Abstract
Nutritional signals have long been implicated in the control of cellular processes that take place in the hypothalamus. This includes food intake regulation and energy balance, inflammation, and most recently, neurogenesis. One of the main glial cells residing in the hypothalamus are tanycytes, radial glial-like cells, whose bodies are located in the lining of the third ventricle, with processes extending to the parenchyma and reaching neuronal nuclei. Their unique anatomical location makes them directly exposed to nutrients in the cerebrospinal fluid. Several research groups have shown that tanycytes can respond to nutritional signals by different mechanisms, such as calcium signaling, metabolic shift, and changes in proliferation/differentiation potential. Despite cumulative evidence showing tanycytes have the molecular components to participate in nutrient detection and response, there are no enough functional studies connecting tanycyte nutrient sensing with hypothalamic functions, nor that highlight the relevance of this process in physiological and pathological context. This review will summarize recent evidence that supports a nutrient sensor role for tanycytes in the hypothalamus, highlighting the need for more detailed analysis on the actual implications of tanycyte-nutrient sensing and how this process can be modulated, which might allow the discovery of new metabolic and signaling pathways as therapeutic targets, for the treatment of hypothalamic related diseases.
Keywords: tanycytes, glucosensing, amino acid detection, fatty acids, vitamins, hypothalamus
Introduction
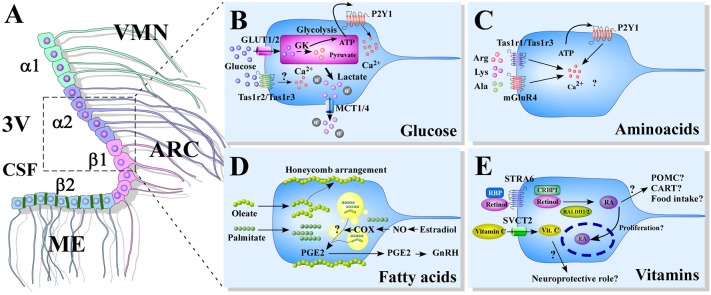
Tanycytes are hypothalamic radial glial-like cells, which are characterized by having their cell bodies located on the basal walls of the third ventricle (3V), in direct contact with the cerebral-spinal fluid (CSF). Histological analysis of rat and mice hypothalamic sections show that tanycytes exhibit long processes that touch blood vessels and neuronal centers located within the ventromedial (VMN) and arcuate nucleus (ARC) (1–3). Tanycytes are classified based on their distribution in the hypothalamic ventricular wall into α1, α2, β1, and β2 (4), (Figure 1A). Basal processes of α1-tanycytes project toward the VMN, while those of α2-tanycytes project to the ARC (1). β1-tanycytes line the infundibular recess, and their basal projections reach the lateral regions of the median eminence (ME) and the ARC; while β2-tanycytes cover the floor of the 3V and extend their projections inside the ME to contact fenestrated capillaries (5–7). This provides privileged access to nutritional signals carried by the bloodstream (8, 9). Also, β2-tanycytes are connected to each other through tight junctions (10), forming a physical barrier for polar molecules that prevent their diffusion into the brain via the CSF, known as the ME-CSF barrier (5, 11, 12).
Figure 1.
Models of nutrient sensing mediated by tanycytes. (A) A schematic representation of the distribution of tanycytes over the wall of the third ventricle (3V). The α1-tanycytes in light green (α1) have long projections that make contact with the neurons of the VMN. α2-tancycytes in purple (α2), have projections to the ARC. β1-tanycytes in pink (β1), make projections to the ARC. Finally, in the floor of the 3V, β2-tanycytes in light blue (β2), are joined by tight junctions forming part of the median eminence (ME)-cerebrospinal fluid (CSF) barrier, and their projections make contact with the ME. (B–E) Scheme based on proposed glucose (B), aminoacids (C), fatty acids (D), and vitamins (E) sensing mechanism mediated by tanycytes. 3V: third ventricle; CSF, cerebral spinal fluid; ME, median eminenece; ARC, arcuate nucleus; VMN, ventromedial nucleus; GK, glucokinase; PGE2, prostaglandin E2; GnRH, Gonadotropin-release hormone; RBP, Retinol binding protein; STRA6, RBP receptor; CRBP1, cellular retinol-binding protein; RALDH, retinaldehyde dehydrogenase; RA, retinoic acid; POMC, pro-opiomelanocortin; CART, cocaine- and amphetamine-regulated transcript; SVCT2, sodium vitamin C co-transporter 2.
