Abstract
Major facilitator superfamily domain containing protein-2a (Mfsd2a) which was considered as an orphan transporter has recently gained attention for its regulatory role in the maintenance of a proper functioning of the blood brain barrier. Besides the major role of Mfsd2a in maintaining the barrier function, increasing evidence has emerged with regards to the contributions of Mfsd2a to various biological processes such as transport, cell fusion, cell cycle, inflammation and regeneration, managing tumor growth, functioning of organs with barrier functions or responses to injury. The purpose of this article is to review the different roles of Mfsd2a and its involvement in the physiological and pathophysiological processes primarily in the central nervous system and throughout the mammalian body under the lights of the current literature.
Keywords: Mfsd2a, blood brain barrier, brain, transport, docosahexaenoic acid
Introduction
Major facilitator superfamily domain containing protein-2a (Mfsd2a) was first identified by Angers et al. in 2008 as a novel member of the major facilitator superfamily (MFS) of membrane proteins (Angers, Uldry, Kong, Gimble, & Jetten, 2008). Since its discovery, various in vivo and in vitro studies have revealed the involvement of Mfsd2a in different systems of the mammalian body (Table 1). One of the exciting breakthroughs has been the identification of the role of Mfsd2a in the mediation of the blood brain barrier (BBB) permeability by selective transportation of the lysophosphatidylcholine (LPC)-binded fatty acids (Nguyen et al., 2014), which brought Mfsd2a forward as a potential therapeutic target for drug delivery into the central nervous system (CNS) (Wang et al., 2016). Additionally, the role of Mfsd2a in tumor development and progression, and maintenance of the functions of placenta and blood retina barrier as well as its contributions to inflammation, organ damage and regeneration in various systems are noteworthy (Tables 1 and 2). Therefore, in this article, we aimed to review the role of Mfsd2a and gain knowledge regarding its involvement in physiological and pathophysiological processes throughout the body under the lights of the current literature.
Table-1:
Summary of studies investigating the major functions of Mfsd2a.
| Tissue/Organ | Function | Deficiency | Species | Reference |
|---|---|---|---|---|
| Liver BAT |
Lipid metabolism Energy expenditure |
Leaner and smaller bodies, increased energy expenditure, hyperactivity, ataxia | Mouse | Berger et al., 2012 |
| Brain | LPC-fatty acid transport Regulation of BBB permeability Brain development |
Reduced uptake of LPC-fatty acids Leaky BBB Microcephaly |
Mouse |
Nguyen et al., 2014
Ben-Zvi et al., 2014 Andreone et al., 2017 |
| Placenta | Receptor for synctin-2 Trophoblast fusion Placental development |
Placental dysfunction Pre-eclampsia |
Human tissues |
Esnault et al., 2008
Toufaily et al., 2013 |
| Eukaryotic Cells | Receptor for Tunicamycin | Reduced sensitivity | Human cells Mouse |
Reiling et al., 2011
Moritake et al., 2017 |
| Eye | DHA transport across the BRB Photoreceptor development |
Slowly progressive retina degeneration Leaky BRB |
Mouse |
Wong et al., 2016
Chow & Gu, 2017 |
BAT: Brown adipose tissue, BBB: Blood brain barrier, BRB: Blood retina barrier, DHA: Docosahexaenoic acid, LPC: Lysophosphatidylcholine.
Table-2:
Summary of studies demonstrating the variations in Mfsd2a levels in response to injury.
| Injury Model | Findings | Species | Reference |
|---|---|---|---|
| ICH | Decreased Mfsd2a, increased BBB permeability | Mouse | Yang et al., 2017 |
| Sepsis induced IPH | Decreased Mfsd2a, increased BBB permeability | Mouse | He et al., 2018 |
| Metastatic Brain Tumors | Decreased Mfsd2a, increased intratumoral BBB permeability | Mouse | Tiwary et al., 2018 |
| Chronic Kidney Disease | Decreased Mfsd2a | Mouse | Zhou et al., 2015 |
| Chronic Liver injury | Increased Mfsd2a | Mouse | Pu et al., 2016 |
| Inflammatory Bowel Disease | Increased Mfsd2a | Mouse | Ungaro et al., 2017 |
BBB: Blood brain barrier, ICH: Intracerebral hemorrhage, IPH: Intraperitoneal hypertension.
Major Facilitator Superfamily and Mfsd2a
Major facilitator superfamily of membrane proteins is one of the largest families of transporters which is constituted of 74 functionally diverse subfamilies and represents almost 25% of all membrane transporters in the prokaryotes (Law, Maloney, & Wang, 2008). Majority of the members of MFS have 12 transmembrane domains composed of two evolutionarily duplicated 6-transmembrane units (Law et al., 2008). The members of the MFS transport a variety of ligands such as sugars, amino acids, nucleotides, organic anions and cations, and drugs across the lipid membranes by using electrochemical potential of the solutes (Lewinson, Adler, Sigal, & Bibi, 2006; Pao, Paulsen, & Saier, 1998; Reddy, Shlykov, Castillo, Sun, & Saier, 2012; Saier et al., 1999). However, ligands and functions of many of the transporters of the MFS remains to be elucidated (Law et al., 2008).
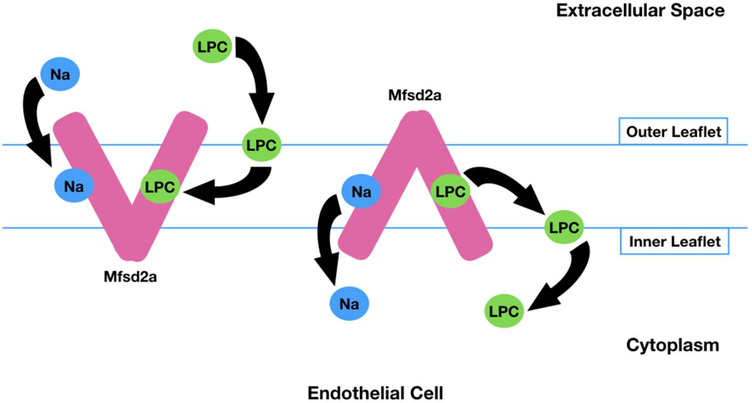
Human Mfsd2a consists of 530 amino acids with glycosylation sites at Asn-217 and Asn-227 and cytogenetically located on chromosome 1p34.2 (Quek, Nguyen, Fan, & Silver, 2016). Mfsd2a is conserved from fish to human whereas it shows 25% and 26% identity in amino acid sequence to melibiose permease (MelB) of S. typhimurium (Angers et al., 2008; Ethayathulla et al., 2014) and lactose permease (LacY) of E. coli (Guan, Mirza, Verner, Iwata, & Kaback, 2007), respectively. In contrast to most of the other members of MFS that transport water-soluble ligands such as sugars and amino acids, Mfsd2a uniquely transports lipids (Nguyen et al., 2014). The transporter function of Mfsd2a is sodium-dependent with a higher specificity for LPC with unsaturated fatty acyl chains (Nguyen et al., 2014). The phosphor-zwitterionic charge of the LPC headgroup is critical for the Mfsd2a-mediated transport. According to the proposed mechanism for Mfsd2a-mediated transport, LPC diffuses laterally into the hydrophobic cleft of Mfsd2a after inserting itself into the outer leaflet of the membrane. Then the conformation of Mfsd2a changes from the outward-open confirmation to inward-open form while the zwitterionic headgroup of LPC is inverted from the outer leaflet to the inner leaflet through a pathway within the transporter (Quek et al., 2016) (Figure 1).
Figure-1:
Proposed mechanism for Na dependent and Mfsd2a mediated LPC transport across the plasma membrane. Na binds to Mfsd2a and LPC inserts itself into the outer leaflet of the membrane. Then, LPC diffuses laterally into the hydrophobic cleft of Mfsd2a, causing a conformational change in Mfsd2a from the outward-open form to the inward-open form. Thus, LPC is inverted from the outer leaflet to the inner leaflet through a pathway within the transporter (Quek et al., 2016).
LPC: Lysophosphatidylcholine, Na: Sodium.
Roles of Mfsd2a in Physiological and Pathological Conditions
We describe here the discoveries that have been made about the role of Mfsd2a in various physiological and pathological states in a chronological sequence.
1. Mfsd2a and Lipid Metabolism
Mfsd2a was first identified in mouse liver and brown adipose tissue where Mfsd2a mRNA was shown to be induced by fasting and during thermogenesis in a β-adrenergic signaling dependent manner in mice, respectively (Angers et al., 2008). Thus, a potential role of Mfsd2a in lipid metabolism and energy expenditure was suggested. This assertion was further supported by the results of a study demonstrating the role of Mfsd2a in lipid metabolism, body growth and motor coordination since Mfsd2a knockout (KO) mice exhibited leaner and smaller bodies with increased whole-body energy expenditure compared to wild-type (Berger, Charron, & Silver, 2012). Knockout of Mfsd2a was also associated with less triglycerides in liver, brown adipose tissue, and serum as well as increased post-natal lethality (Berger et al., 2012). Moreover, fsd2a KO mice had neurologic deficits such as hyperactivity and ataxia. These findings were suggestive of a possible central effect of Mfsd2a, likely a significant role in brain metabolism. However, despite its wide expression in the brain, authors were not able to show any transporter activity of Mfsd2a on any ligands including glucose, fatty acids, pyruvate, amino acids, etc. (Berger et al., 2012).
