Abstract
Background:
Patient derived xenografts (PDXs) provide a unique opportunity for investigators to study tumor cell activity, response to therapeutics, and resistance patterns without exposing the human patient to experimental compounds, and thereby play a crucial role in pre-clinical evaluation of new therapies. It has been reported that PDXs may undergo a transformation to lymphoma, most commonly associated with Epstein Barr virus (EBV). If the character of a xenograft becomes compromised and remains undetected, it could have a detrimental impact on the research community as a whole. Our lab has established a number of pediatric solid tumor PDXs which accurately recapitulate the human tumors following several passages. One particular neuroblastoma PDX was noted to grow quickly and with an unusual phenotype, leading us to hypothesize that this PDX had undergone a transformation.
Methods:
The PDX in question was investigated with histology, immunohistochemistry (IHC), EBER in situ hybridization, and PCR to determine its identity.
Results:
Histology on the tumor revealed a small, round blue cell tumor similar to the original neuroblastoma from which it was derived. IHC staining showed that the tumor was composed of lymphocytes that were CD3 positive, <5% CD4 positive, and CD20 negative. The cells were Epstein Barr virus negative. PCR demonstrated that the tumor was human and not murine in origin.
Conclusion:
These findings indicate that a human T Cell lymphoma developed in place of this neuroblastoma PDX. Changes in PDX identity such as this one will significantly impact studies utilizing pediatric PDXs and the mechanism by which this occurred warrants further investigation.
Keywords: Patient Derived Xenografts, Neuroblastoma, Lymphoma
1. Introduction
Patient derived xenografts (PDXs) have become an extremely important model for the study of malignancy, particularly in the realm of drug discovery. These xenografts recapitulate many aspects of the original tumors in vivo, while providing an ongoing source of cells for future experimentation. It has been documented that one limitation of the xenograft model is that a transformation may occur from one passage to the next, resulting in a tumor that does not resemble the original malignancy [1–4]. Most commonly the resulting malignancy is a B-cell lymphoma, often linked to Epstein Barr Virus, which has known oncogenic properties [3]. While previously documented, development of a human T-cell lymphoma is considerably more rare, and the mechanism by which this occurs is not clear [1,4].
Appropriate identification of PDXs from one passage to the next is critically important due to the role they play in drug development. Crucial data could be potentially nullified by the discovery that a xenograft has transformed without the investigator’s knowledge. The data gleaned from PDXs drive changes in patient therapy and help to develop safety and dosing profiles in preparation for phase I clinical trials. For that reason, it is critical that any investigator using PDX models systematically confirms their identity at regular intervals to prevent this issue.
Our laboratory maintains several lines of pediatric sold tumor PDXs and we routinely sequence them to confirm that a change in identity has not occurred. Recently we noted that one of the PDXs had an abrupt change in phenotype. The tumor had been passed from a confirmed neuroblastoma PDX, that was typically grew slowly in a rounded shape. The new tumor demonstrated a change in appearance (flat, smooth) as well as an accelerated growth rate. For that reason, we suspected a transformation had occurred and initiated the process of elucidating the tumor’s identity.
2. Methods
2.1. PDX maintenance
The PDXs were maintained with approval by the Institutional Animal Care and Use Committee (IACUC 09803). Animals are maintained in 12-hour light/dark cycles with available chow and water 24 hours per day. Six-week-old, athymic nude, female mice are used for passage (Envigo, Pratville, AL). Specimens for passage come directly from the operating room or low-passage freezes. Tumor cells are passaged in a sterile fashion into the right flank of each mouse with 25% Matrigel™ (Corning, Bedford, MA) and Roswell Park Memorial Institute medium (RPMI 1640, Manassas, VA) suspension, and tumors are harvested when they reach a volume of 1500–2000 mm3. At the time of tumor harvest, the tumor was dissected in a sterile fashion, and a portion of the tumor was flash frozen at −80 degrees, while the remaining portion of the tumor was placed in a cassette and formalin fixed and then paraffin-embedded for future studies. For The parent neuroblastoma tumor was used for comparisons for immunohistochemistry and EBER-ISH. A known neuroblastoma PDX (X546) was utilized for PCR comparisons to the tumor in question (X587).