Importantly, accumulating recent data showing increased heterogeneity of tanycytes populations, in terms of neurogenic potential, marker expression, structural differences and transcriptomic profile, poses the need of reviewing the current classification to find new ways of better describing the function of tanycytes subpopulations, so new classification systems have emerged (13).
These anatomical features make it feasible to propose that tanycytes have the properties to detect and respond to different nutritional signals, while being able to functionally couple with neurons of the hypothalamic region. In this review, we will summarize the existing data regarding tanycytes involvement in different nutrients sensing, such as glucose, lipids, vitamins, and amino acids.
Glucosensing Mediated by Tanycytes
Plasma glucose concentrations are known to modulate appetite, and therefore regulate food intake initiation and termination. This is done in a process that is centrally regulated by the hypothalamus, where rises in plasma glucose concentration that occur after a meal signal meal termination (14), although the mechanisms by which it enters the brain are not fully understood. Through microdialysis studies in rats, it has been demonstrated that glucose concentration in the parenchyma does not vary significantly (15), while glucose concentration in CSF changes proportionally to variations in blood glucose concentration (16, 17), suggesting the existence of a mechanism that facilitates the efficient transfer of glucose from the blood to CSF (18). Glucose uptake into the CSF would occur through the circumventricular organs, which are structures that are part of the ventricular walls and have fenestrated capillaries, which gives them high permeability to different nutrients (19).
In the hypothalamus, the ME is a circumventricular organ and, as is was noted before, the ME-CSF barrier is composed of β2-tanycytes, therefore tanycytes would be the main regulators of nutrients accessibility to the CSF (18).
In this context, studies on monkey and rodent hypothalamic slices show that α and β1-tanycytes, express high levels of glucose facilitative transporter GLUT1, exhibiting an intense immunoreaction in their cell processes that reach hypothalamic capillaries and the ARC (1, 20). In addition, β1 and β2-tanycytes express GLUT2, a low affinity/high-capacity glucose transporter. The expression of these two glucose transporters allows tanycytes to incorporate glucose from the CSF (21).
Moreover, rat tanycytes express glucokinase (GK) in situ and in vitro (22), an enzyme that at high glucose concentrations has an efficient glucose phosphorylation activity, and also express the regulatory glucokinase protein (GKRP) (23), which regulates the activity and nuclear compartmentalization of GK (24, 25). The expression of GLUT2, GK, and GKRP in tanycytes puts them in a privileged position to sense glucose variations in the CSF (3).
It has been established that rat primary cultures of tanycytes in response to an increase in extracellular glucose levels, augment intracellular Ca2+ concentration ([Ca2+]i) and ATP levels, a response that is dependent on glycolytic metabolism and P2Y1 receptor activation (26). In situ analysis of hypothalamic slices acutely exposed to glucose or non-metabolizable analogs on the tanycytes bodies also show [Ca2+]i increase in an ATP-dependent manner (27–29) (Figure 1B).
