Abstract
Oxidative stress plays a significant role in the pathophysiology of numerous kidney diseases, generally mediated by reactive oxygen species (ROS). Arsenic (Ar) is known to exert its toxicity through the generation of ROS and inflammation. The current study investigates the protective effects of sulforaphane (SFN) against arsenic-induced renal damage via PI3K/Akt-mediated Nrf2 pathway signaling. Thirty-two male albino Wistar rats were randomly divided into four groups of eight animals each, designated as control, arsenic (Ar), sulforaphane plus Ar (SFN+Ar), and sulforaphane alone (SFN), with oral administration of Ar (5 mg/kg BW) and SFN (80 mg/kg BW) daily for 28 days. Ar administration significantly (P < 0.05) increased the levels of ROS, OHdG, Ar accumulation, and lipid peroxidation, and decreased levels of enzymatic and nonenzymatic antioxidants. Notably, a significant (P < 0.05) increase was observed in markers of apoptosis, DNA damage, TUNEL-positive cells, and dark staining of ICAM-1 in renal tissue with decreased PI3K/Akt/Nrf2 gene expression. The biochemical findings were supported by histopathological and electron microscopy evaluation, which showed severe renal damage in rats treated with Ar. Pretreatment with SFN significantly (P < 0.05) attenuated renal ROS, OHdG, lipid peroxidation, and DNA damage, and increased phase II antioxidants via PI3K/Akt-mediated Nrf2 activation in renal tissue. These results show that dietary supplementation with SFN protects against Ar-induced nephrotoxicity via the PI3K/Akt-mediated Nrf2 signaling pathway in the rat kidney.
Keywords: Sulforaphane, Arsenic, Rat, Nephrotoxicity, PI3K/Akt, Nrf2 pathway
Introduction
Arsenic (Ar) is a major toxicant that is ubiquitous in the environment, and a high level of exposure carries an increased risk of cancers of the skin, liver, lung, and kidney, and cardiovascular and reproductive abnormalities (Yu et al. 2013). Sources of human exposure to heavy metals and metalloids such as arsenic, lead, cadmium, and uranium include both industrial and environmental surroundings. Among many toxicants, arsenic ranks as an important antineoplastic agent that has been used medicinally to treat a variety of ailments for more than 2400 years (Smeester et al. 2011). More than 200 million people worldwide are at risk of arsenic poisoning, among which 100 million live in West Bengal, India (Council 2001), where groundwater arsenic absorption exceeds the maximum permissible level of 50 μg/L, according to the World Health Organization (WHO). Ar generally enters the body via the ingestion of contaminated drinking water, and long-term consumption of Ar-contaminated water results in Ar toxicity in nearly all organ systems of the body (Mandal and Suzuki 2002).
Consumption of arsenic, either through the supply of drinking water or medicinal administration, may cause renal damage. The kidney is a target organ for Ar toxicity, as it has been reported that around 60% of the daily dose of arsenic is excreted through urine. Chronic arsenic exposure has been found to cause alterations in the kidney, with tubular cell vacuolation, interstitial nephritis, and glomerular enlargement (Waalkes et al. 2004a). The most sensitive part of the kidney is the proximal convoluted tubule of epithelial cells with resorptive ability, and the first anatomical segment exposed to filtered toxicants (Peraza et al. 2006). Chronic kidney disease as demonstrated by cloudy urine, proteinuria, glycosuria, and renal cysts is not unusual in arsenic-exposed humans (Council 2001).
A growing body of evidence indicates that numerous mechanisms are involved in heavy metal-induced toxicity, among which the most well known is the generation of free radicals (Shanmugam et al. 2016). While the mechanisms of Ar-induced toxicity are not clearly defined, oxidative stress is generally acknowledged as the underlying mechanism for Ar-induced renal damage (Flora 2011; Yu et al. 2013). Ar induces ROS cycling between oxidation states of metals such as Ar and Fe, or interacts with antioxidants and inflammation, resulting in the accumulation of ROS in cells (Halliwell and Whiteman 2004). Yamanaka et al. (1990) demonstrated that the main mechanism of Ar pathogenesis is associated with reduced antioxidant capacity, leading to cellular macromolecule dysfunction. Ar interference with glutathione (GSH) metabolism initiates depletion of cellular sulfhydryl groups, an essential pathway for arsenic-induced kidney damage (Prabu and Muthumani 2012). However, synthetic chelators exhibit toxic side effects (Gupta et al. 2005). This has led to increased interest in the use of natural dietary supplements with therapeutic potential, free radical scavenging activity, and antioxidant properties against Ar-induced nephrotoxicity (Manna et al. 2008).
Natural antioxidants can help to overcome oxidative stress and free radical-mediated disorders (Thangapandiyan and Miltonprabu 2014). Sulforaphane (SFN) is a naturally occurring isothiocyanate found in cruciferous vegetables such as broccoli, brussels sprouts, and cabbage (Fahey and Talalay 1999; Dinkova-Kostova and Talalay 2008). Innovative research work has confirmed that SFN protects against renal fibrosis (Shin et al. 2010) and intestinal damage (Yoon et al. 2008), and prevents cardiovascular disease (Davis et al. 2009). In addition, there is growing evidence from a range of in vitro studies and animal cancer models that SFN represents a powerful natural antioxidant against cancer (Jeffery and Keck 2008). Likewise, in vivo epidemiological studies have found that consumption of cruciferous vegetables is associated with lower cancer risk in renal, pulmonary, mammary gland, and lymph nodes, highlighting the potential value of SFN in the prevention of a variety of cancers (Cheung and Kong 2010).
Several studies have shown that the PI3K/Akt signaling pathway is involved in the induction of Nrf2/ARE-dependent phase II antioxidant synthesis (Thangapandiyan and Miltonprabu 2014). The PI3K (phosphatidylinositol 3-kinase)/Akt (v-akt murine thymoma viral oncogene homolog 1) is an essential pathway for cell survival, and Akt is activated by PI3K in response to growth factor Ca2+ ions attracted by the phosphate group on the surface of Akt (Yoon et al. 2008). The activated Akt binds with the Keap1 protein of the Nrf2 inhibitor gene and dissociates the Nrf2 from the cytoplasm. The dissociated Nrf2 is translocated to the nucleus to bind with antioxidant response element (ARE) and is involved in the protein synthesis of phase II antioxidants for scavenging of free radicals produced during metabolism of xenobiotic compounds. A wide variety of phytochemicals from natural products, such as epigallocatechin gallate, sulforaphane, and chlorophyllin, protect against oxidative stress-induced cell damage via the PI3K/Akt/Nrf2-dependent pathway (Na et al. 2008).
Negrette-Guzmán et al. (2013) confirmed the protective value of SFN against gentamicin and ischemia/reperfusion renal injury via inhibition of lipid peroxidation and oxidative renal tubular damage in rats. However, the efficacy of SFN against arsenic-induced nephrotoxicity via the PI3K/Akt-mediated Nrf2 pathway has not yet been evaluated. Therefore, our study attempted to demonstrate the molecular therapeutic potential of SFN through serological, biochemical, molecular, and histological analysis to confirm renal protection against Ar toxicity.
Materials and methods
Chemicals
Sodium arsenite (NaAsO2), 1,1,3,3-tetramethoxypropane, and bovine serum albumin were obtained from Sigma-Aldrich (St. Louis, MO, USA). Sulforaphane (SFN) was purchased from Sigma Chemicals (Bangalore). The solvents, chemicals, and other biochemicals were of certified analytical grade and procured from SD Fine chemicals (Mumbai, India) or HiMedia Laboratories (Mumbai). Reagent and kits for this study were obtained from Span Diagnostics, Mumbai, India. Antibodies including caspase-3, caspase-9, cytochrome C, Nrf2, PI3, Akt, and β-actin were purchased from Cell Signaling Technology (Bangalore, India) and Santa Cruz Biotechnology (Santa Cruz, CA).
Experimental animals and diet
Male albino Wistar rats, each weighing 160–200 g, were used for this experiment. They were procured from Animal Science and Veterinary University Mannuthy, Kerala, India. The rats were maintained in a controlled environment under standard conditions of temperature (28 ± 2 °C) and humidity with an alternating light and dark cycle. The animals were fed with commercially available pelleted rat chow (Champaka Feed, Bangalore, India) and water ad libitum. After a week of acclimatization, rats were divided into control and test groups. Eight rats were used in each treatment group. The study was approved by the Institutional Animal Ethics Committee and carried out in accordance with the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Bharathiar University (ref. no BU/IAEC/2017/03), Tamilnadu, India.
Drug treatment
In the current study, NaAsO2 (sodium arsenite) was administered intragastrically at a dose of 5 mg/kg body weight each day for 4 weeks, which is one-eighth of the oral LD50 values in rats (El-Demerdash et al. 2009). SFN fine particles were suspended in double-distilled water and administered intragastrically 90 min prior to the administration of Ar at a dose of 20, 40, or 80 mg/kg/BW daily for 4 weeks.
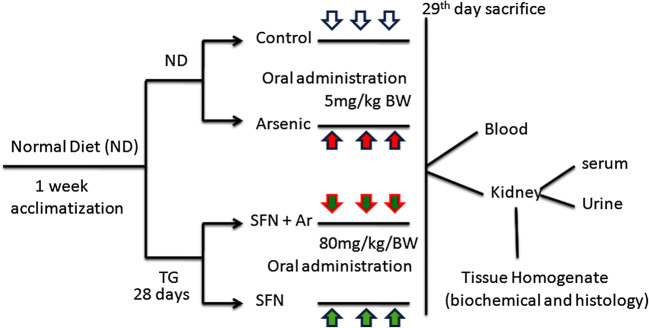
Experimental design (Fig. 1)
Fig. 1.
Experimental design of the study. ND: normal diet. TG: treatment group
An initial investigation was performed with three different doses of SFN (20, 40, and 80 mg/kg/BW) to determine the effective dose for Ar-treated rats. We found that SFN 80 mg/kg BW (body weight) notably reduced the levels of AST, ALT, LDH, and GGT in the serum of arsenic-treated rats in comparison to the other two doses (20 and 40 mg/kg BW). Therefore, we selected 80 mg/kg BW for this study. After an acclimatization period, the animals were divided into four groups of eight rats each (Fig. 1), as follows:
Group 1: normal rats (control)
Group 2: administration of arsenic alone (5 mg/kg BW)
Group 3: treatment with SFN + Ar (80 mg/kg BW + 5 mg/kg BW
Group 4: treatment with SFN alone (80 mg/kg BW)
The total study duration was 4 weeks. Water and food intake was documented regularly. At the end of the period (28 days), the rats were starved overnight and sacrificed under light ether anesthesia by cervical decapitation, and the abdomen was opened. The renal tissue was excised, weighed, and washed in ice-cold saline. The tissue was then pulverized and homogenized in Tris–HCl buffer and centrifuged at 8000×g for 10 min. The major plain supernatant was stored and utilized for various biochemical and molecular assays.
Biochemical analysis
Determination of ROS in blood and tissue
The amount of ROS in blood and renal tissue was quantified by 2′,7′-dichlorofluorescin diacetate (DCF-DA), as outlined previously (Socci et al. 1999).
Estimation of kidney serum markers
The urea, uric acid, and creatinine in serum was quantified spectrophotometrically using a commercial diagnostic kit (Sigma Diagnostics India Pvt. Ltd., Baroda, India). Creatinine clearance as an indicator of the glomerular filtration rate in serum was estimated from serum creatinine in a 24-h urine sample. Bilirubin in serum was calculated using a commercial kit (Medsource Ozone Biomedicals) according to a previously described method (Jung 2008).
Determination of 8-OHdG in urine and kidneys
The concentration of 8-OHdG in the urine and kidney was determined using a highly sensitive ELISA kit (Cell Biolabs, San Diego, CA, USA).
Estimation of arsenic concentration
The arsenic concentration in the kidney was calculated using a hydride vapor generation system (PerkinElmer MHS-10) fitted with an atomic absorption spectrophotometer (AAS, PerkinElmer AAnalyst 100), as described previously (Parker et al. 1967).
