Abstract
A 74-year-old man with lung adenocarcinoma recurrence was admitted to our hospital because of dyspnea 7 days after receiving initial immunotherapy with nivolumab. Electrocardiography revealed ST-segment elevation in V1-6 and echocardiography showed a markedly reduced left ventricular ejection fraction of 9% and akinesis of the anteroseptal wall and apex. He died from acute heart failure 3 days after admission. Microscopically, multiple small foci of myocardial necrosis with few inflammatory cells were scattered in both ventricles. Obstruction of the coronary artery was not identified. We believed that the cause of death was acute heart failure possibly due to nivolumab-induced myocardial necrosis.
Keywords: Nivolumab, Cardiotoxicity, Immune-related adverse events
Abbreviations: irAE, Immune-related adverse event; LVEF, Left ventricular ejection fraction; PD-1, Programmed death-1; PD-L1, Programmed death-ligand 1
1. Case report
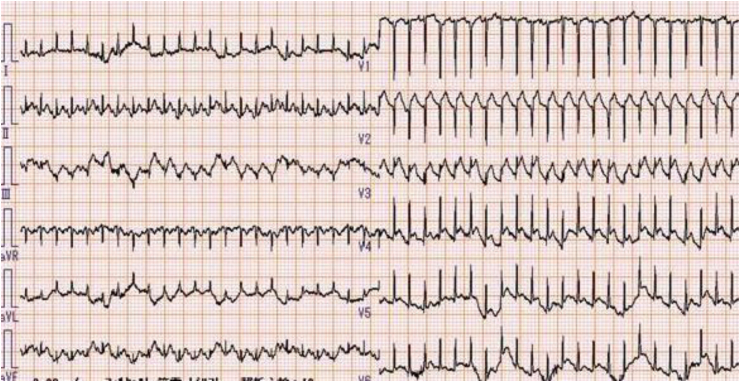
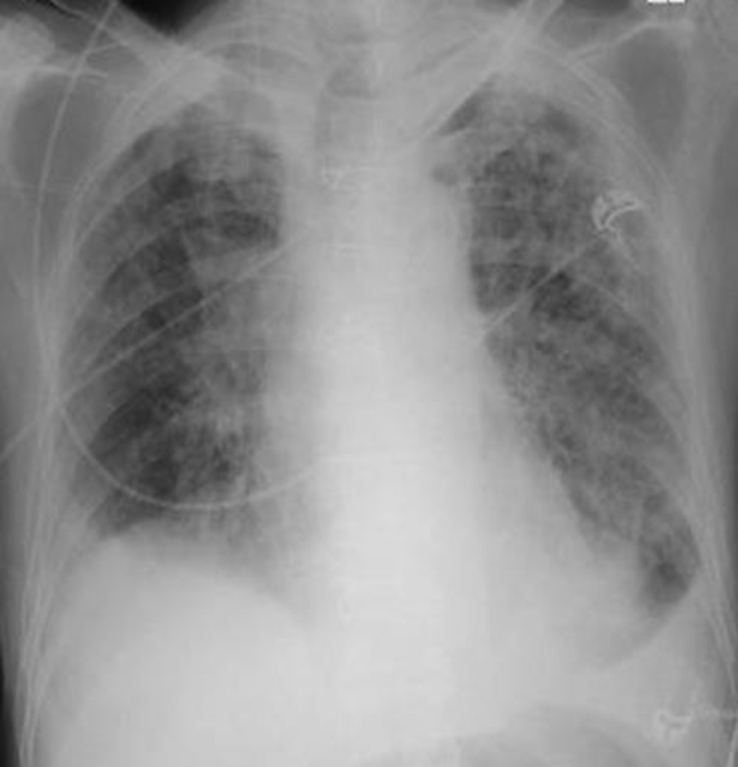
A 74-year-old man underwent lobectomy and lymph node dissection for a primary lung adenocarcinoma (pT1aN3M0, StageⅢB). Computed tomography revealed mediastinal lymph node recurrence 2 months after surgery, and he was treated with 4 cycles of systemic chemotherapy involving carboplatin and pemetrexed. However, as recurrence remarkably progressed after the first-line treatment, he underwent subsequent immunotherapy as second-line treatment and received nivolumab (2 mg/kg body weight) 2 months after the last administration of the chemotherapy drugs. He remained hospitalized for 7 days after the initial nivolumab administration, and blood tests and chest radiography revealed no abnormal findings on the day of discharge. However, he complained of mild general malaise and a decrease in appetite. He was readmitted to our hospital with severe dyspnea 12 hours after discharge. Electrocardiography revealed sinus tachycardia (140 beats/min) and ST-segment elevation in V1-6 (Fig. 1). Echocardiography showed a markedly reduced