Abstract
Patient blood management (PBM) is the timely application of evidence-informed medical and surgical concepts designed to maintain haemoglobin concentration, optimise haemostasis, and minimise blood loss in an effort to improve patient outcomes. The aim of this consensus statement is to provide recommendations on the prevention and treatment of postpartum haemorrhage as part of PBM in obstetrics. A multidisciplinary panel of physicians with expertise in obstetrics, anaesthesia, haematology, and transfusion medicine was convened by the Network for the Advancement of Patient Blood Management, Haemostasis and Thrombosis (NATA) in collaboration with the International Federation of Gynaecology and Obstetrics (FIGO), the European Board and College of Obstetrics and Gynaecology (EBCOG), and the European Society of Anaesthesiology (ESA). Members of the task force assessed the quantity, quality and consistency of the published evidence, and formulated recommendations using the system developed by the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) working group. The recommendations in this consensus statement are intended for use by clinical practitioners managing perinatal care of women in all settings, and by policy-makers in charge of decision making for the update of clinical practice in health care establishments.
Keywords: anaemia, bleeding, blood transfusion, haemostasis, iron deficiency, obstetrics, patient blood management, postpartum haemorrhage
Introduction
Patient blood management (PBM) is the timely application of evidence-informed medical and surgical concepts designed to maintain haemoglobin (Hb) concentration, optimise haemostasis, and minimise blood loss in an effort to improve patient outcomes. The aim of this consensus statement is to provide recommendations on the prevention and treatment of postpartum haemorrhage (PPH) as part of PBM in obstetrics. A previously published consensus statement offers guidance on the management of anaemia and haematinic deficiencies in pregnancy and in the postpartum period1.
Consensus organisation
Scope and purpose
This consensus statement reflects the position of the Network for the Advancement of Patient Blood Management, Haemostasis and Thrombosis (NATA), the International Federation of Gynaecology and Obstetrics (FIGO), the European Board and College of Obstetrics and Gynaecology (EBCOG), and the European Society of Anaesthesiology (ESA). It includes recommendations on the prevention and treatment of PPH.
Target audience
The recommendations in this consensus statement are intended for use by clinical practitioners managing perinatal care of women in all settings, and by policy-makers in charge of decision making for the update of clinical practice in health care establishments. It is important to note that these recommendations need to be tailored to the needs of individual patients or of any given population after consideration of the values and preferences of both healthcare providers and patients, as well as equity issues, explicit assessment of harms and benefits of each recommendation, and feasibility (including resources, capacity and equipment, and implementability). Further involvement of relevant stakeholders on a local level is advised.
Development process
A multidisciplinary panel of physicians with expertise in obstetrics, anaesthesia, haematology, policymaking and epidemiology was convened by NATA in collaboration with FIGO, EBCOG and ESA, with the aim of developing a consensus statement on the prevention and treatment of PPH. The experts were asked to:
determine the scope of the problem;
identify the best methods to detect the problem and establish a differential diagnosis;
assess the evidence and its quality for the detection, evaluation and management of haemorrhage in the postpartum period;
agree on recommended pharmacological and non-pharmacological methods to minimise peripartum blood losses;
define the role of transfusions in PPH; and
issue recommendations on the use of oral iron, intravenous iron and erythropoiesis-stimulating agents to treat anaemia after a PPH.
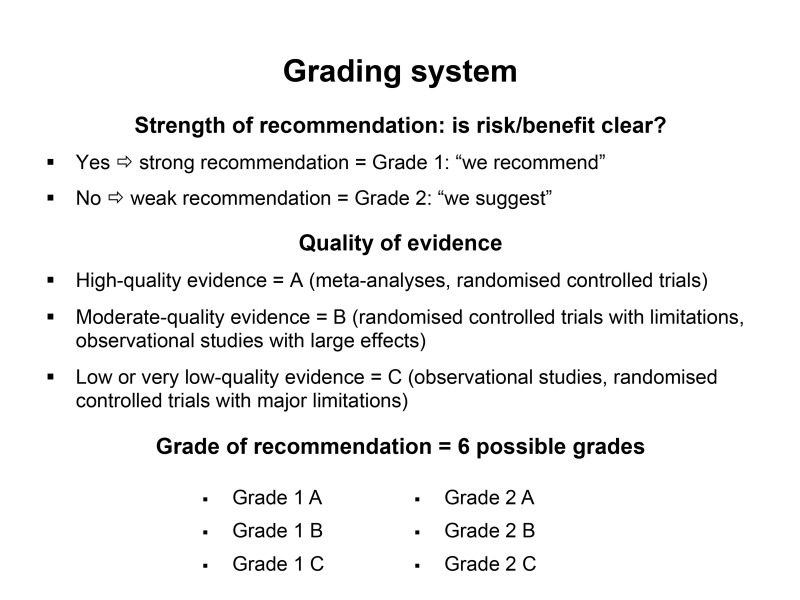
Members of the task force assessed the quality, quantity and consistency of the published evidence, and formulated recommendations using the system developed by the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) working group (Figure 1)2. A preliminary version of the consensus statement was drafted by JS and MM and circulated among the members of the panel for additional input and editing. The document was further reviewed by two anaesthesiologists (IF and SM) who are experts in the field and who were not involved in the first phase of the process. After examination, it was agreed that the recommendations were coherent with ESA recommendations already published in the field3,4.
Figure 1.
The grading system used to evaluate the quality of the evidence and grade recommendations.
Plans for dissemination, adaptation and evaluation
This statement will be disseminated to all members of NATA, FIGO, EBCOG and ESA, and the document will be made available via open access. Additionally, the recommendations will be presented at international forums and specialised events targeting clinicians involved in the prevention and treatment of PPH.
A global view on postpartum haemorrhage: definitions, incidence, risk factors and clinical burden
Postpartum haemorrhage remains a common obstetric emergency and is the leading cause of maternal mortality worldwide. Maternal mortality is defined as the death of a woman whilst pregnant or within 42 days of delivery or termination of pregnancy. According to a systematic analysis, the estimated global number of maternal deaths in 2015 was 275,000, of which 34% were caused by haemorrhage5. The maternal mortality ratio ranged from 15 per 100,000 live births in high socio-demographic index (SDI) countries to 443 per 100,000 livebirths in low SDI countries, where haemorrhage is the leading cause of maternal death5. PPH-related deaths are potentially preventable with timely diagnosis and management6.
There is currently no single satisfactory definition of PPH7. PPH is commonly defined as blood loss of 500 mL or more within 24 hours (h) after birth, while severe PPH is defined as blood loss of 1,000 mL or more and massive life-threatening PPH as ongoing blood loss of more than 2,500 mL or hypovolemic shock within the same timeframe6. These definitions are based on quantifications of blood loss that originated from historical studies in the 1960s that aimed to identify women at high risk of adverse clinical outcomes. The threshold for clinical intervention should take into account maternal health and the clinical context, including pre-delivery Hb concentration and blood flow rate.
PPH is categorised as either primary or secondary: primary PPH occurs in the first 24 h after delivery (early PPH) and secondary PPH occurs 24 h to 12 weeks after delivery (late or delayed PPH).
The overall global incidence of PPH is estimated to be 6–11% and of severe PPH 1–3%, with substantial variations across regions. The incidence of PPH is higher in low-resource countries in Africa and Asia when measured objectively and in the setting of a controlled trial; thus, the true incidence of PPH is likely to be much higher than reported6,8,9. A number of studies have noted an increase in the incidence of PPH in high-resource countries such as Australia, Canada, Ireland, Norway and the United States10–12. Analysis of data from 2,406,784 Dutch women suggests a considerable increase in the incidence of PPH (blood loss of 1,000–1,500 mL) from 2000 to 2013 (4.1 vs 6.1%; p<0.0001)13. This increase was accompanied by a significant decrease in the incidence of PPH-related blood transfusions, suggesting a reduced incidence of massive PPH13.
Uterine atony is the most common cause of PPH and cases have been increasing over recent years. But other causes include genital tract trauma (i.e. vaginal or cervical lacerations), uterine rupture, retained placental tissue and maternal coagulation disorders11,14.
- Recommendation 1. We recommend defining primary PPH as blood loss of more than 500 mL within 24 h, whatever the mode of delivery (1B).
- Recommendation 2. We recommend defining severe PPH as ongoing blood loss of more than 1,000 mL within 24 h or blood loss accompanied by signs/symptoms of hypovolaemia, and massive life-threatening PPH as ongoing blood loss of more than 2,500 mL or hypovolemic shock, whatever the mode of delivery (1B).
Management of women at high risk of postpartum haemorrhage
Identification of women at risk of postpartum haemorrhage
It is important to differentiate risk factors by their influence on the risk of PPH and by their frequency. They can also be classified by the time at which they occur: before pregnancy, during pregnancy, during labour, or after delivery.
Prediction of PPH is inherently difficult and there is no single risk factor (except for abormal placentation, which is discussed below). However, several risk factors increase the risk of PPH, but they are globally not predictive. If only risk factors with an adjusted odds ratio (OR) >2.0 are considered, these are as follows10,12,14:
- multiple pregnancies (OR 2.3–4.7);
- a history of PPH (OR 3.3);
- pregnancy-induced hypertension (OR 1.9–2.5);
- chorioamnionitis (OR 2.5);
- episiotomy (OR 1.4 to 2.2);
- pre-labour caesarean section (OR 1.3–2.3);
- caesarean section during labour (OR 1.7–3.6);
- macrosomia (OR 1.7 to 3.5);
- operative vaginal delivery (OR 2.3).
- Recommendation 3. We recommend that the medical staff be made aware of the risk factors for PPH, allowing prompt action towards its prevention (1C).
Prevention of severe anaemia before and after postpartum haemorrhage
Postpartum anaemia (PPA) is defined as an Hb <10 g/dL within 24–48 h after delivery, although it has also been recommended that PPA be defined as a Hb <11 g/dL at 1 week postpartum and <12 g/dL at 8 weeks15. The prevalence of PPA 48 h after delivery is approximately 50% in Europe and 50–80% in developing countries. PPA should be considered severe if Hb is <7 g/dL16.
Anaemia during the third trimester of pregnancy and PPH are independent risk factors for PPA17. Thus, parturients’ Hb should be determined when labour starts, unless a recent Hb is available and there are no risk factors for PPH. PPA can be aggravated by altered iron homeostasis and reduced erythropoietin secretion and action in women with a higher postpartum inflammatory response (e.g. caesarean section)18. Therefore, every effort should be made to correct anaemia prior to delivery and women with anaemia or at high risk of haemorrhage should be advised only to deliver in hospital where a clear multidisciplinary, multimodal protocol for management of major obstetric haemorrhage is in place. This protocol should be activated as soon as major obstetric haemorrhage is detected or suspected19–22.
Once the haemorrhagic episode is controlled, women should receive intravenous iron for the treatment of moderate-to-severe PPA (Hb 6–9 g/dL)1. Staff should be trained to detect, evaluate and manage hypersensitivity reactions to intravenous iron in accordance with published guidelines23,24. In severely anaemic patients with blunted erythropoiesis secondary to inflammation who do not respond adequately to intravenous iron treatment, as well as in severely anaemic patients who refuse blood transfusion, administration of an erythropoiesis-stimulating agent may be considered after consultation with the haematologist1.
During follow up, a complete blood count plus a serum ferritin level at 8 weeks postpartum are adequate to assess anaemia and iron status in the majority of women with antenatal anaemia and/or significant peripartum bleeding1.
- Recommendation 4. We recommend that parturients should have an Hb determination when labour starts, especially in women with antenatal anaemia (1C).
- Recommendation 5. We recommend that every effort should be made to correct anaemia of pregnancy prior to delivery (1A), and that women with prepartum anaemia be advised to deliver in hospital (1C).
- Recommendation 6. We recommend the administration of intravenous iron to cover individually calculated total iron deficiency in women with moderate-to-severe postpartum anaemia (Hb 6–9 g/dL) (1B).
- Recommendation 7. In severely anaemic patients with blunted erythropoiesis due to infection and/or inflammation who do not respond adequately to intravenous iron treatment, as well as in severely anemic patients who refuse blood transfusion, we suggest the administration of an erythropoiesis-stimulating agent after consultation with the haematologist (2B).
Pre-operative autologous blood donation
The use of autologous blood transfusion in obstetrics is widely debated, but there are little data on the topic. Risks associated with pre-operative autologous blood donation include: iatrogenic anaemia, bacterial contamination, and human errors. A Cochrane review from 2002 points out that pre-operative autologous blood donation may reduce the risk of allogeneic blood transfusion, but it probably increases the risk of receiving either an autologous or allogeneic blood transfusion25.
Except for high-risk situations in which compatible blood is not available, autologous donation is not recommended since severe PPH is rarely anticipated. However, there are reports about autologous blood donation programmes that have reduced the need for allogeneic blood transfusions in high-risk pregnant women26.
- Recommendation 8. We recommend against the routine use of prepartum autologous blood donation to reduce the need for allogeneic blood transfusion in the event of PPH (1C).
Management of placental abnormalities
Prior knowledge of abnormal placentation and its multidisciplinary management seem to reduce the morbidity (blood loss, transfusions, and emergency caesarean sections) associated with such anomalies27,28. This type of approach does not influence gestational age at delivery. In settings with available resources and knowledge, the use of balloon occlusion of the iliac arteries or aorta can be considered as an additional modality to reduce severe bleeding after delivery in case of abnormal placentation29. Previous caesarean section or placenta praevia further increases the risk for adherent placenta and PPH, and the need for hysterectomy30.
- Recommendation 9. We recommend multidisciplinary planning and management for women with abnormal placentation, with or without previous caesarean section (1C).
Pre-delivery management of inherited bleeding disorders and antithrombotic therapy
Inherited bleeding disorders
With the exception of factor XI, all other coagulation factors, especially fibrinogen, increase during pregnancy in normal women, protecting them against excessive blood loss during placental separation31. Women with von Willebrand’s disease have a 1.5-fold risk of PPH, and a 5-fold risk of blood transfusion. Those who are carriers of haemophilia appear more likely to experience primary PPH, and the risk is higher at plasma clotting factor levels below 50 IU/dL, if not supplemented. The risk of secondary PPH in haemophilia carriers is also increased when clotting factor levels return to normal after delivery. An increased risk for PPH has been also reported in women with rare inherited disorders such as congenital hypofibrinogenaemia, deficiencies of factor VII, factor X, factor XI and factor XII, Glanzmann’s thrombasthenia or Bernard-Soulier syndrome32.
Management plans for labour, delivery and postpartum care should be individualised after multidisciplinary antenatal risk assessment by the obstetrician, the anaesthesiologist, and the haematologist during the third trimester of pregnancy32.
- Recommendation 10. We suggest treating patients with inherited blood disorders at risk for or experiencing PPH as standard PPH patients (2B). We also suggest a multidisciplinary anticipation of the risk of bleeding and its specific treatment complementary to standard PPH management for these patients (2B).
Antithrombotics
Due to the lack of data as to their teratogenicy in humans, many antithrombotics are not used in pregnancy. However, the risk of venous thromboembolism (VTE) is considerably increased in pregnancy, and with the current epidemic of obesity in the developed world, many women are considered to require thromboprophylaxis during pregnancy33–36.