Determination of lipid peroxidation markers in kidneys
Lipid peroxidation in renal tissue was estimated calorimetrically by quantification of thiobarbituric acid reactive substances (TBARS) and lipid hydroperoxides (LOOH), as reported previously (Niehaus and Samuelsson 1968; Jiang et al. 1992). Protein carbonyl content (PCC) in the kidney was measured using a spectrophotometric method as described previously (Levine et al. 1990).
Determination of nonenzymatic antioxidants
Reduction of glutathione (GSH) was performed using a previously described technique (Moron et al. 1979) based on reaction with Ellman’s reagent (19.8 mg dithionitrobisbenzoic acid in 100 ml of 0.1% sodium citrate). Total sulfhydryl groups (TSH) in the kidney tissue was estimated following the reaction with dithionitrobisbenzoic acid using the method as previously described (Ellman 1959). Vitamin C and vitamin E levels were calculated according to methods described by Omaye et al. (1979) and Desai (1984), respectively.
Determination of enzymatic antioxidants
The superoxide dismutase (SOD) level was estimated using the method described by Kakkar et al. (1984) in which inhibition of the formation of NADPH-phenazine methosulphate nitroblue tetrazolium formazan was quantified spectrophotometrically at 560 nm. The catalase (CAT) level was measured calorimetrically using dichromate acetic acid reagent, as previously reported (Sinha 1972). Glutathione peroxidase (GPX) activity was estimated following Rotruck et al. (1973) based on the reaction between glutathione remaining after the action of GPX and 5,5′-dithiobis (2-nitrobenzoic acid) to form a complex that absorbs maximally at 412 nm. The glutathione S-transferase (GST) level was measured spectrophotometrically by the method described by Habig et al. (1974) using dichloro-2,4-dinitrobenzene as the substrate. The glutathione reductase (GR) activity was assayed according to the method described by Griffith (1980) using NADPH to convert metabolized glutathione (GSSG) to the reduced form.
Quantitative real time-PCR analysis
RNA was isolated from rat kidney (100 mg) using TriZol Reagent (TaKaRa, Japan) following using the PrimeScript™ RT reagent kit with gDNA Eraser (TaKaRa, Japan), qPCR of kidney was carry out using SYBR Green reagent (TaKaRa, Japan) on PCR detection system (ABI 7500, Applied Biosystems, USA). Rat mRNA from kidney was isolated using TRI Reagent (Sigma-Aldrich) and assayed with TaqMan control reagents (ABI PRISM 7700, Applied Biosystems, Foster City, CA, USA). The following primer pairs were used for this analysis: SOD1: forward: 5′ TAACTGAAGGCCAGCATGGG 3′; reverse: 5′ CATGGACCACCATTGTACGG3′; CAT: forward: 5′ CACTCAGGTGCGGACATTCT 3′; reverse: 5′ TCCGGAGTGGGAGAATCCAT3′; Bax: forward: 5′ GGATGGCTGGGGAGACACCTGAG3′; reverse: 5′ CGGCCCCAGTTGAAGTTGCCATCAG 3′; Bcl-2: forward:5′ TGAGAGCAACCGAACGCCCG3′; reverse:5 ′GCACCCAGAGTGATGCAGGCC 3′; β actin: forward: 5′ AGCCTTCC TTCTTGGGTATGGAATC 3′; reverse: 5′ GGAGCAATGATCTT GATCTTCATGG-3′. These primers were designed using Primer3 and synthesized by Integrated DNA Technologies, Inc. (IDT, Coralville, IA, USA). All real-time PCR assays were performed in triplicate. Relative quantitative analysis was carried out by comparing threshold cycle numbers for target genes and a reference β-actin mRNA.
Immunoblotting technique
The kidney tissue (100 mg) was prepared by homogenization in RIPA lysis buffer (50 mM Tris– HCl, pH 7.4; 150 mM NaCl, 0.25% deoxycholic acid, 1% NP-40, and 1 mM EDTA) containing complete EDTA-free protease inhibitor cocktail (Thermo Scientific, USA). The protein was separated by SDS-PAGE and transferred onto polyvinylidene difluoride (PVDF) membranes. The membranes were incubated with blocking buffer (5% (w/v) skim milk powder in 1× TBS containing 0.1% Tween-20 for 1 h, then incubated with rabbit polyclonal antibodies for (proapoptotic markers) anti-caspase-3, anti-caspase-9, cytochrome-C, anti-PI3K, anti-Akt, anti-Nrf2, and β-actin overnight at 4 °C (Santa Cruz Biotechnology, CA, USA). Membranes were washed and incubated for 1 h at room temperature with HRP-linked secondary antibodies. After washing, membranes were identified by enhanced chemiluminescence (ECL) kit and exposed to X-ray films (Kodak, India). The band intensities were quantified by densitometry analysis using an image analysis system (ImageJ; NIH, Bethesda, MD, USA). The marks were stabilized to the β-actin appearance in each group (mean ± SD) as a percentage of control. No differences were observed in β-actin manifestation between the tentative animal groups.
TUNEL assay
The paraffin pieces were dewaxed, and in situ detection of apoptosis in the renal tissue (100 mg) was performed by terminal deoxynucleotidyl transferase-mediated uridine triphosphate nick-end labeling (TUNEL) assay according to the instructions of the manufacturer (Roche Diagnostics, Risch-Rotkreuz, Switzerland) and the number of apoptotic cells, as defined by chromatin condensation of nuclear fragmentation (apoptotic bodies), was counted. After TUNEL labelling, nuclei were labeled with Hoechst 33258 (Molecular Probes, Invitrogen) and the TUNEL-positive kidney cells were observed using an Olympus IX81 fluorescence microscope with a 20× objective at 2048 × 2048 resolution. The morphometric examination was performed by two independent, blinded investigators. The average number of apoptotic cells in each group was calculated by taking the average of TUNEL-positive apoptotic cells in 10 fields from each kidney sample with 200× magnification.
Immunohistochemistry for renal ICAM-1
Kidney tissues (100 mg) were fixed in 4% buffered formalin. Paraffin-embedded sections were cut, deparaffinized, and hydrated in descending gradations of ethanol, followed by antigen retrieval with citrate buffer. Next, sections were incubated in 0.3% H2O2 in PBS to block endogenous peroxidase activity. The sections were then incubated with anti-intercellular adhesion molecule 1 (ICAM-1) (R&D Systems, Minneapolis, MN, USA) overnight at 4 °C in a moist chamber. Biotinylated secondary antibodies and ABC reagent were added according to manufacturer instructions (Vector Laboratories, Burlingame, CA, USA). Color development was induced by incubation with a DAB kit (Vector laboratories) for 3–5 min, and specific staining was visualized by light microscopy, as described by Pan et al. (2009). After ICAM-1 immunostaining, nuclei were counter-stained using Vector Nuclear Fast Red H-3403 (Vector Laboratories).
Light microscopy examination
For the histopathology investigation, the kidney tissues were dissected and fixed in Bouin’s solution for 14–18 h, processed in a series of graded ethanol to xylene, and embedded in paraffin wax. The paraffin sections were cut at 5–6 μm thickness using a rotary microtome and stained with hematoxylin and eosin for light microscopy examination. The slices were viewed and photographed under an Olympus BX51 light microscope (Olympus, Tokyo, Japan) with an attached digital camera (Olympus C-5050, Tokyo, Japan).
Transmission electron microscopy study (TEM study)
For electron microscopy, kidney tissues were fixed with 2.5% glutaraldehyde in 0.1 M sodium phosphate buffer (pH 7.2) for 3 h at 4 °C, washed in the same buffer for 1 h at 4 °C, and post-fixed with 1% osmium tetroxide in sodium phosphate buffer for 1 h at 4 °C. The tissues were then dehydrated in a graded series of ethanol starting at 50% each step for 10 min and after two changes in propylene oxide. The tissue specimens were embedded in araldite. Ultrathin sections were prepared with magnesium uranyl acetate and lead citrate for the electron microscopic (Jeol JEM-1010) evaluation.
Statistics
Data were analyzed by one-way analysis of variance (ANOVA) followed by Duncan’s multiple range test (DMRT) using a commercially available statistics software package (SPSS for Windows, version 13.0; SPSS Inc., Chicago, IL, USA). Results are expressed as mean ± SD. Values of p < 0.05 were regarded as statistically significant.
Results
SFN ameliorates Ar-induced alterations in body weight, organ weight, and food and water intake
The specific indicators of oxidative stress include body weight, organ weight, and food and water intake. Table 1 illustrate the effects of Ar and SFN on body weight, organ weight ratio (%), and food and water intake in control and experimental rats. In Ar-treated rats, water and pellet consumption and body weight decreased significantly (P < 0.05) with increased organ weight ratio when compared to the control group. However, pretreatment with SFN significantly (P < 0.05) reduced food and water intake, body weight, and organ weight ratio as compared with the Ar-treated group. Administration of SFN alone had no effect on those parameters in the control group.
Table 1.
Effect of SFN on Ar-induced body weight, absolute and relative kidney weight, and food and water intake in control and experimental rats
| Group | Body weight | Absolute kidney weight (g) | Relative kidney weight (g/100 g BW) | Food intake (g/ 100 g BW/day | Water intake (mL/rat/day) | ||
|---|---|---|---|---|---|---|---|
| Initial (g) | Final (g) | %Change | |||||
| Control | 160.00 ± 0.87 | 170.00 ± 4.50 | 10.66 ± 0.47a | 1.47 ± 0.04a | 0.59 ± 0.06a | 13.17 ± 1.19a | 21.18 ± 4.09a |
| Ar | 150.00 ± 4.34 | 144.00 ± 2.56 | 6.54 ± 0.42b | 1.34 ± 0.02b | 0.45 ± 0.07b | 8.20 ± 0.93b | 17.42 ± 1.48b |
| SFN + Ar | 155.00 ± 1.45 | 165.00 ± 2.63 | 9.55 ± 0.57c | 1.58 ± 0.04c | 0.53 ± 0.05c | 10.65 ± 1.20c | 18.60 ± 1.68c |
| SFN | 159.00 ± 2.19 | 172.00 ± 1.76 | 11.21 ± 0.49a | 1.92 ± 0.05a | 0.56 ± 0.07a | 12.83 ± 1.12d | 20.76 ± 1.76d |
Values are given as mean ± SD for eight rats in each group. a-d Values with different superscript letters (a–d) in the same column differ significantly at p < 0.05 (Duncan’s multiple range test)
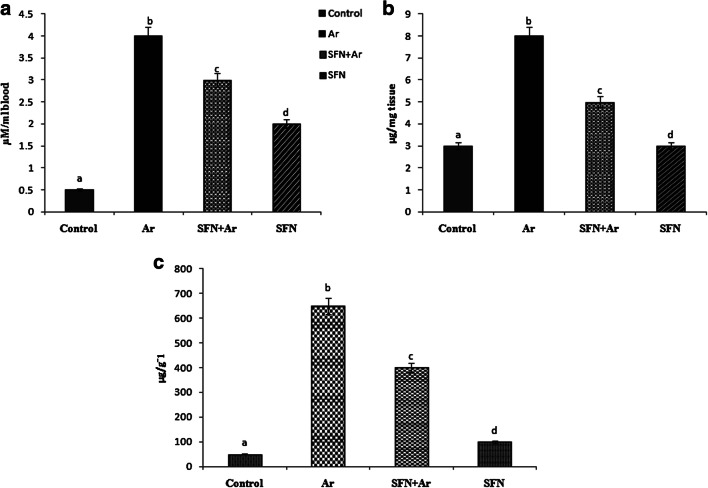
SFN prevents ROS and Ar accumulation in the kidney
The generation of ROS is the primary pathogenic mechanism of Ar-induced oxidative damage to cellular macromolecules. Arsenic treatment of rats resulted in significantly (P < 0.05) increased ROS in blood (Fig. 2a) and renal tissue (Fig. 2b), along with increased Ar levels (Fig. 2c), as compared to controls. Interestingly, SFN pretreatment of Ar-intoxicated rats resulted in a significant (P < 0.05) decrease in levels of ROS and Ar accumulation as compared to the control and Ar groups. Administration of SFN alone produced no change in ROS levels compared to normal control rats.