2. Mfsd2a and Tumors
Mfsd2a is a neighboring gene of TRIT1 (tRNA isopentenyltransferase 1) and MYCL1 on chromosome 1p34.2. Therefore, Mfsd2a was investigated for a possible tumor suppressor effect (Spinola et al., 2010) given the role of TRIT1 and MYCL1 in the development and growth of lung tumors (Spinola et al., 2007; Spinola et al., 2005). Indeed, Mfsd2a mRNA levels were found to be downregulated up to 80-fold in lung cancers including adenocarcinoma and nonsmall cell lung cancer (Spinola et al., 2010). Mfsd2a demonstrated a tumor suppressor function as a result of its ability to block the cell cycle in the G1 phase and to impair adhesive and migratory properties, thereby acting on tumor development and growth through the control of cell cycle, matrix attachment, and cell motility (Spinola et al., 2010).
Contrarily, Mfsd2a was associated with an increased risk of development and progression of gastric cancer, particularly moderately/well-differentiated intestinal-type in Chinese population (S. Chen et al., 2013). Mfsd2a has also been attributed to alleviate the pathways in cell-adhesionmediated drug resistance in hematologic cancers, thereby increasing cancer cell survival (Arvidsson, Henriksson, Sander, & Wright, 2018).
Therefore, based on the very limited data regarding the role of Mfsd2a on tumors, its contributions remain controversial given the tumor suppressor effect on lung cancers in contrast with unfavorable effects on gastric and hematologic malignancies.
3. Mfsd2a and Brain
Blood brain barrier is a highly specialized multicellular membrane which provides an optimal and balanced environment for brain functioning through selective transport systems by regulating the entrance of necessary molecules into the brain while keeping toxins and pathogens away. This specific seal of the brain is primarily provided by the two unique properties of the endothelial cells of the CNS vasculature: specialized tight junctions which form a physical barrier between the blood and brain parenchyma, and very low rates of transcytosis compared to the endothelial cells of other organs (Abbott, Patabendige, Dolman, Yusof, & Begley, 2010; Brightman & Reese, 1969; Knowland et al., 2014; Reese & Karnovsky, 1967; Saunders, iddelow, & Dziegielewska, 2012; Siegenthaler, Sohet, & Daneman, 2013). Despite traditional attention to the importance of tight junctions, recent studies focusing on transcytosis both in physiological and pathologic conditions have emphasized the importance of transcytosis regulation in the maintenance of a properly functioning BBB (Armulik et al., 2010; Ben-Zvi et al., 2014; Knowland et al., 2014). In spite of the knowledge about low rates of transcytosis in brain endothelial cells for decades (Brightman & Reese, 1969; Knowland et al., 2014), its role in BBB functions, and how it is kept at low levels was a mystery until quite recently.
Recent studies have demonstrated the crucial role of Mfsd2a in the formation and functioning of the BBB as well as brain growth without a contribution to morphogenesis.
Mfsd2a and Brain Endothelial Cells
Mfsd2a was shown to be expressed 78.8 times higher in cortical endothelium of the brain than the lung endothelium (Ben-Zvi et al., 2014). The expression of Mfsd2a was selective to the microvessels of the CNS constituting BBB while vasculature of the CNS parts lacking BBB, uch as choroid plexus (Saunders et al., 2012), did not express Mfsd2a (Ben-Zvi et al., 2014; Nguyen et al., 2014). Mfsd2a was not expressed in other cell types of the CNS including pericytes, astrocytes or neurons as well (Ben-Zvi et al., 2014).
Mfsd2a and Lipid Transport Across the BBB
The absence of Mfsd2a was shown to be associated with smaller brain sizes without gross anatomical abnormalities compared to wild-type but with significant loss in Purkinje cells of the cerebellum and neuronal density of CA1 and CA3 regions of the hippocampus in mice (Nguyen et al., 2014). The concomitant presence of severe anxiety and deficits in learning and memory in Mfsd2a KO animals similar to those observed in omega-3 fatty-acid deficiency (Carrie, Clement, de Javel, Frances, & Bourre, 2000; Lafourcade et al., 2011) led the researchers to perform a comprehensive lipidomic analysis in Mfsd2a deficient mice. Indeed, among all fatty-acid 26 species, brain docosahexaenoic acid (DHA), a crucial polyunsaturated omega-3 fatty acid for 27 brain development, motor and cognitive functioning (Connor, 2000; Horrocks & Yeo, 1999; 28 Kidd, 2007; Mozaffarian & Wu, 2011), was decreased more than 50% (Nguyen et al., 2014). Moreover, there was also a significant increase in arachidonic acid levels of brain phospholipids as a result of DHA deficiency (Simopoulos, 2008). Thus, as the specific pattern of localization and expression of Mfsd2a in brain endothelial cells were suggestive of a functional role, further evaluations for a possible DHA transporter activity across the BBB were conducted (Ben-Zvi et al., 2014; Nguyen et al., 2014).
Although brain phospholipids are highly enriched with DHA (Breckenridge, Gombos, & Morgan, 1972; Innis, 2007; Salem, Litman, Kim, & Gawrisch, 2001), DHA cannot be de novo synthetized in brain. Thus, DHA has to be transported across the BBB. The accumulation in the brain is selectively derived from the plasma where DHA is bound to albumin as an unesterified fatty-acid or to LPC (Croset, Brossard, Polette, & Lagarde, 2000). However, Mfsd2a was shown to transport DHA only when it is bound to LPC since Mfsd2a KO mice demonstrated significantly reduced uptake of labeled LPC-DHA from plasma into the brain (Nguyen et al., 2014). Mfsd2a also transported other LPCs with a minimum acyl chain length of 14 carbons with the highest capacity for LPC-DHA followed by LPC-oleate and LPC-palmitate (Nguyen et al., 2014). Mfsd2a transported the fatty-acids in a sodium-dependent manner; thus, transportation function represented high affinity to sodium, as all other Na-dependent transporters (ParoderBelenitsky et al., 2011). Mfsd2a was shown to be essential in maintaining DHA levels during embryogenesis as well. Brain phospholipids demonstrated significantly reduced levels of DHA at (embryonic day) E18.5 in Mfsd2a KO mice. In addition, dietary DHA treatment did not rescue the decreased brain size or neurologic deficits of the Mfsd2a KO mice, thereby emphasizing the importance of plasma-derived LPCs and their transportation across the BBB for normal brain growth and function (Nguyen et al., 2014).
Mfsd2a and Regulation of the BBB Permeability
After being indicated as the major transporter for DHA uptake into the brain, Mfsd2a gained attention for a possible contribution to the formation and functioning of the BBB. The expression of Mfsd2a started as early as E13.5 in the BBB which was shown to gain functionality at E15.5 (Ben-Zvi et al., 2014). Moreover, Mfsd2a KO animals demonstrated a leaky BBB during embryogenesis as well as neonatal period and adulthood without any structural abnormalities in capillary density, capillary diameter, vascular branching or cortical arterial distribution. Together these findings confirmed the functional role of Mfsd2a in BBB rather than a contribution to morphogenesis. Although there were no structural abnormalities in the tight junctions, Mfsd2a KO mice demonstrated increased rates of transcytosis (Ben-Zvi et al., 2014). In other words, the absence of Mfsd2a resulted in a leaky BBB due to increased transcytosis across the endothelial cytoplasm but not due to the opening of the tight junctions (Ben-Zvi et al., 2014). These results were reminiscent of those observed in pericyte-deficient mice (Armulik et al., 2010; Daneman, Zhou, Kebede, & Barres, 2010; Gautam, Zhang, & Yao, 2016). However, Mfsd2a deficient mice id not demonstrate pericyte abnormalities (Ben-Zvi et al., 2014). Hence, increased transcytosis observed in the Mfsd2a KO endothelial cells was not a result of a deficit in the pericyte coverage. In fact, the presence of pericytes was required for the expression of Mfsd2a in the brain endothelial cells. The analysis of previous microarray data of two pericyte-deficient mouse models (Armulik et al., 2010) showed a substantial decrease in Mfsd2a expression in pericyte deficient animals (Ben-Zvi et al., 2014). These findings were consistent with previous studies reporting increased transcytosis following acute stress both in animal models (Nag, 2003) and human subjects (R. D. Bell & Zlokovic, 2009), thereby showing the importance of transcytosis in BBB functions (Nguyen et al., 2014).
After its importance in keeping vesicular trafficking at low levels in the endothelial cells of the CNS vasculature was shown, Mfsd2a underwent evaluation for the mechanisms of transcytosis regulation. For this purpose, Andreone BJ et al. created deficient mice with aspartic acid to alanine point mutation (D96A) to eliminate the transporter function of Mfsd2a without decreasing the protein expression (Andreone et al., 2017). The mutant mice exhibited lipid profiles and microcephaly similar to the Mfsd2a KO mice of previous studies (Nguyen et al., 2014) and the results further confirmed the importance of lipid transport via Mfsd2a for brain development (Andreone et al., 2017). Interestingly, D96A mutant mice also exhibited leaky BBB due to increased vesicular trafficking without an influence on tight junction integrity (Andreone et al., 2017) similar to the previous observations in Mfsd2a KO mice (Ben-Zvi et al., 2014). Thus, lipid transport function of Mfsd2a was implicated as crucial in the suppression of transcytosis.