Low-molecular-weight heparin (LMWH) is the usual thromboprophylaxis used in pregnancy, as recommended by the Royal College of Obstetricians and Gynaecologists (RCOG) and the American College of Chest Physicians (ACCP)33,34. There is no consensus as to the dose of LMWH due to the shortage of clinical trials to assess the appropriate dosage in the prevention and treatment of VTE in pregnancy, and recommended doses vary.
Unfractionated heparin is now used less frequently than previously due to the preference for LMWH which has more predictable pharmacokinetics. Bridging of vitamin K antagonist treatment prior to delivery in a woman with a mechanical heart valve can be an indication for unfractionated heparin.
Fondaparinux is occasionally used when an individual is allergic to LMWHs. This drug should only be used during pregnancy if its use is clearly indicated.
Vitamin K antagonists are rarely used in pregnancy due to their risks of teratogenicity in the first trimester and bleeding risks for the foetus in the second and third trimesters. The most likely indication for vitamin K antagonists in pregnancy is for women with mechanical heart valves, although an LMWH regimen may be used35. For women with prosthetic heart valves, it has been proposed to use an LMWH regimen during the first trimester and after week 36, with strict anti-Xa control. Aspirin 100 mg/day should be added in the presence of a mechanical valve36.
Aspirin is widely used in conditions such as antiphospholipid syndrome and systemic lupus erythematosus. Increased maternal bleeding can occur during delivery when aspirin is used one week prior to and/or during labour and delivery. Prolonged gestation and labour have been reported due to aspirin inhibition of prostaglandin.
Clopidogrel has only been used in a handful of cases. Animal data have failed to reveal evidence of foetotoxicity. There are no controlled data in human pregnancy. Clopidogrel should only be given during pregnancy when the need has been clearly established. There are no data on the excretion of clopidogrel in human milk. Animal data have shown that clopidogrel and/or its metabolites are excreted in rat milk. Because many drugs are excreted in human milk, and because of the potential for serious adverse reactions in nursing infants, a decision should be made as to whether to discontinue nursing or to discontinue the drug, taking into consideration of the level of patient need.
Management of the mother who bleeds on antithrombotics
In general, this does not differ from the management of the non-pregnant patient bleeding on antithrombotics (anticoagulants and/or antiplatelet drugs); i.e., stop the drug, initiate symptomatic treatment, control surgical bleeding, monitor the antithrombotic effect of the drug, and attempt reversal if possible. Reversal should be attempted while remembering the inherent increased risk of thromboembolic complications related to the underlying disease37.
- Recommendation 11. We suggest treating mothers bleeding on antithrombotics in the same way as for non-pregnant women (2B). We also suggest a multidisciplinary anticipation of the increased risk of thromboembolic complications and their specific treatment complementary to standard PPH management for these patients (2B).
Clinical and pharmacological measures for the prevention of postpartum during the third stage of labour
Active management of the third stage of labour to reduce the risk of severe postpartum haemorrhage
Active management of the third stage of labour involves the administration of a prophylactic uterotonic, early cord clamping, and controlled cord traction (CCT) to deliver the placenta6,21,22. Active vs expectant management of the third stage of labour reduces the risk of maternal haemorrhage (blood loss >1,000 mL) at time of birth (average risk ratio [RR] 0.34; 95% confidence interval [CI]: 0.14–0.87) and of a maternal Hb >9 g/dL following birth (average RR 0.50; 95% CI: 0.30–0.83)38. This is also true in the low-risk population with a reduction in blood loss >500 mL (average RR 0.33; 95% CI: 0.20–0.56)38.
- Recommendation 12. We recommend using active management of the third stage of labour to prevent PPH (1A).
Prevention of uterine atony
Prophylactic oxytocin for the third stage of labour reduces the risk of blood loss >500 mL compared with placebo (RR 0.53; 95% CI: 0.38–0.74)39. Prophylactic oxytocin is superior to ergot alkaloids in preventing blood loss >500 mL (RR 0.76; 95% CI: 0.61–0.94), but there is no benefit in combining prophylactic oxytocin and ergot alkaloids for the prevention of PPH39.
Oxytocin dosage and route of administration remain a subject of debate because there is desensitisation due to internalisation of receptors. In addition, whether the clinician is in the presence of a vaginal delivery after labour or a caesaeran section without labour, as well as the presence of cardiovascular disease, should be taken into consideration.
Vaginal delivery
Preventive administration of uterotonics is effective in reducing the incidence of PPH, and oxytocin is the preferred treatment. Intravenous administration of oxytocin during the third stage of labour was found to be associated with a reduced incidence of severe PPH, blood transfusion and admission to a high dependency unit compared with intramuscular administration, with no increase in the number of side effects40. Oxytocin can be administered after delivery of the shoulders or rapidly after birth, or after placental delivery if not administered previously. Administration of oxytocin before or after expulsion of the placenta did not have any significant influence on many clinically important outcomes such as the incidence of PPH, rate of placental retention, and duration of the third stage of labour41.
A dose of 5 or 10 IU of oxytocin can be administered22,39,41. For intravenous administration, a slow injection (lasting approximately 30–60 seconds) is preferable, although no data contraindicate intravenous bolus injections (rapid intravenous injection of 1–2 seconds) in women without cardiovascular risk factors. It must be remembered that exposure to oxytocin during labour is an independent risk factor for severe PPH, secondary to uterine atony, and requires blood transfusion42.
In women at cardiovascular risk, a very slow intravenous administration (over >5 min) is recommended in order to limit haemodynamic effects21. Alternatively, successive doses of 0.05–0.5 IU, preceded by phenylephrine administration, can be used. Routine maintenance infusion of oxytocin is not recommended for vaginal delivery.
In a recent randomised controlled trial (RCT) that enrolled 29,645 women after vaginal birth, heat-stable carbetocin, an oxytocin analogue, was not inferior to oxytocin in preventing PPH (blood loss ≥500 mL) or the use of additional uterotonic agents43. Non-inferiority was not shown for the prevention of severe PPH (blood loss ≥1,000 mL), but the low event rate for this outcome reduced the power of the trial. These data inform the care of women in parts of the world where a lack of heat stability is a barrier to the use of oxytocin43.
Obstetric teams may choose whether or not to implement routine use graduated blood collection bags. Only one randomised trial has examined this and showed no difference in the risk of severe PPH44. However, accurate assessment of the amount of blood loss is important21.
Routine cord drainage, CCT, uterine massage, and routine voiding after delivery do not impact the incidence or severity of PPH. Moreover, there is no scientific evidence to justify a recommendation that any of the following will prevent PPH: early or late cord clamping, any particular maternal position during labour, or very early breastfeeding21. Similarly, tranexamic acid should not be used routinely for the prevention of PPH21. In a recent placebo-controlled trial, administration of tranexamic acid in addition to oxytocin did not decrease the incidence of PPH in women with vaginal delivery45.
In case of placental retention, oxytocin administration is not effective, whether administered by an intrafunicular, intravenous (IV) or intramuscular (IM) route. Should placental delivery not occur, manual removal is recommended between 30 and 60 min after delivery in the absence of bleeding, and earlier in case of ongoing bleeding42. Routine manual uterine examination is not recommended after vaginal delivery for women with a previous caesarean section21.
- Recommendation 13. We recommend preventive administration of uterotonics after vaginal delivery. Adjusted oxytocin doses (5–10 IU) administered intravenously are the preferred prophylactic treatment (1A).
Caesarean delivery
There is no evidence to justify preferring one caesarean technique over another with respect to prevention of PPH. Placental delivery by CCT is associated with less blood loss than manual removal46. A slow (at least 1 min) intravenous injection of 5–10 IU of oxytocin is recommended except for women with overt cardiovascular risks in whom the injection must last at least 5 mins to limit its haemodynamic effects. Initial oxytocin dosage should be followed by a maintenance infusion as long as it does not exceed 10 IU/hour21. The treatment can be stopped at the end of two hours if uterine tone is satisfactory and there is no abnormal bleeding21.
The effective dose of oxytocin infusion to prevent uterine atony for an elective cesarean delivery may be lower than the clinical infusions currently in use. The “rule of 3” algorithm consists of IV oxytocin (3 IU/3 mL), followed by uterine tone assessment at 3, 6, 9, and 12 min, and if inadequate, additional uterotonic agents are administered. A recent RCT showed that the “rule of 3” algorithm resulted in lower oxytocin doses compared with continuous-infusion oxytocin in women undergoing elective caesarean delivery47. No differences in uterine tone, maternal haemodynamics, side effects, or blood loss were observed.
Carbetocin reduces the risk of PPH, but in the absence of a non-inferiority trial, oxytocin remains the gold standard for preventing PPH after caesarean delivery48,49. Whereas there is no evidence of the efficacy of tranexamic acid for the prevention of PPH in women with vaginal delivery, a meta-analysis of four studies found a reduced risk (RR 0.43, 95% CI: 0.23–0.78) of severe PPH (blood loss ≥1,000 mL) in women undergoing caesarean section50. Tranexamic acid should not be used routinely for PPH prevention in the setting of caesarean delivery but should be considered in case of antepartum bleeding and in women at increased risk of PPH22. Estimation of blood loss during caesarean section is essential and should be mentioned in the surgical report.
- Recommendation 14. We recommend preventive administration of uterotonics after caesarean section. Adjusted oxytocin doses (5–10 IU) administered intravenously are the preferred prophylactic treatment (1A). Intravenous administration of tranexamic acid (0.5–1.0 g) in addition to oxytocin should be considered in women at increased risk of PPH (1C).
Summary of the WHO 2012 guidelines for the prevention of PPH
The guidelines developed by the World Health Organization (WHO)6 include the following recommendations for the prevention of PPH:
- the use of uterotonics for the prevention of PPH during the third stage of labour is recommended for all births.
- Oxytocin (10 IU, IV/IM) is the recommended uterotonic drug for the prevention of PPH.
- In settings where oxytocin is unavailable, the use of other injectable uterotonics (if appropriate, ergometrine/methylergometrine or the fixed-dose combination of oxytocin and ergometrine) or oral misoprostol (600 μg) is recommended.
- In settings where skilled birth attendants are not present and oxytocin is unavailable, the administration of misoprostol (600 μg orale administration [PO]) by community health care workers and lay health workers is recommended for the prevention of PPH.
- In settings where skilled birth attendants are available, CCT is recommended for vaginal births if the care provider and the parturient regard a small reduction in blood loss and a small reduction in the duration of the third stage of labour as important.
- In settings where skilled birth attendants are unavailable, CCT is not recommended.
- Late cord clamping (performed after 1 to 3 min after birth) is recommended for all births while initiating simultaneous essential newborn care.
- Early cord clamping (<1 min after birth) is not recommended unless the neonate is asphyxiated and needs to be moved immediately for resuscitation.
- Sustained uterine massage is not recommended as an intervention to prevent PPH in women who have received prophylactic oxytocin.
- Postpartum abdominal uterine tonus assessment for early identification of uterine atony is recommended for all women.
- CCT is the recommended method for removal of the placenta after caesarean section.
Initial management of postpartum haemorrhage
Early recognition, activation of protocols and resource mobilisation are critical in the management of severe bleeding. Therapeutic measures should be targeted at stabilising the patient and controlling the cause of PPH. The “four Ts” (i.e. the causes of primary PPH) should be considered as principles of care: Tone (uterine atony), Trauma (genital trauma including damage to the vulva, vagina, cervix and uterus), Tissue (retained and invasive placenta), and Thrombin (coagulopathy)22.
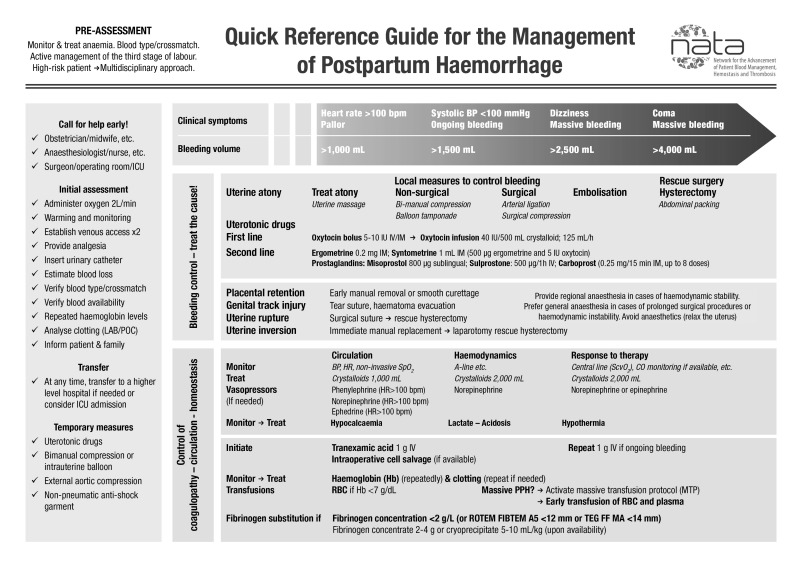
Obstetric units in hospitals should have a PPH management algorithm, and healthcare professionals trained on its activation and use (see Figures 2 and 3). To this end, regular emergency drills for the management of PPH should be implemented. Once introduced, such emergency drills are invaluable for keeping all staff up-dated and alert to the emergency treatment of PPH20.
Figure 2.
Quick reference guide for the management of postpartum haemorrhage.
ICU: intensive care unit; LAB: central laboratory; POC: point-of-care; BP: blood pressure; HR: heart rate; RBC: red blood cell; PPH: postpartum haemorrhage; TEG: thromboelastography; ROTEM: rotational thromboelastometry.
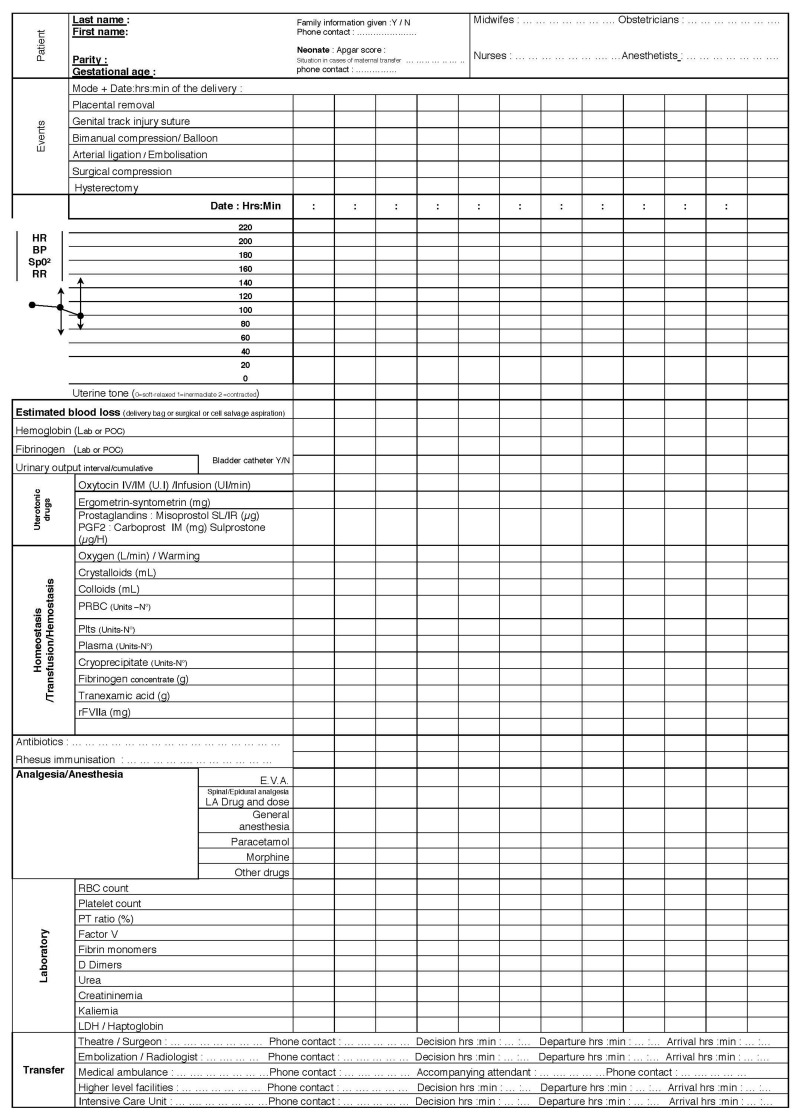
Figure 3.