Fig. 2.
Effect of SFN on ROS levels in blood (a) and kidney (b) and Ar accumulation (c) in the control and experimental rats. Values are expressed as mean ± SD for four groups of eight rats each. Statistical significance was determined by one-way ANOVA (Duncan’s multiple range test). Values not sharing a common superscript letter (a–c) differ significantly at p < 0.05
Ar-induced alterations in kidney serum parameters is attenuated by SFN treatment
The levels of urea, uric acid, creatinine, and bilirubin were determined under Ar and SFN treatment in kidney serum samples depicted in Table 2. Rats treated with Ar exhibited significantly (P < 0.05) improved kidney serum parameters (urea, uric acid, creatinine, and bilirubin) as compared to control and SFN groups. Prior administration of SFN led to a significant (P < 0.05) decrease in the above estimated parameters as compared to the Ar group. No statistically significant difference was observed between the group receiving SFN alone and the control group.
Table 2.
Effect of sulforaphane (SFN) on arsenic-induced renal markers in control and experimental rats
| Group | Control | Arsenic | SFN + Ar | SFN |
|---|---|---|---|---|
| Urea (mg/dL) | 46.95 ± 5.85a | 180.89 ± 18.55b | 71.93 ± 22.51 | 45.93 ± 5.67a |
| Uric acid (mg/dL) | 7.36 ± 0.460a | 20.99 ± 0.942b | 10.28 ± 0.610c | 7.67 ± 0.465a |
| Creatinine (mg/dL) | 0.140 ± 0.0047a | 0.340 ± 0.0017b | 0.191 ± 0.010c | 0.149 ± 0.006a |
| Bilirubin (mg/dL) | 41.15 ± 3.87a | 91.09 ± 6.63b | 47.07 ± 4.14c | 43.09 ± 4.12a |
Values are given as the mean ± SD for eight rats in each group. Values not sharing a common superscript letter (a–c) differ significantly at P < 0.05 (Duncan’s multiple range test)
SFN sulforaphane, Ar arsenic
SFN treatment prevents Ar-induced 8-OHdG in urine and kidney
To investigate how SFN prevents Ar-induced renal damage, 8-OHdG, as a hallmark DNA marker, was measured in control and experimental animals (Fig. 3). There was a significant (P < 0.05) increase in renal 8-OHdG in urine and kidney tissue after Ar exposure when compared to controls. Interestingly, SFN pre-administration significantly (P < 0.05) reduced 8-OHdG in urine and kidney tissue as compared to that of control and SFN groups.
Fig. 3.
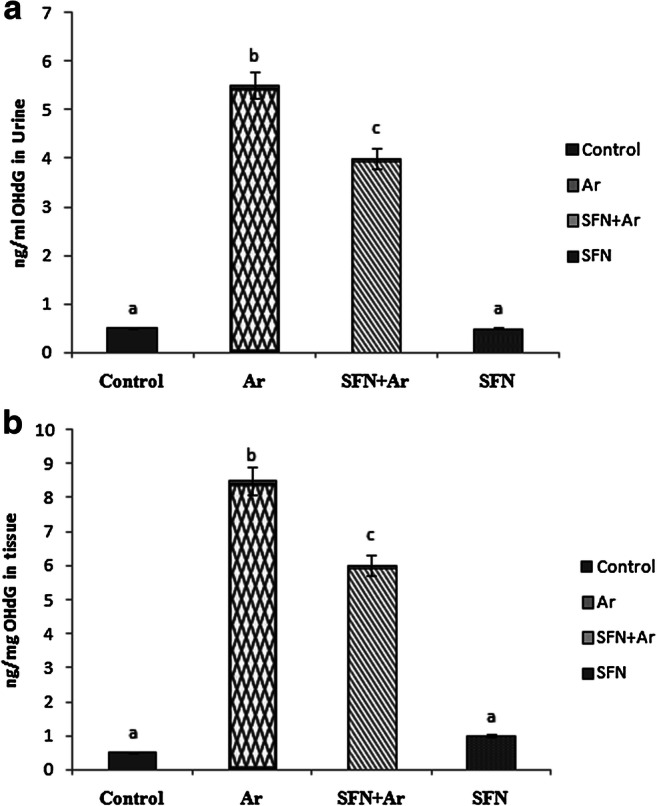
Effect of SFN and Ar on OHdG in the urine and renal tissue of control and experimental rats. Values are expressed as mean ± SD for 4 groups of eight rats in each. Statistical significance was determined by one-way ANOVA (Duncan’s multiple range test). Values not sharing a common superscript letter (a–c) differ significantly at p < 0.05
SFN prevents Ar-induced lipid peroxidation in rat kidneys
Lipid peroxidation is the oxidative degradation of lipids in which free radicals steal electrons from the normal cells. In the Ar-treated group, lipid peroxidation (Fig. 4) markers TBARS (Fig. 4a), LOOH (Fig. 4b), and PCC (Fig. 4c) were significantly (P < 0.05) increased compared with the control group, while SFN pretreatment significantly (P < 0.05) decreased these lipid peroxidation markers in comparison with the Ar group. Rats treated with SFN alone exhibited no significant differences in lipid oxidation markers compared with controls.
Fig. 4.
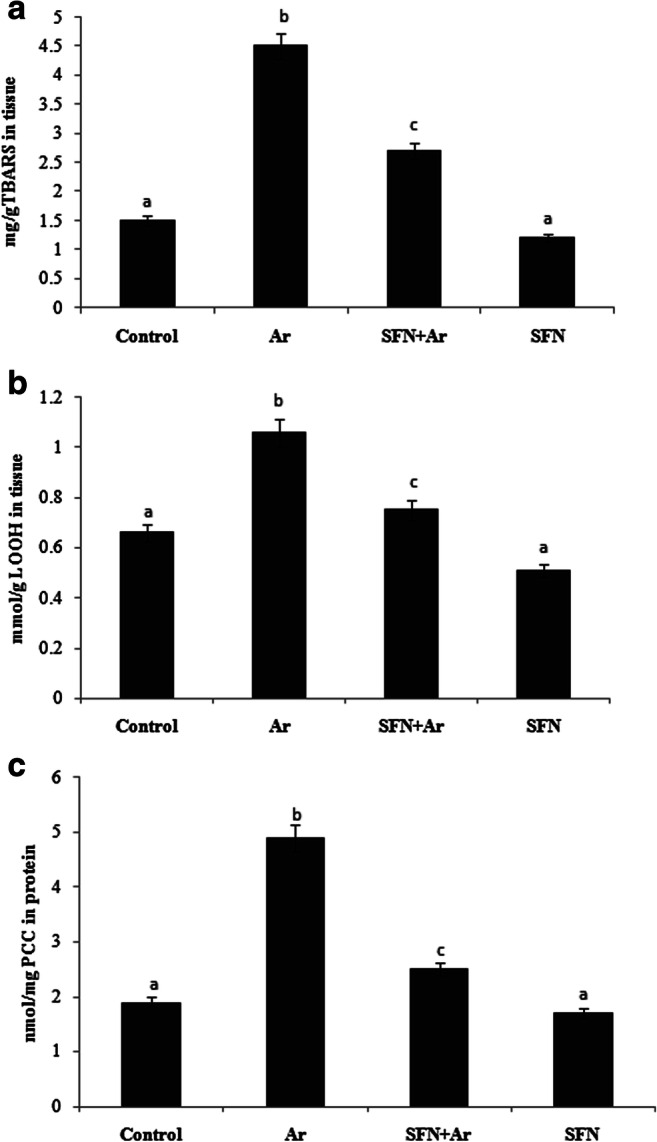
Effect of SFN and Ar in lipid peroxidation markers TBARS, LOOH, and PCC in control and experimental rats. Values are expressed as mean ± SD for four groups of eight rats in each. Statistical significance was determined by one-way ANOVA (Duncan’s multiple range test). Values not sharing a common superscript letter (a–c) differ significantly at p < 0.05
SFN treatment increases nonenzymatic antioxidant levels in rat kidneys
Table 3 shows the nonenzymatic antioxidant levels in control and experimental groups. The levels of GSH, TSH, vitamin C, and vitamin E were significantly (P < 0.05) lower in the arsenic-treated group than the control group. In the group with SFN pre-administration, however, levels of nonenzymatic antioxidants were significantly (P < 0.05) higher than in the Ar-treated group. The SFN and control groups showed similar activity.
Table 3.
Effect of sulforaphane (SFN) on arsenic-induced renal nonenzymatic antioxidant in control and experimental rats
| Group | Control | Arsenic | SFN + Ar | SFN |
|---|---|---|---|---|
| GSH (μg/g protein) | 5.78 ± 0.34a | 12.80 ± 0.14b | 4.53 ± 0.22c | 4.82 ± 0.36d |
| TSH (μg/g protein) | 12.21 ± 0.70a | 9.69 ± 0.50b | 11.99 ± 0.77c | 12.24 ± 0.74a |
| Vitamin C (μmol/mg/tissue) | 0.94 ± 0.09a | 0.57 ± 0.06b | 0.78 ± 0.07c | 0.95 ± 0.06a |
| Vitamin E (μmol/mg/tissue) | 0.58 ± 0.07a | 0.38 ± 0.02b | 0.49 ± 0.03c | 0.64 ± 0.04d |
Values are given as the mean ± SD for eight rats in each group. Values not sharing a common superscript letter (a–d) they differ significantly at P < 0.05 (Duncan’s multiple range test)
SFN sulforaphane, Ar arsenic
SFN treatment increases enzymatic antioxidant levels in rat kidneys
The effect of Ar and SFN on the enzymatic antioxidant levels in the kidney was determined, as shown in Table 4. Activity levels of enzymatic antioxidants SOD, CAT, GPx, GST, and GR in the kidney were significantly (P < 0.05) decreased with Ar administration compared to controls. SFN pre-administration in Ar-treated rats significantly (P < 0.05) increased the levels of enzymatic antioxidants compared with controls and rats treated with Ar alone.
Table 4.
Effect of sulforaphane (SFN) on arsenic-induced renal enzymatic antioxidant levels in control and experimental rats
| Groups | Control | Arsenic | SFN + Ar | SFN |
|---|---|---|---|---|
| SOD | 13.7 ± 0.10a | 9.35 ± 4.29b | 11.4 ± 0.11c | 14.5 ± 0.08a |
| CAT | 46.3 ± 5.5a | 28.7 ± 4.5b | 42.5 ± 4.9c | 47.8 ± 3.8a |
| GPx | 7.37 ± 0.30a | 5.56 ± 0.18b | 6.68 ± 0.41c | 7.24 ± 0.32a |
| GST | 7.19 ± 0.39a | 4.35 ± 0.34b | 5.38 ± 0.27c | 7.33 ± 0.36a |
| GR | 0.52 ± 0.07a | 0.32 ± 0.06b | 0.42 ± 0.04c | 0.56 ± 0.07a |
Values are given as mean ± SD for eight rats in each group. Values with different superscript letters (a–c) in the same column differ significantly at P < 0.05 (Duncan’s multiple range test)
SOD one unit of enzyme activity was defined as the enzyme reaction that gave 50% inhibition of NBT reduction per minute/mg protein, CAT μmol of H2O2 consumed/min/mg protein, GPX μmol of GSH consumed/min/mg protein, GST μmol of CDNB-GSH conjugate formed/min/mg protein, GR μmol of NADPH oxidized/min/mg protein
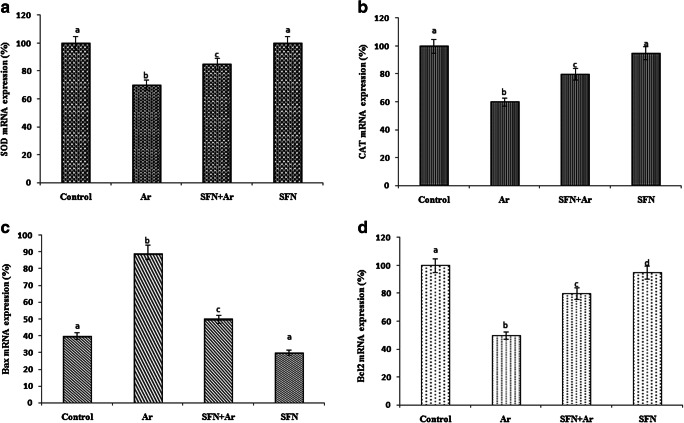
Effect of SFN on RT-PCR analysis
As shown in Fig. 5, there was a significant (P < 0.05) decrease in mRNA including SOD (Fig. 5a), CAT (Fig. 5b), and antiapoptotic B-cell lymphoma 2 (Bcl-2) (Fig. 5d) with an increase in proapoptotic Bax (Fig. 5c) in Ar-intoxicated rats when compared to controls. SFN treatment in Ar-treated rats resulted in a significant (P < 0.05) increase in SOD (Fig. 5a), CAT (Fig. 5b), and Bcl-2 (Fig. 5d), with a decrease in Bax (Fig. 5c), compared to that of controls. However, SFN alone showed no statistically significant difference compared to control.