The physiological relevance of glucose or glucose analogs-induced responses on tanycytes has not been fully studied. However, some groups have made progress on demonstrating the importance of glucose metabolism in tanycytes and how this can impact the activity of hypothalamic neuronal nuclei. For instance, in vitro and in situ studies in rodents, showing monocarboxylate transporter (MCT) expression in tanycytes have led to propose that glucose would be preferentially captured by tanycytes, generating other metabolic substrates, like lactate (3, 30, 31), that once released, it could be used by nearby neurons in a mechanism similar to one originally described by Pellerin and Magistretti, known as the astrocyte-neuron lactate shuttle hypothesis (32). In vivo studies in rats, inhibiting monocarboxylate transporter 1 (MCT1), GLUT2 or GK in tanycytes, through adenoviral injections into the 3V, show impaired neuronal response to fasting and acute glucose administration, given by altered expression of orexigenic and anorexigenic neuropeptides, accompanied by altered feeding behavior (33–35). These recent data support the notion that metabolic coupling between tanycytes and neurons can modulate the neuronal activity in hypothalamic areas associated with regulation of eating behavior.
Interestingly, a study in male siberian hamster showed, through in situ hybridization, that MCTs could be subject to photoperiodic regulation (36), suggesting that the amount of lactate released by tanycytes could be affected by daylight duration. In addition, the authors propose that lactate can be used for the synthesis of glutamine, modulating the supply of glutamate neurotransmitter to hypothalamic neurons (36).
It is important to note that a recent study has shown that tanycytes are able to sense glucose by a different mechanism, through the sweet taste receptor Tas1r2/Tas1r3, whose activation with glucose and other analogs produces [Ca2+]i increase (28). It is necessary to further explore if this mechanism is truly relevant under physiological conditions.
Amino Acid Detection by Tanycytes
Different studies have analyzed the impact of protein-rich or protein-deprived diets on brain functions, such as central regulation of energy balance and food intake showing that protein consumption is homeostatically regulated by central amino acid sensing (37, 38). One of the sensing centers is localized in the hypothalamus. This is shown in studies where hypothalamic microinjections of either balanced amino-acid solutions or just leucine suppresses appetite and inhibits feeding in rats (39–43). Other studies with leucine supplementation in the diet also show feeding inhibition, similar to that observed with high-protein diets (44, 45). In addition, plasma leucine levels increase rapidly after food intake (46), also increasing its concentration in the brain (47), exercising a suppressive role on food intake.
Regarding amino acid entry into the brain, it has been determined that endothelial cells from the BBB express diverse systems of amino acid transporters in situ. So far, four facilitative carriers have been identified in the luminal (blood) membrane; system “L1” (for large essential neutral amino acid), “y+” (for cationic amino acids), “xG−” (for acidic amino acid) and “n” (for glutamine). The abluminal (parenchyma) membrane, on the other hand, only expresses system L1 and y+, (with lower and higher expression compared to the luminal membrane, respectively) (48–50). This differential distribution allows for the concentration of essential neutral amino acids, while promoting the elimination of non-essential amino acids and toxic amino acids such as glutamate.
The abluminal membrane, in addition, expresses 5 systems of Na+ -dependent amino acid transporters that allow their export from the brain (51–53). System A (for small nonessential neutral amino acids, that preferentially transport alanine) (54–56); ASC system (for some large and small neutral amino acids) (57–59), system N (for nitrogen rich amino acids) (60), the excitatory acidic amino acid transporter (EAAT) family (61, 62), and finally the Na+ -LNAA system (for large neutral amino acids) (50).
Most of the literature describes hypothalamic amino acid sensing relying on some discrete groups of neurons, located at the basal and lateral hypothalamus. For instance, it has been shown, in rodents, that leucine increases the frequency of POMC action potentials ex vivo and c-Fos immunoreactivity in situ, in a mTOR dependent manner (40, 42, 63). In addition, leucine effects on food intake are neutralized with mTORC1 inhibitor rapamycin (63). Other reports using mice show that hypocretin/orexin neurons also respond to non-essential amino acids through a dual mechanism involving inhibition of K+(ATP) channels and activation of amino acid transporters from system A ex vivo (64).