An example of the postpartum haemorrhage chart.
HR: heart rate; BP: blood pressure; RR: respiratory rate; Lab: central laboratory; POC: point-of-care; PGF2: prostaglandin F2; PRBC: packed red blood cells; rFVIIa: recombinant activated factor VII; E.V.A.: expiratory ventilation assistance;
LA: local anaesthetic; LDH: lactate dehydrogenase.
- Recommendation 15. We recommend that all healthcare professionals be trained to prevent PPH, to recognise the early signs of PPH, and to use pharmacological, mechanical and surgical methods to arrest PPH, according to the causative factor (1C).
- Recommendation 16. We recommend that obstetric units in hospitals have an easy-to-use, clear-cut PPH management algorithm and introduce regular emergency drills for care of PPH (1C).
Obstetric aspects
Diagnosis, blood loss evaluation, assessment of severity
Visual estimation tends to under-estimate blood loss as blood mixes with other fluids and a significant volume of blood can be retained within the uterus. Thus, additional methods are needed. All sponges and sanitary pads should be saved and weighed to measure blood loss. This should continue whilst the patient is being monitored for ongoing losses. The use of graduated blood collection bags may contribute to the accurate assessment of blood loss (although it does not predict the severity of PPH)21. Cumulative losses should be clearly documented in the notes whenever possible.
Clinical signs and symptoms of hypovolaemia should be included in the assessment of PPH severity. However, clinical signs of hypovolaemia (tachycardia and hypotension) are misleading in pregnancy due to plasmatic volume expansion, and might not become manifest until blood losses reach 1,000–1,500 mL51,52. A systolic blood pressure below 80 mmHg associated with worsening tachycardia, tachypnoea and altered mental state usually indicates a PPH in excess of 1,500 mL51. The shock index (heart rate/systolic pressure) identifies women at risk of adverse outcomes secondary to PPH53. The relative haemodilution and increased cardiac output inherent to normal pregnancy allow important blood loss to occur before a drop in Hb concentration is identified51.
- Recommendation 17. We recommend using clinical signs and symptoms together with visual estimation of peripartum blood loss in the assessment of PPH (1C).
Mechanical measures
As uterine atony is the most common cause of primary PPH, simple mechanical and physiological measures such as palpating and rubbing the uterine fundus (uterine massage) and emptying the bladder to stimulate uterine contractions represent first-line management of PPH. This practice is based on professional consensus rather than published evidence, and is usually followed by administration of uterotonics22.
- Recommendation 18. We recommend uterine massaging and bladder emptying to stimulate uterine contractions as first-line management of PPH (1C).
Interventional placental delivery
When the placenta is not spontaneously delivered within 30 min after parturition, the parturient should be diagnosed as having a retained placenta. After observation for a further 30 min, it should be manually removed in the delivery room if epidural analgesia is sufficient, or the patient should be taken to the operating room for manual removal of the placenta if additional analgesia is required. The use of additional oxytocin (10 IU, IV or IM) with CCT is the method of choice and should be accompanied by a single dose of antibiotics6. The use of ergometrine or prostaglandin E2 is not recommended6. Of note, the use of CCT is paramount in treating a retained placenta; therefore, it is essential to teach CCT in medical and midwifery training programmes6. CCT is not recommended in the absence of uterotonic drugs or prior to signs of separation of the placenta because this can cause partial placental separation, a ruptured cord, excessive bleeding, and/or uterine inversion6.
- Recommendation 19. For retained placenta, we suggest manual removal using additional oxytocin in combination with controlled cord traction, followed by a single dose of antibiotics (2C).
Genital tract examination
Damage to the genital tract may occur spontaneously or through manipulation during delivery. Uterine trauma may occur following very prolonged or vigorous labour, extra-uterine or intra-uterine manipulation of the foetus, or attempts to remove a retained placenta manually or with instrumentation. Uterine inversion can also occur. In this scenario, the clinician should attempt to reposition the uterus. Uterotonics should not be administered. Nor should any attempt be made to remove the placenta until the uterus is repositioned. If these attempts fail, arrangements should be made to transfer the patient to the operating room6. Cervical laceration is most commonly associated with forceps delivery, although it may occur spontaneously. Vaginal sidewall laceration is also most commonly associated with operative vaginal delivery, but it may occur spontaneously, especially if a foetal hand presents with the head (compound presentation). Lower vaginal trauma occurs either spontaneously or because of episiotomy. Spontaneous lacerations usually involve the posterior fourchette; however, trauma to the periurethral and clitoral region may occur and can be problematic.
All these factors may cause significant blood losses and should be appropriately identified by manual uterine examination and careful visual assessment of the genital tract21. Local guidelines from the University College London Hospitals National Health Service Foundation Trust provide the following recommendations54:
- check for obvious genital trauma. Ensure good lighting and visualise the cervix;
- visualise the apex; apply pressure to the bleeding point and suture in the room if it is easily identified; transfer to the operating room if not;
- examine the anus, vulva, vagina and cervix under direct vision;
- manually explore the uterine cavity to exclude trauma or retained placental tissue (if the uterus is explored, give intravenous antibiotics);
- consider laparotomy. If the abdomen is not open and the maternal condition suggests blood loss greater than visible, consider intra-abdominal bleeding. Ultrasound scan may be helpful to look for intra-abdominal blood (e.g. between liver and right kidney). However, do not allow scanning to delay laparotomy.
- Recommendation 20. We recommend careful examination of the genital tract to ascertain whether PPH after vaginal delivery is of traumatic origin (1C).
Anaesthetics
The anaesthesia staff should be systematically alerted and involved at the initial stage of PPH management as soon as the bleeding appears to be higher than expected, even in the absence of cardiovascular instability. Whether the alert should be triggered when blood loss is moderate (500 mL) or severe (>1,000 mL) depends on the woman’s clinical condition and local resources. Close collaboration between the obstetrician and anaesthetist, together with the obstetric team, is a major component of PPH management, as stated by ESA guidelines for the management of severe perioperative bleeding3. Indeed, the first steps in PPH management by the anaesthetist and the obstetrician take place simultaneously. Initially, the goals of anaesthetic management of PPH are for rapid assessment of the parturient, to initiate monitoring, to restore intravascular volume to achieve normovolaemia and haemodynamic stability, and to provide adequate anaesthesia and analgesia.
- Recommendation 21. We recommend that the anaesthesia staff be systematically alerted and involved at the initial stage of severe PPH or as soon as the bleeding appears to be higher than expected, even in the absence of cardiovascular instability (1C).
Monitoring
In the context of PPH, non-invasive cardiovascular monitoring should be rapidly put in place to check for signs of hypovolaemia. Furthermore, it is important to monitor for potential maternal cardiovascular side effects of uterotonic treatment. Initial monitoring includes continuous heart rate (HR), blood pressure (BP) recording every 15 min, respiratory rate (RR) and peripheral oxygen saturation. If severe PHH develops, monitoring should be intensified with BP every 3–5 min and electrocardiography (ECG). Invasive monitoring with an arterial line (continuous invasive BP), central line (central venous pressure and saturation), and non-invasive or minimally invasive cardiac output monitoring may be considered according to severity of PPH and availability.
Persistent or recurrent maternal hypotension despite active fluid resuscitation and/or use of vasopressive drugs (ephedrine or phenylephrine) indicates severe PPH. Inconsistency between minor or moderate bleeding and its haemodynamic impact suggests hypovolaemia due to non-externalised bleeding.
- Recommendation 22. We recommend that non-invasive cardiovascular monitoring be placed rapidly to check for the occurrence of signs of hypovolaemia (1C).
The initial Hb level does not accurately reflect blood loss because compensatory mechanisms that move fluids from the interstitial space require time and are not apparent in the initial Hb measurement. However, the initial Hb measurement is useful to determine a baseline Hb level as anaemia is very frequent in parturients. Use of point-of-care testing to perform serial measurements provides information on the degree of developing anaemia and the severity of bleeding.
- Recommendation 23. We recommend evaluation of anaemia by measuring Hb levels early and repeatedly in patients with ongoing PPH, preferably by use of point-of-care testing (1C).
Haemodynamic management
The goal of haemodynamic management is to sustain normovolaemia, thus securing normal perfusion during the active phase of PPH. Using blood pressure as a single marker for signs of hypovolaemia is not sufficient and other measures such as HR, RR, peripheral oxygen saturation, skin colour, and temperature should be considered. In the case of severe ongoing PPH, the perfusion status can be evaluated with arterial or venous blood gases, looking for lactic acidosis.
The 2016 update of the ESA guidelines for the management of severe perioperative bleeding recommends against the use of central venous pressure (CVP) and pulmonary artery occlusion pressure as the only variables to guide fluid therapy and the optimisation of pre-load during severe bleeding4. Instead, dynamic assessment of fluid responsiveness and non-invasive measurement of cardiac output should be considered (1B)4.
Normalisation of blood pressure by fluid resuscitation may actually increase hydrostatic pressure on the wound and cause blood clots to dislodge (a mechanism referred to in the trauma literature as “popping the clot”). Moreover, fluid resuscitation can cause dilution of coagulation factors, hypothermia and further aggravation of coagulopathy55. Over the last ten years, in bleeding trauma patients, the concept of damage control resuscitation has been developed and has improved survival by focusing on permissive hypotensive resuscitation, limiting the use of fluids and advocating the early use of blood products to prevent the lethal triad of acidosis, coagulopathy and hypothermia56.
Regarding the management of PPH, permissive hypotensive resuscitation has not been evaluated in RCTs, but use of permissive hypotension during the bleeding phase (mean arterial pressure [MAP] between 55–65 mmHg) might be a goal associated with better maternal outcomes.
- Recommendation 24. We suggest considering permissive hypotensive resuscitation during the bleeding phase of severe PPH, aiming for a MAP of 55–65 mmHg, remembering to normalise MAP upon control of PPH or when bleeding becomes acceptable (2C).
Fluid resuscitation
Fluid resuscitation with either crystalloids or colloids has never been compared in the context of PPH. Compared with crystalloids, haemodynamic stabilisation with iso-oncotic colloids such as human albumin and hydroxyethyl starch (HES) causes less tissue oedema. However, one RCT including women undergoing elective caesarean section did not find any difference in haemodynamic stability between crystalloids and colloids57.
In critically ill patients, systematic reviews did not identify any benefit in using colloids rather than crystalloids; in addition, they raised the point that some colloids appear to increase mortality58,59. Infusion of colloids in patients with severe bleeding can aggravate dilutional coagulopathy by additional effects on fibrin polymerisation and platelet aggregation. The European guideline on management of major bleeding and coagulopathy following trauma recommends that crystalloids be used initially to treat the hypotensive bleeding trauma patient60. One RCT in severe PPH found a very low incidence of fibrinogen depletion and coagulopathy when patients with an estimated blood loss in the range of 1,400–2,000 mL were resuscitated with crystalloids (substitution of 1 mL crystalloid for 1 mL blood loss)61.
By analogy with surgical, trauma and critically ill patients, initial fluid replacement with a balanced crystalloid solution is recommended. The use of colloids is not recommended as part of the initial management of PPH since there are no data to support this approach. Hypervolaemia secondary to crystalloids or colloids (to a level exceeding the interstitial space in steady state and beyond an optimal cardiac pre-load) should be avoided4.
- Recommendation 25. We recommend restrictive crystalloid resuscitation (1–2 mL of crystalloid for every 1 mL of blood loss) as initial fluid resuscitation according to the clinical condition and the estimated blood loss (1C).
Associated measures
- One (or more) large venous access of good quality should be available. If the bleeding is not rapidly controlled, we recommend that two large peripheral cannulae (14 or 16 G) be placed.
- Blood type should be identified and antibody screening should be secured, and the blood bank should be contacted if bleeding is not rapidly controlled.
- PPH management protocols must include the immediate issue of group O, RhD-negative and K-negative units, followed by group-specific blood22.
- Oxygen therapy via a standard nasal cannula or facemask is recommended.
Prevention of hypothermia
The impact of hypothermia on blood loss in the context of PPH has never been explored. In trauma patients without traumatic brain injury, hypothermia is associated with increased morbidity and mortality62. A meta-analysis of RCTs in surgical patients reports that even mild hypothermia significantly increases blood loss (by approximately 16%) and the relative risk of transfusion (by approximately 22%)63. One RCT showed that in women undergoing caesarean delivery the perioperative Hb difference was lower in women who received pre-warmed intravenous fluids than in those who did not64. Active and early warming of the patient is recommended, including cutaneous warming and active warming of fluid infusions, especially in the context of rapid fluid resuscitation and blood transfusion.
- Recommendation 26. We recommend active temperature management with the goal of maintaining normothermia (1B).
Correction of acidosis
Acidosis, together with hypothermia, alters the process of haemostasis and contributes to the morbidity and mortality of bleeding patients. No relevant clinical studies are available in obstetric patients. Acidosis impairs the coagulation process in a progressive manner, and its timely correction is important to limit its contribution to coagulation dysfunctions4,65. During acidosis, the coagulation process starts normally, but there is a delay in clot formation and a reduction in clot strength. In animal models, acidosis (pH 7.1) was shown to cause a significant depletion in fibrinogen concentration, platelet count, and thrombin generation; coagulation remained impaired after pH correction with bicarbonate infusion66–68. The latter effect could be attributed to the reduction of fibrinogen levels and platelet count. In fact, an increase in fibrinogen degradation rate without effects on its synthesis has been observed in acidosis; moreover, fibrinogen levels remain depleted after correction of acidosis68–70. As for platelets, their accelerated removal from the circulation, due to changes in structure and shape when the pH drops below 7.4, or an altered aggregation have been hypothesised as the cause of a decrease in platelet count68. Accordingly, coagulation function may not be restored only by the correction of acidosis, since other haemostatic factors (such as fibrinogen) are important. It is important to bear in mind that most of the literature is based on in vitro or animal models which do not necessarily reflect the complex pathophysiological mechanisms behind the impairment of coagulation in patients with severe bleeding.
The effect of acidosis on the efficacy of tranexamic acid and recombinant activated factor VII (rFVIIa) has been investigated. In vitro and animal studies confirmed the efficacy of tranexamic acid during severe metabolic acidosis71,72. Administration of rFVIIa in acidotic conditions has been shown to improve thrombin generation73,74. However, other studies indicate that acidosis reduces the activity and effectiveness of rFVIIa75,76. In adult surgical patients, rFVIIa may be less effective in the presence of acidotic coagulopathy77. These observations suggest that rFVIIa should only be considered alongside correction of acidosis.