Fig. 5.
Effect of SFN on the impact of Ar-induced RT-PCR changes in SOD1 (a), CAT (b), Bax (c), and Bad (d) in control and experimental rats. Values are expressed as mean ± SD for four groups of eight rats each. Statistical significance was determined by one-way ANOVA (Duncan’s multiple range test). Values not sharing a common superscript letter (a–c) differ significantly at p < 0.05
SFN pretreatment inhibits apoptotic markers in kidney
Figure 6a represents the western blot analysis of caspase-3, caspase-9, and cytochrome-C in the kidneys of control and experimental groups. Densitometric analysis of western blots is given in Fig. 6b. The expression of Cas-3, Cas-9, and cytochrome C were significantly (P < 0.05) up-regulated in Ar-affected rats when compared with controls. Co-administration of SFN and Ar resulted in significantly (P < 0.05) down-regulated expression of those apoptotic markers when compared to rats treated with Ar alone.
Fig. 6.
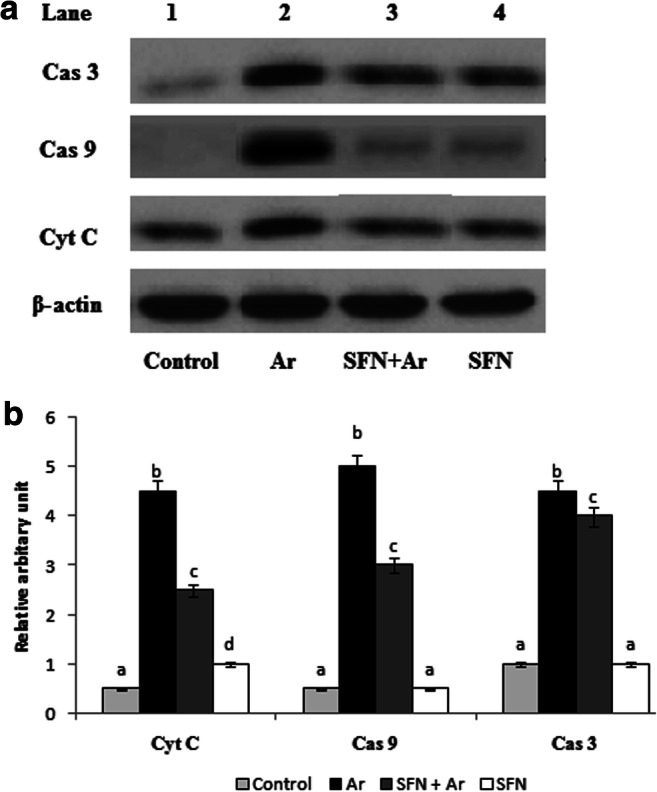
a Effect of SFN on Ar-induced changes in the expression of apoptotic markers Cas3, Cas9, and cytochrome C in control and experimental rats by western blot. Lane 1: control. Lane 2: Ar (5 mg/kg/BW). Lane 3: SFN (80 mg/kg/BW) + Ar. Lane 4: SFN alone. b Effect of SFN on Cas3, Cas9, and cytochrome C protein band intensities scanned by densitometer. Values are expressed as mean ± SD for four groups of eight rats in each. Statistical significance was determined by one-way ANOVA (Duncan’s multiple range test). Values not sharing a common superscript letter (a–c) differ significantly at p < 0.05
Effect of SFN on activation of Nrf2 via PI3K/Akt pathway
The effect of SFN on PI3K/Akt-mediated Nrf2 activation in the kidney of control and Ar-treated rats is presented in Fig. 7a. The expression of PI3/Akt was significantly (P < 0.05) decreased with respect to the decrease in Nrf2 gene expression when compared with controls. Densitometric analysis (Fig. 7b) of western blots revealed significantly (P < 0.05) up-regulated Nrf2 gene expression in Ar-treated rats after SFN exposure. Rats treated with SFN alone also showed significantly increased levels of PI3K when compared to the Ar-treated group.
Fig. 7.
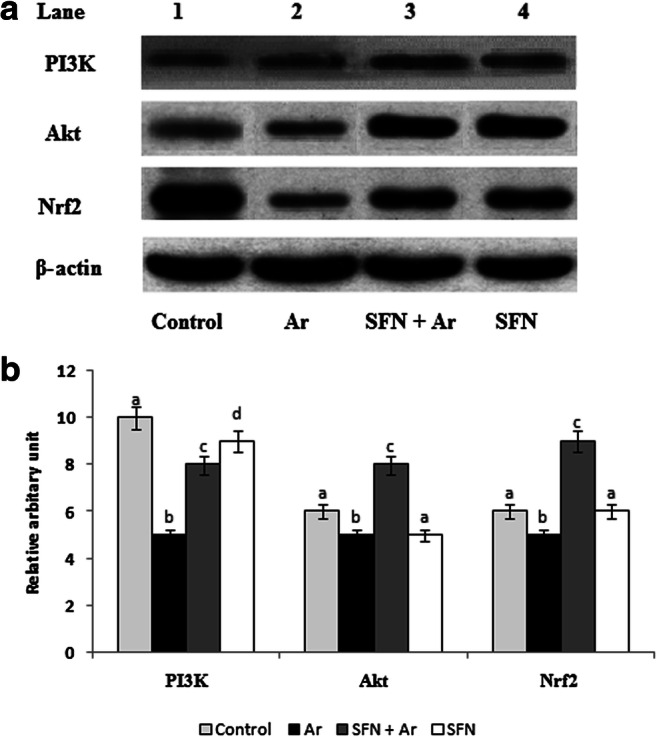
a Effect of SFN on Ar-induced changes in the expression of cell survival proteins Nrf2, Akt, and PI3K in control and experimental rats by western blot. Lane 1: Control. Lane 2: Ar (5 mg/kg/BW). Lane 3: SFN (80 mg/kg/BW) + Ar. Lane 4: SFN alone. b Effect of SFN on Nrf2, Akt, and PI3K protein band intensities scanned by densitometer. Values are expressed as mean ± SD for four groups of eight rats in each. Statistical significance was determined by one-way ANOVA (Duncan’s multiple range test). Values not sharing a common superscript letter (a–c) differ significantly at p < 0.05
SFN prevents Ar-induced cellular death by TUNEL assay
Ar-induced cell death was evaluated by TUNEL assay in control and experimental rats. As shown in Fig. 8, the TUNEL-positive cell numbers and DNA fragmentation were significantly (P < 0.05) increased in the kidneys of Ar-treated rats (Fig. 8b) when compared with controls (Fig. 8a). SFN pretreatment significantly (P < 0.05) prevented Ar-induced enhanced cell death (Fig. 8c) when compared with rats treated with Ar alone and controls. SFN alone had no effect compared with control (Fig. 8d).
Fig. 8.
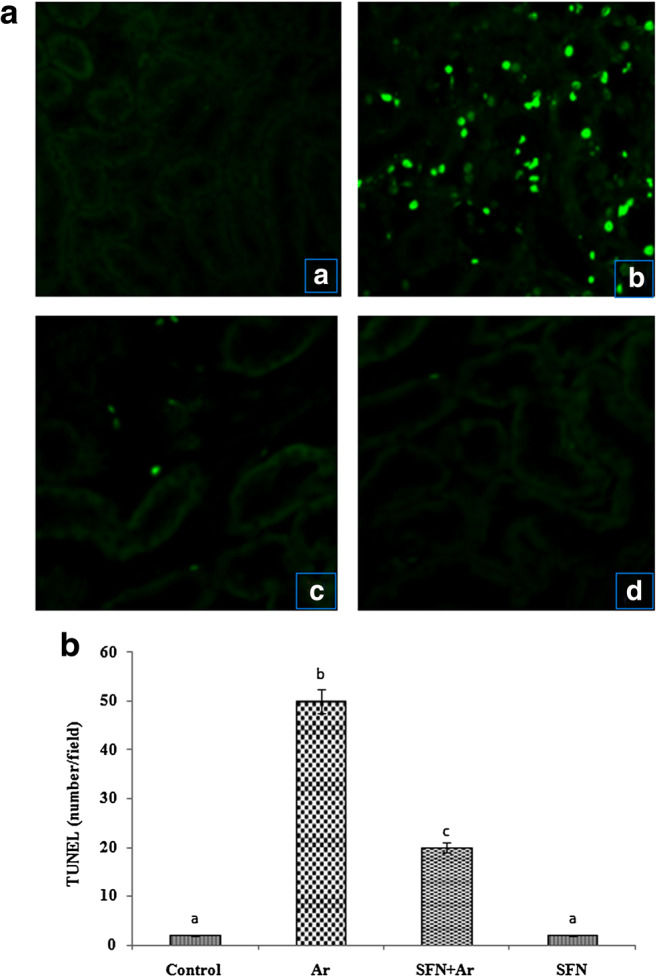
a Effect of SFN on Ar-induced changes in DNA damage in control and experimental rats by TUNEL assay. (A) Control: no DNA damage. (B) Ar (5 mg/kg/BW): DNA damage with TUNEL positive. (C) SFN (80 mg/kg/BW) + Ar: minimal DNA damage. (D) SFN alone: no DNA damage. (b) Effect of SFN on Ar-induced DNA damage band intensities scanned by densitometer. Values are expressed as mean ± SD for four groups of eight rats in each. Statistical significance was determined by one-way ANOVA (Duncan’s multiple range test). Values not sharing a common superscript letter (a–c) differ significantly at p < 0.05
Effect of SFN on Ar-induced renal adhesion molecule (ICAM)
As shown in Fig. 9, Ar treatment significantly (P < 0.05) increased the expression of kidney inflammatory intercellular adhesion molecule (ICAM) (Fig. 9b and c) compared with the control group (Fig. 9a). This increased expression of inflammatory ICAM staining was significantly (P < 0.05) attenuated by SFN pretreatment (Fig. 9d). SFN alone had no effect on ICAM compared with control (Fig. 9e).
Fig. 9.