left ventricular ejection fraction (LVEF) of 9% and akinesis of the anteroseptal wall and apex (Video 1). Chest radiography revealed acute pulmonary edema (Fig. 2). The troponin I level was elevated at 0.40 ng/mL (reference level, <0.03 ng/mL), creatine phosphokinase level peaked at 251 U/L (reference level, 29–168 U/L), creatine kinase-myocardial band level peaked at 35 U/L (reference level, <5.99 U/L), and brain natriuretic peptide level was elevated at 250 pg/mL (reference level, <18.4 pg/mL). An extracorporeal circulation-assisting device was necessary to maintain the hemodynamics although large amount of catecholamine was used; however, he did not desire aggressive lifesaving approaches because of cancer progression died from acute heart failure 3 days after admission. No coronary risk factors were noted, normal cardiac function was observed before the immunotherapy, and no new drug, except nivolumab, was administered within the last 2 months.
Fig. 1.
Electrocardiography on admission, showing sinus tachycardia (140 beats/min) and ST-segment elevation in V1-6.
Fig. 2.
Chest radiography on admission, showing acute pulmonary edema in both lungs.
Supplementary video related to this article can be found at https://doi.org/10.1016/j.rmcr.2019.100839.
The following is the supplementary data related to this article:
Echocardiography findings. Echocardiography on admission shows a markedly reduced left ventricular ejection fraction of 9% and akinesis of the anteroseptal wall and apex.1
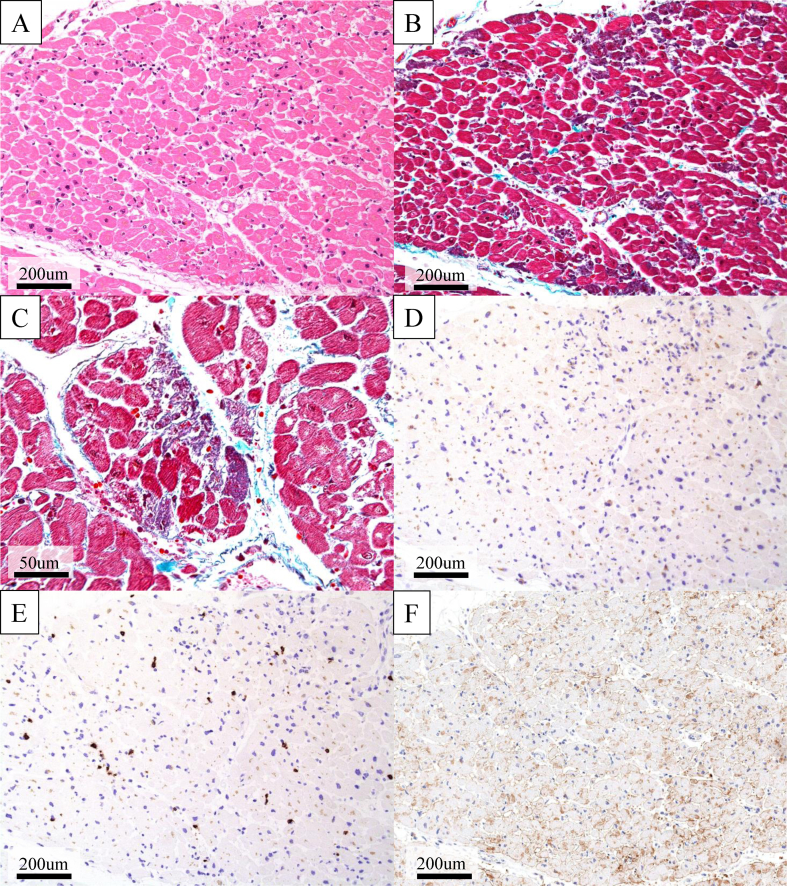
Autopsy revealed multiple small foci of myocardial necrosis with few inflammatory cells scattered in both ventricles (Fig. 3A–E). Overexpression of programmed death-ligand 1 (PD-L1) was not found in cardiomyocytes (Fig. 3F). Additionally, atherosclerotic or thrombotic obstruction of the coronary artery and immune-related pathological findings, such as rhabdomyolysis, were not found. Therefore, we believed that the cause of death was acute heart failure possibly due to nivolumab-induced myocardial necrosis.