- Recommendation 27. We recommend correcting acidosis, although this correction alone cannot immediately ameliorate acidosis-induced coagulopathy (1C).
Adequate anaesthesia and analgesia
It is important to provide adequate anaesthesia and analgesia to carry out uterine revision and genital tract examination in the context of PPH.
Local and regional anaesthesia
Due to the higher incidence of difficult airway management78 and of pulmonary aspiration in the obstetric population, local and regional techniques are recommended if cardiovascular stability is preserved and if there is no evidence of coagulopathy. If an epidural catheter has been placed during labour and the level of analgesia is not sufficient, epidural titration with a local anaesthetic is recommended. In the absence of an epidural catheter, spinal anaesthesia with a low dose of local anaesthetic and an opioid can be performed.
General anaesthesia
In the context of haemodynamic instability, active bleeding or suspicion of coagulation disorders, a general anaesthetic with oro-tracheal intubation is recommended. Rapid sequence induction to reduce the risk of pulmonary aspiration during induction of general anaesthesia is recommended in this context.
Uterotonic drugs
Uterotonic drugs can be divided into a first-line treatment with oxytocin and a second-line treatment with misoprostol, sulprostone, carbetocin, etc. The points for discussion in uterotonic treatment are the dosage and the administration route of oxytocin. In a German survey including over 300,000 deliveries, a bolus injection of oxytocin was the most frequent therapy (85.3%)79. In nearly 32% of the cases, the dose of oxytocin ranged between 5 and 10 units, illustrating a general heterogeneity in the use of uterotonics. We also found regional differences in the therapeutic use of uterotonics among Western countries. In general, ergotamin or misoprostol is not used in many countries because of the severe side effects (ergotamine) or the limited method of administration (misoprostol). Misoprotol is not recommended as a second-line treatment21. There is a general use of IV prostaglandins (sulprostone, carboprost) as a second-line option to oxytocin or longer-acting carbetocin30,80. Sulprostone must be administered within 30 min of the diagnosis of PPH, should oxytocin be ineffective; this time limit can be shortened according to the severity of the bleeding21.
Potential maternal side effects of uterotonic drugs are a matter of concern. Oxytocin can cause hypotension, arrhythmias, and an increased risk of myocardial infarction that seems to have an effect on maternal outcome after childbirth80. Second-line uterotonic treatment with prostaglandins, such as the E2-acting sulprostone, leads to uterine contraction via G protein-mediated calcium channel activation. A review of the literature supports the use of oral or sublingual misoprostol in reducing the risk of severe PPH and blood transfusion81. Compared with conventional injectable uterotonics, oral misoprostol was associated with a higher risk of severe PPH and use of additional uterotonics, but with a trend towards fewer blood transfusions81. Misoprostol appears to be effective and safe in geographic areas with little access to facilities and skilled healthcare providers81. However, in a retrospective study, Schmitz et al. observed that prostaglandins were underused in PPH treatment, especially when a standardised protocol for PPH management was not in place82. These findings underline the need to focus on issues of implementation and to provide adequate training to use this effective treatment option.
Continuous uterotonic therapy with oxytocin infusion can be considered according to the condition of the uterus and prior exposure to oxytocin; it requires a higher infusion rate in the treatment of uterine atony leading to PPH (40 IU in 500 mL crystalloid; 125 mL/hour)83. In settings in which the use of oxytocin is not feasible, misoprostol or ergometrine can be used as alternatives treatment for PPH6,84–86.
- Recommendation 28. We recommend first-line uterotonic treatment for PPH with the use of oxytocin 5–10 IU IV or IM given as a slow infusion/injection (1A).
Vasopressors
Vasopressors should be used while paying very close attention to the aggressive treatment of hypovolaemia, which is to be corrected first or in parallel. Phenylephrine and ephedrine are widely used in the obstetric setting. Data mostly come from studies that investigated the reactive hypotension due to spinal anaesthesia and its treatment with a vaso- or cardioactive agent. Restrictive use of ephedrine has been advocated due to a possible association with foetal acidosis; however, the clinical importance of this observation is unclear87.
A phenylephrine infusion (25–50 μg/min) appears to be more effective than phenylephrine boluses to prevent hypotension and can be conveniently titrated to maintain the targeted BP88,89. Norepinephrine (an initial 10 μg bolus, followed by the infusion of 200 μg/hour, if needed) has now been validated as the inotropic support after spinal anaesthesia for caesarean section and is the recommended inotropic support for haemorrhagic shock90.
- Recommendation 29. We recommend using phenylephrine (100 μg bolus or 25–50 μg/min infusion) or norepinephrine (10 μg bolus, followed by a 200 μg/hour infusion if needed) when tachycardia is predominant (HR >100 bpm), or ephedrine if normocardia is predominant (HR <100 bpm), as first-line treatment for unacceptable maternal hypotension in PPH (1C).
Calcium homeostasis
Calcium has an important role in the pathophysiology of haemorrhage and coagulopathy. Calcium concentration drops further in the case of massive transfusion due to citrate-induced calcium binding and should be monitored, and levels corrected accordingly, in severe PPH91.
- Recommendation 30. We recommend correcting calcium levels, especially in cases of massive transfusion, in order to optimise coagulation as well as uterine muscle contraction (1C).
Antifibrinolytic drugs
Tranexamic acid is an antifibrinolytic agent that has been shown to reduce blood loss and transfusion in several types of surgery, and also reduce mortality in trauma patients92,93. The primary mechanism is related to its antifibrinolytic effect, as an inhibitor of fibrinolysis to stabilise the clot, hence reducing bleeding, transfusions and, potentially, morbidity and mortality. In the setting of PPH, there are currently 12 RCTs ongoing including more than 3,000 patients showing reductions in blood loss, risk of PPH, and blood transfusions following vaginal and caesarean delivery50,94,95. However, there is insufficient evidence regarding the safety aspects (serious side effects, thromboembolic events) of tranexamic acid and its effect on mortality.
The WOrld MAternal ANtifibrinolytic (WOMAN) trial aimed to determine the effect of early administration of tranexamic acid on mortality, hysterectomy and other morbidities in women with PPH after vaginal delivery. The investigators enrolled 20,060 women who were randomly assigned to receive 1 g intravenous tranexamic acid (with a second dose given after 30 min, if needed) or placebo. Although the diagnosis was clinical, investigators specified that diagnosis of primary PPH could be based on clinically estimated blood loss of more than 500 mL after vaginal birth or 1,000 mL after caesarean section or any blood loss sufficient to compromise haemodynamic stability. Death due to bleeding was significantly reduced in women given tranexamic acid compared with placebo (1.5 vs 1.9%; p=0.045), especially in women treated within three hours of giving birth. There were no between-group differences in all other causes of death, hysterectomy rates or the incidence of adverse events (including thromboembolic events)95. Analysis of the effects of timing of administration on bleeding survival indicates that delay in tranexamic acid administration appears to reduce the benefit (by approximately 10% per every 15 min of delay) with no benefit seen beyond three hours96. The 2017 WHO recommendation on tranexamic acid for the treatment of PPH states that tranexamic acid should be recognised as a life-saving intervention and be made readily available for the management of PPH in settings where emergency obstetric care is provided, regardless of the level of healthcare system resources97.
- Recommendation 31. We recommend the administration of tranexamic acid (1 g by intravenous route) as soon as possible within the first 3 hours after PPH onset. This dose can be repeated after 30 min if bleeding continues (1B).
Antibiotics for the prevention of secondary infection
No data from randomised trials are available on the efficacy of antibiotic prophylaxis in the case of manual uterine exploration. One retrospective cohort study of more than 2,100 vaginal deliveries reported that manual removal of the placenta is a risk factor for endometritis98. Two case-control studies did not find an association between manual removal of the placenta and endometritis; however, less than 150 women were included in each of these two studies99,100.
- Recommendation 32. We recommend using antibiotic prophylaxis according to the local protocol during the initial management of PPH for endo-uterine procedures (1C).
Intraoperative cell salvage
Intraoperative cell salvage (ICS) has been used in caesarean and vaginal delivery. Some observational studies have reported its efficacy and safety101–104. Elagamy et al. used ICS in 20 patients at high risk of PPH during caesarean delivery because of abnormal placentation101. Of the 15 women who received autologous blood, 13 (86.7%) did not require allogeneic red blood cell (RBC) transfusion, and there were no in vivo or ex vivo safety concerns. Milne et al. followed 884 PPH patients during caesarean and vaginal delivery with the use of ICS102. Sufficient blood was collected to permit reinfusion in 189 of 884 (21%) patients without any safety concerns. Teare et al. performed an ex vivo analysis of 50 vaginal blood collections, showing a median residual contamination similar to that found in caesarean section, and concluded that this was not significant103.
Intraoperative cell salvage should be available in high-level maternal care centres and used in cases of massive PPH. Standard ICS lavage and reinfusion procedures should be used as part of the resuscitation strategy while remembering that the ICS output for reinfusion consists predominantly of erythrocytes. Reinfusion can be considered if the Hb drops below 8–10 g/dL or if blood loss increases from >800 to 1,000 mL. The use of a leucocyte depletion filter has been recommended by several groups65,101,102,104.
- Recommendation 33. We recommend using intraoperative cell salvage (with leucocyte-depletion filters in the infusion line) for patients at high risk of severe PPH or ongoing PPH, or when packed RBCs are unavailable (due to immunological reasons) or are declined by the patient (1C).
Management of severe or ongoing postpartum haemorrhage
Continuation of uterotonics
In persisting life-threatening bleeding related to uterine atonia, a switch to prostaglandins is recommended (e.g. sulprostone, carboprost) but maternal side effects must be monitored. A concomitant use of oxytocin and prostaglandins is not recommended due to potentiation of their cardiovascular side effects. Methergin is not a first-line agent in PPH but can be used as a second-line therapy. The use of methergin is associated with a high risk of cardiovascular side effects105.
- Recommendation 34. We recommend second-line uterotonic treatment for PPH with the use of ergometrin (0.2 mg, IM), misoprostol (800 μg, sublingual), sulprostone (500 μg/1 hour, IV) or carboprost (0.25 mg/15 min IM, up to 8 doses) if bleeding is not controlled after the administration of oxytocin (1B).
Coagulation monitoring
Methods to assess haemostatic impairment during PPH include clinical observation, laboratory-based tests (prothrombin time [PT], activated partial thromboplastin time [aPTT], Clauss fibrinogen and complete blood count, including platelets) and point-of-care viscoelastic tests22,30,106.
The usefulness of laboratory-based tests (the “gold standard”) has not been fully been validated in the context of PPH, and turnaround time may be significant. Thus, laboratory-based tests must be performed serially looking for a trend, and haemostatic blood components substituted accordingly37. In the context of rapid bleeding, their clinical usefulness of laboratory-based tests is rather limited. Clauss fibrinogen should always be measured as it falls early and reaches levels below 2 g/L before any change in PT or aPTT22,30,106. Data from a retrospective cohort study among 1,312 women experiencing severe PPH requiring blood transfusion suggest that detection of prolonged aPTT and, especially, low levels of fibrinogen (<2 g/L) during early PPH can help identify women who may benefit from targeted haemostatic treatment107.
Assays of clot viscoelasticity (thromboelastography [TEG] and rotational thromboelastometry [ROTEM]) monitor developing clot strength and subsequent fibrinolysis, although they are not a sensitive measure of the latter108. Specific parameters have been used to identify impaired platelet function, low fibrinogen levels and low coagulation factors, and have been used to guide blood component therapy37. A Cochrane review concluded that transfusion guided by TEG or ROTEM appears to reduce bleeding, but there is no evidence that it improves morbidity or mortality109. The NICE diagnostic guidance on the use of viscoelastic devices recommends that such devices be used to manage and monitor haemostasis during and after cardiac surgery but notes that outside cardiac surgery there is little supporting evidence and the evidence available is variable110. For the moment, no specific TEG or ROTEM algorithm can be recommended to guide transfusion practice in the setting of PPH37.
- Recommendation 35. We recommend assessing haemostatic competence and risk of coagulopathy in severe ongoing PPH through laboratory tests (platelet count, PT, international normalised ratio [INR], aPTT, fibrinogen level) or viscoelastic haemostatic tests to guide appropriate, goal-directed use of haemostatic blood components and pro-haemostatic agents (1B).
Administration of coagulation factor concentrates
Fibrinogen replacement therapy
In massive haemorrhage, fibrinogen is the first factor to fall to critical levels in patients resuscitated with RBC concentrates, colloids and crystalloids111. Outside PPH, fibrinogen substitution has been shown to reduce bleeding and transfusion requirements112. In PPH, the severity of bleeding is associated with fibrinogen levels. Furthermore, a level of fibrinogen below 2 g/L increases the risk for severe PPH (with a 100% positive predictive value)113. Fibrinogen can be replaced by either cryoprecipitate or fibrinogen concentrate, depending on local availability114. Wikkelsø et al. performed a prospective, multicentre RCT in severe PPH comparing pre-emptive use of 2 g fibrinogen concentrate (26 mg/kg) with placebo. They showed a significant increase in fibrinogen levels but no reduction in RBC transfusion rates61. Patients enrolled in the trial had severe but not massive PPH (only 10% had a systolic BP <100 mmHg); in addition, 75% had normofibrinogenaemia and only 2% had fibrinogen levels below 2 g/L after 1.5 L bleeding resuscitated with crystalloids (1 mL crystalloid for 1 mL bleeding)61.
In a retrospective national survey, Makino et al. reported on 99 cases of fibrinogen concentrate therapy; a median dose of 3 g was not sufficient to restore fibrinogen plasma levels (which increased from 0.7 to 1.9 g/L) at a late stage of massive PPH (mean 3.6 L)115. Mallaiah et al. prospectively observed the effect of switching from an RBC:plasma 1:1 ratio strategy to an RBC:fibrinogen concentrate replacement strategy guided by ROTEM diagnosis of hypofibrinogenaemia (trigger FIBTEM A5 <12 mm corresponding approximately to a fibrinogen concentration <2 g/L) in severe PPH116. They observed a reduction in the number of patients requiring transfusion of >6 RBC units (p=0.015), in total blood product use (p<0.0001), and in the incidence of transfusion-associated circulatory overload (TACO), but not in hysterectomy rates or total RBC use116.
Several ongoing RCTs are currently evaluating the effect of fibrinogen concentrate in severe PPH (trial identifiers: NCT02155725, NCT01910675 and ISRCTN46295339). Ducloy-Bouthors et al. are investigating the effect of 3 g fibrinogen in patients requiring second-line uterotonics, and Aawar et al. are investigating the effect of goal-directed therapy using ROTEM, increasing fibrinogen levels to >4 g/L (FIBTEM A5 >23 mm) from a trigger fibrinogen concentration <3.0 g/L (FIBTEM A5 <16 mm)117,118.
- Recommendation 36. We recommend against pre-emptive fibrinogen supplementation (1B). Instead, fibrinogen levels should be monitored early in severe ongoing PPH in order to consider cryoprecipitate or fibrinogen concentrate substitution at a plasma level <2 g/L or FIBTEM A5 <12 mm (1C).