New evidence, however, suggests that tanycytes might also contribute to hypothalamic amino acid sensing, highlighting the involvement of non-neuronal cells in this process. Benford et al. (28) showed in rodents that glucosensing mediated by a group of tanycytes, was dependent on the expression of the sweet taste receptor Tas1r2/Tas1r3 (28), which shares structural similarities with the Tas1r1/Tas1r3 heterodimer associated with the umami taste or non-aromatic L-amino acids detection (65, 66). 67, on the other hand, showed using rodent brain slices that L-amino acids such as Arg, Lys, and Ala increased [Ca2+]i in a ATP-dependent manner in tanycytes, a response also dependent on Tas1r1 expression and mGluR4 function, which is another umami taste receptor (67).
These new findings show that tanycytes have at least two mechanisms of amino acid sensing through umami taste receptors, proposing tanycytes as a new cell type for hypothalamic amino acid detection (Figure 1C). More studies are needed to address the relevance and implications of amino acid glial detection on regulating food intake, as well as whether these mechanisms might be altered in pathologies associated with diabetes and obesity.
Fatty Acid Sensing Mediated by Tanycytes
Although several studies have emerged showing high fat diet (HFD) or specific fatty acid administration can induce hypothalamic inflammation (68–70) and/or hypothalamic neurogenesis (71, 72), not many studies have addressed the particular involvement of tanycytes in fatty acid incorporation and response.
It has been shown that the BBB limits the free passage of lipid components into the brain in a selective way (73, 74), allowing for example, the entry of linoleic acid to the brain parenchyma, while restricting the entry of oleic acid (74). Recent work by Hofmann et al. (75), using click chemistry to trace fatty acids in the hypothalamus, shows a differential distribution when slices are acutely incubated with saturated (SFA) vs. poly-unsaturated fatty acids (PUFAs). For instance, SFAs palmitate and stearate were highly detected in tanycytes and in a lesser degree in the ARC, while PUFAs oleate and linoleate were found in both areas, with signal diffusing from the ventricular wall to the parenchyma (75). Oleate staining in tanycytes was mostly isolated to the plasma membrane or spread in between tanycytes, resulting in a honeycomb-like arrangement, while palmitate was highly detected in the whole tanycytic layer (75).
This hypothalamic fatty acid distribution seems to change when mice are kept on a HFD for 10 weeks, observing a reduction of oleic acid presence in the ARC with a more concentrated expression in the tanycyte layer. In these same mice, HFD increased the number and size of lipid droplets (75), indicating tanycytes capability to store and perhaps metabolize these lipids. In a recent review, Rodriguez et al., suggest that lipid droplets present in tanycytes could be used in the production of prostaglandin E2 (PGE2) (76), which has been describe to promotes the retraction of tanycytes endfeet, facilitating the secretion of gonadotrophin releasing hormone (GnRH) into the pericapillary space and fenestrated capillaries, reviewed in (77) (Figure 1D).
However, how these observations relate to tanycytes role in hypothalamic energy balance control, inflammation or neurogenesis are still lacking. Because fatty acids can act as signaling molecules and regulators of the above processes, more studies are needed to elucidate if they can alter the way tanycytes sense other molecules or change their responses.
Tanycytes and Vitamins
Although several specific roles related to brain function have been attributed to different vitamins (78), only vitamin A has been largely studied in the hypothalamus. More specifically in describing their key role relating to proteins for uptake and metabolism. However, not much is known about vitamin A possible effects on hypothalamic regulated functions.
This liposoluble vitamin, known as retinol, travels through the bloodstream bound to the retinol binding protein (RBP) and can enter cells through the interaction with RBP receptor (STRA6) (79). Once inside the cell, it interacts with cellular retinol-binding protein (CRBP1), allowing the proximity to enzymes, called retinaldehyde dehydrogenases (RALDHs), that convert retinol into its metabolic active form, retinoic acid (RA) (80, 81). RA later interacts with nuclear receptors (RARs), which act as ligand-activated transcription factors that when bound to retinoid X receptors (RXRs), translocate to the nucleus, interact with RA response elements (RARE) and regulate gene expression (82).