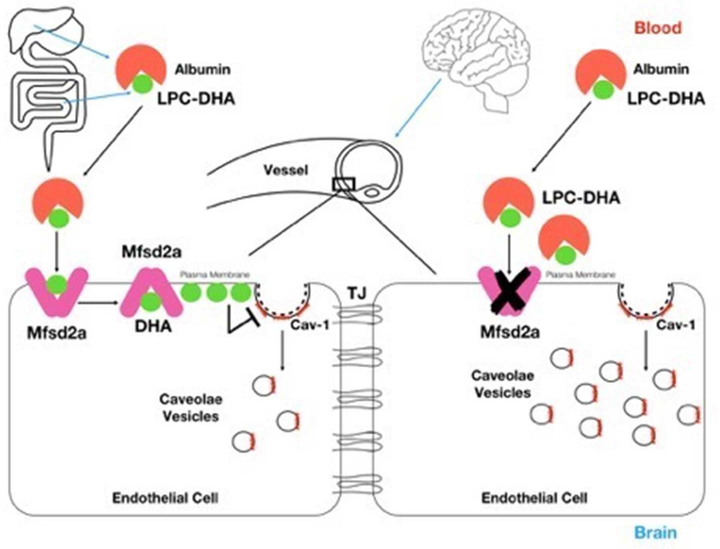
Interestingly, increased vesicles in Mfsd2a deficient mice were positive for caveolin-1 (cav-1) which is the mandatory coat protein for the formation of caveolae vesicles from the plasma membrane. Thus, authors performed a genetic rescue experiment by crossing Mfsd2a KO with cav-1 KO mice to test whether Mfsd2a suppresses caveolae-mediated transcytosis in the regulation of BBB permeability. Indeed, double KOs of Mfsd2a and cav-1 completely reversed the increased number of cytoplasmic vesicles and rescued BBB leakage which was evident in single Mfsd2a KO mice (Andreone et al., 2017). These findings were consistent with former studies revealing the impact of lipid composition of the plasma membrane on the formation and functionality of caveolae vesicles (Lingwood & Simons, 2010; Parton & del Pozo, 2013). Similarly, DHA was previously shown to displace cav-1 from the plasma membrane as well, thereby decreasing the formation of caveolae vesicles in its presence (W. Chen, Jump, Esselman, & Busik, 2007; Q. Li et al., 2007; D. W. Ma et al., 2004). Thus, these studies showed that lipid transport function of Mfsd2a played a critical role in the regulation of permeability and in the maintenance of integrity of the BBB by acting as a suppressor on caveolae-mediated transcytosis in the endothelial cells of the CNS vasculature (Figure 2). However, double KOs of Mfsd2a and cav-1 did not rescue the development of microcephaly which was primarily associated with the transport function, thus inhibition of transcytosis was not required for DHA uptake into the brain via Mfsd2a (Andreone et al., 2017).
Figure-2:
Regulation of BBB permeability by Mfsd2a mediated suppression of caveolae mediated transcytosis in endothelial cells. DHA, which is either absorbed from the intestines or de novo synthetized, is conjugated to LPC in the liver and is bound to albumin forming LPC DHA in the plasma. LPC-DHA uptake across the endothelial cells of the BBB is mediated by Mfsd2a. The lipid composition of the plasma membrane which is highly enriched by DHA impairs the formation of caveolae vesicles. In the absence of Mfsd2a, cav-1 positive caveolae vesicles and thus, transcytosis across the endothelial cell increases.
Cav-1: Caveolin-1, DHA: Docosahexaenoic acid, LPC: Lysophosphatidylcholine, TJ: Tight junction.
Mfsd2a and BBB Disruption
Disruption of the BBB has been implicated in the pathogenesis of various acute and chronic neurological conditions (R. D. Bell & Zlokovic, 2009; S. Chen et al., 2014; Claassen et al., 2002; Jiang, Ewing, & Chopp, 2012; Z. Zhao, Nelson, Betsholtz, & Zlokovic, 2015; Zlokovic, 2008). Consistently, attenuation of increased BBB permeability following different types of brain injury has been shown to be associated with better neurological outcomes and decreased mortality rates (Hasegawa, Suzuki, Uekawa, Kawano, & Kim-Mitsuyama, 2015; Hu et al., 2017; E. Kim, Yang, Park, & Cho, 2017; L. Li et al., 2015; Merali, Leung, Mikulis, Silver, & Kassner, 2015; Pang et al., 2017; Rolland et al., 2017; Shi et al., 2016; H. Zhao et al., 2016; Zuo et al., 2017).
Currently, there are only a few studies revealing the response of Mfsd2a to pathological processes involving the brain. For instance, Mfsd2a was shown to be decreased in the perihematomal tissue in a mice model of intracerebral hemorrhage (ICH) (Yang et al., 2017). As Mfsd2a was selectively expressed in the endothelial cells of the CNS vasculature, microvessels surrounding the perihematomal tissues were suggested to be involved in BBB disruption following ICH (Yang et al., 2017). Consistently, downregulation of Mfsd2a protein levels by siRNA was associated with increased BBB permeability and worse neurological outcomes whilst overexpression of Mfsd2a by AAV-CMV-Mfsd2a virus injection was associated with decreased BBB permeability. Moreover, ICH increased the complexity of the transcytotic vesicles in Mfsd2a KO animals compared to wild-type without structural differences in tight junctions. Consistent with previous studies, this study revealed that Mfsd2a had a protective effect on BBB by decreasing transcytosis. In other terms, Mfsd2a precluded abundant vesicular trafficking which leads to increased transcellular passage and thus, attenuated ICH induced BBB disruption while improving neurological function (Yang et al., 2017).
In a more recent study of lipopolysaccharide induced sepsis in mice, increased pressure in the jugular vein was associated with increased pressure in the capillary bed of the BBB which then in turn disrupted the BBB leading to sepsis associated encephalopathy (He, Xu, Fu, Lin, & Zhang, 2018). Interestingly, dysfunction of the BBB in response to sepsis associated intraperitoneal hypertension was at least partly due to the decreased levels of Mfsd2a, which was concluded to be a result of endothelial cells’ feeling the pressure changes (He et al., 2018).
Decreased Mfsd2a expression levels as well as impaired LPC-fatty acid transporter function were shown to be associated with disrupted brain vascular endothelium leading to intratumoral BBB dysfunction in metastatic tumors of the brain as well (Tiwary et al., 2018). In contrast with the findings of previous studies that proposed pericyte dependent expression of Mfsd2a (Ben-Zvi et al., 2014), astrocytes were shown to be responsible for the maintenance of Mfsd2a expression in brain endothelial cells through TGF-beta1 and bFGF signaling pathways in this novel patient derived xenograft (PDX) mouse model of brain metastases (Tiwary et al., 2018).
Inactivating Mutations of Mfsd2a
Mutations causing partial or complete inactivation of Mfsd2a with autosomal recessive inheritance have been reported recently, causing mild and severe microcephaly syndromes, respectively (Alakbarzade et al., 2015; Guemez-Gamboa et al., 2015).
The partial inactivating mutation of Mfsd2a was described by Alakbarzade et al. in a large Pakistani pedigree harboring 10 affected members who suffered from microcephaly, spastic quadriparesis, intellectual disability, and aphasia (Alakbarzade et al., 2015). The affected individuals were homozygous for the variant encoding p.Ser339Leu while the heterozygous parents of these individuals did not exhibit any of the symptoms. Moreover, cell surface localization of the Ser339Leu mutant was shown to be similar to that of Mfsd2a in the wild-type. Consistently, mass spectrometric analysis demonstrated increased total LPC levels in the affected individuals’ plasmas compared to their unaffected siblings and age-matched controls. These results confirmed that the transport of LPCs in the affected individuals was impaired due to partial inactivating mutation of Mfsd2a, leading to aforementioned neurological findings (Alakbarzade et al., 2015). Thus, a clinical spectrum with partial loss of Mfsd2a transport function was described (Alakbarzade et al., 2015).
A more severe group of signs and symptoms including microcephaly, developmental delay, intellectual disability, hypotonia, spastic quadriparesis, hydrocephalus, cerebellar and brainstem hypoplasia/atrophy, and seizures were defined by Guemez-Gamboa et al. in four members of two families, one of which was from Libya and the other was from Egypt (Guemez-Gamboa et al., 2015). The exome sequences of the affected individuals exhibited homozygous, altering mutations of p.Thr159Met and p.Ser166Leu variants in the Mfsd2a gene. The mutant proteins’ expressions and localizations to the plasma membrane were similar to the wild-type. Overall protein levels were not affected, thereby suggesting that p.Thr159Met and p.Ser166Leu mutations did not destabilize the protein. However, LPC transport function of Mfsd2a was completely impaired by these mutations and the affected individuals did not survive beyond the first years of life. Consistent with a defect in LPC uptake in the BBB, lipidomic analyses showed higher levels of LPC in the affected individuals’ plasmas compared to their heterozygous parents and the control group (Guemez-Gamboa et al., 2015).
