delayed response. This would suggest that, in line with our predictions, generating lie responses required greater cognitive control than truthful responses. Another interpretation is that this effect represents the negative slow wave (NSW) that has been associated with working memory load (Perez, Vogel, Luck, & Kappenman, 2012; Ruchkin, Johnson Jr, Canoune, & Ritter, 1990). In prior work testing verbal stimuli, NSW increased as the memory load increased. In other words, the waveform became more negative as people held more items in working memory and this effect was maximal at frontal sites similar to the present finding (see Ruchkin, Johnson Jr, Grafman, Canoune, & Ritter, 1992). This interpretation suggests that responding to a verbal statement with a deceptive response requires greater working memory resources than that of an honest response, perhaps because of increased sources that must be maintained in memory (e.g., truth and lie).
Under either explanation, the frontal negativity effect we observed would indicate that preparing a lie response required greater cognitive resources than responding truthfully, which is broadly consistent with our hypotheses. However, in contrast to our hypotheses, this effect did not differ by age. Given the behavioral results discussed above, this finding may suggest that older adults engaged cognitive resources to the same degree as younger adults, but that this cognitive effort was less effective in separating truth and lie information, leading to more confusion during the later memory test. This interpretation is also supported by the findings of our regression analyses, where the interaction of frontal negativity and age did not predict correct memory for truth or lie items. This would suggest that, although younger and older adults perform differently on behavioral measures at later test, they are engaging potentially the same processes to the same extent during the task. However, this cognitive exertion is not as effective for older adults, as they show reduced correct memory for lie items later on. Alternatively, it may be that given the decline in frontal regions associated with executive function discussed above, the equivalent effects in younger and older adults actually represent a greater expenditure of cognitive resources on the part of older adults. Previous work has shown that trials with a larger NSW were more likely to be remembered in a later test (Rösler, Heil, & Röder, 1997). Similarly, if older adults expended more cognitive effort to generate lie responses, they may have been more likely to misremember lies as being true, thus explaining behavioral age differences for lie items.
It is important to note that the present study is not without limitations. Although we argue that lying is a stressful process, we lack a direct measure of stress in the task. Indices such as heart rate (Gillie et al., 2014) or salivary cortisol (Lupien, Maheu, Tu, Fiocco, & Schramek, 2007; Wolf, 2009) have previously been used to measure the effects of stress on memory. Future work would benefit from including these measures during the task to assess whether the stress response from lying actually tracks with memory performance (i.e., does increased stress from lying track with decreased correct memory performance?).
4.1. Conclusions
The present study investigated the role of cognitive processes in determining whether people misremember items to which they previously lied. We did not find the expected MFN effect. However, results revealed frontal negativity, greater for lie than truth items, in the time window between cue and response. This suggests that greater cognitive resources were required to respond in the lie condition than the truth condition, but the magnitude of this effect did not differ by age. Our findings extend the prior literature by showing that age influences the effects of lying on memory. Results revealed that older adults had reduced correct memory for lie items compared to younger adults. Future work should further investigate the influence cognitive processes like working memory and cognitive burden have on the effects of lying on memory, perhaps by manipulating working memory capacity or cognitive load.
Supplementary Material
Highlights.
-
-
Lying requires cognitive control, which may impair memory at later test.
-
-
Participants lied about completing actions while EEG data were collected.
-
-
Older adults had reduced correct memory for items to which they lied.
-
-
Frontal negativity emerged prior to response, greater for lie than truth responses.
-
-
Results suggest neural activation is associated with working memory.
Acknowledgments
We gratefully acknowledge support from the NIA Cognitive Training in Social Context Training Grant (T32AG000204; for LEP). We would like to thank members of the Aging, Culture, & Cognition lab for their feedback.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Looking at response rates across age in a 2 (Between-subjects factor: Age Group- younger adults, older adults) × 2 (Within-subjects factor: Response type- “yes,” “no”) mixed ANOVA, there was no significant Age Group by Response type interaction, F(1,40 )=1.77, p=.19, partial ɳ2=.04. There was a main effect of Response type, F(1,40)=6.59, p=.01, partial ɳ2=.14, reflecting more “yes” (M = 52.26, SD = 5.51) than “no” responses (M = 48.02, SD = 6.22), t(41)=2.48, p=.02. There was a marginal main effect of Age Group, F(1,40)=3.00, p=.09, partial ɳ2=.07. However, younger adults and older adults did not significantly differ in the number of either “yes,” t(40)=.71, p=.48, or “no” responses, t(40)=1.76, p=.09 [“Yes:” (YA: M = 51.68, SD = 5.67; OA: M = 52.9, SD = 5.4); “No:” (YA: M = 49.59, SD = 5.5; OA = 46.3, SD = 6.64)].