Vitamin A immunohistochemistry on rat brain tissue, with an antiserum developed against RA shows that vitamin A is almost exclusively detected in hypothalamic cell bodies and some dendrites of the paraventricular nuclei and the dorsal perifornical region (83). Interestingly, studies have shown that some of the crucial proteins involved in vitamin A uptake and nuclear transportation, as well as RA synthetizing enzymes are expressed by tanycytes and that expression can be regulated by melatonin and thyroid hormones (84–86). (84), showed in adult rats that tanycytes were the only cells in the hypothalamus positive for RALDH1 expression, by immunofluorescence (84, 86). Moreover, RALDH2 was shown to be expressed in tanycytes at the protein level, but not mRNA in situ, and because RALDH2 proteins were detected by western blotting also in the choroid plexus and CSF, the authors suggested that tanycytes might be able to endocyte it from the 3V (86). They also showed the expression of STRA6 and CRBP1 in tanycytes by in situ hybridization (84) (Figure 1E).
In rodents, numerous studies have seen that RA related proteins are under photoperiodic control in tanycytes. For instance, in the siberian hamster, exposure to long photoperiod (LD) correlates to increase levels of CRBP1 in situ, particularly in the lining of the 3V, while short photoperiod (SD) correlates with very low levels of CRBP1 (87). In addition, pinealectomized animals maintained higher levels of CRBP1, mimicking LD, showing at least in some way CRBP1 expression was modulated by melatonin, which has also been shown to regulate the expression of other proteins on tanycytes, such as cytoskeleton intermediate filament protein vimentin (88).
In the photosensitive rat strain F344/N, LD induces high expression of STRA6, CRBP1, RALDH1, and CYP26b1, a monooxygenase involved in RA metabolism, in the lining of the 3V, while low or undetectable expression is detected under SD in situ (85). This photoperiod regulation of RA related genes can also be achieved by melatonin injections under LD, recapitulating the results obtained with pinealectomized mice by Ross et al. (87).
Another study, using 8 week/old rats, RALDH1 levels in the hypothalamus increase at the transcriptional level after 4 h of receiving a triiodothyronine (T3) subcutaneous injection. They also increase when hypothalamic slices are directly incubated for 48 h with T3 ex vivo or when primary cultures of tanycytes are treated with T3 in vitro (89). In addition, CYP26b1, known to be upregulated by RA, increases its expression by 2-fold in the hypothalamic area when T3 is administered in vivo, suggesting RA synthesis occurred on tanycytes (89).
All of this data shows tanycytes are able to synthesize and metabolize RA, in a process regulated by both melatonin and T3, however, only few studies correlate RA proteins expression patterns to cellular processes that could be in part regulated by tanycytes. For example, in hypothalamic organotypic slice cultures, the addition of RA blocks tanycytes proliferation induced by epidermal growth factor. While in vivo, RALDH1 expression in tanycytes during LD correlates with significantly lower number of cells positive for proliferation marker KI67 (86). No further studies have seen if this anti-proliferative effect modulates hypothalamic neurogenesis, as it has been shown for RA in other neurogenic niches as the subventricular zone and the dentate gyrus (90–92).
Finally, rat hypothalamic slices cultured with 1 μM RA increased the transcriptional expression of AgRP and POMC neuropeptides, but the same experiment in the photosensitive rat strain F344/N showed only significant increase for POMC expression (89). More studies aiming to suppress or overexpress RA related proteins in tanycytes are needed to study whether endogenous RA synthesis and metabolism can modulate energy balance and food intake processes, as well as other cellular processes involving tanycytes participation.