Pharmacological Strategies Targeting Mfsd2a
The medical treatment of CNS diseases remains challenging due to the very high selectivity of the BBB. Consequently, only 2% of the small-molecular drugs are allowed to enter the brain while 98% and 100% of the small-molecular and high-molecular drugs are excluded, respectively (Pardridge, 2005; Peluffo et al., 2015). The drugs which can cross the BBB in pharmacologically significant amounts usually have a molecular mass under 400-Da (Pardridge, 2012) and exhibit high lipid solubility (Pardridge, 2012) whereas only a few with a molecular mass over 600-Da can cross the BBB (Pan & Kastin, 2001; Pardridge, 2005). However, a small number of drugs can cross the BBB by carrier-mediated transport such as levodopa, the dopamine precursor used in the medical treatment of Parkinson disease, which is carried by an amino acid transporter, LAT1 (Gomes & Soares-da-Silva, 1999). This restricted transport into the brain is more severe than that into any other organs, partly due to the extremely suppressed transcytosis in the endothelial cells of the CNS (Armulik et al., 2010; Strazielle & Ghersi-Egea, 2016). Therefore, one of the key strategies for the treatment of CNS disorders has been enhancing the drug delivery across the BBB. Targeting the transcytotic transport is considered as a promising therapeutic approach since there are no known restrictions regarding the molecular mass or physicochemical properties of a substrate for its transportation by this route (Armulik et al., 2010; Strazielle & Ghersi-Egea, 2016). Thus, Mfsd2a is suggested as a potential target for drug delivery across the BBB, given the strong evidence demonstrating Mfsd2a as a key player both in the formation and functioning of the BBB through the regulation of transcytosis (Ben-Zvi et al., 2014; Nguyen et al., 2014).
Transient opening of the BBB via concomitant administration of an Mfsd2a inhibitor and the therapeutic drug is a possible way of drug delivery into the CNS (Wang et al., 2016). However, there are no currently available and proven inhibitors of Mfsd2a. Construction of LPC-drug complexes which will act as ligands for Mfsd2a is another proposed mechanism for Mfsd2a based pharmacological strategies (Wang et al., 2016). Moreover, conjugation of drugs with higher lipophilicity with Mfsd2a would facilitate their Mfsd2a-mediated transport since increasing the lipophilicity of drugs enhances their penetration to the BBB (Loryan et al., 2015; Pardridge, 2005; Wang et al., 2016). Proposed strategies are not without risks such that enhancing transcytosis might facilitate the uptake of substances other than the target drug and might potentiate further damage to the CNS tissues (Wang et al., 2016). Hence, coupling drugs with LPC seems to be a more reasonable option.
4. Mfsd2a and Blood-Retina Barrier
The blood-retina barrier (BRB) consists of an inner and outer barrier which are composed of the endothelial cells of the retinal microvasculature and the tight junctions of the retinal pigment epithelial cells, respectively (Fliesler & Anderson, 1983).
Similar to the brain, eye is highly enriched in DHA which is primarily found in the phospholipids of the photoreceptor membrane discs in outer rod segments (SanGiovanni & Chew, 2005). DHA is localized with rhodopsin, a crucial photoreceptor for disc membrane formation along with the phospholipids. As the eye cannot synthetize DHA de novo, its uptake across the BRB is mandatory (SanGiovanni & Chew, 2005). Based on the results of previous studies regarding the functional role of Mfsd2a in the BBB and high expression of Mfsd2a in retina pigment epithelium, Wong et al. evaluated Mfsd2a as a potential transporter for DHA uptake into the eye across the BRB. However, despite the importance of DHA for photoreceptor health (Cunha-Vaz, 1979), Mfsd2a KO mice demonstrated only a slowly progressive retina degeneration due to the decreased length of the outer segment without morphological abnormalities and significant visual impairment (Wong et al., 2016). This situation was concluded to be either due to alternative mechanisms of uptake or triggered de novo synthesis of DHA in the eye of Mfsd2a KO mice. Moreover, retina pigment epithelium was the major site of the eye for Mfsd2a expression as genetic deletion of Mfsd2a in vascular endothelium did not affect DHA levels in the eye (Wong et al., 2016). To be noted, Mfsd2a dependent suppression of transcytosis was important in the development of functional BRB while persistence of transcytosis in Mfsd2a KO mice resulted in leaky BRB both in the early postnatal period and adulthood (Chow & Gu, 2017).
Mfsd2a was also studied as a potential mechanism for crossing the BRB and expression patterns of systemically injected adeno-associated virus serotype 9 (AAV9) which is considered as a non-invasive route for gene delivery (Bostick, Ghosh, Yue, Long, & Duan, 2007; Dalkara et al., 2012; Rahim et al., 2011). Consistent with previous studies mentioning transcytosis as a possible mechanism for AAV9 to cross the epithelial barriers (C. L. Bell et al., 2011; Di Pasquale & Chiorini, 2006; Shen, Bryant, Brown, Randell, & Asokan, 2011), AAV9 was shown to start crossing the developing BRB through transcytosis with the onset of Mfsd2a expression. Moreover, the suppression of transcytosis limited retinal transduction (Byrne, Lin, Lee, Schaffer, & Flannery, 2015).
5. Mfsd2a and Placenta
The multinucleated cell layer on the outer surface of the human placental villi, syncytiotrophoblast, is the main component of placenta, ensuring gas and nutrient exchange between the mother and fetus while also providing hormone production and immunomodulation (Benirschke & Driscoll, 1967). The maintenance of syncytiotrophoblast during pregnancy depends on continuous cell-cell fusion of the subjacent cytotrophoblasts. Mfsd2a was introduced as the receptor of syncytin-2, a second placental fusogenic protein after syncytin 1 (Blaise, de Parseval, Benit, & Heidmann, 2003), for cell-cell fusion in placenta where it is highly expressed (Esnault et al., 2008). Reduced Mfsd2a expression was noted in severe pre-eclamptic placentas in vitro which was consistent with previous studies demonstrating reduced syncytin-2 levels in patients with severe pre-eclampsia (Langbein et al., 2008; Vargas et al., 2011). Based on these data, Mfsd2a was suggested to have an important role in trophoblast fusion and placenta development (Toufaily et al., 2013).
In gestational diabetes mellitus (GDM), the high blood glucose levels of the mother are inversely correlated with behavioral and intellectual development of the offspring (Rizzo, Metzger, Burns, & Burns, 1991). Maternal-fetal transfer of DHA has been shown to be impaired in humans with GDM (Pagan et al., 2013). Thus, a possible disturbance in the maternal-fetal LPC fatty-acid transport system with potential adverse effects on fetal neural development was proposed to occur in GDM. Therefore, researchers evaluated Mfsd2a for a potential role in DHA transportation in placenta since DHA is preferably and selectively transferred across the placenta from the maternal circulation to fetus, compared to other fatty-acids (Gil-Sanchez et al., 2010). Indeed, a significant decrease in placental Mfsd2a levels with concomitant lower DHA levels in the cord blood was demonstrated in pregnant women with GDM (Prieto-Sanchez et al., 2017). Moreover, DHA levels in the cord blood of these pregnancies correlated with psychomotor skills at postnatal 6 months of age (Prieto-Sanchez et al., 2017). Interestingly, Mfsd2a was shown to be downregulated to almost undetectable levels in the off springs of mothers receiving DHA enriched diet in mice, which was an evidence of negative feedback control of DHA on Mfsd2a (Pauter et al., 2017).
6. Mfsd2a and Tunicamycin Induced Endoplasmic Reticulum Stress
The capacity of endoplasmic reticulum (ER) of folding the luminal proteins may be compromised by various stimuli such as hypoxia, glucose deprivation, or infection. Resultantly, unfolded or misfolded proteins accumulate in the ER lumen and lead to a condition termed ‘ER stress’ which then activates a cytoprotective adaptive response termed ‘unfolded protein response’ (UPR) (Hetz, 2012; Lynch et al., 2012; Marchi, Patergnani, & Pinton, 2014; Meusser, Hirsch, Jarosch, & Sommer, 2005; Torres-Peraza et al., 2013). The aim of UPR is to attenuate the ER stress and reestablish the hemostasis by several mechanisms such as inhibition of protein influx and protein synthesis while aggravating their degradation in the ER (Grootjans, Kaser, Kaufman, & Blumberg, 2016; Ron & Walter, 2007; Vincenz-Donnelly & Hipp, 2017). In cases of persistent stress, prolonged activation or failure of UPR to regain the hemostasis in the ER leads to apoptotic cell death through the induction of DNA damage. ER stress, misregulated protein homeostasis and UPR signaling have been implicated in a variety of diseases including diabetes, cancer, inflammation and neurodegeneration (I. Kim, Xu, & Reed, 2008; Y. Ma & Hendershot, 2004; Ron & Walter, 2007; Zhang & Kaufman, 2006).
Tunicamycin is a drug with antibiotic and antiviral properties which is widely used in models of ER stress (Takatsuki, Arima, & Tamura, 1971) as it inhibits the asparagine (N)-linked glycosylation of proteins in the ER (Brandish et al., 1996; Heifetz, Keenan, & Elbein, 1979; Keller, Boon, & Crum, 1979). Thus, tunicamycin exposure results in misfolding of the proteins and leads to extensive activation of the UPR. However, how tunicamycin enters the cells was unknown until Mfsd2a was shown to have an important role as tunicamycin transporter (Reiling et al., 2011). Mfsd2a was a critical determinant of tunicamycin sensitivity as cells without Mfsd2a were resistant to tunicamycin while Mfsd2a overexpression resulted in increased intracellular tunicamycin accumulation and increased sensitivity (Reiling et al., 2011). Cterminal residues of Mfsd2a were found to be important for sensitization to tunicamycin. Consistent with a role in transport, Mfsd2a was shown to be localized to the plasma membrane (Reiling et al., 2011). The role of Mfsd2a as a tunicamycin transporter was further supported by in vivo studies as well (Moritake et al., 2017).