Prothrombin complex concentrate
Prothrombin complex concentrates (PCCs) contain a concentrate of coagulation factors II, VII, IX, X and proteins S and C (and some heparin), and are recommended for urgent reversal of the effect of vitamin K antagonists119–121. Successful use of PCC was described in a case report of massive PPH122. In an ongoing trial, PCC and fibrinogen are being compared to plasma in PPH (trial identifier: NCT01910675). At the moment, there is no evidence to support the use of PCCs in the management of PPH, and their use should be limited to clinical trials in order to gather evidence on their efficacy and, in particular, on their safety.
- Recommendation 37. We recommend against using prothrombin complex concentrates in PPH because of safety concerns and a lack of evidence to support their efficacy (1C).
Recombinant activated factor VII
Some benefits have been reported to be associated with the off-label use of recombinant activated factor VII administered to treat life-threatening bleeding; however, data from 35 RCTs were unable to confirm a positive effect123. Furthermore, an analysis of the available RCTs indicates that treatment with high doses of rFVIIa significantly increased the risk of arterial (but not venous) thromboembolic events124. Several studies have described the use of rFVIIa in PPH. Non-randomised cohort studies have shown efficacy and have not identified any safety concerns125–127.
- Recommendation 38. We suggest considering the use of recombinant activated factor VII, alongside acidosis correction, only as a last resort in massive ongoing PPH because of safety concerns and lack of evidence supporting its efficacy (2C).
Blood component therapy
Despite an increased incidence of PPH in both low- and high-income countries, the need for transfusion of blood components remains infrequent (0.9 to 2.5% of all deliveries)128. Blood component therapy includes allogeneic or autologous packed RBCs, plasma/fresh frozen plasma (FFP), single-donor apheresis platelet units (PLT-A) or platelet concentrate pools (PLT-P), and cryoprecipitate containing plasma with high levels of fibrinogen, von Willebrand factor, factor VIII and factor XIII. Blood products are an essential part of the management of severe or massive PPH and must be readily available in centres treating these patients.
The treatment of acute anaemia is a major component of PPH management. Transfusion management in PPH is guided by the clinical symptoms of the patient, and postpartum anaemia remains under-appreciated85. Severe maternal morbidity, such as myocardial ischaemia, has been described as a frequent complication of PPH, and suboptimal transfusion therapy may lead to maternal death7,129,130. The focus is, therefore, on identifying the patients who need transfusion support and ensuring this is adequate and appropriate, dividing the patients into non-massive bleeders and massive bleeders; alternatives to transfusion should always be considered.
Red blood cell transfusion
In non-massive bleeders, or when bleeding is controllable, several RCTs and systematic reviews outside PPH support a restrictive transfusion strategy, i.e. considering RBC transfusion in most patients only when the Hb concentration is below 7 g/dL131. The transfusion requirements in septic shock (TRISS) trial showed that severely ill patients with septic shock can safely benefit from an Hb trigger of 7 g/dL132. Furthermore, an RCT of upper gastrointestinal bleeding in 921 patients, of whom one-third were admitted with signs of hypovolemic shock (systolic BP <100 mmHg), showed that an Hb trigger of 7 g/dL was safe and increased 45-day survival when applied from the earliest phase133. In severely anaemic PPH patients, an RCT looked at the influence of a restrictive strategy on clinically assessed fatigue134. There was not clinical significance between the two arms and a restrictive strategy seemed equally safe and clinically justified. However, if massive PPH develops, it is important to shift to the local massive transfusion protocol (MTP).
It should be borne in mind that, due to the use of restrictive transfusion protocols, once the bleeding episode is controlled, the parturient will have moderate-to-severe anaemia. Anaemia is associated with important maternal morbidities, including depression, fatigue, and impaired cognition1,16. These adverse events can negatively impact maternal-child bonding and the mother’s ability to care for the new-born child1,16. Therefore, pharmacological treatment of anaemia should be targeted at these patients (see recommendations 6 and 7).
- Recommendation 39. We recommend using a restrictive RBC transfusion trigger (only considering transfusion when Hb concentration is less than 7 g/dL) in non-massively bleeding patients with symptoms of anaemia (1C).
Fresh frozen plasma transfusion
There are very little data on the use of fresh frozen plasma transfusion (FFP) in non-massive PPH, but FFP is part of the MTP in massive PPH. According to several guidelines, FFP transfusion should be considered in massive ongoing PPH when there is a clinical suspicion of coagulopathy and laboratory tests are not in the norm37,135.
If laboratory results are not available and bleeding continues after administration of 4 RBC units, then FFP may be transfused, at least in a 1:2 FFP:RBC ratio, until haemostatic test results are known136. Earlier FFP transfusion could be considered for placental abruption, amniotic fluid embolism or delayed diagnosis of bleeding136. If massive PPH develops, it is important to shift to the local MTP.
- Recommendation 40. We suggest transfusing a standard dose of plasma (15–20 mL/kg) in severe ongoing PPH guided by abnormalities in coagulation tests (e.g. PT, INR and/or aPTT >1.5 times normal or CT prolongation in ROTEM or R-time prolongation in TEG) (2C).
- Recommendation 41. If laboratory results are not available and bleeding continues after administration of 4 RBC units, we suggest FFP transfusion at least in a 1:2 FFP:RBC ratio until haemostatic test results are known (2C).
Platelet concentrate transfusion
Platelet transfusion is part of the MTP in massive PPH, but there is very little evidence on when to use platelets in severe ongoing PPH. Thrombocytopenia develops in women who have low platelet counts prior to labour (pre-eclampsia/eclampsia or inherited/immune/gestational thrombocytopenia), are bleeding secondary to placental abruption or amniotic fluid embolism, or have severe or major bleeding, but this may be very much affected by the resuscitation strategy111,136. Platelet count has been associated with survival in major haemorrhage137, and the early use of platelet transfusion in trauma is supported by evidence as part of an MTP138.
There is a consensus that platelets should be transfused at platelet counts <75×109/L (or when point-of-care testing indicates impaired platelet function), aiming to maintain a level >50×109/L during ongoing PPH4,136. If massive PPH develops, it is important to shift to the local MTP.
- Recommendation 42. We recommend transfusing a standard dose of platelets (5–10 mL/kg) in severe ongoing PPH guided by abnormalities in laboratory tests (e.g. platelet count <75×109/L, reduced clot strength related to impaired platelet function as measured by TEG or ROTEM or reduced platelet function as measured by a platelet function test) (1C).
Transfusion management of massive PPH
Massive or life-threatening PPH, defined by a blood loss >2.5 L, is a clinical condition characterised by severe ongoing bleeding causing hypovolaemia and shock. The focus is on stopping the bleeding, performing resuscitation, and avoiding further complications. In massive PPH, there is a risk of coagulopathy associated with an increased risk of morbidity and mortality105,117,139,140.
There are no prospective RCTs in the obstetric setting investigating optimal transfusion management in patients with massive PPH, but we have observational evidence in this and other conditions. In an observational study, Gutierrez et al. collected data from 31 patients with massive PPH treated with an MTP allowing rapid access to RBC, plasma and platelet transfusions141. Patients received a median of 18 blood products in total. Post-resuscitation blood samples revealed normal fibrinogen levels and platelet counts, and no coagulopathy. In a 4-year observational study of 142 patients with massive PPH, Pasquier et al. observed a change in transfusion practices towards an increase in the plasma:RBC ratio (from 1:1.8 to 1:1.1) and found that a higher ratio was associated with a significant reduction in the rate of invasive procedures such as arterial ligation, B-Lynch suture, and hysterectomy142. The limited available evidence precludes any clear guidance on transfusion practice during severe PPH, and until more data are available clinicians may choose to use different transfusion options according to the local MTP. The main benefit of an MTP is to reduce delays in transfusion administration and correction of coagulopathy.
In a mixed population of 832 massively transfused patients (surgery, trauma and PPH), Johansson et al. observed an increase in survival in patients treated with an MTP using a high ratio of readily available plasma and platelets, as guided by thromboelastography137. Markers of resuscitation, such as increased platelet count post-resuscitation, were associated with improved 30-day and 90-day survival. In contrast, an analysis of massive transfusion ratios in 601 non-trauma, surgical and critically ill patients (carried out from 2011 to 2015) found that FFP:RBC and PLT:RBC ratios were not associated with 30-day mortality hazard ratios after controlling for baseline characteristics and disease severity143. These data suggest that in the setting of massive haemorrhage FFP:RBC or PLT:RBC transfusion ratios greater than 1:2 may not be appropriate for all patients, and that further research to guide appropriate resuscitation strategies in non-trauma patients is warranted.
Recent guidelines from the International Society of Thrombosis and Haemostasis on the management of coagulopathy associated with PPH recommend against a 1:1:1 RBC:FFP:PLT ratio and suggest that if 8 units of RBC and FFP have been infused and tests of haemostasis remain unavailable, fibrinogen supplementation (cryoprecipitate or fibrinogen concentrate) and platelet transfusion should be considered136.
- Recommendation 43. We suggest that all obstetric units have a clear-cut massive transfusion protocol for the initial management of life-threatening PPH, considering early transfusion therapy with RBCs and FFP (2C).
Mechanical and surgical options
Physical measures
If bleeding persists after uterine massage and administration of uterotonics, the obstetrician should consider the potentially life-saving procedures mentioned below.
Bimanual compression of the uterus (external or internal) consists in applying pressure to stop or slow the bleeding. The uterus should be kept compressed until medical support becomes available19.
Aortic compression is a life-saving intervention in the presence of severe PPH, whatever its aetiology. By cutting off the blood supply to the pelvis, blood is spared while preparing for any necessary intervention without delay19.
Hydrostatic intrauterine balloon tamponade may arrest or stop bleeding in over 50% of women, with few complications reported, and without any further need for surgical treatment6,19,144,145. At caesarean section (particularly after placenta previa), the procedure consists of inserting a catheter and inflating the balloon with 500 mL sterile water/saline if bleeding persists from placental bed vessels146. This relatively simple technique may be part of existing protocols as a second-line option for management of atonic PPH6,19,22.
Non-pneumatic anti-shock garment (NASG) can buy time for women with haemorrhage-related hypovolemic shock regardless of the cause. It can be used to treat shock or during transfer from lower-level facilities to tertiary facilities, or while waiting for a caesarean section19. The NASG is made of neoprene and Velcro and compresses the lower body with nine articulated segments that close tightly around the legs, pelvis and abdomen. There is a foam ball in the abdominal segment which increases compression. It works through the application of counter-pressure to the lower body, which may reverse shock by returning blood to the vital organs. The NASG allows perineal access for performing vaginal procedures, and the abdominal segment can be removed for abdominal surgery. A systematic review evaluated the effects of NASG compared with standard care in the management of PPH (five observational studies, one cluster-randomised trial)147. The pooled analysis of observational studies showed that use of a NASG resulted in a significant reduction in severe maternal outcomes and death (but not in transfusion), although the quality of evidence was low.
- Recommendation 44. We suggest the use of bimanual uterine compression as a temporising measure until appropriate care is available for the treatment of PPH due to uterine atony after vaginal delivery (2C).
- Recommendation 45. We suggest the use of external aortic compression as a temporising measure until appropriate care is available for the treatment of PPH due to uterine atony after vaginal delivery (2C).
- Recommendation 46. Should a woman not respond to uterotonics or should uterotonics not be available, we suggest the use of intrauterine balloon tamponade as first-line treatment for PPH mostly due to uterine atony (2C).
- Recommendation 47. We suggest the use of non-pneumatic anti-shock garment as a temporising measure until appropriate care is available (2B).
Selective arterial embolisation
Uterine artery embolisation is considered less invasive than arterial ligation and has contributed to reduced rates of hysterectomy for intractable PPH in high-income countries (it is a resource-intensive intervention). Success rates (control of bleeding without further procedures or surgeries) for embolisation as the initial procedure after conservative management ranged from 58 to 98% (success in 1,251 of 1,435 women), with a median success rate of 89%148. Reporting of potential harmful effects was poor; one literature review found 19 cases of uterine necrosis149. The strength of the evidence is low for the ability of embolisation to control bleeding without additional procedures or surgeries6,148.
- Recommendation 48. If other measures have failed and if the necessary resources are available, we suggest the use of uterine artery embolisation as a treatment for PPH due to uterine atony (2B).
Surgical options
A wide range of surgical interventions can be used, such as uterine compression sutures, selective arterial ligation or hysterectomy (subtotal or total). These are mostly used as a last resort as they may eliminate or adversely affect future fertility and pregnancy.
Laparotomy to apply compression sutures
If laparotomy is considered, a hysterectomy set and Robinson drains must be available. A B-Lynch suture or Hayman uterine compression suture should be considered, particularly if there is an element of uterine atony. The success rate is about 60–70% but given the few studies available (two cohort studies, n=220 women) the strength of the evidence is insufficient to evaluate the efficacy of uterine compression sutures in controlling bleeding148.
Bilateral uterine artery ligation
Rates of successful control of bleeding without further procedures or surgeries ranged from 36 to 96% with a median of 92% in three studies. The strength of the evidence is low for ligation as a means to control bleeding without further procedures or surgeries148.
Hysterectomy (total or subtotal)
The proportion of women with severe PPH requiring a hysterectomy for bleeding control is decreasing in favour of more conservative surgical techniques. The decision to proceed should be made by an experienced clinician (preferably two) and performed early (especially in cases of placenta accreta or uterine rupture)22.
Pelvic tamponade (abdominal packing)
This may have a role after hysterectomy in women with consumption coagulopathy or diffuse haemorrhage150.
- Recommendation 49. If bleeding does not stop despite treatment with uterotonics and other available conservative interventions (e.g. uterine massage, balloon tamponade), we recommend the use of invasive surgical interventions (1C).
Thromboprophylaxis
Venous thromboembolism (VTE) is a preventable serious maternal complication most likely to occur during the postpartum period. The analysis of a large linked primary and secondary care database containing 222,334 pregnancies showed that women with PPH (including intrapartum haemorrhage) had significantly increased VTE rates in the first three weeks postpartum151. This also applies to women receiving RBC transfusion152. Therefore, in women with severe or massive PPH and coagulopathy, thromboprophylaxis should be started as soon as possible after bleeding has been controlled and coagulopathy has been corrected136. Thromboprophylaxis should be continued for at least ten days or until other risk factors are no longer present (e.g. until the post-PPH inflammatory syndrome disappears); the duration of thromboprophylaxis required should be determined by the attending physician. Guidelines for thromboprophylaxis should be included in the locally agreed PPH management protocol.
- Recommendation 50. In patients experiencing severe or massive PPH and/or transfusion, we recommend starting thromboprophylaxis as soon as possible once bleeding has been controlled (1B).
Management of secondary postpartum haemorrhage
Secondary or late PPH (0.5–2% of deliveries) is defined as haemorrhage occurring from 24 h to six weeks after delivery and requiring therapeutic action21. The most frequent cause is retention of placental fragments and/or endometritis, which may be associated with incomplete uterine involution; other causes include false-aneurysms of the uterine artery, arteriovenous fistulae (vascular abnormalities), choriocarcinoma, and coagulation disorders21.
The following recommendations for the management of secondary PPH have been developed by the UK Royal College of Obstetricians and Gynecologists22:
- in women presenting with secondary PPH, an assessment of vaginal microbiology should be performed (high vaginal and endocervical swabs) and appropriate use of antimicrobial therapy should be initiated when endometritis is suspected.
- A pelvic ultrasound may help to exclude the presence of retained products of conception, although the diagnostic procedures of retained products are not reliable.