Regarding other vitamins, it might be worth mentioning that sodium- vitamin C co-transporter 2 (SVCT2) has been detected in rat and mice tanycytes, by in-situ hybridization, confocal microscopy and ultrastructural immunohistochemistry, showing a specific location in β-1 tanycytes (21, 93). Although vitamin C have long been known to participate in numerous brain functions, such as neurotransmission, neuroprotection and differentiation (78), there are no studies exploring the specific role that vitamin C might have in tanycytes, or if tanycytes only accumulate vitamin C to later release it into the parenchyma as a neuroprotective agent. Taking into consideration that tanycytes are proposed as hypothalamic stem cells, and that vitamin C increases neurogenesis in vitro (94, 95) it would be interesting to analyze how vitamin C uptake by tanycytes can modulate this process in vivo.
Future Directions
Due to their localization in the hypothalamus, tanycytes are in a privileged position to detect different signals, such as hormones, nutrients and growth factors, coming either from the CSF or the blood. Nevertheless, demonstrating that tanycytes act as a sensory cell, able to modulate other brain cell types during regulatory complex processes, has taken a long time. In this context, energy homeostasis and neuroendocrine regulation mediated by hormonal responses that increase energy expenditure or change feeding behavior, require fine-tune regulation, in which brain detection of nutrients plays a key role. For a long time, studies that have tried to understand the regulatory mechanisms involved in nutrient detection have focused on the neuronal component, neglecting the participation of a cell type that, molecularly and histologically, has the necessary characteristics to participate in these processes. Here, we present a small compilation of the evidence supporting the micronutrient sensor role of tanycytes, which altogether suggest that it changes the nutrient sensing ability, which could contribute to the physiopathological responses observed in metabolic disease like obesity and diabetes.
However, as it can be extracted from the literature, for some micronutrients, scientific evidence is still scarce, and further studies are needed to delve into the molecular and cellular mechanisms behind tanycytes nutrients sensing, as well as to explore their physiological or pathological relevance. It is necessary to incorporate genetic tools in these studies that allow for specific tracing of the different tanycytes populations, complemented with in vivo analysis of metabolic related responses, in order to improve tanycyte sensing related knowledge.
Author Contributions
RE-V and KO wrote the manuscript with the input of AR.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would also like to thank Rachael Fasnacht for kindly reviewing our manuscript.
Footnotes
Funding. This work was supported by Programa de Cooperación Internacional, Apoyo a la Formación de Redes Internacionales para investigadores en etapa inicial, de CONICYT, REDI 170306 and Universidad San Sebastián, Campus Tres Pascualas, Concepción, Chile.
References
- 1.Garcia MA, Carrasco M, Godoy A, Reinicke K, Montecinos VP, Aguayo LG, et al. Elevated expression of glucose transporter-1 in hypothalamic ependymal cells not involved in the formation of the brain-cerebrospinal fluid barrier. J Cell Biochem. (2001) 80:491–503. [DOI] [PubMed] [Google Scholar]
- 2.Rodriguez EM, Blazquez JL, Pastor FE, Pelaez B, Pena P, Peruzzo B, et al. Hypothalamic tanycytes: a key component of brain-endocrine interaction. Int Rev Cytol. (2005) 247:89–164. 10.1016/S0074-7696(05)47003-5 [DOI] [PubMed] [Google Scholar]