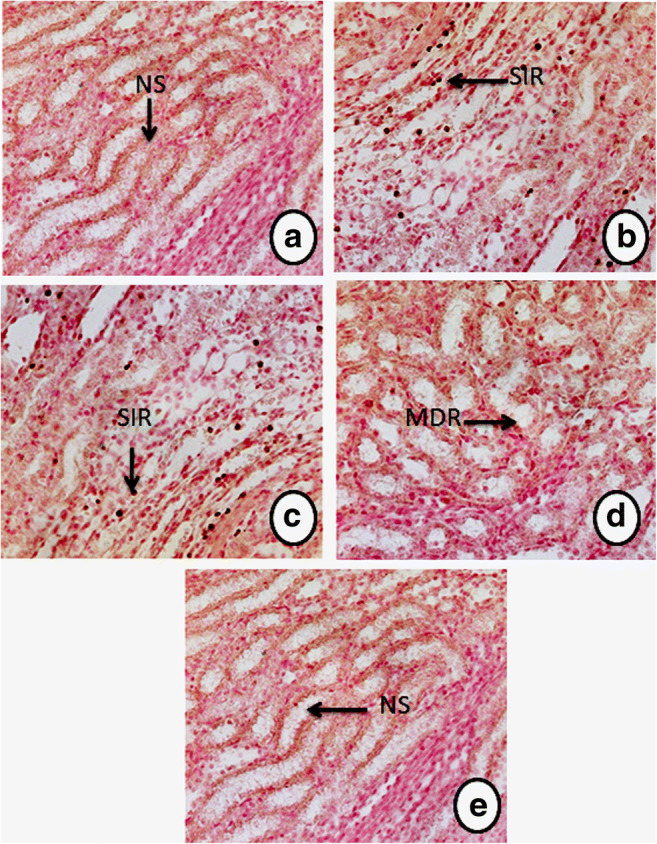
Effect of SFN and Ar on the immunohistochemistry expression of kidney intercellular adhesion molecules (ICAM) in control and experimental rats. a Control rats: no staining (NS) with the ICAM antibody. b, c Ar-treated rats: strong immunoreactivity (SIR) of ICAM antibody in the renal tissue. d Ar-treated rats pre-treated with SFN: markedly decreased immunoreactivity (MDR) of ICAM in renal tissue. e SFN alone: treated rats show tissue appearance similar to controls (NS)
Effect of SFN on Ar-induced histopathological changes
Figure 10 shows the histopathological evaluation of Ar- and SFN-treated control and experimental rats. The kidneys of the control rats (Fig. 10a) and SFN-treated rats (Fig. 10e) show a normal histoarchitecture. Arsenic exposure (Fig. 11b and c) resulted in extensive degeneration of renal tissue, with globular space, tubular degeneration, vacuolization, hemorrhage, glomerular atrophy, sinusoidal dilation, and necrosis in the medullary area of Ar-treated rats. Pre-administration of SFN in Ar-treated rats showed significant attenuation of podocytes to near normal, with no pathological cipher as compared to control and Ar-treated rats (Fig. 10d).
Fig. 10.
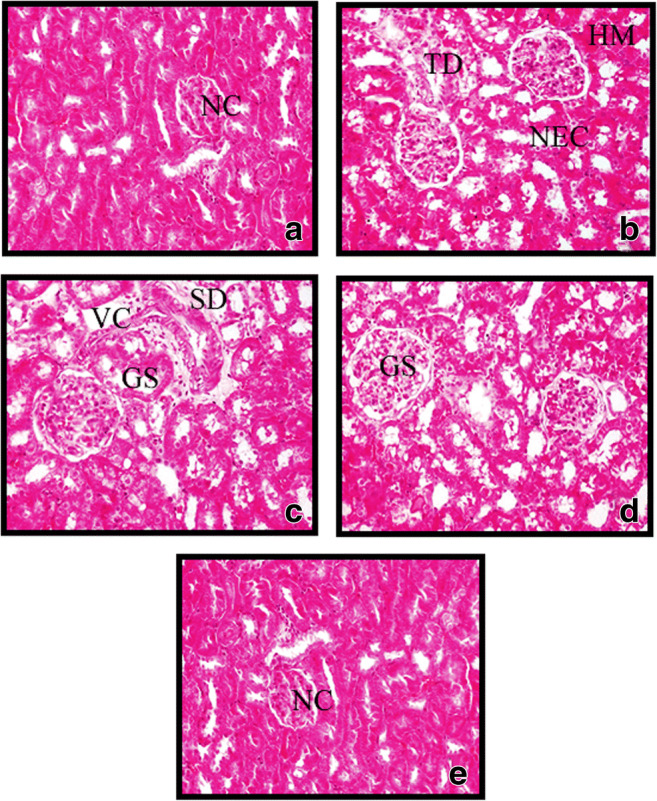
Effect of SFN on the Ar-induced histological changes in the kidneys of control and experimental animals. a Control rats: normal control (NC) renal tissue histology is seen. b, c Ar-treated rats show degenerative changes including glomerular space (GS), tubular degeneration (TD), vacuolization (VC), hemorrhage (HM), glomerular atrophy, sinusoidal dilation (SD), and necrosis (NEC) in the medullary area of Ar-treated rats. d Ar-treated rats with SFN: effective prevention of degenerative changes and shrunken cytoplasm and nuclei of renal tissue. e Rats treated with SFN alone: renal tissue histology similar to control was observed. (H&E, ×40)
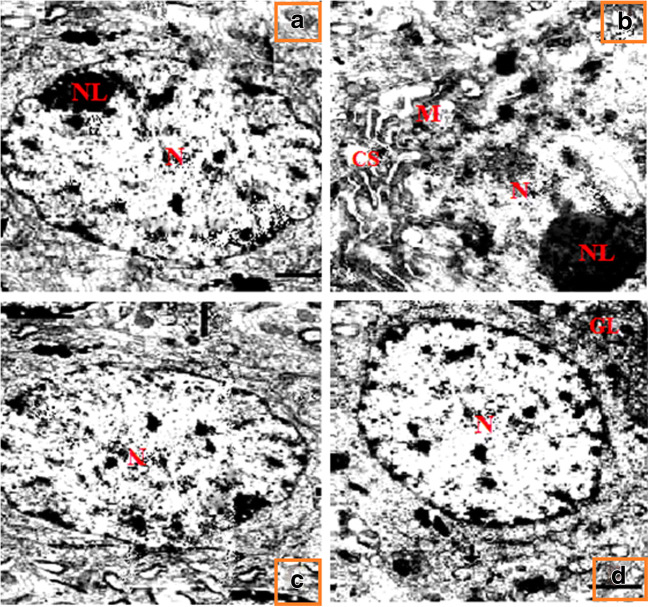
Fig. 11.
Effect of SFN on Ar-induced changes in podocytes by electron microscopy study of control and experimental rats. In the control and SFN groups, the ultrastructure of podocytes was normal (a, d). Ar-treated group showed severe degenerative changes including dilated cisternae of endoplasmic reticulum (CS), mitochondrial swelling (M), and degeneration of the nucleolus (NL) were observed in podocytes of the kidney as compared with controls (b). Pre-administration of SFN was effective in preventing all pathological changes compared with controls and rats treated with SFN alone (c)
Effect of SFN on electron microscopy study
Figure 11 shows the protective effect of SFN on Ar-induced changes in podocytes by electron microscopy study of control and experimental rats. In the control and SFN groups, the ultrastructure of the podocytes was normal (Fig. 11a and d). The Ar-treated group revealed severe degenerative changes including dilated cisternae of the endoplasmic reticulum, mitochondrial swelling, and degeneration of the nucleolus observed in podocytes of the kidney compared with controls (Fig. 11b). Pre-administration of SFN was effective in preventing all pathological changes compared with control group and SFN treatment alone (Fig. 11c).
Discussion
Arsenic (Ar) is known to induce oxidative nephropathy via production of reactive oxygen species (ROS), reactive nitrogen species (RNS), and dimethyl arsenic peroxyl radicals (Flora 2011). Ar is also linked to carcinogenic effects in the skin, lungs, liver, kidneys, and bladder (Waalkes et al. 2004b). Therefore, a key approach for ameliorating Ar-induced renal toxicity is the use of antioxidant agents. Sulforaphane is regarded as a potent natural antioxidant that scavenges superoxide, hydroxyl, hydrogen peroxide, and peroxyl radicals, and also promotes the upregulation of various cytoprotective antioxidant genes (Fahey and Talalay 1999). In this context, the current study also confirmed kidney function against the oxidative toxic insult by Ar.
Ar is known to inhibit growth by interference with a number of metabolic processes (Tu and Ma 2002), and has also been shown to induce death of various cells in vitro and in vivo through activation of apoptosis pathways (Dong 2002). In the present study, we calculated food and water intake and relative and absolute kidney weight of the control and experimental rats, and observed that Ar-exposed rats exhibited critically decreased body weight and intake of food and water compared with controls. Sinha et al. (2008) reported that oxidative stress is an essential factor in the development of Ar nephropathy and damage to renal tubular cells, which causes a reduction in body weight. In our study, Ar-treated rats showed lower water and food intake, along with retarded growth and changes in absolute and relative kidney weight. The morphological changes observed in Ar-intoxicated rats were effectively attenuated by pretreatment with SFN. These results corroborate those of a previous report by Shang et al. (2015), where SFN provided strong antioxidant activity against Ar-induced nephrotoxicity.
It has long been believed that ROS are involved in several pathogenic infection-linked inflammatory process (Li et al. 2002). One organ typically affected by Ar-induced ROS production is the kidney (Prabu and Muthumani 2012). In the current study, a notable increase in ROS was found in the blood and renal tissue of Ar-intoxicated rats versus controls, which confirms earlier reports (Jain et al. 2015). This may be due to the induction by Ar of a complex chain of one-electron reduction of molecular oxygen, which first leads to the formation of superoxide radicals (O2) in the kidney. The O2 is unstable and readily accepts a second electron to form H2O2, and at the same time disturbing a large amount of an important cellular waste. Pre-administration of SFN drastically diminished ROS on average in Ar-exposed rats. This is likely explained by the successful free radical scavenging and potent hydrogen-donating properties of SFN, which minimize the inflammatory process.
Increased levels of markers such as urea, uric acid, creatinine, and creatinine clearance are an indication of renal damage (Sener et al. 2007). Earlier studies have reported that arsenic induces renal dysfunction upon continuous exposure and causes nephrotoxicity in rats (Sener et al. 2007; Bhattacharya and Haldar 2012). Because the primary waste products of protein metabolism are urea, uric acid, and creatinine, which must be excreted through the kidneys, an increase in these parameters in blood is considered a major marker of renal injury by Ar-induced protein oxidation (Silva 2004). SFN pretreatment in Ar-intoxicated rats reduced the elevated levels of renal markers as compared to controls. Therefore, SFN had been established as an excellent scavenger of reactive oxygen and nitrogen species, preventing lipid peroxidation and protein carbonylation, and thereby modulating serum and urinary markers in the kidneys (Cui et al. 2012; Kensler et al. 2013).
8-Hydroxy-2’deoxyguanosine (8-OHdG) is one of the predominant forms of free radical-induced oxidative lesions, and has therefore been widely used as a biomarker for oxidative stress and carcinogenesis and as an indicator of oxidative DNA damage promoted by different xenobiotics in the kidney (Li et al. 2008). Our study confirmed that the levels of 8-OHdG in urine and kidney tissue were significantly increased with higher Ar concentrations in intoxicated rats as compared to controls. These findings are in agreement with an earlier report by Prabhulkar and Li (2010), who observed greater DNA damage with increased ROS levels due to the accumulation of Ar in renal tissue. This DNA damage from exposure to Ar deposition will affect the proximal tubular epithelial cells, leading to tubular dysfunction and critical sign of undesirable consequence in DNA strands. However, pretreatment with SFN resulted in significantly less DNA damage, with decreased Ar accumulation in renal tissue as compared to the Ar group, which agreed with previous reports (Shang et al. 2015). This could be due to the presence of glucosinolate, which is hydrolyzed by myrosinase to scavenge the ROS in renal tissue and reduce DNA damage.
Lipid peroxidation (LPO) is the process in which free radicals steal electrons from the lipids in cell membranes, resulting in cell damage. It typically affects polyunsaturated fatty acids, since they contain multiple double bonds, between which are methylene bridges (-CH2-) that possess reactive hydrogen atoms (Ostrea et al. 1985). In a recent study, Ar-treated rats displayed elevated kidney lipid peroxides, hydroperoxides, and protein carbonyls, and a striking demur in antioxidant skeleton, clearly denotes the persistence of oxidative pressure. The outcome of the current study is in good agreement with the results reported by Ostrea et al. (1985), in which Ar administration markedly increased renal lipid peroxidation in rats. This could be due to progressive ROS that decreasing the glomerular function and increase growth of extracellular matrix proteins in the glomerular capillaries and mesangium, ultimately leading to glomerulosclerosis in renal tissues (Ostrea et al. 1985). Rats pretreated with SFN exhibited significantly reduced levels of lipid peroxides, hydroperoxides, and protein carbonyls in comparison to normal controls. This normalization may be achieved by the antioxidant and free radical suppressive properties of SFN, which protects the cells from oxidative stress and lipid peroxidation (Robles-Osorio et al. 2015).