7. Mfsd2a and Other Organs
Recently, Mfsd2a gene was found to be one of the differentially expressed genes which were involved in chronic kidney disease models in a Smad3-dependent manner (Zhou et al., 2015). Interestingly, Mfsd2a was specifically expressed in the periportal hepatocytes in the adult mouse liver as well. Moreover, liver regeneration following partial hepatectomy as well as chronic liver injury significantly stimulated the expansion of Mfsd2a positive periportal hepatocytes which replaced the pericentral hepatocytes (Pu et al., 2016).
Mfsd2a was also shown to be specifically expressed in the epithelium of the human large intestine in healthy and inflammatory conditions. In addition, the expression of Mfsd2a was upregulated in inflammatory mucosa compared to healthy mucosa. TNF-alpha activated Mfsd2a expression while other interleukins and interferon-gamma had no effect. Interestingly, Mfsd2a was more involved in the resolving phase of inflammation as patients in this phase of the inflammatory bowel disease demonstrated even higher levels of Mfsd2a compared to healthy subjects or patients with the active disease. TNF-alpha could activate Mfsd2a progressively even in healthy tissues up to 24 hours with a peak effect at 8 hours post-stimulation as well (Ungaro et al., 2017). Additionally, Mfsd2a silencing exacerbated endothelial TNF-alpha-induced response in vitro, thereby suggesting an Mfsd2a-mediated control of the intestinal endothelial response to inflammatory stimulus. Thus, induction of this pathway was suggested as a novel target for the resolution of intestinal inflammatory diseases (Ungaro et al., 2017).
Conclusion and Clinical Perspectives
Mfsd2a, a novel LPC transporter selectively expressed in the endothelial cells of the CNS, provides significant contributions to the formation and functioning of the BBB. Possible pharmacologic application in order to facilitate drug delivery across the BBB seems to be the most exciting translational value of the identification of Mfsd2a. However, specific therapeutic strategies targeting BBB disruption are yet to be determined. Changes in Mfsd2a expression levels following different types of brain injury may be unique to the pathology, which will be an important factor to consider while creating specific targeted therapeutic strategies. Nonetheless, large numbers of in vivo and in vitro studies are warranted in order to corroborate the practicality and applicability of pharmacologic strategies based on the modulation of BBB permeability via Mfsd2a. Additionally, growing evidence shows considerable functions of Mfsd2a in different organs in response to a variety of stimuli including inflammation, hemorrhage, and regeneration. Therefore, it will be encouraging to evaluate the role of Mfsd2a in physiological and pathophysiological processes throughout the body following various types of injuries and tumor formation, by further pharmacological, translational and clinical studies.
Significance statement.
Mfsd2a has recently gained attention for its crucial role in the regulation of the blood brain barrier permeability. Moreover, emerging evidence support considerable contributions of Mfsd2a in various organs in response to a variety of stimuli including inflammation, hemorrhage, and regeneration. In this comprehensive review, different roles of Mfsd2a have been discussed in detail particularly focusing on its role in the regulation of blood brain barrier permeability. This manuscript is important since, to the best to our knowledge, it is the first systematic review regarding the involvement of Mfsd2a in the physiological and pathophysiological processes throughout the mammalian body.
Acknowledgement
This work is supported by NIH P01NS082184 to JHZ, NIH R21NS101284 to JT, AHA 17GRNT33670031 to JT, and 17POST33320005 to PS.
Footnotes
Conflict of Interest Statement
All the authors declare that they have no conflicts of interest.
References
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, & Begley DJ (2010). Structure 3 and function of the blood-brain barrier. Neurobiol Dis, 37(1), 13–25. doi: 10.1016/j.nbd.2009.07.030 [DOI] [PubMed] [Google Scholar]
- Alakbarzade V, Hameed A, Quek DQ, Chioza BA, Baple EL, Cazenave-Gassiot A, Crosby AH (2015). A partially inactivating mutation in the sodium-dependent lysophosphatidylcholine transporter MFSD2A causes a non-lethal microcephaly syndrome. Nat Genet, 47(7), 814–817. doi: 10.1038/ng.3313 [DOI] [PubMed] [Google Scholar]
- Andreone BJ, Chow BW, Tata A, Lacoste B, Ben-Zvi A, Bullock K, . . . Gu C (2017). Blood-Brain Barrier Permeability Is Regulated by Lipid Transport-Dependent Suppression of Caveolae-Mediated Transcytosis. Neuron, 94(3), 581–594 e585. doi: 10.1016/j.neuron.2017.03.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angers M, Uldry M, Kong D, Gimble JM, & Jetten AM (2008). Mfsd2a encodes a 14 novel major facilitator superfamily domain-containing protein highly induced in brown 15 adipose tissue during fasting and adaptive thermogenesis. Biochem J, 416(3), 347–355. doi: 10.1042/BJ20080165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, . . . Betsholtz C (2010). Pericytes regulate the blood-brain barrier. Nature, 468(7323), 557–561. doi: 10.1038/nature09522 [DOI] [PubMed] [Google Scholar]
- Arvidsson G, Henriksson J, Sander B, & Wright AP (2018). Mixed-species RNAseq analysis of human lymphoma cells adhering to mouse stromal cells identifies a core gene set that is also differentially expressed in the lymph node microenvironment of mantle cell lymphoma and chronic lymphocytic leukemia patients. Haematologica, 103(4), 666–678. doi: 10.3324/haematol.2017.182048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell CL, Vandenberghe LH, Bell P, Limberis MP, Gao GP, Van Vliet K, Wilson JM (2011). The AAV9 receptor and its modification to improve in vivo lung gene transfer in mice. J Clin Invest, 121(6), 2427–2435. doi: 10.1172/JCI5736728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RD, & Zlokovic BV (2009). Neurovascular mechanisms and blood-brain barrier 29 disorder in Alzheimer’s disease. Acta Neuropathol, 118(1), 103–113. doi: 10.1007/s00401-009-0522-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Zvi A, Lacoste B, Kur E, Andreone BJ, Mayshar Y, Yan H, & Gu C (2014). Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature, 509(7501), 507–511. doi: 10.1038/nature13324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benirschke K, & Driscoll SG (1967). The pathology of the human placenta. Berlin, New York,: Springer-Verlag. [Google Scholar]
- Berger JH, Charron MJ, & Silver DL (2012). Major facilitator superfamily domain3 containing protein 2a (MFSD2A) has roles in body growth, motor function, and lipid metabolism. PLoS One, 7(11), e50629. doi: 10.1371/journal.pone.0050629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaise S, de Parseval N, Benit L, & Heidmann T (2003). Genomewide screening for fusogenic human endogenous retrovirus envelopes identifies syncytin 2, a gene conserved on primate evolution. Proc Natl Acad Sci U S A, 100(22), 13013–13018. doi: 10.1073/pnas.2132646100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostick B, Ghosh A, Yue Y, Long C, & Duan D (2007). Systemic AAV-9 transduction in mice is influenced by animal age but not by the route of administration. Gene Ther, 14(22), 1605–1609. doi: 10.1038/sj.gt.3303029 [DOI] [PubMed] [Google Scholar]
- Brandish PE, Kimura KI, Inukai M, Southgate R, Lonsdale JT, & Bugg TD (1996). Modes of action of tunicamycin, liposidomycin B, and mureidomycin A: inhibition of phospho-N-acetylmuramyl-pentapeptide translocase from Escherichia coli. Antimicrob Agents Chemother, 40(7), 1640–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breckenridge WC, Gombos G, & Morgan IG (1972). The lipid composition of adult rat brain synaptosomal plasma membranes. Biochim Biophys Acta, 266(3), 695–707. [DOI] [PubMed] [Google Scholar]