2.2. Immunohistochemistry
Formalin-fixed paraffin-embedded specimens were sectioned into 6 μm sections and baked at 70°C for one hour on positive slides. The specimens were deparaffinized, steamed, and standard hematoxylin and eosin staining was performed. Slides were stained by the University of Alabama (UAB) IHC Core for CD3 (LN10), CD4 (4B12), and CD20 (L26) (Leica Biosystems, Nussloch, Germany) per manufacturers protocol (IHC Protocol F) on the automated BOND system in combination with Bond Polymer Refine Detection (Leica Biosystems). Theses slides were reviewed by a board-certified pathologist (EMM).
2.3. EBER-ISH
Due to the prevalence of EBV-related lymphoma in transformed PDXs, additional formalin-fixed paraffin-embedded slides were sent to the UAB IHC Core to undergo EBV-encoded RNA in-situ hybridization (EBER-ISH). Slides were stained per manufacturer’s protocol on the Ventana Benchmark instrument using the INFORM EBER Probe (Ventana Medical Systems, CAT#790–2842, Tucson, AZ). All slides were reviewed with board-certified a pathologist (EMM).
2.4. PCR
PCR for both mouse and human GAP-DH was used to determine whether the tumor was mouse or human in origin and was performed by the UAB TRENDD Center RNA/DNA Isolation and TaqMan QPCR/Genotyping Core Facility. Frozen tumor specimens were provided and RNA extracted via RNeasy® Mini Kit (Quiagen, Valencia, CA) and QIAcube (Quiagen). Primescrpt™ RT Master Mix (Takara/Clontech, Mountain View, CA) was used to synthesize cDNA, and PCR was performed using Premix EX Taq (Probe qPCR) (Takara/Clontech) and Rotor-Gene Q (Quiagen). Primers for human GAPDH (4325792) and mouse GAPDH (4351309) were used (Applied Biosystem, Thermofisher).
3. Results
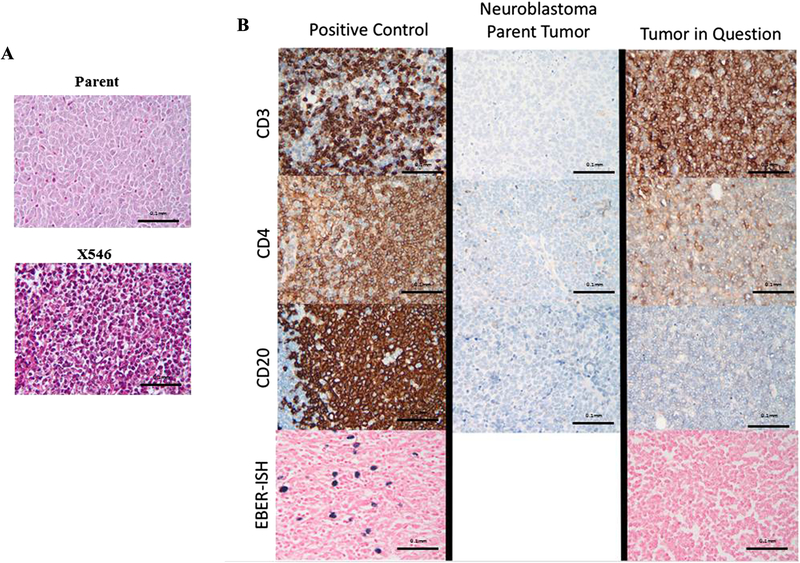
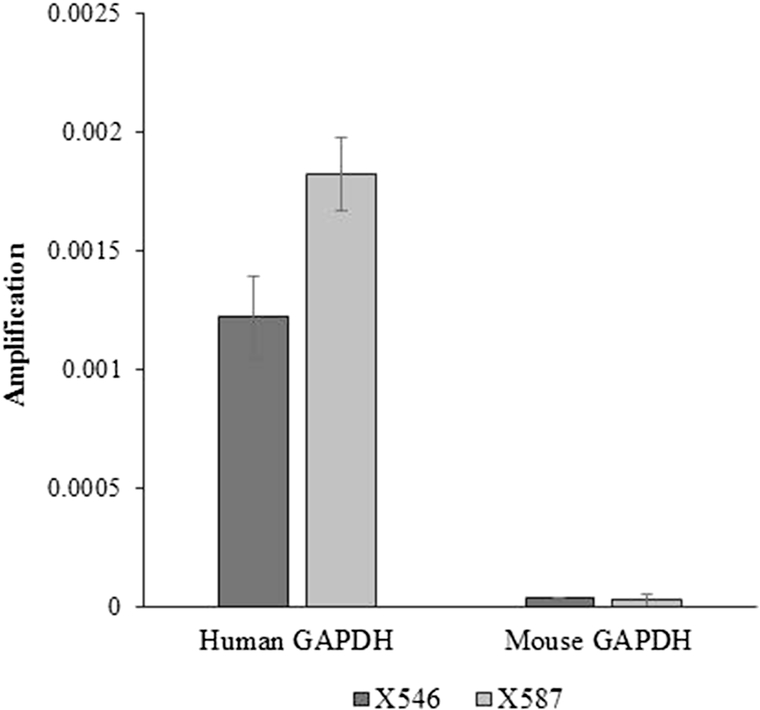
H&E staining demonstrated a tumor composed of small blue round cells, not significantly different from the parent PDX (Fig. 1 A). Immunohistochemistry revealed that the parent tumor, a confirmed neuroblastoma, was negative for CD3, CD4, and CD20 (Fig. 1 B, middle panels). The tumor in question was positive for CD3, <5% positive for CD4, and negative for CD20 (Fig. 1 B, top three right panels). This profile resembles that of a T-cell lymphoma. EBER-ISH (Fig. 1 B, bottom right panel) confirmed that the tumor cells were negative for Epstein Barr Virus. Positive control slides were included for each antigen (Fig. 1 B, left panels). PCR demonstrated amplification of human GAP-DH in a known human neuroblastoma PDX (X587) and the tumor in question (X546), suggesting the tumor was human in origin rather than murine (Fig. 2).