Declarations of interest: none.
References
- Abe N, Suzuki M, Tsukiura T, Mori E, Yamaguchi K, Itoh M, & Fujii T (2006). Dissociable roles of prefrontal and anterior cingulate cortices in deception. Cerebral Cortex, 16(2), 192–199. [DOI] [PubMed] [Google Scholar]
- Anderson MC, Bjork EL, & Bjork RA (2000). Retrieval-induced forgetting: Evidence for a recall-specific mechanism. Psychonomic Bulletin & Review, 7(3), 522–530. [DOI] [PubMed] [Google Scholar]
- Anderson MC, & Green C (2001). Suppressing unwanted memories by executive control. Nature, 410(6826), 366–369. [DOI] [PubMed] [Google Scholar]
- Anderson MC, & Huddleston E (2012). Towards a cognitive and neurobiological model of motivated forgetting. In True and false recovered memories (pp. 53–120): Springer. [DOI] [PubMed] [Google Scholar]
- Anderson MC, Ochsner KN, Kuhl B, Cooper J, Robertson E, Gabrieli SW, Gabrieli JD (2004). Neural systems underlying the suppression of unwanted memories. Science, 303(5655), 232–235. [DOI] [PubMed] [Google Scholar]
- Anderson MC, Reinholz J, Kuhl BA, & Mayr U (2011). Intentional suppression of unwanted memories grows more difficult as we age. Psychology and Aging, 26(2), 397. [DOI] [PubMed] [Google Scholar]
- Balota DA, Dolan PO, & Duchek JM (2000). Memory changes in healthy older adults. The Oxford handbook of memory, 395–409.
- Bayen UJ, & Murnane K (1996). Aging and the use of perceptual and temporal information in source memory tasks. Psychology and Aging, 11(2), 293. [DOI] [PubMed] [Google Scholar]
- Bayen UJ, Murnane K, & Erdfelder E (1996). Source discrimination, item detection, and multinomial models of source monitoring. Journal of experimental psychology: Learning, Memory, and Cognition, 22(1), 197. [Google Scholar]
- Bergström ZM, de Fockert JW, & Richardson-Klavehn A (2009). ERP and behavioural evidence for direct suppression of unwanted memories. Neuroimage, 48(4), 726–737. [DOI] [PubMed] [Google Scholar]
- Blair RC, & Karniski W (1993). An alternative method for significance testing of waveform difference potentials. Psychophysiology, 30(5), 518–524. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, & Cohen JD (2001). Conflict monitoring and cognitive control. Psychological Review, 108(3), 624. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Nystrom LE, Fissell K, Carter CS, & Cohen JD (1999). Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature, 402(6758), 179–181. [DOI] [PubMed] [Google Scholar]
- Buchanan TW, & Lovallo WR (2001). Enhanced memory for emotional material following stress-level cortisol treatment in humans. Psychoneuroendocrinology, 26(3), 307–317. [DOI] [PubMed] [Google Scholar]
- Cahill L, Gorski L, & Le K (2003). Enhanced human memory consolidation with post-learning stress: interaction with the degree of arousal at encoding. Learning & Memory, 10(4), 270–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell KL, Grady CL, Ng C, & Hasher L (2012). Age differences in the frontoparietal cognitive control network: implications for distractibility. Neuropsychologia, 50(9), 2212–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ SE, Van Essen DC, Watson JM, Brubaker LE, & McDermott KB (2009). The contributions of prefrontal cortex and executive control to deception: evidence from activation likelihood estimate meta-analyses. Cerebral Cortex, 19(7), 1557–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson SÅ, & Bylin S (1999). Does simulating amnesia mediate genuine forgetting for a crime event? Applied Cognitive Psychology, 13(6), 495–511. [Google Scholar]
- Crowne DP, & Marlowe D (1960). A new scale of social desirability independent of psychopathology. Journal of consulting psychology, 24(4), 349. [DOI] [PubMed] [Google Scholar]
- Delorme A, & Makeig S (2004). EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of neuroscience methods, 134(1), 9–21. [DOI] [PubMed] [Google Scholar]