Ar toxicity in the kidney is reflected by a reduction in cellular co-enzymatic oxidative inhibitors such as GSH, TSH, vitamin C, and vitamin E. Excess production of ROS induced by Ar damages the proximal tubular cells (PTC) of the glomerulus that mediate the efflux Ar to be excreted by urine (Li et al. 2010; Psurski et al. 2017). In the current study, Ar intoxication reduced the level of nonenzymatic antioxidants due to increased pro-oxidative and electrophilic matter causing renal failure. This may be due to the arrest of glutathione synthesizing precursor enzymes such as gamma-glutamyl cysteinyl synthetase and GSH synthetase by Ar-induced excess ROS in treated animals. Our results were in good accord with (Li et al. 2010), who observed reduced levels of nonenzymatic antioxidants in cases of Ar-induced renal damage in mice. On the other hand, pre-administration of SFN significantly rescued the decreased nonenzymatic antioxidants when compared with the control group. This may be because of the occurrence of electrophilic isothiocyanate and free cysteine-SH groups in the SFN, as a natural primary target for activation of antioxidant levels in Ar-induced toxicity in renal tissue (Mi et al. 2008).
Antioxidants that are capable of neutralizing the harmful oxidative effects of xenobiotics, such as SOD, CAT, and GPx., are known as enzymatic antioxidants. Unstable molecules such as free radicals are able to readily donate or remove electrons from adjacent molecules that adversely alter the physiology of organs (Robbins and Zhao 2011). The destructive properties of free radicals acquired as products of metabolism are inhibited by these substances (Ramasarma 2007). The superoxide dismutase (SOD) is a metalloprotein that accelerates the transformation of superoxide radicals. The heme protein catalase (CAT) accelerates the reduction of H2O2 to water and oxygen, and thus protects the cell against oxidative damage from OH- (McCord et al. 1971). Glutathione peroxidase (GPx) is a selenium-containing enzyme that plays a key role in reducing H2O2 and hydroperoxide to harmless materials. Glutathione S-transferases (GSTs) are a family of phase II detoxifying enzymes that shield cellular macromolecules from unstable electrophiles (free radicals). In particular, GSTs influence the conjugation of glutathione (GSH) to a wide array of internal and external electrophilic complexes. Glutathione reductase (GR) can act as a scavenger for singlet oxygen in various electrophiles, which accelerates the reduction of GSSG to GSH. In the current study, Ar administration significantly reduced enzymatic antioxidants such as SOD, CAT, GPx, GST, and GR in comparison to controls. This result was well in line with an earlier report by Jain et al. (2015), who observed lower levels of enzymatic antioxidants in arsenic-treated rat kidneys. Similarly, Gong et al. (2015) observed a reduction in enzymatic antioxidants in Ar-treated rats, mainly due to oxidative stress and unpaired electrons in the renal tissue. Pre-administration of SFN to Ar-treated rats resulted in significantly increased levels of enzymatic substances in renal tissue, primarily due to the presence of functional groups in SFN (isothiocyanates). The C=C double bonds and hydroxyl groups (OH) in SFN may bind with the antioxidant response element (ARE) in the promoter region of genes coding for the activation of Nrf2 and phase II antioxidant and subsequent antioxidant enzymes in the animal body (Beltran et al. 2012). SFN is a well-studied antioxidant for its inhibition of the Keap-1 protein associated with Nrf2 and activation of phase II antioxidant enzymes under stress conditions (Myzak and Dashwood 2006).
It has been confirmed that Ar accumulation in the podocytes commonly promotes the production of ROS and decreases calcium absorption in the kidney, ultimately leading to the release of proapoptotic Bax (B-cell-associated x protein) leading to tubular cell death (Santos et al. 2008). Bcl-2 genes are key regulators of functional and pathological effects in cell death. This family consists of both cell death promoter (Bax and Bad) and cell inhibitor (Bcl-2 and Bcl-xL) proteins. Peraza et al. (2006) demonstrated that elevated levels of Bax/Bad or Bcl-2/Bcl-XL are directly linked with the initiation of apoptosis in Ar-treated renal proximal tubular cells. In the present study, RT-PCR results clearly showed that the level of the proapoptotic marker Bax was significantly increased in Ar-treated animals compared with Bcl-2, SOD1, and CAT of normal controls. Our results confirmed that the administration of Ar increased Bax and reduced the antiapoptotic Bcl-2 gene responsible for the activation of phase II antioxidants such as SOD1 and CAT, which was in sequence with the earlier account of Peraza et al. (2006). Pretreatment with SFN increased Bcl-2 and decreased Bax, indicating that SFN provided antiapoptotic benefits against Ar-induced cell death via the modulation of Nrf2, a member of the basic region–leucine zipper NF-E2 family, which binds to cis-acting elements in the 5′-flanking region of the genes for activating phase II enzymes such as SOD1 and CAT.
The protease enzymes (caspase group) play essential role in apoptosis and inflammation. Cytochrome C aids in the activation of Apaf-1 and caspase-9 in the apoptosome. Once activated, caspase-9 cleaves and activates caspase-3 and caspase-7, ensuing in extensive proteolysis and cell death (Prabu and Muthumani 2012). In the current work, an increase in cytochrome C, Cas-9, and Cas-3 were observed in Ar-treated rats compared with controls. This could be due to the release of cytochrome C in the renal mitochondria due to Ar-mediated intrinsic apoptosis pathway that cleaves the pro-apoptosome and releases the Cas-9 cell death proteins that activate the final apoptotic protein Cas-3, leading to renal damage (Negrette-Guzmán et al. (2013). Pretreatment of Ar-treated animals with SFN demonstrated a notable decline in all apoptotic markers, as evidenced from the previous report of Guerrero-Beltrán et al. (2012) who observed the antiapoptotic efficacy of SFN against cisplatin induced mitochondrial damage in rat kidney. Our results also well accommodate with the previous report suggesting that SFN exerts antiapoptotic effects, suppressing apoptosome initiation during Ar-induced oxidative stress in mitochondrial renal tissue.
The PI3K (phosphatidylinositol-4,5-bis-phosphate 3-kinase)/Akt(v-akt murine thymoma viral oncogene homolog) pathway is involved in cell survival, proliferation, and metabolism, and is commonly associated with harmful modification processes in a variety of cancers, including kidney (Linehan et al. 2010). Activation of the PI3K/Akt pathway may be fundamental in considering apoptotic and proapoptotic genes. Akt, also known as protein kinase B (PKB), is the downstream serine threonine kinase target of PI3K (Franke et al. 1995). Akt plays a vital role in regulating numerous downstream signaling pathways including those that inhibit apoptosis and stimulate proliferation, protein synthesis, and glucose metabolism (Manning and Toker 2017). Akt is stimulated by PI3K in reaction to growth factors, Ca2+ entry. According to Franke et al. (1995), the downstream levels of PI3K/Akt significantly decreased the expression of the Nrf2 gene and its downstream proteins, which are essential for cell survival and proliferation in normal cells. In the current work, our results revealed decreased point of PI3K/Akt and Nrf2 protein was found in Ar-exposed rats compared with normal controls. Pretreatment with SFN significantly increased PI3K, Akt, and Nrf2 in Ar-treated rats as evidenced by RT-PCR, western blotting, and other biochemical activity observed in Ar-treated rats. The underlying mechanism for the activation of Nrf2 by SFN is not clear, but it may be related to its high chemical electrophilicity of the central carbon of the isothiocyanate (-N=C=S) group. This isothiocyanate group readily reacts with sulfur-, nitrogen-, and oxygen-centered enzymes such as cysteine and leucine residues of Nrf2 gene (Zhang 2012). Sulforaphane (SFN) has been proven to activate Nrf2, which protects the kidney from oxidative damage (Gao and Talalay 2004). The phosphatidylinositol 3-kinase (PI3K)/protein kinase B (PKB) are vital signaling enzymes involved in the transduction of a variety of signals from the cell surface to the nucleus. Both of their pathways are associated with the modulation of ARE-induced gene expression via Nrf2 activation in renal tissue (Li Chew et al. 2015). This leads to the de novo synthesis of phase II antioxidant enzymes that strongly protect cells from Ar-induced oxidative stress (Zhang 2012).
Several studies have demonstrated the use of TUNEL (terminal deoxynucleotidyl transferase dUTP nick end labelling) assay as a technique for identifying apoptotic DNA fragmentation, quantifying apoptotic cells, or detecting excessive DNA breakage in individual cells (Dong 2002; Peraza et al. 2006). It has been confirmed that chronic exposure to small doses (<100 μg/L) of arsenic in drinking water is associated with increased risk of DNA mutation in skin, lungs, and kidney (Saint-Jacques et al. 2014). Bau et al. (2002) reported that Ar and other oxidative stress resulted in DNA damage in the liver, kidney, and multiple other organs. In the current study, there was extensive DNA damage observed in Ar-intoxicated rats compared to controls. This result further confirmed our previous 8-OHdG results showing that Ar was able to generate ROS during its biotransformation and caused DNA strand breaks and chromosomal aberrations (Bau et al. 2002). This may be due to Ar-released 8-oxoguanine, which leads to nucleotide mutation (G:C to T:A), causing DNA strand breaks even at low concentrations (Eder et al. 2006). Pre-administration of SFN potentially abrogates Ar-induced DNA damage by its potent hydrogen-donating property and free radical scavenging ability in co-treated animal groups. Cheung and Kong (2010) demonstrated DNA protective effects of SFN against various xenobiotics. Hence, SFN is at least in part responsible for DNA protection.
Inflammation is part of a complex biological response of body tissues. Ar exposure has been found to be an important cause of renal failure associated with oxidative stress, proximal tubular dysfunction, and necrosis of proximal tubular epithelial cells in the kidneys of rats (Li et al. 2010). Kidney injury intracellular adhesion molecule (ICAM) is a member of the immunoglobulin superfamily responsible for immunity against pathogens or xenobiotics. In the present study, Ar-induced oxidative stress increased ICAM within 48 h in damaged renal tissues, as evidenced by an increase in basic renal markers urea, creatinine, and 8-OHdG in the kidney compared with controls. Ar administration may stimulate interleukin-1 (IL-1) and tumor necrosis factor (TNF) in lymphocytes via LFA-1 (integrin), a receptor found on leukocytes. The activated IL-1 and TNF cause inflammation in renal tissue by forming an ICAM-1/LFA-1 bond and then transmigrating into renal tissues, causing severe inflammation as supported by strong expression (ICAM) of immunohistochemistry (Prabu and Muthumani 2012). In comparison with controls, SFN pre-administration significantly attenuated renal inflammation owing to a battery of antioxidants and detoxification enzymes by binding ARE in the promoter regions of Nrf2 genes and scavenging inflammation-causing negative electrons in renal tissue.
In the present study, histopathological examination of Ar-treated rats revealed alterations in renal tissue including tubular necrosis, inflammatory cell infiltration, tubular degeneration, hemorrhage, and vacuolization. Similarly, electron microscopy study confirmed Ar-induced degeneration of podocytes including dilated cisternae of the endoplasmic reticulum, mitochondrial swelling, and degeneration of the nucleolus. This may be due to the accumulation of free radicals and increased lipid peroxidation by activated free Ar ions in the Ar-intoxicated animals. The increased generation of lipid peroxides associated with ROS leads to loss of membrane integrity and other pathological changes including nucleolus disintegration in kidney tissue as confirmed by electron microscopy study. Administration of SFN reduced Ar-induced histopathological and TEM alterations quite substantially in both organs and tissues via its antioxidant and membrane-stabilizing effects in rat kidneys.