- Brightman MW, & Reese TS (1969). Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol, 40(3), 648–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne LC, Lin YJ, Lee T, Schaffer DV, & Flannery JG (2015). The expression pattern of systemically injected AAV9 in the developing mouse retina is determined by age. Mol Ther, 23(2), 290–296. doi: 10.1038/mt.2014.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrie I, Clement M, de Javel D, Frances H, & Bourre JM (2000). Phospholipid supplementation reverses behavioral and biochemical alterations induced by n-3 polyunsaturated fatty acid deficiency in mice. J Lipid Res, 41(3), 473–480. [PubMed] [Google Scholar]
- Chen S, Feng H, Sherchan P, Klebe D, Zhao G, Sun X, . . . Zhang JH (2014). Controversies and evolving new mechanisms in subarachnoid hemorrhage. Prog Neurobiol, 115, 64–91. doi: 10.1016/j.pneurobio.2013.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Zheng Z, Tang J, Lin X, Wang X, & Lin J (2013). Association of polymorphisms and haplotype in the region of TRIT1, MYCL1 and MFSD2A with the risk and clinicopathological features of gastric cancer in a southeast Chinese population. Carcinogenesis, 34(5), 1018–1024. doi: 10.1093/carcin/bgt010 [DOI] [PubMed] [Google Scholar]
- Chen W, Jump DB, Esselman WJ, & Busik JV (2007). Inhibition of cytokine signaling in human retinal endothelial cells through modification of caveolae/lipid rafts by docosahexaenoic acid. Invest Ophthalmol Vis Sci, 48(1), 18–26. doi: 10.1167/iovs.060619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow BW, & Gu C (2017). Gradual Suppression of Transcytosis Governs Functional BloodRetinal Barrier Formation. Neuron, 93(6), 1325–1333 e1323. doi: 10.1016/j.neuron.2017.02.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claassen J, Carhuapoma JR, Kreiter KT, Du EY, Connolly ES, & Mayer SA (2002). Global cerebral edema after subarachnoid hemorrhage: frequency, predictors, and impact on outcome. Stroke, 33(5), 1225–1232. [DOI] [PubMed] [Google Scholar]
- Connor WE (2000). Importance of n-3 fatty acids in health and disease. Am J Clin Nutr, 71(1 Suppl), 171S–175S. doi: 10.1093/ajcn/71.1.171S [DOI] [PubMed] [Google Scholar]
- Croset M, Brossard N, Polette A, & Lagarde M (2000). Characterization of plasma unsaturated lysophosphatidylcholines in human and rat. Biochem J, 345 Pt 1, 61–67. [PMC free article] [PubMed] [Google Scholar]
- Cunha-Vaz J (1979). The blood-ocular barriers. Surv Ophthalmol, 23(5), 279–296. [DOI] [PubMed] [Google Scholar]
- Dalkara D, Byrne LC, Lee T, Hoffmann NV, Schaffer DV, & Flannery JG (2012). Enhanced gene delivery to the neonatal retina through systemic administration of tyrosine-mutated AAV9. Gene Ther, 19(2), 176–181. doi: 10.1038/gt.2011.163 [DOI] [PubMed] [Google Scholar]
- Daneman R, Zhou L, Kebede AA, & Barres BA (2010). Pericytes are required for bloodbrain barrier integrity during embryogenesis. Nature, 468(7323), 562–566. doi: 10.1038/nature09513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pasquale G, & Chiorini JA (2006). AAV transcytosis through barrier epithelia and endothelium. Mol Ther, 13(3), 506–516. doi: 10.1016/j.ymthe.2005.11.007 [DOI] [PubMed] [Google Scholar]
- Esnault C, Priet S, Ribet D, Vernochet C, Bruls T, Lavialle C, . . . Heidmann T (2008). A placenta-specific receptor for the fusogenic, endogenous retrovirus-derived, human syncytin-2. Proc Natl Acad Sci U S A, 105(45), 17532–17537. doi: 10.1073/pnas.0807413105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethayathulla AS, Yousef MS, Amin A, Leblanc G, Kaback HR, & Guan L (2014).Structure-based mechanism for Na(+)/melibiose symport by MelB. Nat Commun, 5, 3009. doi: 10.1038/ncomms4009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliesler SJ, & Anderson RE (1983). Chemistry and metabolism of lipids in the vertebrate retina. Prog Lipid Res, 22(2), 79–131. [DOI] [PubMed] [Google Scholar]
- Gautam J, Zhang X, & Yao Y (2016). The role of pericytic laminin in blood brain barrier integrity maintenance. Sci Rep, 3(6), 36450. doi: 10.1038/srep36450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Sanchez A, Larque E, Demmelmair H, Acien MI, Faber FL, Parrilla JJ, & Koletzko B (2010). Maternal-fetal in vivo transfer of [13C]docosahexaenoic and other 1fatty acids across the human placenta 12 h after maternal oral intake. Am J Clin Nutr, 92(1), 115–122. doi: 10.3945/ajcn.2010.29589 [DOI] [PubMed] [Google Scholar]
- Gomes P, & Soares-da-Silva P (1999). L-DOPA transport properties in an immortalised cell line of rat capillary cerebral endothelial cells, RBE 4. Brain Res, 829(1–2), 143–150. [DOI] [PubMed] [Google Scholar]
- Grootjans J, Kaser A, Kaufman RJ, & Blumberg RS (2016). The unfolded protein response in immunity and inflammation. Nat Rev Immunol, 16(8), 469–484. doi: 10.1038/nri.2016.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan L, Mirza O, Verner G, Iwata S, & Kaback HR (2007). Structural determination of wild-type lactose permease. Proc Natl Acad Sci U S A, 104(39), 15294–15298. doi: 10.1073/pnas.0707688104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guemez-Gamboa A, Nguyen LN, Yang H, Zaki MS, Kara M, Ben-Omran T, . . . Gleeson JG (2015). Inactivating mutations in MFSD2A, required for omega-3 fattyacid transport in brain, cause a lethal microcephaly syndrome. Nat Genet, 47(7), 809–813. doi: 10.1038/ng.3311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa Y, Suzuki H, Uekawa K, Kawano T, & Kim-Mitsuyama S (2015). Characteristics of Cerebrovascular Injury in the Hyperacute Phase After Induced Severe Subarachnoid Hemorrhage. Transl Stroke Res, 6(6), 458–466. doi: 10.1007/s12975-015-0423-9 [DOI] [PubMed] [Google Scholar]
- He YJ, Xu H, Fu YJ, Lin JY, & Zhang MW (2018). Intraperitoneal hypertension, a 31 novel risk factor for sepsis-associated encephalopathy in sepsis mice. Sci Rep, 8(1), 8173. doi: 10.1038/s41598-018-26500-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heifetz A, Keenan RW, & Elbein AD (1979). Mechanism of action of tunicamycin on the UDP-GlcNAc:dolichyl-phosphate Glc-NAc-1-phosphate transferase. Biochemistry,18(11), 2186–2192. [DOI] [PubMed] [Google Scholar]
- Hetz C (2012). The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol, 13(2), 89–102. doi: 10.1038/nrm3270 [DOI] [PubMed] [Google Scholar]
- Horrocks LA, & Yeo YK (1999). Health benefits of docosahexaenoic acid (DHA). Pharmacol Res, 40(3), 211–225. doi: 10.1006/phrs.1999.0495 [DOI] [PubMed] [Google Scholar]
- Hu Q, Manaenko A, Bian H, Guo Z, Huang JL, Guo ZN, . . . Zhang JH (2017). Hyperbaric Oxygen Reduces Infarction Volume and Hemorrhagic Transformation Through ATP/NAD(+)/Sirt1 Pathway in Hyperglycemic Middle Cerebral Artery Occlusion Rats. Stroke, 48(6), 1655–1664. doi: 10.1161/STROKEAHA.116.015753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis SM (2007). Dietary (n-3) fatty acids and brain development. J Nutr, 137(4), 855–859. doi: 10.1093/jn/137.4.855 [DOI] [PubMed] [Google Scholar]
- Jiang Q, Ewing JR, & Chopp M (2012). MRI of blood-brain barrier permeability in cerebral ischemia. Transl Stroke Res, 3(1), 56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller RK, Boon DY, & Crum FC (1979). N-Acetylglucosamine- 1 -phosphate transferase from hen oviduct: solubilization, characterization, and inhibition by tunicamycin. Biochemistry, 18(18), 3946–3952. [DOI] [PubMed] [Google Scholar]
- Kidd PM (2007). Omega-3 DHA and EPA for cognition, behavior, and mood: clinical findings and structural-functional synergies with cell membrane phospholipids. Altern Med Rev, 12(3), 207–227. [PubMed] [Google Scholar]
- Kim E, Yang J, Park KW, & Cho S (2017). Inhibition of VEGF Signaling Reduces Diabetes-Exacerbated Brain Swelling, but Not Infarct Size, in Large Cerebral Infarction in Mice. Transl Stroke Res. doi: 10.1007/s12975-017-0601-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I, Xu W, & Reed JC (2008). Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov, 7(12), 1013–1030. doi: 10.1038/nrd2755 [DOI] [PubMed] [Google Scholar]
- Knowland D, Arac A, Sekiguchi KJ, Hsu M, Lutz SE, Perrino J, . . . Agalliu D (2014). Stepwise recruitment of transcellular and paracellular pathways underlies bloodbrain barrier breakdown in stroke. Neuron, 82(3), 603–617. doi: 10.1016/j.neuron.2014.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafourcade M, Larrieu T, Mato S, Duffaud A, Sepers M, Matias I, . . . Manzoni OJ (2011). Nutritional omega-3 deficiency abolishes endocannabinoid-mediated neuronal functions. Nat Neurosci, 14(3), 345–350. doi: 10.1038/nn.2736 [DOI] [PubMed] [Google Scholar]