- DePaulo BM, Lindsay JJ, Malone BE, Muhlenbruck L, Charlton K, & Cooper H (2003). Cues to deception. Psychological Bulletin, 129(1), 74. [DOI] [PubMed] [Google Scholar]
- Dominique J-F, Aerni A, Schelling G, & Roozendaal B (2009). Glucocorticoids and the regulation of memory in health and disease. Frontiers in neuroendocrinology, 30(3), 358–370. [DOI] [PubMed] [Google Scholar]
- Drivdahl SB, Zaragoza MS, & Learned DM (2009). The role of emotional elaboration in the creation of false memories. Applied Cognitive Psychology, 23(1), 13–35. [Google Scholar]
- Eysenck SB, & Eysenck HJ (1964). An improved short questionnaire for the measurement of extraversion and neuroticism. Life sciences, 3(10), 1103–1109. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Christ S, & Hohnsbein J (2000). ERP components on reaction errors and their functional significance: a tutorial. Biological psychology, 51(2), 87–107. [DOI] [PubMed] [Google Scholar]
- Fields EC (2017). Factorial Mass Univariate ERP Toolbox [Computer software] [Google Scholar]
- Fields EC, & Kuperberg GR (2018). Having your cake and eating it too: Flexibility and power with mass univariate statistics for ERP data. Poster presented at the 25th Annual Meeting of the Cognitive Neuroscience Society, Boston, MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, & McHugh PR (1975). “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research, 12(3), 189–198. [DOI] [PubMed] [Google Scholar]
- Friedman D, Nessler D, Johnson R Jr, Ritter W, & Bersick M (2007). Age-related changes in executive function: an event-related potential (ERP) investigation of task-switching. Aging, Neuropsychology, and Cognition, 15(1), 95–128. [DOI] [PubMed] [Google Scholar]
- Ganis G, Kosslyn SM, Stose S, Thompson WL, & Yurgelun-Todd DA (2003). Neural Correlates of Different Types of Deception: An fMRI Investigation. Cerebral Cortex, 13(8), 830–836. doi: 10.1093/cercor/13.8.830 [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Cooney JW, Rissman J, & D’Esposito M (2005). Top-down suppression deficit underlies working memory impairment in normal aging. Nature neuroscience, 8(10), 1298–1300. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MG, Meyer DE, & Donchin E (1993). A neural system for error detection and compensation. Psychological Science, 4(6), 385–390. [Google Scholar]
- Gillie BL, Vasey MW, & Thayer JF (2014). Heart rate variability predicts control over memory retrieval. Psychological Science, 25(2), 458–465. [DOI] [PubMed] [Google Scholar]
- Goff LM, & Roediger HL (1998). Imagination inflation for action events: Repeated imaginings lead to illusory recollections. Memory & Cognition, 26(1), 20–33. [DOI] [PubMed] [Google Scholar]
- Groppe DM, Urbach TP, & Kutas M (2011). Mass univariate analysis of event‐related brain potentials/fields I: A critical tutorial review. Psychophysiology, 48(12), 1711–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasher L, Stoltzfus ER, Zacks RT, & Rypma B (1991). Age and inhibition. Journal of experimental psychology: Learning, Memory, and Cognition, 17(1), 163. [DOI] [PubMed] [Google Scholar]
- Henkel LA, Johnson MK, & De Leonardis DM (1998). Aging and source monitoring: Cognitive processes and neuropsychological correlates. Journal of Experimental Psychology: General, 127(3), 251. [DOI] [PubMed] [Google Scholar]
- Hoscheidt SM, LaBar KS, Ryan L, Jacobs WJ, & Nadel L (2014). Encoding negative events under stress: High subjective arousal is related to accurate emotional memory despite misinformation exposure. Neurobiology of Learning and Memory, 112(0), 237–247 doi: 10.1016/j.nlm.2013.09.008 [DOI] [PubMed] [Google Scholar]
- Johnson R Jr, Barnhardt J, & Zhu J (2004). The contribution of executive processes to deceptive responding. Neuropsychologia, 42(7), 878–901. [DOI] [PubMed] [Google Scholar]