- Surgical evacuation of retained placental tissue should be undertaken or supervised by an experienced clinician.
Protocol- vs clinical judgement-based management of postpartum haemorrhage
The introduction of clinical protocols/guidelines which aim to identify and adopt strategies based on the best evidence has become the norm. We are aware of strong recommendations in several guidelines which certainly help clinicians to provide the best available health care options. In contrast, we found mostly weak recommendations in most recent guidelines for the prevention and treatment of PPH6,19,21,22, probably because the implementation of these recommendations is a more complex process.
Compared with protocol-based therapy, clinical judgment-based therapy involves the appropriate application of clinician knowledge and individual expertise to the care of individual patients153. The expert uses the sum of all of his/her cognitive processes in the making of the clinical decision. The problem is that in this context there is often an associated lack of interdisciplinary teamwork. This is unfortunate, because the field of obstetrics requires educated experts in all related disciplines154.
A clinical protocol proposes a multidisciplinary care plan which is not dependent on individual experts. It aims to standardise care, improve outcomes and reduce costs. The problem with protocols is the huge variations between different guidelines/protocols and lack of implementation, despite evidence showing that they improve outcomes and the strong support they receive from international organisations6,154–156.
Conclusions
This consensus statement provides recommendations on the management of PPH as part of PBM in obstetrics. The aim of the multidisciplinary panel of physicians with expertise in obstetrics, anaesthesia, haematology and transfusion medicine has been to generate recommendations based on the best available evidence to assist clinical decision making by practitioners managing the perinatal care of women in all settings, putting patient safety at the forefront at all times. This document does not intend to replace the individual judgment of the physician who has all the clinical data relevant to a particular patient.
Acknowledgements
Financial support for this project was provided by the pharmaceutical group LFB through an unrestricted educational grant to NATA. The sponsor had no influence on the development process or the content of the manuscript.
Footnotes
A multidisciplinary consensus statement developed by the Network for the Advancement of Patient Blood Management, Haemostasis and Thrombosis (NATA) in collaboration with the International Federation of Gynaecology and Obstetrics (FIGO), the European Board and College of Obstetrics and Gynaecology (EBCOG) and the European Society of Anaesthesiology (ESA)
Disclaimer
The Authors accept responsibility for the views expressed in this article, which do not necessarily represent the views, decisions or policies of the institutions to whom they are affiliated. They have aimed high and have provided what they consider to be the “optimal” guidelines according to current knowledge. However, they also acknowledge that the evidence is not clear-cut for all circumstances. Feasibility, costs, values and preferences and a balance of harms:benefits need to be considered when adopting and adapting these recommendations; available budget, equipment and capacity also need to be taken into consideration. Therefore, these recommendations have to be adapted to the specific situations, the resources and the strategies of the individual countries and regions.
Authors’ contributions
The Authors’ contributions to the development of this consensus statement included: participation in a one-day face-to-face meeting (all Authors except IF, WH and SM), preparation of a detailed meeting agenda (FC and FG), writing one or several sections of the manuscript (JS, ASD-B, M-PB, IF, FG, SH, SM and JN), preparing Figures 2 and 3 (ASD-B), compiling contributions and preparing a first complete draft of the manuscript (JS and MM), reviewing and providing input on several draft versions of the complete manuscript (all Authors), copyediting the latest versions of the manuscript (FC), and approving the final version for submission (all Authors).
Disclosure of conflicts of interest
ASD-B has received consulting fees and research support from LFB. IF has received travel support for congress attendance and/or financial support for educational activities from Vifor Pharma, Fresenius Kabi and CSL Behring. MM has received funding for consultancies, lectures and/or travel from Vifor Pharma, Wellspect HealthCare, Pharmacosmos, Ferrer Pharma, CSL Behring, PharmaNutra and Zambon. SH has received speaker fees and consulting fees from CSL Behring and speaker fees from Vifor Pharma. CMS has received consulting fees from LFB and Octapharma. The other Authors have reported no conflicts of interest relevant to this article.
References
- 1.Muñoz M, Peña-Rosas JP, Robinson S, et al. Patient blood management in obstetrics: management of anaemia and haematinic deficiencies in pregnancy and in the post-partum period: NATA consensus statement. Transfus Med. 2018;28:22–39. doi: 10.1111/tme.12443. [DOI] [PubMed] [Google Scholar]
- 2.Guyatt GH, Cook DJ, Jaeschke R, et al. Grades of recommendation for antithrombotic agents: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133:123S–31S. doi: 10.1378/chest.08-0654. [DOI] [PubMed] [Google Scholar]
- 3.Kozek-Langenecker SA, Afshari A, Albaladejo P, et al. Management of severe perioperative bleeding: Guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol. 2013;30:270–382. doi: 10.1097/EJA.0b013e32835f4d5b. [DOI] [PubMed] [Google Scholar]
- 4.Kozek-Langenecker SA, Ahmed AB, Afshari A, et al. Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology: first update 2016. Eur J Anaesthesiol. 2017;34:332–95. doi: 10.1097/EJA.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 5.GBD 2015 Maternal Mortality Collaborators. Global, regional, and national levels of maternal mortality, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1775–812. doi: 10.1016/S0140-6736(16)31470-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. WHO recommendations for the prevention and treatment of postpartum haemorrhage. Geneva: WHO; 2012. [PubMed] [Google Scholar]
- 7.Abdul-Kadir R, McLintock C, Ducloy AS, et al. Evaluation and management of postpartum hemorrhage: consensus from an international expert panel. Transfusion. 2014;54:1756–68. doi: 10.1111/trf.12550. [DOI] [PubMed] [Google Scholar]
- 8.Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2:e323–33. doi: 10.1016/S2214-109X(14)70227-X. [DOI] [PubMed] [Google Scholar]
- 9.Souza JP, Gulmezoglu AM, Vogel J, et al. Moving beyond essential interventions for reduction of maternal mortality (the WHO Multicountry Survey on Maternal and Newborn Health): a cross-sectional study. Lancet. 2013;381:1747–55. doi: 10.1016/S0140-6736(13)60686-8. [DOI] [PubMed] [Google Scholar]
- 10.Knight M, Callaghan WM, Berg C, et al. Trends in postpartum hemorrhage in high resource countries: a review and recommendations from the International Postpartum Hemorrhage Collaborative Group. BMC Pregnancy Childbirth. 2009;9:55. doi: 10.1186/1471-2393-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bateman BT, Berman MF, Riley LE, Leffert LR. The epidemiology of postpartum hemorrhage in a large, nationwide sample of deliveries. Anesth Analg. 2010;110:1368–73. doi: 10.1213/ANE.0b013e3181d74898. [DOI] [PubMed] [Google Scholar]
- 12.Kramer MS, Berg C, Abenhaim H, et al. Incidence, risk factors, and temporal trends in severe postpartum hemorrhage. Am J Obstet Gynecol. 2013;209:449.e1–7. doi: 10.1016/j.ajog.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 13.van Stralen G, von Schmidt Auf Altenstadt JF, Bloemenkamp KW, et al. Increasing incidence of postpartum hemorrhage: the Dutch piece of the puzzle. Acta Obstet Gynecol Scand. 2016;95:1104–10. doi: 10.1111/aogs.12950. [DOI] [PubMed] [Google Scholar]
- 14.Lutomski JE, Byrne BM, Devane D, Greene RA. Increasing trends in atonic postpartum haemorrhage in Ireland: an 11-year population-based cohort study. BJOG. 2012;119:306–14. doi: 10.1111/j.1471-0528.2011.03198.x. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and Mineral Nutrition Information System. Geneva: WHO; 2011. [Google Scholar]
- 16.Milman N. Postpartum anemia I: definition, prevalence, causes, and consequences. Ann Hematol. 2011;90:1247–53. doi: 10.1007/s00277-011-1279-z. [DOI] [PubMed] [Google Scholar]
- 17.Allary J, Soubirou JF, Michel J, et al. An individual scoring system for the prediction of postpartum anaemia. Ann Fr Anesth Reanim. 2013;32:e1–7. doi: 10.1016/j.annfar.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Krafft A, Huch R, Breymann C. Impact of parturition on iron status in nonanaemic iron deficiency. Eur J Clin Invest. 2003;33:919–23. doi: 10.1046/j.1365-2362.2003.01244.x. [DOI] [PubMed] [Google Scholar]
- 19.Rajan PV, Wing DA. Postpartum hemorrhage: evidence-based medical interventions for prevention and treatment. Clin Obstet Gynecol. 2010;53:165–81. doi: 10.1097/GRF.0b013e3181ce0965. [DOI] [PubMed] [Google Scholar]
- 20.Lalonde A. Prevention and treatment of postpartum hemorrhage in low-resource settings. International Federation of Gynecology and Obstetrics. Int J Gynaecol Obstet. 2012;117:108–18. doi: 10.1016/j.ijgo.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Sentilhes L, Vayssière C, Deneux-Tharaux C, et al. Postpartum hemorrhage: guidelines for clinical practice from the French College of Gynaecologists and Obstetricians (CNGOF): in collaboration with the French Society of Anesthesiology and Intensive Care (SFAR) Eur J Obstet Gynecol Reprod Biol. 2016;198:12–21. doi: 10.1016/j.ejogrb.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 22.Royal College of Obstetricians and Gynaecologists. Prevention and management of postpartum haemorrhage. Green-top guideline No. 52. BJOG. 2017;124:e106–49. doi: 10.1111/1471-0528.14178. [DOI] [PubMed] [Google Scholar]
- 23.Gómez-Ramírez S, Shander A, et al. Prevention and management of acute reactions to intravenous iron in surgical patients. Blood Transfus. 2019;17:137–45. doi: 10.2450/2018.0156-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rampton D, Folkersen J, Fishbane S, et al. Hypersensitivity reactions to intravenous iron: guidance for risk minimization and management. Haematologica. 2014;99:1671–6. doi: 10.3324/haematol.2014.111492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henry DA, Carless PA, Moxey AJ, et al. Pre-operative autologous donation for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev. 2002:CD003602. doi: 10.1002/14651858.CD003602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamamoto Y, Yamashita T, Tsuno NH, et al. Safety and efficacy of preoperative autologous blood donation for high-risk pregnant women: experience of a large university hospital in Japan. J Obstet Gynaecol Res. 2014;40:1308–16. doi: 10.1111/jog.12348. [DOI] [PubMed] [Google Scholar]
- 27.Shamshirsaz AA, Fox KA, Salmanian B, et al. Maternal morbidity in patients with morbidly adherent placenta treated with and without a standardized multidisciplinary approach. Am J Obstet Gynecol. 2015;212:218.e1–9. doi: 10.1016/j.ajog.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 28.Royal College of Obstetricians and Gynaecologists. Placenta praevia, placenta praevia accreta and vasa praevia: diagnosis and management. London UK: RCOG; 2011. (Green-top guideline No. 27). [Google Scholar]
- 29.Clausen C, Stensballe J, Albrechtsen CK, et al. Balloon occlusion of the internal iliac arteries in the multidisciplinary management of placenta percreta. Acta Obstet Gynecol Scand. 2013;92:386–91. doi: 10.1111/j.1600-0412.2012.01451.x. [DOI] [PubMed] [Google Scholar]
- 30.Knight M. Peripartum hysterectomy in the UK: management and outcomes of the associated haemorrhage. BJOG. 2007;114:1380–7. doi: 10.1111/j.1471-0528.2007.01507.x. [DOI] [PubMed] [Google Scholar]
- 31.Allard S, Green L, Hunt BJ. How we manage the haematological aspects of major obstetric haemorrhage. Br J Haematol. 2014;164:177–88. doi: 10.1111/bjh.12605. [DOI] [PubMed] [Google Scholar]
- 32.Makris M, Van Veen JJ, Tait CR, et al. Guideline on the management of bleeding in patients on antithrombotic agents. Br J Haematol. 2013;160:35–46. doi: 10.1111/bjh.12107. [DOI] [PubMed] [Google Scholar]
- 33.Royal College of Obstetricians and Gynaecologists. Reducing the risk of venous thromboembolism during pregnancy and the puerperium. London, UK: RCOG; 2015. (Green-top guideline No. 37a). [Google Scholar]
- 34.Bates SM, Greer IA, Middeldorp S, et al. VTE, thrombophilia, antithrombotic therapy, and pregnancy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(Suppl 2):e691S–736S. doi: 10.1378/chest.11-2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu Z, Fan J, Luo X, et al. Anticoagulation regimens during pregnancy in patients with mechanical heart valves: a systematic review and meta-analysis. Can J Cardiol. 2016;32:1248.e1–9. doi: 10.1016/j.cjca.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 36.Dennis A. Valvular heart disease in pregnancy. Int J Obstet Anesth. 2016;25:4–8. doi: 10.1016/j.ijoa.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 37.Hunt BJ, Allard S, Keeling D, et al. A practical guideline for the haematological management of major haemorrhage. Br J Haematol. 2015;170:788–803. doi: 10.1111/bjh.13580. [DOI] [PubMed] [Google Scholar]
- 38.Begley CM, Gyte GM, Devane D, et al. Active versus expectant management for women in the third stage of labour. Cochrane Database Syst Rev. 2015:CD007412. doi: 10.1002/14651858.CD007412.pub4. [DOI] [PubMed] [Google Scholar]
- 39.Westhoff G, Cotter AM, Tolosa JE. Prophylactic oxytocin for the third stage of labour to prevent postpartum haemorrhage. Cochrane Database Syst Rev. 20131808:CD00. doi: 10.1002/14651858.CD001808.pub2. [DOI] [PubMed] [Google Scholar]