- 3.Elizondo-Vega R, Cortes-Campos C, Barahona MJ, Oyarce KA, Carril CA, Garcia-Robles MA. The role of tanycytes in hypothalamic glucosensing. J Cell Mol Med. (2015) 19:1471–82. 10.1111/jcmm.12590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akmayev IG, Popov AP. Morphological aspects of the hypothalamic-hypophyseal system. VII The tanycytes: their relation to the hypophyseal adrenocorticotrophic function an ultrastructural study. Cell Tissue Res. (1977) 180:263–82. 10.1007/BF00231958 [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez EM, Gonzalez CB, Delannoy L. Cellular organization of the lateral and postinfundibular regions of the median eminence in the rat. Cell Tissue Res. (1979) 201:377–408. 10.1007/BF00236998 [DOI] [PubMed] [Google Scholar]
- 6.Flament-Durand J, Brion JP. Tanycytes: morphology and functions: a review. Int Rev Cytol. (1985) 96:121–55. 10.1016/S0074-7696(08)60596-3 [DOI] [PubMed] [Google Scholar]
- 7.Ciofi P, Garret M, Lapirot O, Lafon P, Loyens A, Prevot V, et al. Brain-endocrine interactions: a microvascular route in the mediobasal hypothalamus. Endocrinology. (2009) 150:5509–19. 10.1210/en.2009-0584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balland E, Dam J, Langlet F, Caron E, Steculorum S, Messina A, et al. Hypothalamic tanycytes are an ERK-gated conduit for leptin into the brain. Cell Metab. (2014) 19:293–301. 10.1016/j.cmet.2013.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Langlet F. Tanycytes: a gateway to the metabolic hypothalamus. J Neuroendocrinol. (2014) 26:753–60. 10.1111/jne.12191 [DOI] [PubMed] [Google Scholar]
- 10.Mullier A, Bouret SG, Prevot V, Dehouck B. Differential distribution of tight junction proteins suggests a role for tanycytes in blood-hypothalamus barrier regulation in the adult mouse brain. J Comp Neurol. (2010) 518:943–62. 10.1002/cne.22273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brightman MW, Reese TS. Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol. (1969) 40:648–77. 10.1083/jcb.40.3.648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peruzzo B, Pastor FE, Blazquez JL, Amat P, Rodriguez EM. Polarized endocytosis and transcytosis in the hypothalamic tanycytes of the rat. Cell Tissue Res. (2004) 317:147–64. 10.1007/s00441-004-0899-1 [DOI] [PubMed] [Google Scholar]
- 13.Prevot V, Dehouck B, Sharif A, Ciofi P, Giacobini P, Clasadonte J. The versatile tanycyte: a hypothalamic integrator of reproduction and energy metabolism. Endocr Rev. (2018) 39:333–68. 10.1210/er.2017-00235 [DOI] [PubMed] [Google Scholar]
- 14.Mayer J. Glucostatic mechanism of regulation of food intake. N Engl J Med. (1953) 249:13–6. 10.1056/NEJM195307022490104 [DOI] [PubMed] [Google Scholar]
- 15.de Vries MG, Arseneau LM, Lawson ME, Beverly JL. Extracellular glucose in rat ventromedial hypothalamus during acute and recurrent hypoglycemia. Diabetes. (2003) 52:2767–73. 10.2337/diabetes.52.11.2767 [DOI] [PubMed] [Google Scholar]
- 16.Lewis LD, Ljunggren B, Ratcheson RA, Siesjo BK. Cerebral energy state in insulin-induced hypoglycemia, related to blood glucose and to EEG. J Neurochem. (1974) 23:673–9. 10.1111/j.1471-4159.1974.tb04390.x [DOI] [PubMed] [Google Scholar]
- 17.Shram NF, Netchiporouk LI, Martelet C, Jaffrezic-Renault N, Cespuglio R. Brain glucose: voltammetric determination in normal and hyperglycaemic rats using a glucose microsensor. Neuroreport. (1997) 8:1109–12. 10.1097/00001756-199703240-00009 [DOI] [PubMed] [Google Scholar]
- 18.Langlet F, Levin BE, Luquet S, Mazzone M, Messina A, Dunn-Meynell AA, et al. Tanycytic VEGF-A boosts blood-hypothalamus barrier plasticity and access of metabolic signals to the arcuate nucleus in response to fasting. Cell Metab. (2013) 17:607–17. 10.1016/j.cmet.2013.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ganong WF. Circumventricular organs: definition and role in the regulation of endocrine and autonomic function. Clin Exp Pharmacol Physiol. (2000) 27:422–7. 10.1046/j.1440-1681.2000.03259.x [DOI] [PubMed] [Google Scholar]