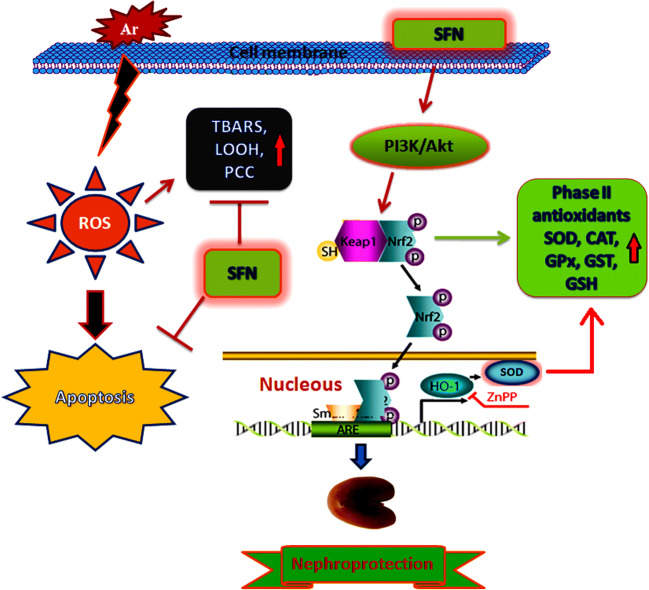
In summary, SFN showed a variety of beneficial properties, including free radical scavenging, anti-inflammatory, membrane-stabilizing, and antiapoptotic activity as possible key factors in the nephroprotective efficacy against Ar-induced oxidative renal injury. Ultimately, SFN was effective in preventing Ar-induced nephrotoxicity by activating phase II antioxidant enzymes via modulation of Nrf2 gene expression through the PI3K/Akt signaling pathway, and thus may prove beneficial in patients at high risk of arsenic toxicity (Fig. 12). Further comprehensive studies are in progress to clarify the specific mechanism of SFN against Ar-induced nephrotoxicity in rats.
Fig. 12.
Graphical abstract shows the protective mechanism of SFN against Ar-induced damage in the kidney tissue via PI3K/Akt-mediated Nrf2 signaling pathway
Acknowledgements
This project was funded by the DST-SERB, India, under NPDF scheme (PDF/2016/003063), New Delhi, India. The authors would also like to thank the professor and head, Department of Zoology, Bharathiar University, for providing necessary facilities and support to carry out this work.
Compliance with ethical standards
Conflict of interest
The authors have declared no conflict of interest.
Footnotes
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1007/s11356-024-34526-w
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
8/1/2024
This article has been retracted. Please see the Retraction Notice for more detail: 10.1007/s11356-024-34526-w
References
- Bau DT, Wang TS, Chung CH et al (2002) Oxidative DNA adducts and DNA-protein cross-links are the major DNA lesions induced by arsenite. Environ Health Perspect 110:753–756. 10.1289/ehp.02110s5753 10.1289/ehp.02110s5753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltran CEG, Oliver MC, Chaverri JP, Chirino YI (2012) Protective effect of sulforaphane against oxidative stress: recent advances. Exp Toxicol Pathol 64:503–508. 10.1016/j.etp.2010.11.005 10.1016/j.etp.2010.11.005 [DOI] [PubMed] [Google Scholar]
- Bhattacharya S, Haldar PK (2012) Trichosanthes dioica fruit ameliorates experimentally induced arsenic toxicity in male albino rats through the alleviation of oxidative stress. Biol Trace Elem Res 148:232–241. 10.1007/s12011-012-9363-3 10.1007/s12011-012-9363-3 [DOI] [PubMed] [Google Scholar]
- Cheung KL, Kong A-N (2010) Molecular targets of dietary Phenethyl Isothiocyanate and Sulforaphane for Cancer chemoprevention. AAPS J 12:87–97. 10.1208/s12248-009-9162-8 10.1208/s12248-009-9162-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Council NR (2001) Arsenic in drinking water: 2001 update. The National Academies Press, Washington, DC. 10.17226/10194 [Google Scholar]
- Cui W, Bai Y, Miao X et al (2012) Prevention of diabetic nephropathy by sulforaphane: possible role of Nrf2 upregulation and activation. Oxidative Med Cell Longev. 10.1155/2012/821936 [DOI] [PMC free article] [PubMed]
- Davis R, Singh KP, Kurzrock R, Shankar S (2009) Sulforaphane inhibits angiogenesis through activation of FOXO transcription factors. Oncol Rep 22:1473–1478. 10.3892/or_00000589 10.3892/or_00000589 [DOI] [PubMed] [Google Scholar]
- Desai ID (1984) Vitamin E analysis methods for animal tissues. In: Methods in enzymology, pp 138–147. 10.1016/S0076-6879(84)05019-9 [DOI] [PubMed]
- Dinkova-Kostova AT, Talalay P (2008) Direct and indirect antioxidant properties of inducers of cytoprotective proteins. Mol Nutr Food Res 52:S128–S138. 10.1002/mnfr.200700195 10.1002/mnfr.200700195 [DOI] [PubMed] [Google Scholar]
- Dong Z (2002) The molecular mechanisms of arsenic-induced cell transformation and apoptosis. Environ Health Perspect 110:757–759. 10.1289/ehp.02110s5757 10.1289/ehp.02110s5757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eder E, Wacker M, Lutz U et al (2006) Oxidative stress related DNA adducts in the liver of female rats fed with sunflower-, rapeseed-, olive- or coconut oil supplemented diets. Chem Biol Interact 159:81–89. 10.1016/j.cbi.2005.09.004 10.1016/j.cbi.2005.09.004 [DOI] [PubMed] [Google Scholar]
- El-Demerdash FM, Yousef MI, Radwan FME (2009) Ameliorating effect of curcumin on sodium arsenite-induced oxidative damage and lipid peroxidation in different rat organs. Food Chem Toxicol 47:249–254. 10.1016/j.fct.2008.11.013 10.1016/j.fct.2008.11.013 [DOI] [PubMed] [Google Scholar]
- Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77. 10.1016/0003-9861(59)90090-6 10.1016/0003-9861(59)90090-6 [DOI] [PubMed] [Google Scholar]
- Fahey JW, Talalay P (1999) Antioxidant functions of sulforaphane: a potent inducer of phase II detoxication enzymes. Food Chem Toxicol 37:973–979 10.1016/S0278-6915(99)00082-4 [DOI] [PubMed] [Google Scholar]
- Flora SJS (2011) Arsenic-induced oxidative stress and its reversibility. Free Radic Biol Med 51:257–281. 10.1016/j.freeradbiomed.2011.04.008 10.1016/j.freeradbiomed.2011.04.008 [DOI] [PubMed] [Google Scholar]
- Franke TF, Il YS, TO C et al (1995) The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell 81:727–736. 10.1016/0092-8674(95)90534-0 10.1016/0092-8674(95)90534-0 [DOI] [PubMed] [Google Scholar]
- Gao X, Talalay P (2004) Induction of phase 2 genes by sulforaphane protects retinal pigment epithelial cells against photooxidative damage. Proc Natl Acad Sci U S A (Medical Sciences) 101:10446–10451. 10.1073/pnas.0403886101 10.1073/pnas.0403886101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong X, Ivanov VN, Davidson MM, Hei TK (2015) Tetramethylpyrazine (TMP) protects against sodium arsenite-induced nephrotoxicity by suppressing ROS production, mitochondrial dysfunction, pro-inflammatory signaling pathways and programed cell death. Arch Toxicol 89:1057–1070. 10.1007/s00204-014-1302-y 10.1007/s00204-014-1302-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith OW (1980) Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem 106:207–212. 10.1016/0003-2697(80)90139-6 10.1016/0003-2697(80)90139-6 [DOI] [PubMed] [Google Scholar]
- Guerrero-Beltrán CE, Calderón-Oliver M, Pedraza-Chaverri J, Chirino YI (2012) Protective effect of sulforaphane against oxidative stress: recent advances. Exp Toxicol Pathol 64:503–508. 10.1016/j.etp.2010.11.005 10.1016/j.etp.2010.11.005 [DOI] [PubMed] [Google Scholar]
- Gupta R, Kannan GM, Sharma M, Flora SJS (2005) Therapeutic effects of Moringa oleifera on arsenic-induced toxicity in rats. Environ Toxicol Pharmacol 20:456–464. 10.1016/j.etap.2005.05.005 10.1016/j.etap.2005.05.005 [DOI] [PubMed] [Google Scholar]
- Habig WH, Pabst MJ, Jakoby WB (1974) The first enzymatic stepo in mercapturic acid formation. J Biol Chem 249:7130–7139. 10.14026/j.cnki.0253-9705.2010.23.013 10.14026/j.cnki.0253-9705.2010.23.013 [DOI] [PubMed] [Google Scholar]
- Halliwell B, Whiteman M (2004) Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol 142:231–255. 10.1038/sj.bjp.0705776 10.1038/sj.bjp.0705776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A, Agrawal S, Flora SJS (2015) Arsenic and nicotine co-exposure lead to some synergistic effects on oxidative stress and apoptotic markers in young rat blood, liver, kidneys and brain. Toxicol Rep 2:1334–1346. 10.1016/j.toxrep.2015.09.003 10.1016/j.toxrep.2015.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery EH, Keck AS (2008) Translating knowledge generated by epidemiological and in vitro studies into dietary cancer prevention. Mol Nutr Food Res 52:S7–S17 [DOI] [PubMed] [Google Scholar]
- Jiang ZY, Hunt JV, Wolff SP (1992) Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low density lipoprotein. Anal Biochem 202:384–389. 10.1016/0003-2697(92)90122-N 10.1016/0003-2697(92)90122-N [DOI] [PubMed] [Google Scholar]
- Jung K (2008) Tietz fundamentals of clinical chemistry, 6th edition. Carl A. Burtis, Edward R. Ashwood, and David E. Bruns, editors. St Louis, MO: Saunders/Elsevier, 2008, 976 pp. Clin Chem 54:1933–1933 10.1373/clinchem.2007.101378 [DOI] [Google Scholar]
- Kakkar P, Das B, Viswanathan PN (1984) A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys 21:130–132. 10.1097/YCO.0b013e3280117733 10.1097/YCO.0b013e3280117733 [DOI] [PubMed] [Google Scholar]
- Kensler TW, Egner PA, Agyeman AS et al (2013) Keap1-Nrf2 signaling: a target for cancer prevention by sulforaphane. Top Curr Chem 329:163–178. 10.1007/128-2012-339 10.1007/128-2012-339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine RL, Garland D, Oliver CN et al (1990) Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 186:464–478. 10.1016/0076-6879(90)86141-H 10.1016/0076-6879(90)86141-H [DOI] [PubMed] [Google Scholar]
- Li N, Wang M, Oberley TD et al (2002) Comparison of the pro-oxidative and proinflammatory effects of organic diesel exhaust particle chemicals in bronchial epithelial cells and macrophages. J Immunol 169:4531–4541. 10.4049/jimmunol.169.8.4531 10.4049/jimmunol.169.8.4531 [DOI] [PubMed] [Google Scholar]
- Li X, Pi J, Li B et al (2008) Urinary arsenic speciation and its correlation with 8-OHdG in Chinese residents exposed to arsenic through coal burning. Bull Environ Contam Toxicol 81:406–411. 10.1007/s00128-008-9471-0 10.1007/s00128-008-9471-0 [DOI] [PubMed] [Google Scholar]
- Li Z, Piao F, Liu S et al (2010) Subchronic exposure to arsenic trioxide-induced oxidative DNA damage in kidney tissue of mice. Experimental and toxicologic pathology : official journal of the Gesellschaft für Toxikologische Pathologie 62:543–547. 10.1016/j.etp.2009.07.003 10.1016/j.etp.2009.07.003 [DOI] [PubMed] [Google Scholar]
- Li Chew C, Lunardi A, Gulluni F et al (2015) In vivo role of INPP4B in tumor and metastasis suppression through regulation of PI3K-AKT signaling at endosomes. Cancer discovery 5:740–751. 10.1158/2159-8290.CD-14-1347 10.1158/2159-8290.CD-14-1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linehan WM, Srinivasan R, Schmidt LS (2010) The genetic basis of kidney cancer: a metabolic disease. Nature Reviews Urology 7:277–285. 10.1038/nrurol.2010.47 10.1038/nrurol.2010.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal BK, Suzuki KT (2002) Arsenic round the world: a review. Talanta 58:201–235. 10.1016/S0039-9140(02)00268-0 10.1016/S0039-9140(02)00268-0 [DOI] [PubMed] [Google Scholar]