- 40.Adnan N, Conlan-Trant R, McCormick C, et al. Intramuscular versus intravenous oxytocin to prevent postpartum haemorrhage at vaginal delivery: randomised controlled trial. BMJ. 2018;362:k3546. doi: 10.1136/bmj.k3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soltani H, Hutchon DR, Poulose TA. Timing of prophylactic uterotonics for the third stage of labour after vaginal birth. Cochrane Database Syst Rev. 2010:CD006173. doi: 10.1002/14651858.CD006173.pub2. [DOI] [PubMed] [Google Scholar]
- 42.Grotegut CA, Paglia MJ, Johnson LN, et al. Oxytocin exposure during labor among women with postpartum hemorrhage secondary to uterine atony. Am J Obstet Gynecol. 2011;204:56.e1–6. doi: 10.1016/j.ajog.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Widmer M, Piaggio G, Nguyen TMH, et al. Heat-stable carbetocin versus oxytocin to prevent hemorrhage after vaginal birth. N Engl J Med. 2018;379:743–52. doi: 10.1056/NEJMoa1805489. [DOI] [PubMed] [Google Scholar]
- 44.Zhang WH, Deneux-Tharaux C, Brocklehurst P, et al. Effect of a collector bag for measurement of postpartum blood loss after vaginal delivery: cluster randomised trial in 13 European countries. BMJ. 2010;340:c293. doi: 10.1136/bmj.c293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sentilhes L, Winer N, Azria E, et al. Tranexamic acid for the prevention of blood loss after vaginal delivery. N Engl J Med. 2018;379:731–42. doi: 10.1056/NEJMoa1800942. [DOI] [PubMed] [Google Scholar]
- 46.Dupont C, Ducloy-Bouthors AS, Huissoud C. [Clinical and pharmacological procedures for the prevention of postpartum haemorrhage in the third stage of labor]. J Gynecol Obstet Biol Reprod (Paris) 2014;43:966–97. doi: 10.1016/j.jgyn.2014.09.025. [In French.] [DOI] [PubMed] [Google Scholar]
- 47.Kovacheva VP, Soens MA, Tsen LC. A randomized, double-blinded trial of a “rule of threes” algorithm versus continuous infusion of oxytocin during elective cesarean delivery. Anesthesiology. 2015;123:92–100. doi: 10.1097/ALN.0000000000000682. [DOI] [PubMed] [Google Scholar]
- 48.Su LL, Chong YS, Samuel M. Carbetocin for preventing postpartum haemorrhage. Cochrane Database Syst Rev. 2012:CD005457. doi: 10.1002/14651858.CD005457.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ortiz-Gómez JR, Morillas-Ramírez F, Fornet-Ruiz I, et al. [Clinical and pharmacological study of the efficacy of carbetocin in elective caesareans compared to low and usual doses of oxytocin]. Rev Esp Anestesiol Reanim. 2013;60:7–15. doi: 10.1016/j.redar.2012.06.013. [In Spanish.] [DOI] [PubMed] [Google Scholar]
- 50.Novikova N, Hofmeyr GJ, Cluver C. Tranexamic acid for preventing postpartum haemorrhage. Cochrane Database Syst Rev. 2015:CD007872. doi: 10.1002/14651858.CD007872.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rath WH. Postpartum hemorrhage--update on problems of definitions and diagnosis. Acta Obstet Gynecol Scand. 2011;90:421–8. doi: 10.1111/j.1600-0412.2011.01107.x. [DOI] [PubMed] [Google Scholar]
- 52.Guasch E, Gilsanz F. Massive obstetric hemorrhage: current approach to management. Med Intensiva. 2016;40:298–310. doi: 10.1016/j.medin.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 53.Nathan HL, El Ayadi A, Hezelgrave NL, et al. Shock index: an effective predictor of outcome in postpartum haemorrhage? BJOG. 2015;122:268–75. doi: 10.1111/1471-0528.13206. [DOI] [PubMed] [Google Scholar]
- 54.University College London Hospitals NHS Foundation Trust. Guideline for the management of postpartum haemorrhage and massive obstetric haemorrhage. London: UCLH; 2009. [Google Scholar]
- 55.Johansson PI, Stensballe J, Oliveri R, et al. How I treat patients with massive hemorrhage. Blood. 2014;124:3052–8. doi: 10.1182/blood-2014-05-575340. [DOI] [PubMed] [Google Scholar]
- 56.Holcomb JB, Jenkins D, Rhee P, et al. Damage control resuscitation: directly addressing the early coagulopathy of trauma. J Trauma. 2007;62:307–10. doi: 10.1097/TA.0b013e3180324124. [DOI] [PubMed] [Google Scholar]
- 57.McDonald S, Fernando R, Ashpole K, Columb M. Maternal cardiac output changes after crystalloid or colloid coload following spinal anesthesia for elective cesarean delivery: a randomized controlled trial. Anesth Analg. 2011;113:803–10. doi: 10.1213/ANE.0b013e31822c0f08. [DOI] [PubMed] [Google Scholar]
- 58.Haase N, Perner A, Hennings LI, et al. Hydroxyethyl starch 130/0.38–0.45 versus crystalloid or albumin in patients with sepsis: systematic review with meta-analysis and trial sequential analysis. BMJ. 2013;346:f839. doi: 10.1136/bmj.f839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Perel P, Roberts I, Ker K. Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database Syst Rev. 2013;2:CD000567. doi: 10.1002/14651858.CD000567.pub6. [DOI] [PubMed] [Google Scholar]
- 60.Rossaint R, Bouillon B, Cerny V, et al. The European guideline on management of major bleeding and coagulopathy following trauma: fourth edition. Crit Care. 2016;20:100. doi: 10.1186/s13054-016-1265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wikkelsø AJ, Edwards HM, Afshari A, et al. Pre-emptive treatment with fibrinogen concentrate for postpartum haemorrhage: randomized controlled trial. Br J Anaesth. 2015;114:623–33. doi: 10.1093/bja/aeu444. [DOI] [PubMed] [Google Scholar]
- 62.Bernabei AF, Levison MA, Bender JS. The effects of hypothermia and injury severity on blood loss during trauma laparotomy. J Trauma. 1992;33:835–9. doi: 10.1097/00005373-199212000-00007. [DOI] [PubMed] [Google Scholar]
- 63.Rajagopalan S, Mascha E, Na J, Sessler DI. The effects of mild perioperative hypothermia on blood loss and transfusion requirement. Anesthesiology. 2008;108:71–7. doi: 10.1097/01.anes.0000296719.73450.52. [DOI] [PubMed] [Google Scholar]
- 64.Yokoyama K, Suzuki M, Shimada Y, et al. Effect of administration of pre-warmed intravenous fluids on the frequency of hypothermia following spinal anesthesia for Cesarean delivery. J Clin Anesth. 2009;21:242–8. doi: 10.1016/j.jclinane.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 65.De Robertis E, Kozek-Langenecker SA, Tufano R, et al. Coagulopathy induced by acidosis, hypothermia and hypocalcaemia in severe bleeding. Minerva Anestesiol. 2015;81:65–75. [PubMed] [Google Scholar]
- 66.Darlington DN, Kheirabadi BS, Delgado AV, et al. Coagulation changes to systemic acidosis and bicarbonate correction in swine. J Trauma. 2011;71:1271–7. doi: 10.1097/TA.0b013e318214f522. [DOI] [PubMed] [Google Scholar]
- 67.Engström M, Schött U, Romner B, Reinstrup P. Acidosis impairs the coagulation: a thromboelastographic study. J Trauma. 2006;61:624–8. doi: 10.1097/01.ta.0000226739.30655.75. [DOI] [PubMed] [Google Scholar]
- 68.Martini WZ, Dubick MA, Pusateri AE, et al. Does bicarbonate correct coagulation function impaired by acidosis in swine? J Trauma. 2006;61:99–106. doi: 10.1097/01.ta.0000215574.99093.22. [DOI] [PubMed] [Google Scholar]
- 69.Martini WZ, Dubick MA, Wade CE, Holcomb JB. Evaluation of tris-hydroxymethylaminomethane on reversing coagulation abnormalities caused by acidosis in pigs. Crit Care Med. 2007;35:1568–74. doi: 10.1097/01.CCM.0000266682.74602.6A. [DOI] [PubMed] [Google Scholar]
- 70.Martini WZ, Holcomb JB. Acidosis and coagulopathy: the differential effects on fibrinogen synthesis and breakdown in pigs. Ann Surg. 2007;246:831–5. doi: 10.1097/SLA.0b013e3180cc2e94. [DOI] [PubMed] [Google Scholar]
- 71.Dirkmann D, Radü-Berlemann J, Görlinger K, Peters J. Recombinant tissue-type plasminogen activator-evoked hyperfibrinolysis is enhanced by acidosis and inhibited by hypothermia but still can be blocked by tranexamic acid. J Trauma Acute Care Surg. 2013;74:482–8. doi: 10.1097/TA.0b013e318280dec1. [DOI] [PubMed] [Google Scholar]
- 72.Porta CR, Nelson D, McVay D, et al. The effects of tranexamic acid and prothrombin complex concentrate on the coagulopathy of trauma: an in vitro analysis of the impact of severe acidosis. J Trauma Acute Care Surg. 2013;75:954–60. doi: 10.1097/TA.0b013e31829e20bf. [DOI] [PubMed] [Google Scholar]
- 73.Darlington DN, Kheirabadi BS, Scherer MR, et al. Acidosis and correction of acidosis does not affect rFVIIa function in swine. Int J Burns Trauma. 2012;2:145–57. [PMC free article] [PubMed] [Google Scholar]
- 74.Darlington DN, Kremenevskiy I, Pusateri AE, et al. Effects of in vitro hemodilution, hypothermia and rFVIIa addition on coagulation in human blood. Int J Burns Trauma. 2012;2:42–50. [PMC free article] [PubMed] [Google Scholar]
- 75.Hall K, Forrest P, Sawyer C. The effects of acidosis and hypothermia on blood transfusion requirements following factor VII administration. Anaesth Intensive Care. 2007;35:494–7. doi: 10.1177/0310057X0703500405. [DOI] [PubMed] [Google Scholar]
- 76.Meng ZH, Wolberg AS, Monroe DM, 3rd, Hoffman M. The effect of temperature and pH on the activity of factor VIIa: implications for the efficacy of high-dose factor VIIa in hypothermic and acidotic patients. J Trauma. 2003;55:886–91. doi: 10.1097/01.TA.0000066184.20808.A5. [DOI] [PubMed] [Google Scholar]
- 77.Viuff D, Lauritzen B, Pusateri AE, et al. Effect of haemodilution, acidosis, and hypothermia on the activity of recombinant factor VIIa (NovoSeven) Br J Anaesth. 2008;101:324–31. doi: 10.1093/bja/aen175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Djabatey EA, Barclay PM. Difficult and failed intubation in 3430 obstetric general anaesthetics. Anaesthesia. 2009;64:1168–71. doi: 10.1111/j.1365-2044.2009.06060.x. [DOI] [PubMed] [Google Scholar]
- 79.Marcus HE, Fabian A, Lier H, et al. Survey on the use of oxytocin for cesarean section. Minerva Anestesiol. 2010;76:890–5. [PubMed] [Google Scholar]
- 80.Knapp J, Hofer S, Lier H. [Anesthesiological approach to postpartum hemorrhage]. Anaesthesist. 2016;65:225–40. doi: 10.1007/s00101-016-0148-5. [In German.] [DOI] [PubMed] [Google Scholar]
- 81.Tunçalp Ö, Hofmeyr GJ, Gülmezoglu AM. Prostaglandins for preventing postpartum haemorrhage. Cochrane Database Syst Rev. 2012:CD000494. doi: 10.1002/14651858.CD000494.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schmitz T, Tararbit K, Dupont C, et al. Prostaglandin E2 analogue sulprostone for treatment of atonic postpartum hemorrhage. Obstet Gynecol. 2011;118:257–65. doi: 10.1097/AOG.0b013e3182255335. [DOI] [PubMed] [Google Scholar]
- 83.Lavoie A, McCarthy RJ, Wong CA. The ED90 of prophylactic oxytocin infusion after delivery of the placenta during cesarean delivery in laboring compared with nonlaboring women: an up-down sequential allocation dose-response study. Anesth Analg. 2015;121:159–64. doi: 10.1213/ANE.0000000000000781. [DOI] [PubMed] [Google Scholar]
- 84.Blum J, Winikoff B, Raghavan S, et al. Treatment of post-partum haemorrhage with sublingual misoprostol versus oxytocin in women receiving prophylactic oxytocin: a double-blind, randomised, non-inferiority trial. Lancet. 2010;375:217–23. doi: 10.1016/S0140-6736(09)61923-1. [DOI] [PubMed] [Google Scholar]
- 85.Butwick AJ, Aleshi P, Fontaine M, et al. Retrospective analysis of transfusion outcomes in pregnant patients at a tertiary obstetric center. Int J Obstet Anesth. 2009;18:302–8. doi: 10.1016/j.ijoa.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 86.Winikoff B, Dabash R, Durocher J, et al. Treatment of post-partum haemorrhage with sublingual misoprostol versus oxytocin in women not exposed to oxytocin during labour: a double-blind, randomised, non-inferiority trial. Lancet. 2010;375:210–6. doi: 10.1016/S0140-6736(09)61924-3. [DOI] [PubMed] [Google Scholar]
- 87.Ngan Kee WD, Lee A, Khaw KS, et al. A randomized double-blinded comparison of phenylephrine and ephedrine infusion combinations to maintain blood pressure during spinal anesthesia for cesarean delivery: the effects on fetal acid-base status and hemodynamic control. Anesth Analg. 2008;107:1295–302. doi: 10.1213/ane.0b013e31818065bc. [DOI] [PubMed] [Google Scholar]
- 88.Langesaeter E, Dyer RA. Maternal haemodynamic changes during spinal anaesthesia for caesarean section. Curr Opin Anaesthesiol. 2011;24:242–8. doi: 10.1097/ACO.0b013e32834588c5. [DOI] [PubMed] [Google Scholar]
- 89.Ngan Kee WD. Prevention of maternal hypotension after regional anaesthesia for caesarean section. Curr Opin Anaesthesiol. 2010;23:304–9. doi: 10.1097/ACO.0b013e328337ffc6. [DOI] [PubMed] [Google Scholar]
- 90.Ngan Kee WD, Lee SW, Ng FF, et al. Randomized double-blinded comparison of norepinephrine and phenylephrine for maintenance of blood pressure during spinal anesthesia for cesarean delivery. Anesthesiology. 2015;122:736–45. doi: 10.1097/ALN.0000000000000601. [DOI] [PubMed] [Google Scholar]
- 91.Ho KM, Leonard AD. Concentration-dependent effect of hypocalcaemia on mortality of patients with critical bleeding requiring massive transfusion: a cohort study. Anaesth Intensive Care. 2011;39:46–54. doi: 10.1177/0310057X1103900107. [DOI] [PubMed] [Google Scholar]
- 92.Henry DA, Carless PA, Moxey AJ, et al. Anti-fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev. 20111886:CD00. [Google Scholar]
- 93.Shakur H, Roberts I, Bautista R, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010;376:23–32. doi: 10.1016/S0140-6736(10)60835-5. [DOI] [PubMed] [Google Scholar]
- 94.Wang HY, Hong SK, Duan Y, Yin HM. Tranexamic acid and blood loss during and after cesarean section: a meta-analysis. J Perinatol. 2015;35:818–25. doi: 10.1038/jp.2015.93. [DOI] [PubMed] [Google Scholar]
- 95.WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389:2105–16. doi: 10.1016/S0140-6736(17)30638-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gayet-Ageron A, Prieto-Merino D, Ker K, et al. Effect of treatment delay on the effectiveness and safety of antifibrinolytics in acute severe haemorrhage: a meta-analysis of individual patient-level data from 40,138 bleeding patients. Lancet. 2018;391:125–32. doi: 10.1016/S0140-6736(17)32455-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.World Health Organization. WHO recommendation on tranexamic acid for the treatment of postpartum haemorrhage. Geneva: WHO; 2017. [PubMed] [Google Scholar]
- 98.Ely JW, Rijhsinghani A, Bowdler NC, Dawson JD. The association between manual removal of the placenta and postpartum endometritis following vaginal delivery. Obstet Gynecol. 1995;86:1002–6. doi: 10.1016/0029-7844(95)00327-n. [DOI] [PubMed] [Google Scholar]
- 99.Titiz H, Wallace A, Voaklander DC. Manual removal of the placenta--a case control study. Aust N Z J Obstet Gynaecol. 2001;41:41–4. doi: 10.1111/j.1479-828x.2001.tb01292.x. [DOI] [PubMed] [Google Scholar]