- Johnson R Jr, Barnhardt J, & Zhu J (2005). Differential effects of practice on the executive processes used for truthful and deceptive responses: An event-related brain potential study. Cognitive Brain Research, 24(3), 386–404. [DOI] [PubMed] [Google Scholar]
- Johnson R Jr, Henkell H, Simon E, & Zhu J (2008). The self in conflict: The role of executive processes during truthful and deceptive responses about attitudes. Neuroimage, 39(1), 469–482. doi: 10.1016/j.neuroimage.2007.08.032 [DOI] [PubMed] [Google Scholar]
- Johnson MK, De Leonardis DM, Hashtroudi S, & Ferguson SA (1995). Aging and single versus multiple cues in source monitoring. Psychology and Aging, 10(4), 507. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Hashtroudi S, & Lindsay DS (1993). SOURCE MONITORING. Psychological Bulletin, 114(1), 3–28. doi: 10.1037//0033-2909.114.1.3 [DOI] [PubMed] [Google Scholar]
- Langleben DD, Schroeder L, Maldjian J, Gur R, McDonald S, Ragland JD, Childress AR (2002). Brain activity during simulated deception: an event-related functional magnetic resonance study. Neuroimage, 15(3), 727–732. [DOI] [PubMed] [Google Scholar]
- Lee T-W, Girolami M, & Sejnowski TJ (1999). Independent component analysis using an extended infomax algorithm for mixed subgaussian and supergaussian sources. Neural computation, 11(2), 417–441. [DOI] [PubMed] [Google Scholar]
- Levy BJ, & Anderson MC (2008). Individual differences in the suppression of unwanted memories: the executive deficit hypothesis. Acta psychologica, 127(3), 623–635. [DOI] [PubMed] [Google Scholar]
- Loftus EF (2005). Planting misinformation in the human mind: A 30-year investigation of the malleability of memory. Learning & Memory, 12(4), 361–366. doi: 10.1101/lm.94705 [DOI] [PubMed] [Google Scholar]
- Lopez-Calderon J, & Luck SJ (2014). ERPLAB: an open-source toolbox for the analysis of event-related potentials. Frontiers in human neuroscience, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, Maheu F, Tu M, Fiocco A, & Schramek TE (2007). The effects of stress and stress hormones on human cognition: Implications for the field of brain and cognition. Brain and cognition, 65(3), 209–237. [DOI] [PubMed] [Google Scholar]
- Maris E, & Oostenveld R (2007). Nonparametric statistical testing of EEG-and MEG-data. Journal of neuroscience methods, 164(1), 177–190. [DOI] [PubMed] [Google Scholar]
- Martin AJ, Friston KJ, Colebatch JG, & Frackowiak RS (1991). Decreases in regional cerebral blood flow with normal aging. Journal of Cerebral Blood Flow & Metabolism, 11(4), 684–689. [DOI] [PubMed] [Google Scholar]
- Meade ML, & Roediger HL (2009). Age differences in collaborative memory: The role of retrieval manipulations. Memory & Cognition, 37(7), 962–975. [DOI] [PubMed] [Google Scholar]
- Oorsouw KV, & Giesbrecht T (2008). Minimizing culpability increases commission errors in a mock crime paradigm. Legal and Criminological Psychology, 13(2), 335–344. [Google Scholar]
- Oorsouw KV, & Merckelbach H (2004). Feigning amnesia undermines memory for a mock crime. Applied Cognitive Psychology: The Official Journal of the Society for Applied Research in Memory and Cognition, 18(5), 505–518. [Google Scholar]
- Oorsouw KV, & Merckelbach H (2006). Simulating amnesia and memories of a mock crime. Psychology, Crime & Law, 12(3), 261–271. [Google Scholar]
- Otgaar H, & Baker A (2018). When lying changes memory for the truth. Memory, 26(1), 2–14. [DOI] [PubMed] [Google Scholar]
- Pardo JV, Lee JT, Sheikh SA, Surerus-Johnson C, Shah H, Munch KR, Dysken MW (2007). Where the brain grows old: decline in anterior cingulate and medial prefrontal function with normal aging. Neuroimage, 35(3), 1231–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulhus DL, & Reid DB (1991). Enhancement and denial in socially desirable responding. Journal of personality and social psychology, 60(2), 307. [Google Scholar]
- Perez VB, Vogel EK, Luck S, & Kappenman E (2012). What ERPs can tell us about working memory. The Oxford handbook of event-related potential components, 361–372.