Fig. 3.
Histopathological findings on autopsy. (A) Hematoxylin-eosin staining and (B, C) Elastica-Masson staining. Multiple small foci of myocardial necrosis are scattered in the left ventricle (B; lower magnification, C; higher magnification). (D–E) Few inflammatory cells infiltrate in the necrotic area of the myocardium (D; CD4 E; CD8). (F) Programmed death-ligand 1 (PD-L1; 22C3) staining. Overexpression of PD-L1 is not seen in cardiomyocytes.
2. Discussion
Immune checkpoint inhibitors enhance anti-tumor responses, but they can cause unwanted side effects. A previous study reported irAEs in various organs [1]. Cardiotoxicity is not a common irAE, but several recent articles have mentioned the occurrence of myocarditis and acute heart failure in cancer patients treated with immune checkpoint inhibitors shown in Table 1 [[2], [3], [4], [5], [6]]. According to the Bristol–Myers Squibb corporate pharmacovigilance database up to August 2016, 18 drug-related myocarditis were reported among 20,594 patients (0.09%). Additionally, only 1 patients treated with nivolumab alone had fatal myocarditis [7].
Table 1.
Summary of review articles reporting ICI-associated cardiotoxicities.
| Author | No. of cases | Cardiac toxicity | Time of onset | Treatment | Outcome |
|---|---|---|---|---|---|
| Heinzerling (2016) | 8 | Various | 16 weeks (median) | Steroids (63%) | 3 patients (38%) died of a side effect |
| Escudier (2017) | 30 | Various | 65 days (median) | Not listed | 8 patients (27%) died of a cardiovascular event |
| Moslehi (2018) | 101 | Myocarditis | 27 days (median) | Not listed | 46 patients (46%) died of severe myocarditis |
| Mahmood (2018) | 35 | Myocarditis | 34 days (median) | Steroids (89%) | 6 patients (17%) died of a cardiovascular event |
| Yang (Case reports review) (2018) | 13 | Myocarditis, Pericarditis, Takotsubo cardiomyopathy | 15 days- 24 weeks | Steroids (92%) | 2 patients (15%) died of myocarditis |
Studies on programmed death-1 (PD-1) revealed its involvement in cancer escape from the host immune system [8,9] and the suppression of immune cell-mediated inflammation [10,11]. One study showed fatal myocarditis with infiltration of T cells in the myocardium and a high titer of autoantibodies against cardiac myosin in PD-1-deficient MRL mice [12]. Additionally, a recent case report on immunotherapy-induced myocarditis showed significant infiltration of T cells and macrophages in the myocardium, suggesting that T cells drive the immunotherapy reaction [13].
On the other hand, in our case, multiple small foci of myocardial necrosis with few inflammatory cells (macrophages and lymphocytes) were scattered in both ventricles. The LVEF reduced enough to cause multiple organ dysfunction. These clinical and pathological findings were significantly different from those of previously reported immunotherapy-induced myocarditis, suggesting that the cardiotoxicity in the present patient was caused by a mechanism different from that associated with previously reported myocarditis.