- 100.Tandberg A, Albrechtsen S, Iversen OE. Manual removal of the placenta. Incidence and clinical significance. Acta Obstet Gynecol Scand. 1999;78:33–6. [PubMed] [Google Scholar]
- 101.Elagamy A, Abdelaziz A, Ellaithy M. The use of cell salvage in women undergoing cesarean hysterectomy for abnormal placentation. Int J Obstet Anesth. 2013;22:289–93. doi: 10.1016/j.ijoa.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 102.Milne ME, Yazer MH, Waters JH. Red blood cell salvage during obstetric hemorrhage. Obstet Gynecol. 2015;125:919–23. doi: 10.1097/AOG.0000000000000729. [DOI] [PubMed] [Google Scholar]
- 103.Teare KM, Sullivan IJ, Ralph CJ. Is cell salvaged vaginal blood loss suitable for re-infusion? Int J Obstet Anesth. 2015;24:103–10. doi: 10.1016/j.ijoa.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 104.Albright CM, Rouse DJ, Werner EF. Cost savings of red cell salvage during cesarean delivery. Obstet Gynecol. 2014;124:690–6. doi: 10.1097/AOG.0000000000000465. [DOI] [PubMed] [Google Scholar]
- 105.Butwick AJ, Carvalho B, Blumenfeld YJ, et al. Second-line uterotonics and the risk of hemorrhage-related morbidity. Am J Obstet Gynecol. 2015;212:642.e1–7. doi: 10.1016/j.ajog.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Solomon C, Collis RE, Collins PW. Haemostatic monitoring during postpartum haemorrhage and implications for management. Br J Anaesth. 2012;109:851–63. doi: 10.1093/bja/aes361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gillissen A, van den Akker T, Caram-Deelder C, et al. Coagulation parameters during the course of severe postpartum hemorrhage: a nationwide retrospective cohort study. Blood Adv. 2018;2:2433–42. doi: 10.1182/bloodadvances.2018022632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Raza I, Davenport R, Rourke C, et al. The incidence and magnitude of fibrinolytic activation in trauma patients. J Thromb Haemost. 2013;11:307–14. doi: 10.1111/jth.12078. [DOI] [PubMed] [Google Scholar]
- 109.Afshari A, Wikkelsø A, Brok J, et al. Thrombelastography (TEG) or thromboelastometry (ROTEM) to monitor haemotherapy versus usual care in patients with massive transfusion. Cochrane Database Syst Rev. 2011:CD007871. doi: 10.1002/14651858.CD007871.pub2. [DOI] [PubMed] [Google Scholar]
- 110.National Institute for Health and Care Excellence. Detecting, managing and monitoring haemostasis: viscoelastometric point-of-care testing (ROTEM, TEG and Sonoclot systems) NICE diagnostic guidance. 2014:13. [Google Scholar]
- 111.Hiippala ST, Myllylä GJ, Vahtera EM. Hemostatic factors and replacement of major blood loss with plasma-poor red cell concentrates. Anesth Analg. 1995;81:360–5. doi: 10.1097/00000539-199508000-00026. [DOI] [PubMed] [Google Scholar]
- 112.Wikkelsø A, Lunde J, Johansen M, et al. Fibrinogen concentrate in bleeding patients. Cochrane Database Syst Rev. 2013:CD008864. doi: 10.1002/14651858.CD008864.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Charbit B, Mandelbrot L, Samain E, et al. The decrease of fibrinogen is an early predictor of the severity of postpartum hemorrhage. J Thromb Haemost. 2007;5:266–73. doi: 10.1111/j.1538-7836.2007.02297.x. [DOI] [PubMed] [Google Scholar]
- 114.Jensen NH, Stensballe J, Afshari A. Comparing efficacy and safety of fibrinogen concentrate to cryoprecipitate in bleeding patients: a systematic review. Acta Anaesthesiol Scand. 2016;60:1033–42. doi: 10.1111/aas.12734. [DOI] [PubMed] [Google Scholar]
- 115.Makino S, Takeda S, Kobayashi T, et al. National survey of fibrinogen concentrate usage for post-partum hemorrhage in Japan: investigated by the Perinatology Committee, Japan Society of Obstetrics and Gynecology. J Obstet Gynaecol Res. 2015;41:1155–60. doi: 10.1111/jog.12708. [DOI] [PubMed] [Google Scholar]
- 116.Mallaiah S, Barclay P, Harrod I, et al. Introduction of an algorithm for ROTEM-guided fibrinogen concentrate administration in major obstetric haemorrhage. Anaesthesia. 2015;70:166–75. doi: 10.1111/anae.12859. [DOI] [PubMed] [Google Scholar]
- 117.Ducloy-Bouthors AS, Mignon A, Huissoud C, et al. Fibrinogen concentrate as a treatment for postpartum haemorrhage-induced coagulopathy: A study protocol for a randomised multicentre controlled trial. The fibrinogen in haemorrhage of DELivery (FIDEL) trial. Anaesth Crit Care Pain Med. 2016;35:293–8. doi: 10.1016/j.accpm.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 118.Aawar N, Alikhan R, Bruynseels D, et al. Fibrinogen concentrate versus placebo for treatment of postpartum haemorrhage: study protocol for a randomised controlled trial. Trials. 2015;16:169. doi: 10.1186/s13063-015-0670-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Frontera JA, Lewin JJ, 3rd, Rabinstein AA, et al. Guideline for reversal of antithrombotics in intracranial hemorrhage: a Statement for healthcare professionals from the Neurocritical Care Society and Society of Critical Care Medicine. Neurocrit Care. 2016;24:6–46. doi: 10.1007/s12028-015-0222-x. [DOI] [PubMed] [Google Scholar]
- 120.Goldstein JN, Refaai MA, Milling TJ, Jr, et al. Four-factor prothrombin complex concentrate versus plasma for rapid vitamin K antagonist reversal in patients needing urgent surgical or invasive interventions: a phase 3b, open-label, non-inferiority, randomised trial. Lancet. 2015;385:2077–87. doi: 10.1016/S0140-6736(14)61685-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sarode R, Milling TJ, Jr, Refaai MA, et al. Efficacy and safety of a 4-factor prothrombin complex concentrate in patients on vitamin K antagonists presenting with major bleeding: a randomized, plasma-controlled, phase IIIb study. Circulation. 2013;128:1234–43. doi: 10.1161/CIRCULATIONAHA.113.002283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Glynn JC, Plaat F. Prothrombin complex for massive obstetric haemorrhage. Anaesthesia. 2007;62:202–3. doi: 10.1111/j.1365-2044.2007.04972.x. [DOI] [PubMed] [Google Scholar]
- 123.Simpson E, Lin Y, Stanworth S, et al. Recombinant factor VIIa for the prevention and treatment of bleeding in patients without haemophilia. Cochrane Database Syst Rev. 2012:CD005011. doi: 10.1002/14651858.CD005011.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Levi M, Levy JH, Andersen HF, Truloff D. Safety of recombinant activated factor VII in randomized clinical trials. N Engl J Med. 2010;363:1791–800. doi: 10.1056/NEJMoa1006221. [DOI] [PubMed] [Google Scholar]
- 125.Barillari G, Frigo MG, Casarotto M, et al. Use of recombinant activated factor VII in severe post-partum haemorrhage: data from the Italian Registry: a multicentric observational retrospective study. Thromb Res. 2009;124:e41–7. doi: 10.1016/j.thromres.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 126.Phillips LE, McLintock C, Pollock W, et al. Recombinant activated factor VII in obstetric hemorrhage: experiences from the Australian and New Zealand Haemostasis Registry. Anesth Analg. 2009;109:1908–15. doi: 10.1213/ANE.0b013e3181c039e6. [DOI] [PubMed] [Google Scholar]
- 127.Murakami M, Kobayashi T, Kubo T, et al. Experience with recombinant activated factor VII for severe post-partum hemorrhage in Japan, investigated by Perinatology Committee, Japan Society of Obstetrics and Gynecology. J Obstet Gynaecol Res. 2015;41:1161–8. doi: 10.1111/jog.12712. [DOI] [PubMed] [Google Scholar]
- 128.Butwick AJ, Goodnough LT. Transfusion and coagulation management in major obstetric hemorrhage. Curr Opin Anaesthesiol. 2015;28:275–84. doi: 10.1097/ACO.0000000000000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Karpati PC, Rossignol M, Pirot M, et al. High incidence of myocardial ischemia during postpartum hemorrhage. Anesthesiology. 2004;100:30–6. doi: 10.1097/00000542-200401000-00009. discussion 5A. [DOI] [PubMed] [Google Scholar]
- 130.Bonnet MP, Deneux-Tharaux C, Bouvier-Colle MH. Critical care and transfusion management in maternal deaths from postpartum haemorrhage. Eur J Obstet Gynecol Reprod Biol. 2011;158:183–8. doi: 10.1016/j.ejogrb.2011.04.042. [DOI] [PubMed] [Google Scholar]
- 131.Holst LB, Petersen MW, Haase N, et al. Restrictive versus liberal transfusion strategy for red blood cell transfusion: systematic review of randomised trials with meta-analysis and trial sequential analysis. BMJ. 2015;350:h1354. doi: 10.1136/bmj.h1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Holst LB, Haase N, Wetterslev J, et al. Lower versus higher hemoglobin threshold for transfusion in septic shock. N Engl J Med. 2014;371:1381–91. doi: 10.1056/NEJMoa1406617. [DOI] [PubMed] [Google Scholar]
- 133.Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:11–21. doi: 10.1056/NEJMoa1211801. [DOI] [PubMed] [Google Scholar]
- 134.Prick B, Jansen A, Steegers E, et al. Transfusion policy after severe postpartum haemorrhage: a randomised non-inferiority trial. BJOG. 2014;121:1005–14. doi: 10.1111/1471-0528.12531. [DOI] [PubMed] [Google Scholar]
- 135.Stainsby D, MacLennan S, Thomas D, et al. Guidelines on the management of massive blood loss. Br J Haematol. 2006;135:634–41. doi: 10.1111/j.1365-2141.2006.06355.x. [DOI] [PubMed] [Google Scholar]
- 136.Collins P, Abdul-Kadir R, Thachil J. Management of coagulopathy associated with postpartum hemorrhage: guidance from the SSC of the ISTH. J Thromb Haemost. 2016;14:205–10. doi: 10.1111/jth.13174. [DOI] [PubMed] [Google Scholar]
- 137.Johansson PI, Stensballe J. Effect of Haemostatic Control Resuscitation on mortality in massively bleeding patients: a before and after study. Vox Sang. 2009;96:111–8. doi: 10.1111/j.1423-0410.2008.01130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Holcomb JB, Tilley BC, Baraniuk S, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313:471–82. doi: 10.1001/jama.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wikkelsø AJ, Hjortøe S, Gerds TA, et al. Prediction of postpartum blood transfusion - risk factors and recurrence. J Matern Fetal Neonatal Med. 2014;27:1661–7. doi: 10.3109/14767058.2013.872095. [DOI] [PubMed] [Google Scholar]
- 140.Johansson PI, Stensballe J, Rosenberg I, et al. Proactive administration of platelets and plasma for patients with a ruptured abdominal aortic aneurysm: evaluating a change in transfusion practice. Transfusion. 2007;47:593–8. doi: 10.1111/j.1537-2995.2007.01160.x. [DOI] [PubMed] [Google Scholar]
- 141.Gutierrez MC, Goodnough LT, Druzin M, Butwick AJ. Postpartum hemorrhage treated with a massive transfusion protocol at a tertiary obstetric center: a retrospective study. Int J Obstet Anesth. 2012;21:230–5. doi: 10.1016/j.ijoa.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 142.Pasquier P, Gayat E, Rackelboom T, et al. An observational study of the fresh frozen plasma: red blood cell ratio in postpartum hemorrhage. Anesth Analg. 2013;116:155–61. doi: 10.1213/ANE.0b013e31826f084d. [DOI] [PubMed] [Google Scholar]
- 143.Etchill EW, Myers SP, McDaniel LM, et al. Should all massively transfused patients be treated equally? An analysis of massive transfusion ratios in the nontrauma setting. Crit Care Med. 2017;45:1311–6. doi: 10.1097/CCM.0000000000002498. [DOI] [PubMed] [Google Scholar]
- 144.Georgiou C. Balloon tamponade in the management of postpartum haemorrhage: a review. BJOG. 2009;116:748–57. doi: 10.1111/j.1471-0528.2009.02113.x. [DOI] [PubMed] [Google Scholar]
- 145.Sathe NA, Likis FE, Young JL, et al. Procedures and uterine-sparing surgeries for managing postpartum hemorrhage: a systematic review. Obstet Gynecol Surv. 2016;71:99–113. doi: 10.1097/OGX.0000000000000273. [DOI] [PubMed] [Google Scholar]
- 146.Johanson R, Kumar M, Obhrai M, Young P. Management of massive postpartum haemorrhage: use of a hydrostatic balloon catheter to avoid laparotomy. BJOG. 2001;108:420–2. doi: 10.1111/j.1471-0528.2001.00102.x. [DOI] [PubMed] [Google Scholar]
- 147.Pileggi-Castro C, Nogueira-Pileggi V, Tunçalp Ö, et al. Non-pneumatic anti-shock garment for improving maternal survival following severe postpartum haemorrhage: a systematic review. Reprod Health. 2015;12:28. doi: 10.1186/s12978-015-0012-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Likis FE, Sathe NA, Morgans AK, et al. Management of postpartum hemorrhage AHRQ Comparative Effectiveness Reviews. Rockville, MD, USA: Agency for Healthcare Research and Quality (US); 2015. [PubMed] [Google Scholar]
- 149.Poujade O, Ceccaldi PF, Davitian C, et al. Uterine necrosis following pelvic arterial embolization for post-partum hemorrhage: review of the literature. Eur J Obstet Gynecol Reprod Biol. 2013;170:309–14. doi: 10.1016/j.ejogrb.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 150.Deffieux X, Vinchant M, Wigniolle I, et al. Maternal outcome after abdominal packing for uncontrolled postpartum hemorrhage despite peripartum hysterectomy. PLoS One. 2017;12:e0177092. doi: 10.1371/journal.pone.0177092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Abdul Sultan A, Grainge MJ, West J, et al. Impact of risk factors on the timing of first postpartum venous thromboembolism: a population-based cohort study from England. Blood. 2014;124:2872–80. doi: 10.1182/blood-2014-05-572834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Abbasi N, Balayla J, Laporta DP, et al. Trends, risk factors and mortality among women with venous thromboembolism during labour and delivery: a population-based study of 8 million births. Arch Gynecol Obstet. 2014;289:275–84. doi: 10.1007/s00404-013-2923-8. [DOI] [PubMed] [Google Scholar]
- 153.Karthikeyan G, Pais P. Clinical judgement & evidence-based medicine: time for reconciliation. Indian J Med Res. 2010;132:623–6. doi: 10.4103/0971-5916.73418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gholitabar M, Ullman R, James D, Griffiths M. Caesarean section: summary of updated NICE guidance. BMJ. 2011;343:d7108. doi: 10.1136/bmj.d7108. [DOI] [PubMed] [Google Scholar]
- 155.Dahlke JD, Mendez-Figueroa H, Maggio L, et al. Prevention and management of postpartum hemorrhage: a comparison of 4 national guidelines. Am J Obstet Gynecol. 2015;213:76.e1–10. doi: 10.1016/j.ajog.2015.02.023. [DOI] [PubMed] [Google Scholar]
- 156.Kacmar RM, Mhyre JM, Scavone BM, et al. The use of postpartum hemorrhage protocols in United States academic obstetric anesthesia units. Anesth Analg. 2014;119:906–10. doi: 10.1213/ANE.0000000000000399. [DOI] [PubMed] [Google Scholar]