- Langbein M, Strick R, Strissel PL, Vogt N, Parsch H, Beckmann MW, & Schild RL (2008). Impaired cytotrophoblast cell-cell fusion is associated with reduced Syncytin and increased apoptosis in patients with placental dysfunction. Mol Reprod Dev, 75(1), 175183. doi: 10.1002/mrd.20729 [DOI] [PubMed] [Google Scholar]
- Law CJ, Maloney PC, & Wang DN (2008). Ins and outs of major facilitator superfamily antiporters. Annu Rev Microbiol, 62, 289–305. doi: 10.1146/annurev.micro.61.080706.093329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinson O, Adler J, Sigal N, & Bibi E (2006). Promiscuity in multidrug recognition and transport: the bacterial MFS Mdr transporters. Mol Microbiol, 61(2), 277–284. doi: 10.1111/j.1365-2958.2006.05254.x [DOI] [PubMed] [Google Scholar]
- Li L, Tao Y, Tang J, Chen Q, Yang Y, Feng Z, . . . Chen Z (2015). A Cannabinoid Receptor 2 Agonist Prevents Thrombin-Induced Blood-Brain Barrier Damage via the Inhibition of Microglial Activation and Matrix Metalloproteinase Expression in Rats. Transl Stroke Res, 6(6), 467–477. doi: 10.1007/s12975-015-0425-7 [DOI] [PubMed] [Google Scholar]
- Li Q, Zhang Q, Wang M, Liu F, Zhao S, Ma J, . . . Li J (2007). Docosahexaenoic acid affects endothelial nitric oxide synthase in caveolae. Arch Biochem Biophys, 466(2), 250259. doi: 10.1016/j.abb.2007.06.023 [DOI] [PubMed] [Google Scholar]
- Lingwood D, & Simons K (2010). Lipid rafts as a membrane-organizing principle. Science, 327(5961), 46–50. doi: 10.1126/science.1174621 [DOI] [PubMed] [Google Scholar]
- Loryan I, Sinha V, Mackie C, Van Peer A, Drinkenburg WH, Vermeulen A, . . . Wassvik CM (2015). Molecular properties determining unbound intracellular and extracellular brain exposure of CNS drug candidates. Mol Pharm, 12(2), 520–532. doi: 10.1021/mp5005965 [DOI] [PubMed] [Google Scholar]
- Lynch JM, Maillet M, Vanhoutte D, Schloemer A, Sargent MA, Blair NS, . . . Molkentin JD (2012). A thrombospondin-dependent pathway for a protective ER stress response. Cell, 149(6), 1257–1268. doi: 10.1016/j.cell.2012.03.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma DW, Seo J, Davidson LA, Callaway ES, Fan YY, Lupton JR, & Chapkin RS (2004). n-3 PUFA alter caveolae lipid composition and resident protein localization in mouse colon. FASEB J, 18(9), 1040–1042. doi: 10.1096/fj.03-1430fje [DOI] [PubMed] [Google Scholar]
- Ma Y, & Hendershot LM (2004). The role of the unfolded protein response in tumour development: friend or foe? Nat Rev Cancer, 4(12), 966–977. doi: 10.1038/nrc1505 [DOI] [PubMed] [Google Scholar]
- Marchi S, Patergnani S, & Pinton P (2014). The endoplasmic reticulum-mitochondria connection: one touch, multiple functions. Biochim Biophys Acta, 1837(4), 461–469. doi: 10.1016/j.bbabio.2013.10.015 [DOI] [PubMed] [Google Scholar]
- Merali Z, Leung J, Mikulis D, Silver F, & Kassner A (2015). Longitudinal assessment of imatinib’s effect on the blood-brain barrier after ischemia/reperfusion injury with permeability MRI. Transl Stroke Res, 6(1), 39–49. doi: 10.1007/s12975-014-0358-6 [DOI] [PubMed] [Google Scholar]
- Meusser B, Hirsch C, Jarosch E, & Sommer T (2005). ERAD: the long road to destruction. Nat Cell Biol, 7(8), 766–772. doi: 10.1038/ncb0805-766 [DOI] [PubMed] [Google Scholar]
- Moritake H, Obara M, Saito Y, Kashimada A, Takagi M, Funakoshi-Tago M, . . . Nunoi H (2017). A mouse model reveals that Mfsd2a is critical for unfolded protein response upon exposure to tunicamycin. Hum Cell, 30(2), 88–97. doi: 10.1007/s13577-016-0153-7 [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, & Wu JH (2011). Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol, 58(20), 2047–2067. doi: 10.1016/j.jacc.2011.06.063 [DOI] [PubMed] [Google Scholar]
- Nag S (2003). Pathophysiology of blood-brain barrier breakdown. Methods Mol Med, 89, 9724119. doi: 10.1385/1-59259-419-0:97 [DOI] [PubMed] [Google Scholar]
- Nguyen LN, Ma D, Shui G, Wong P, Cazenave-Gassiot A, Zhang X, . . . Silver DL (2014). Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature, 509(7501), 503–506. doi: 10.1038/nature13241 [DOI] [PubMed] [Google Scholar]
- Pagan A, Prieto-Sanchez MT, Blanco-Carnero JE, Gil-Sanchez A, Parrilla JJ, Demmelmair H, . . . Larque E (2013). Materno-fetal transfer of docosahexaenoic acid is impaired by gestational diabetes mellitus. Am J Physiol Endocrinol Metab, 305(7), E826–833. doi: 10.1152/ajpendo.00291.2013 [DOI] [PubMed] [Google Scholar]
- Pan W, & Kastin AJ (2001). Changing the chemokine gradient: CINC1 crosses the blood33 brain barrier. J Neuroimmunol, 115(1–2), 64–70. [DOI] [PubMed] [Google Scholar]
- Pang J, Chen Y, Kuai L, Yang P, Peng J, Wu Y, . . . Jiang Y (2017). Inhibition of Blood-Brain Barrier Disruption by an Apolipoprotein E-Mimetic Peptide Ameliorates Early Brain Injury in Experimental Subarachnoid Hemorrhage. Transl Stroke Res, 8(3), 37257–272. doi: 10.1007/s12975-016-0507-1 [DOI] [PubMed] [Google Scholar]
- Pao SS, Paulsen IT, & Saier MH Jr. (1998). Major facilitator superfamily. Microbiol Mol Biol Rev, 62(1), 1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge WM (2005). The blood-brain barrier: bottleneck in brain drug development. NeuroRx, 2(1), 3–14. doi: 10.1602/neurorx.2.1.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge WM (2012). Drug transport across the blood-brain barrier. J Cereb Blood Flow Metab, 32(11), 1959–1972. doi: 10.1038/jcbfm.2012.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paroder-Belenitsky M, Maestas MJ, Dohan O, Nicola JP, Reyna-Neyra A, Follenzi A, . . . Carrasco N (2011). Mechanism of anion selectivity and stoichiometry of the Na+/I- symporter (NIS). Proc Natl Acad Sci U S A, 108(44), 17933–17938. doi: 10.1073/pnas.1108278108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton RG, & del Pozo MA (2013). Caveolae as plasma membrane sensors, protectors and organizers. Nat Rev Mol Cell Biol, 14(2), 98–112. doi: 10.1038/nrm3512 [DOI] [PubMed] [Google Scholar]
- Pauter AM, Trattner S, Gonzalez-Bengtsson A, Talamonti E, Asadi A, Dethlefsen O, & Jacobsson A (2017). Both maternal and offspring Elovl2 genotypes determine systemic DHA levels in perinatal mice. J Lipid Res, 58(1), 111–123. doi: 10.1194/jlr.M070862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluffo H, Unzueta U, Negro-Demontel ML, Xu Z, Vaquez E, Ferrer-Miralles N, & Villaverde A (2015). BBB-targeting, protein-based nanomedicines for drug and nucleic acid delivery to the CNS. Biotechnol Adv, 33(2), 277–287. doi: 10.1016/j.biotechadv.2015.02.004 [DOI] [PubMed] [Google Scholar]
- Prieto-Sanchez MT, Ruiz-Palacios M, Blanco-Carnero JE, Pagan A, Hellmuth C, Uhl O, . . . Larque E (2017). Placental MFSD2a transporter is related to decreased DHA in cord blood of women with treated gestational diabetes. Clin Nutr, 36(2), 513–521. doi: 10.1016/j.clnu.2016.01.014 [DOI] [PubMed] [Google Scholar]
- Pu W, Zhang H, Huang X, Tian X, He L, Wang Y, . . . Zhou B (2016). Mfsd2a+ hepatocytes repopulate the liver during injury and regeneration. Nat Commun, 7, 13369. doi: 10.1038/ncomms13369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quek DQ, Nguyen LN, Fan H, & Silver DL (2016). Structural Insights into the Transport Mechanism of the Human Sodium-dependent Lysophosphatidylcholine Transporter MFSD2A. J Biol Chem, 291(18), 9383–9394. doi: 10.1074/jbc.M116.721035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahim AA, Wong AM, Hoefer K, Buckley SM, Mattar CN, Cheng SH, . . . Waddington SN (2011). Intravenous administration of AAV2/9 to the fetal and neonatal mouse leads to differential targeting of CNS cell types and extensive transduction of the nervous system. FASEB J, 25(10), 3505–3518. doi: 10.1096/fj.11182311 [DOI] [PubMed] [Google Scholar]