Figure 1.
A. Hematoxylin and eosin staining of the PDX tumor in question revealed a tumor composed of small blue round cells (bottom panel), not significantly different from the parent (top panel). B. Side by side comparison of the PDX in question (X546), a known human neuroblastoma, and positive control demonstrated marked differences in staining between the transformed PDX and the parent tumor. The PDX in question (right panels) stains positive for CD3, about 5% positive for CD4, and negative for CD20, while the patent tumor (middle panels) stains negative for all three immunomarkers. EBER-ISH for the PDX was negative (bottom right panel) compared to positive control (bottom left panel), indicating that this lymphoma was not EBV related.
Figure 2.
PCR results for the PDX in question (X546) when compared to a known human neuroblastoma (X587) demonstrated similarly high amplification of human GAPDH and little to no amplification of mouse GAPDH, suggesting the tumor was human in origin.
4. Discussion
Due to the overall success of PDX programs in a multitude of solid cancer types, investigators are finding increasing evidence of pitfalls and limitations to their use. As with any new technique, these issues are often only identified with prolonged and expanded use. Decades ago, investigators realized the potential benefit of using PDXs as an experimental model for chemotherapeutics [5]. It wasn’t until much later that it was reported that the PDXs had the potential to transform from one passage to the next from a carcinoma or sarcoma to a human lymphoma [2]. The rates of this occurrence vary between the different types of murine models, but Bondarenko et.al found rates as high as 32% in NOD scid gamma mice [1]. In looking for an explanation for this occurrence, the earliest evidence pointed to Epstein Barr Virus as the primary cause [2]. The virus remained latent in human B-cells that often accompanied the transplanted tumor tissue, and took advantage of the immunodeficient environment to re-emerge as a lymphoma [2]. The mechanism by which Epstein Barr Virus infection leads to B-cell lymphoma is well documented and understood [6].