- Pickel K (2004). When a lie becomes the truth: The effects of self‐generated misinformation on eyewitness memory. Memory, 12(1), 14–26. [DOI] [PubMed] [Google Scholar]
- Podlesny JA, & Raskin DC (1977). Physiological measures and the detection of deception. Psychological Bulletin, 84(4), 782. [PubMed] [Google Scholar]
- Roediger HL, & McDermott KB (1995). Creating false memories: Remembering words not presented in lists. Journal of experimental psychology: Learning, Memory, and Cognition, 21(4), 803. [Google Scholar]
- Rösler F, Heil M, & Röder B (1997). Slow negative brain potentials as reflections of specific modular resources of cognition. Biological psychology, 45(1–3), 109–141. [DOI] [PubMed] [Google Scholar]
- Rotello CM, & Zeng M (2008). Analysis of RT distributions in the remember—know paradigm. Psychonomic Bulletin & Review, 15(4), 825–832. [DOI] [PubMed] [Google Scholar]
- Ruchkin DS, Johnson R Jr, Canoune H, & Ritter W (1990). Short-term memory storage and retention: An event-related brain potential study. Electroencephalography and clinical Neurophysiology, 76(5), 419–439. [DOI] [PubMed] [Google Scholar]
- Ruchkin DS, Johnson R Jr, Grafman J, Canoune H, & Ritter W (1992). Distinctions and similarities among working memory processes: An event-related potential study. Cognitive Brain Research, 1(1), 53–66. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, & Babcock RL (1991). Decomposing adult age differences in working memory. Developmental psychology, 27(5), 763. [Google Scholar]
- Schacter DL (2002). The seven sins of memory: How the mind forgets and remembers: Houghton Mifflin Harcourt [Google Scholar]
- Scheffers MK, & Coles MG (2000). Performance monitoring in a confusing world: error-related brain activity, judgments of response accuracy, and types of errors. Journal of Experimental Psychology: Human Perception and Performance, 26(1), 141. [DOI] [PubMed] [Google Scholar]
- Schultz SK, O’Leary DS, Ponto LLB, Watkins GL, Hichwa RD, & Andreasen NC (1999). Age‐related changes in regional cerebral blood flow among young to midlife adults. Neuroreport, 10(12), 2493–2496. [DOI] [PubMed] [Google Scholar]
- Spence SA, Farrow TF, Herford AE, Wilkinson ID, Zheng Y, & Woodruff PW (2001). Behavioural and functional anatomical correlates of deception in humans. Neuroreport, 12(13), 2849–2853. [DOI] [PubMed] [Google Scholar]
- Sporer SL, & Schwandt B (2007). Moderators of nonverbal indicators of deception: A meta-analytic synthesis. Psychology, Public Policy, and Law, 13(1), 1. [Google Scholar]
- Tays WJ, Dywan J, Mathewson KJ, & Segalowitz SJ (2008). Age differences in target detection and interference resolution in working memory: an event-related potential study. Journal of Cognitive Neuroscience, 20(12), 2250–2262. [DOI] [PubMed] [Google Scholar]
- Verschuere B, Spruyt A, Meijer EH, & Otgaar H (2011). The ease of lying. Consciousness and cognition, 20(3), 908–911. [DOI] [PubMed] [Google Scholar]
- Vieira KM, & Lane SM (2013). How you lie affects what you remember. Journal of Applied Research in Memory and Cognition, 2(3), 173–178. [Google Scholar]
- Von Hippel W, & Trivers R (2011). The evolution and psychology of self-deception. Behavioral and Brain Sciences, 34(01), 1–16. [DOI] [PubMed] [Google Scholar]
- Vru A, & Heaven S (1999). Vocal and verbal indicators of deception as a function of lie complexity. Psychology, crime and law, 5(3), 203–215. [Google Scholar]
- Walczyk JJ, Roper KS, Seemann E, & Humphrey AM (2003). Cognitive mechanisms underlying lying to questions: Response time as a cue to deception. Applied Cognitive Psychology, 17(7), 755–774. [Google Scholar]
- Watson D, Clark LA, & Tellegen A (1988). Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of personality and social psychology, 54(6), 1063. [DOI] [PubMed] [Google Scholar]
- Wechsler D (1997). WAiS-iii: Psychological Corporation San Antonio, TX. [Google Scholar]
- Wells GL, & Gavanski I (1989). Mental simulation of causality. Journal of personality and social psychology, 56(2), 161. [Google Scholar]
- West R, Jakubek K, Wymbs N, Perry M, & Moore K (2005). Neural correlates of conflict processing. Experimental brain research, 167(1), 38–48. [DOI] [PubMed] [Google Scholar]
- Wolf OT (2009). Stress and memory in humans: twelve years of progress? Brain research, 1293, 142–154. [DOI] [PubMed] [Google Scholar]
- Zaragoza MS, Belli RF, & Payment KE (2007). Misinformation effects and the suggestibility of eyewitness memory. Do justice and let the sky fall: Elizabeth Loftus and her contributions to science, law, and academic freedom, 35–63.
- Zuckerman M, DePaulo BM, & Rosenthal R (1981). Verbal and nonverbal communication of deception. Advances in experimental social psychology, 14(1), 59. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.