- Reddy VS, Shlykov MA, Castillo R, Sun EI, & Saier MH Jr. (2012). The major facilitator superfamily (MFS) revisited. FEBS J, 279(11), 2022–2035. doi: 10.1111/j.1742-4658.2012.08588.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese TS, & Karnovsky MJ (1967). Fine structural localization of a blood-brain barrier to exogenous peroxidase. J Cell Biol, 34(1), 207–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiling JH, Clish CB, Carette JE, Varadarajan M, Brummelkamp TR, & Sabatini DM (2011). A haploid genetic screen identifies the major facilitator domain containing 2A (MFSD2A) transporter as a key mediator in the response to tunicamycin. Proc Natl Acad Sci U S A, 108(29), 11756–11765. doi: 10.1073/pnas.1018098108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo T, Metzger BE, Burns WJ, & Burns K (1991). Correlations between antepartum maternal metabolism and intelligence of offspring. N Engl J Med, 325(13), 911–916. doi: 10.1056/NEJM199109263251303 [DOI] [PubMed] [Google Scholar]
- Rolland WB, Krafft PR, Lekic T, Klebe D, LeGrand J, Weldon AJ, . . . Zhang JH (2017). Fingolimod confers neuroprotection through activation of Rac1 after experimental germinal matrix hemorrhage in rat pups. J Neurochem, 140(5), 776–786. doi: 10.1111/jnc.13946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D, & Walter P (2007). Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol, 8(7), 519–529. doi: 10.1038/nrm2199 [DOI] [PubMed] [Google Scholar]
- Saier MH Jr., Beatty JT, Goffeau A, Harley KT, Heijne WH, Huang SC, . . . Virk PS (1999). The major facilitator superfamily. J Mol Microbiol Biotechnol, 1(2), 257–279. [PubMed] [Google Scholar]
- Salem N Jr., Litman B, Kim HY, & Gawrisch K (2001). Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids, 36(9), 945–959. [DOI] [PubMed] [Google Scholar]
- SanGiovanni JP, & Chew EY (2005). The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog Retin Eye Res, 24(1), 87–138. doi: 10.1016/j.preteyeres.2004.06.002 [DOI] [PubMed] [Google Scholar]
- Saunders NR, Liddelow SA, & Dziegielewska KM (2012). Barrier mechanisms in the developing brain. Front Pharmacol, 3, 46. doi: 10.3389/fphar.2012.00046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S, Bryant KD, Brown SM, Randell SH, & Asokan A (2011). Terminal N-linked galactose is the primary receptor for adeno-associated virus 9. J Biol Chem, 286(15), 13532–13540. doi: 10.1074/jbc.M110.210922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Doycheva DM, Xu L, Tang J, Yan M, & Zhang JH (2016). Sestrin2 induced by hypoxia inducible factor1 alpha protects the blood-brain barrier via inhibiting VEGF after severe hypoxic-ischemic injury in neonatal rats. Neurobiol Dis, 95, 111–121. doi: 10.1016/j.nbd.2016.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegenthaler JA, Sohet F, & Daneman R (2013). ‘Sealing off the CNS’: cellular and molecular regulation of blood-brain barriergenesis. Curr Opin Neurobiol, 23(6), 1057–1064. doi: 10.1016/j.conb.2013.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simopoulos AP (2008). The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med (Maywood), 233(6), 674–688. doi: 10.3181/0711-MR-311 [DOI] [PubMed] [Google Scholar]
- Spinola M, Falvella FS, Colombo F, Sullivan JP, Shames DS, Girard L, . . . Dragani TA (2010). MFSD2A is a novel lung tumor suppressor gene modulating cell cycle and matrix attachment. Mol Cancer, 9, 62. doi: 10.1186/1476-4598-9-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinola M, Falvella FS, Galvan A, Pignatiello C, Leoni VP, Pastorino U, . . . Dragani TA (2007). Ethnic differences in frequencies of gene polymorphisms in the MYCL1 region and modulation of lung cancer patients’ survival. Lung Cancer, 55(3), 271–277. doi: 10.1016/j.lungcan.2006.10.023 [DOI] [PubMed] [Google Scholar]
- Spinola M, Galvan A, Pignatiello C, Conti B, Pastorino U, Nicander B, . . . Dragani TA (2005). Identification and functional characterization of the candidate tumor suppressor gene TRIT1 in human lung cancer. Oncogene, 24(35), 5502–5509. doi: 10.1038/sj.onc.1208687 [DOI] [PubMed] [Google Scholar]
- Strazielle N, & Ghersi-Egea JF (2016). Potential Pathways for CNS Drug Delivery Across the Blood-Cerebrospinal Fluid Barrier. Curr Pharm Des, 22(35), 5463–5476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatsuki A, Arima K, & Tamura G (1971). Tunicamycin, a new antibiotic. I. Isolation and 38 characterization of tunicamycin. J Antibiot (Tokyo), 24(4), 215–223. [DOI] [PubMed] [Google Scholar]
- Tiwary S, Morales JE, Kwiatkowski SC, Lang FF, Rao G, & McCarty JH (2018). Metastatic Brain Tumors Disrupt the Blood-Brain Barrier and Alter Lipid Metabolism by Inhibiting Expression of the Endothelial Cell Fatty Acid Transporter Mfsd2a. Sci Rep, 8(1), 8267. doi: 10.1038/s41598-018-26636-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Peraza JF, Engel T, Martin-Ibanez R, Sanz-Rodriguez A, Fernandez-Fernandez MR, Esgleas M, . . . Lucas JJ (2013). Protective neuronal induction of ATF5 in endoplasmic reticulum stress induced by status epilepticus. Brain, 136(Pt 4), 1161–1176. doi: 10.1093/brain/awt044 [DOI] [PubMed] [Google Scholar]
- Toufaily C, Vargas A, Lemire M, Lafond J, Rassart E, & Barbeau B (2013). MFSD2a, the Syncytin-2 receptor, is important for trophoblast fusion. Placenta, 34(1), 85–88. doi: 10.1016/j.placenta.2012.10.012 [DOI] [PubMed] [Google Scholar]
- Ungaro F, Tacconi C, Massimino L, Corsetto PA, Correale C, Fonteyne P, . . . Danese, S. (2017). MFSD2A Promotes Endothelial Generation of Inflammation-Resolving Lipid Mediators and Reduces Colitis in Mice. Gastroenterology, 153(5), 1363–1377 e1366. doi: 10.1053/j.gastro.2017.07.048 [DOI] [PubMed] [Google Scholar]
- Vargas A, Toufaily C, LeBellego F, Rassart E, Lafond J, & Barbeau B (2011). Reduced expression of both syncytin 1 and syncytin 2 correlates with severity of preeclampsia. Reprod Sci, 18(11), 1085–1091. doi: 10.1177/1933719111404608 [DOI] [PubMed] [Google Scholar]
- Vincenz-Donnelly L, & Hipp MS (2017). The endoplasmic reticulum: A hub of protein quality control in health and disease. Free Radic Biol Med, 108, 383–393. doi: 10.1016/j.freeradbiomed.2017.03.031 [DOI] [PubMed] [Google Scholar]
- Wang JZ, Xiao N, Zhang YZ, Zhao CX, Guo XH, & Lu LM (2016). Mfsd2a-based pharmacological strategies for drug delivery across the blood-brain barrier. Pharmacol Res, 104, 124–131. doi: 10.1016/j.phrs.2015.12.024 [DOI] [PubMed] [Google Scholar]
- Wong BH, Chan JP, Cazenave-Gassiot A, Poh RW, Foo JC, Galam DL, . . . Silver DL (2016). Mfsd2a Is a Transporter for the Essential omega-3 Fatty Acid Docosahexaenoic Acid (DHA) in Eye and Is Important for Photoreceptor Cell Development. J Biol Chem, 291(20), 10501–10514. doi: 10.1074/jbc.M116.721340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YR, Xiong XY, Liu J, Wu LR, Zhong Q, Zhou K, . . . Yang QW (2017). Mfsd2a (Major Facilitator Superfamily Domain Containing 2a) Attenuates Intracerebral Hemorrhage-Induced Blood-Brain Barrier Disruption by Inhibiting Vesicular Transcytosis. J Am Heart Assoc, 6(7). doi: 10.1161/JAHA.117.005811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, & Kaufman RJ (2006). The unfolded protein response: a stress signaling pathway critical for health and disease. Neurology, 66(2 Suppl 1), S102–109. doi: 10.1212/01.wnl.0000192306.98198.ec [DOI] [PubMed] [Google Scholar]
- Zhao H, Zhang X, Dai Z, Feng Y, Li Q, Zhang JH, . . .Feng H (2016). P2X7 Receptor Suppression Preserves Blood-Brain Barrier through Inhibiting RhoA Activation after Experimental Intracerebral Hemorrhage in Rats. Sci Rep, 6, 23286. doi: 10.1038/srep23286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Nelson AR, Betsholtz C, & Zlokovic BV (2015). Establishment and Dysfunction of the Blood-Brain Barrier. Cell, 163(5), 1064–1078. doi: 10.1016/j.cell.2015.10.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Xiong Y, Huang XR, Tang P, Yu X, & Lan HY (2015). Identification of Genes Associated with Smad3-dependent Renal Injury by RNA-seq-based Transcriptome Analysis. Sci Rep, 5, 17901. doi: 10.1038/srep17901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV (2008). The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron, 57(2), 178–201. doi: 10.1016/j.neuron.2008.01.003 [DOI] [PubMed] [Google Scholar]
- Zuo S, Ge H, Li Q, Zhang X, Hu R, Hu S, . . . Feng H (2017). Artesunate Protected Blood-Brain Barrier via Sphingosine 1 Phosphate Receptor 1/Phosphatidylinositol 3 Kinase Pathway After Subarachnoid Hemorrhage in Rats. Mol Neurobiol, 54(2), 12131228. doi: 10.1007/s12035-016-9732-6 [DOI] [PubMed] [Google Scholar]