In very rare cases PDXs have grown human T-cell lymphomas at the site of implantation that are unrelated to EBV [1,4]. This phenomenon is quite rare and the mechanism by which it takes place is not clear. Other investigators have hypothesized human T cell expansion as a result of graft versus host disease to serve as the initiating event [1,7]. Data from von Bonin et al. supports this hypothesis, demonstrating T cell lymphoma development in AML xenografts that was prevented by depleting the number of T cells co-transplanted with the graft [7].
Regardless of the mechanism by which it occurs, the development of a mass at the xenografts site of injection that is histologically distinctive from the parent tumor poses a problematic situation for researches. Growth of an imposter tumor could lead to false data, erroneous conclusions, and ultimately dangerous changes in patient care based on those conclusions. To our knowledge, this phenomenon has not been described in the pediatric literature. With the constant development of new PDXs from the pediatric population, it is critical that investigators be aware of this issue and implement a systemic approach to maintaining the authenticity of their xenografts.
Acknowledgements:
The authors would like to thank Changchun Ren PhD. and the TRENDD Center RNA/DNA isolation and TaqMan QPCR/Genotyping Core Facility and Debra Horton in the University of Alabama Immunohistochemistry Core for their contributions.
Funding:
This work was funded, in part, by National Institutes of Health T32 CA183926 (APW), T32 CA091078 (LLS), Kaul Pediatric Research Award (EAB), Open Hands Overflowing Hearts (KW), Sid Strong Foundation (KW), and the Elaine Roberts Foundation (KW).
Abbreviations:
- PDX
Patient Derived Xenografts
- EBV
Epstein Barr Virus
- IHC
immunohistochemistry
- EBER-ISH
EBV-encoded RNA in-situ hybridization
- PCR
Polymerase Chain Reaction
- IACUC
Institutional Animal Care and Use Committee
- UAB
University of Alabama at Birmingham
- AML
Acute Myeloid Leukemia
Footnotes
Declaration of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Bondarenko G, Ugolkov A, Rohan S, Kulesza P, Dubrovskyi O, Gursel D, et al. Patient-Derived Tumor Xenografts Are Susceptible to Formation of Human Lymphocytic Tumors. Neoplasia. 2015; 17(9), 735–741. doi: 10.1016/j.neo.2015.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chen K, Ahmed S, Adeyi O, Dick JE, Ghanekar A. Human solid tumor xenografts in immunodeficient mice are vulnerable to lymphomagenesis associated with Epstein-Barr virus. PLOS ONE. 2012; 7(6), e39294. doi: 10.1371/journal.pone.0039294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Taurozzi AJ, Beekharry R, Wantoch M, Labarthe M C, Walker H F, Seed RI, et al. Spontaneous development of Epstein-Barr Virus associated human lymphomas in a prostate cancer xenograft program. PLOS ONE. 2017; 12(11), e0188228. doi: 10.1371/journal.pone.0188228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wetterauer C, Vlajnic T, Schüler J, Gsponer JR, Thalmann GN, Cecchini M, et al. Early development of human lymphomas in a prostate cancer xenograft program using triple knock-out immunocompromised mice. Prostate. 2015; 75(6), 585–592. doi: 10.1002/pros.22939 [DOI] [PubMed] [Google Scholar]
- [5].Giovanella BC, Stehlin JS, Williams LJ, Lee SS, Shepard RC. Heterotransplantation of human cancers into nude mice: a model system for human cancer chemotherapy. Cancer. 1978; 42(5), 2269–2281. [DOI] [PubMed] [Google Scholar]
- [6].Brady G, MacArthur GJ, Farrell PJ. Epstein-Barr virus and Burkitt lymphoma. J Clin Pathol. 2007; 60(12), 1397–1402. doi: 10.1136/jcp.2007.047977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].von Bonin M, Wermke M, Cosgun KN, Thiede C, Bornhauser M, Wagemaker G, et al. In vivo expansion of co-transplanted T cells impacts on tumor re-initiating activity of human acute myeloid leukemia in NSG mice. PLOS ONE. 2013; 8(4), e60680. doi: 10.1371/journal.pone.0060680 [DOI] [PMC free article] [PubMed] [Google Scholar]