- Manna P, Sinha M, Sil PC (2008) Arsenic-induced oxidative myocardial injury: protective role of arjunolic acid. Arch Toxicol 82:137–149. 10.1007/s00204-007-0272-8 10.1007/s00204-007-0272-8 [DOI] [PubMed] [Google Scholar]
- Manning BD, Toker A (2017) AKT/PKB signaling: navigating the network. Cell 169:381–405. 10.1016/j.cell.2017.04.001 10.1016/j.cell.2017.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord JM, Keele BB, Fridovich I (1971) An enzyme-based theory of obligate anaerobiosis: the physiological function of superoxide dismutase. Proc Natl Acad Sci U S A 68:1024–1027. 10.1073/pnas.68.5.1024 10.1073/pnas.68.5.1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi L, Xiao Z, Hood BL et al (2008) Covalent binding to tubulin by isothiocyanates. A mechanism of cell growth arrest and apoptosis. J Biol Chem 283:22136–22146. 10.1074/jbc.M802330200 10.1074/jbc.M802330200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moron MS, Depierre JW, Mannervik B (1979) Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta 582:67–78. 10.1016/0304-4165(79)90289-7 10.1016/0304-4165(79)90289-7 [DOI] [PubMed] [Google Scholar]
- Myzak MC, Dashwood RH (2006) Chemoprotection by sulforaphane: keep one eye beyond Keap1. Cancer Lett 233:208–218. 10.1016/j.canlet.2005.02.033 10.1016/j.canlet.2005.02.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na HK, Kim EH, Jung JH, Lee HH, Hyun JW, Surh YJ (2008) Epigallocatechin gallate induces Nrf2-mediated antioxidant enzyme expression via activation of PI3K and ERK in human mammary epithelial cells. Arch Biochem Biophys 476:171–177 10.1016/j.abb.2008.04.003 [DOI] [PubMed] [Google Scholar]
- Negrette-Guzmán M, Huerta-Yepez S, Medina-Campos ON et al (2013) Sulforaphane attenuates gentamicin-induced nephrotoxicity: role of mitochondrial protection. Evid Based Complement Alternat Med 2013:17. 10.1155/2013/135314 10.1155/2013/135314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehaus WG, Samuelsson B (1968) Formation of Malonaldehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur J Biochem 6:126–130. 10.1111/j.1432-1033.1968.tb00428.x 10.1111/j.1432-1033.1968.tb00428.x [DOI] [PubMed] [Google Scholar]
- Omaye ST, David Turnbull J, Sauberlich HE (1979) [1] selected methods for the determination of ascorbic acid in animal cells, tissues, and fluids. Methods Enzymol 62:3–11. 10.1016/0076-6879(79)62181-X 10.1016/0076-6879(79)62181-X [DOI] [PubMed] [Google Scholar]
- Ostrea EM, Cepeda EE, C a F, Balun JE (1985) Red cell membrane lipid peroxidation and hemolysis secondary to phototherapy. Acta Paediatr Scand 74:378–381. 10.1111/j.1651-2227.1985.tb10987 10.1111/j.1651-2227.1985.tb10987 [DOI] [PubMed] [Google Scholar]
- Pan H, Mukhopadhyay P, Rajesh M, Patel V, Mukhopadhyay B, Gao B, et al. (2009) Cannabidiol attenuates cisplatin-induced nephrotoxicity by decreasing oxidative/nitrosative stress, inflammation, and cell death. J Pharmacol Exp Ther 328:708–714 [DOI] [PMC free article] [PubMed]
- Parker MM, Humoller FL, Mahler DJ (1967) Determination of copper and zinc in biological material. Clin Chem 13:40–48 10.1093/clinchem/13.1.40 [DOI] [PubMed] [Google Scholar]
- Peraza MA, Cromey DW, Carolus B et al (2006) Morphological and functional alterations in human proximal tubular cell line induced by low level inorganic arsenic: evidence for targeting of mitochondria and initiated apoptosis. J Appl Toxicol 26:356–367. 10.1002/jat.1149 10.1002/jat.1149 [DOI] [PubMed] [Google Scholar]
- Prabhulkar S, Li CZ (2010) Assessment of oxidative DNA damage and repair at single cellular level via real-time monitoring of 8-OHdG biomarker. Biosens Bioelectron 26:1743–1749. 10.1016/j.bios.2010.08.029 10.1016/j.bios.2010.08.029 [DOI] [PubMed] [Google Scholar]
- Prabu SM, Muthumani M (2012) Silibinin ameliorates arsenic induced nephrotoxicity by abrogation of oxidative stress, inflammation and apoptosis in rats. Mol Biol Rep 39:11201–11216. 10.1007/s11033-012-2029-6 10.1007/s11033-012-2029-6 [DOI] [PubMed] [Google Scholar]
- Psurski M, Janczewski Ł, Świtalska M et al (2017) Novel phosphonate analogs of sulforaphane: synthesis, in-vitro and in-vivo anticancer activity. Eur J Med Chem 132:63–80. 10.1016/j.ejmech.2017.03.028 10.1016/j.ejmech.2017.03.028 [DOI] [PubMed] [Google Scholar]
- Ramasarma T (2007) Many faces of superoxide dismutase, originally known as erythrocuprein. Curr Sci 92:184–191 [Google Scholar]
- Robbins D, Zhao Y (2011) The role of manganese superoxide dismutase in skin cancer. Enzyme Research 2011:1–7. 10.4061/2011/409295 10.4061/2011/409295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles-Osorio ML, Sabath-Silva E, Sabath E (2015) Arsenic-mediated nephrotoxicity. Ren Fail 37(4):542–547. 10.3109/0886022X.2015 10.3109/0886022X.2015 [DOI] [PubMed] [Google Scholar]
- Rotruck JT, Pope AL, Ganther HE et al (1973) Selenium: biochemical role as a component of glatathione peroxidase. Science 179:588–590. 10.1126/science.179.4073.588 10.1126/science.179.4073.588 [DOI] [PubMed] [Google Scholar]
- Saint-Jacques N, Parker L, Brown P, Dummer TJ (2014) Arsenic in drinking water and urinary tract cancers: a systematic review of 30 years of epidemiological evidence. Environmental Health: A Global Access Science Source 13:44. 10.1186/1476-069X-13-44 10.1186/1476-069X-13-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos NAG, Bezerra CSC, Martins NM et al (2008) Hydroxyl radical scavenger ameliorates cisplatin-induced nephrotoxicity by preventing oxidative stress, redox state unbalance, impairment of energetic metabolism and apoptosis in rat kidney mitochondria. Cancer Chemother Pharmacol 61:145–155. 10.1007/s00280-007-0459-y 10.1007/s00280-007-0459-y [DOI] [PubMed] [Google Scholar]
- Sener G, Toklu HZ, Cetinel S (2007) β-Glucan protects against chronic nicotine-induced oxidative damage in rat kidney and bladder. Environ Toxicol Pharmacol 23:25–32. 10.1016/j.etap.2006.06.003 10.1016/j.etap.2006.06.003 [DOI] [PubMed] [Google Scholar]
- Shang G, Tang X, Gao P et al (2015) Sulforaphane attenuation of experimental diabetic nephropathy involves GSK-3 beta/Fyn/Nrf2 signaling pathway. J Nutr Biochem 26:596–606. 10.1016/j.jnutbio.2014.12.008 10.1016/j.jnutbio.2014.12.008 [DOI] [PubMed] [Google Scholar]
- Shanmugam T, Selvaraj M, Poomalai S (2016) Epigallocatechin gallate potentially abrogates fluoride induced lung oxidative stress, inflammation via Nrf2/Keap1 signaling pathway in rats: an in-vivo and in-silico study. Int Immunopharmacol 39:128–139. 10.1016/j.intimp.2016.07.022 10.1016/j.intimp.2016.07.022 [DOI] [PubMed] [Google Scholar]
- Shin DH, Park HM, Jung KA et al (2010) The NRF2-heme oxygenase-1 system modulates cyclosporin A-induced epithelial-mesenchymal transition and renal fibrosis. Free Radic Biol Med 48:1051–1063. 10.1016/j.freeradbiomed.2010.01.021 10.1016/j.freeradbiomed.2010.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva FG (2004) Chemical-induced nephropathy: a review of the renal tubulointerstitial lesions in humans. Toxicol Pathol 32(2):71–84 10.1080/01926230490457530 [DOI] [PubMed] [Google Scholar]
- Sinha AK (1972) Colorimetric assay of catalase. Anal Biochem 47:389–394. 10.1016/0003-2697(72)90132-7 10.1016/0003-2697(72)90132-7 [DOI] [PubMed] [Google Scholar]
- Sinha M, Manna P, Sil PC (2008) Arjunolic acid attenuates arsenic-induced nephrotoxicity. Pathophysiology 15:147–156. 10.1016/j.pathophys.2008.03.001 10.1016/j.pathophys.2008.03.001 [DOI] [PubMed] [Google Scholar]
- Smeester L, Rager JE, Bailey KA et al (2011) Epigenetic changes in individuals with arsenicosis. Chem Res Toxicol 24:165–167. 10.1021/tx1004419 10.1021/tx1004419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socci DJ, Bjugstad KB, Jones HC et al (1999) Evidence that oxidative stress is associated with the pathophysiology of inherited hydrocephalus in the H-Tx rat model. Exp Neurol 155:109–117. 10.1006/exnr.1998.6969 10.1006/exnr.1998.6969 [DOI] [PubMed] [Google Scholar]
- Thangapandiyan S, Miltonprabu S (2014) Epigallocatechin gallate supplementation protects against renal injury induced by fluoride intoxication in rats: role of Nrf2/HO-1 signaling. Toxicol Rep 1:12–30. 10.1016/j.toxrep.2014.01.002 10.1016/j.toxrep.2014.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Tu C, Ma LQ (2002) Effects of arsenic concentrations and forms on arsenic uptake by the hyperaccumulator ladder brake. J Environ Qual 31:641–647. 10.2134/jeq2002.0641 10.2134/jeq2002.0641 [DOI] [PubMed] [Google Scholar]
- Waalkes MP, Liu J, Ward JM, Diwan BA (2004a) Mechanisms underlying arsenic carcinogenesis: hypersensitivity of mice exposed to inorganic arsenic during gestation. Toxicology 198:31–38. 10.1016/j.tox.2004.01.017 10.1016/j.tox.2004.01.017 [DOI] [PubMed] [Google Scholar]
- Waalkes MP, Liu J, Ward JM, Diwan BA (2004b) Animal models for arsenic carcinogenesis: inorganic arsenic is a transplacental carcinogen in mice. Toxicol Appl Pharmacol 198:377–384. 10.1016/j.taap.2003.10.028 10.1016/j.taap.2003.10.028 [DOI] [PubMed] [Google Scholar]
- Yamanaka K, Hoshino M, Okamoto M et al (1990) Induction of DNA damage by dimethylarsine, a metabolite of inorganic arsenics, is for the major part likely due to its peroxyl radical. Biochem Biophys Res Commun 168:58–64. 10.1016/0006-291X(90)91674-H 10.1016/0006-291X(90)91674-H [DOI] [PubMed] [Google Scholar]
- Yoon HY, Kang NI, Lee HK et al (2008) Sulforaphane protects kidneys against ischemia-reperfusion injury through induction of the Nrf2-dependent phase 2 enzyme. Biochem Pharmacol 75:2214–2223. 10.1016/j.bcp.2008.02.029 10.1016/j.bcp.2008.02.029 [DOI] [PubMed] [Google Scholar]
- Yu M, Xue J, Li Y et al (2013) Resveratrol protects against arsenic trioxide-induced nephrotoxicity by facilitating arsenic metabolism and decreasing oxidative stress. Arch Toxicol 87:1025–1035. 10.1007/s00204-013-1026-4 10.1007/s00204-013-1026-4 [DOI] [PubMed] [Google Scholar]
- Zhang Y (2012) The molecular basis that unifies the metabolism, cellular uptake and chemopreventive activities of dietary isothiocyanates. Carcinogenesis 33:2–9 10.1093/carcin/bgr255 [DOI] [PMC free article] [PubMed] [Google Scholar]