3. Conclusion
We experienced a case of fatal acute heart failure caused by myocardial necrosis possibly associated with nivolumab-induced cardiotoxicity. Cardiotoxicity is a rare irAE, but it sometimes can be fatal. The mechanism of cardiotoxicity should be clarified, and cardiac function should be assessed and monitored following immune checkpoint inhibitor therapies to prevent severe irAEs.
Conflicts of interest
The authors have declared no conflict of interest.
Informed consent
Informed consent was obtained from the patient/family.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgements
Writing, review and/or revision of the manuscript: TS, KY, TH, TY, KT, MM, and SI; administrative, technical, or material support: KY and MM; study supervision: KY, MM and SI.
We thank Dr. Masashi Mikubo for immunohistochemistry staining.
References
- 1.Michot J.M., Bigenwald C., Champiat S. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur. J. Cancer. 2016;54:139–148. doi: 10.1016/j.ejca.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 2.Heinzerling L., Ott P.A., Hodi F.S. Cardiotoxicity associated with CTLA4 and PD1 blocking immunotherapy. J. Immunother. Canc. 2016;4 doi: 10.1186/s40425-016-0152-y. 50-5016-0152-y. eCollection 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Escudier M., Cautela J., Malissen N. Clinical features, management, and outcomes of immune checkpoint inhibitor-related cardiotoxicity. Circulation. 2017;136(21):2085–2087. doi: 10.1161/CIRCULATIONAHA.117.030571. [DOI] [PubMed] [Google Scholar]
- 4.Moslehi J.J., Salem J.E., Sosman J.A., Lebrun-Vignes B., Johnson D.B. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet. 2018;391(10124) doi: 10.1016/S0140-6736(18)30533-6. 933-6736(18)30533-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahmood S.S., Fradley M.G., Cohen J.V. Myocarditis in patients treated with immune checkpoint inhibitors. J. Am. Coll. Cardiol. 2018;71(16):1755–1764. doi: 10.1016/j.jacc.2018.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang S., Asnani A. Cardiotoxicities associated with immune checkpoint inhibitors. Curr. Probl. Cancer. 2018;42(4):422–432. doi: 10.1016/j.currproblcancer.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Johnson D.B., Balko J.M., Compton M.L. Fulminant myocarditis with combination immune checkpoint blockade. N. Engl. J. Med. 2016;375(18):1749–1755. doi: 10.1056/NEJMoa1609214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishimura H., Okazaki T., Tanaka Y. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291(5502):319–322. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 9.Chikuma S., Terawaki S., Hayashi T. PD-1-mediated suppression of IL-2 production induces CD8+ T cell anergy in vivo. J. Immunol. 2009;182(11):6682–6689. doi: 10.4049/jimmunol.0900080. [DOI] [PubMed] [Google Scholar]
- 10.Iwai Y., Ishida M., Tanaka Y., Okazaki T., Honjo T., Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl. Acad. Sci. U. S. A. 2002;99(19):12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okazaki T., Tanaka Y., Nishio R. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat. Med. 2003;9(12):1477–1483. doi: 10.1038/nm955. [DOI] [PubMed] [Google Scholar]
- 12.Wang J., Okazaki I.M., Yoshida T. PD-1 deficiency results in the development of fatal myocarditis in MRL mice. Int. Immunol. 2010;22(6):443–452. doi: 10.1093/intimm/dxq026. [DOI] [PubMed] [Google Scholar]
- 13.Tadokoro T., Keshino E., Makiyama A. Acute lymphocytic myocarditis with anti-PD-1 antibody nivolumab. Circ. Heart Fail. 2016;9(10) doi: 10.1161/CIRCHEARTFAILURE.116.003514. 10.1161/CIRCHEARTFAILURE.116.003514. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Echocardiography findings. Echocardiography on admission shows a markedly reduced left ventricular ejection fraction of 9% and akinesis of the anteroseptal wall and apex.1