- 20.Harik SI, Kalaria RN, Andersson L, Lundahl P, Perry G. Immunocytochemical localization of the erythroid glucose transporter: abundance in tissues with barrier functions. J Neurosci. (1990) 10:3862–72. 10.1523/JNEUROSCI.10-12-03862.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia M, Millan C, Balmaceda-Aguilera C, Castro T, Pastor P, Montecinos H, et al. Hypothalamic ependymal-glial cells express the glucose transporter GLUT2, a protein involved in glucose sensing. J Neurochem. (2003) 86:709–24. 10.1046/j.1471-4159.2003.01892.x [DOI] [PubMed] [Google Scholar]
- 22.Millan C, Martinez F, Cortes-Campos C, Lizama I, Yanez MJ, Llanos P, et al. Glial glucokinase expression in adult and post-natal development of the hypothalamic region. ASN Neuro. (2010) 2:e00035. 10.1042/AN20090059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salgado M, Tarifeno-Saldivia E, Ordenes P, Millan C, Yanez MJ, Llanos P, et al. Dynamic localization of glucokinase and its regulatory protein in hypothalamic tanycytes. PLoS ONE. (2014) 9:e94035. 10.1371/journal.pone.0094035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vandercammen A, Van Schaftingen E. The mechanism by which rat liver glucokinase is inhibited by the regulatory protein. Eur J Biochem. (1990) 191:483–9. 10.1111/j.1432-1033.1990.tb19147.x [DOI] [PubMed] [Google Scholar]
- 25.Vandercammen A, Van Schaftingen E. Competitive inhibition of liver glucokinase by its regulatory protein. Eur J Biochem. (1991) 200:545–51. 10.1111/j.1432-1033.1991.tb16217.x [DOI] [PubMed] [Google Scholar]
- 26.Orellana JA, Saez PJ, Cortes-Campos C, Elizondo RJ, Shoji KF, Contreras-Duarte S, et al. Glucose increases intracellular free Ca(2+) in tanycytes via ATP released through connexin 43 hemichannels. Glia. (2012) 60:53–68. 10.1002/glia.21246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frayling C, Britton R, Dale N. ATP-mediated glucosensing by hypothalamic tanycytes. J Physiol. (2011) 589:2275–86. 10.1113/jphysiol.2010.202051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benford H, Bolborea M, Pollatzek E, Lossow K, Hermans-Borgmeyer I, Liu B, et al. A sweet taste receptor-dependent mechanism of glucosensing in hypothalamic tanycytes. Glia. (2017) 65:773–89. 10.1002/glia.23125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ebling FJP, Lewis JE. Tanycytes and hypothalamic control of energy metabolism. Glia. (2018) 66:1176–84. 10.1002/glia.23303 [DOI] [PubMed] [Google Scholar]
- 30.Cortes-Campos C, Elizondo R, Llanos P, Uranga RM, Nualart F, Garcia MA. MCT expression and lactate influx/efflux in tanycytes involved in glia-neuron metabolic interaction. PLoS ONE. (2011) 6:e16411. 10.1371/journal.pone.0016411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cortes-Campos C, Elizondo R, Carril C, Martinez F, Boric K, Nualart F, et al. MCT2 expression and lactate influx in anorexigenic and orexigenic neurons of the arcuate nucleus. PLoS ONE. (2013) 8:e62532. 10.1371/journal.pone.0062532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci USA. (1994) 91:10625–9. 10.1073/pnas.91.22.10625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elizondo-Vega R, Cortes-Campos C, Barahona MJ, Carril C, Ordenes P, Salgado M, et al. Inhibition of hypothalamic MCT1 expression increases food intake and alters orexigenic and anorexigenic neuropeptide expression. Sci Rep. (2016) 6:33606. 10.1038/srep33606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uranga RM, Millan C, Barahona MJ, Recabal A, Salgado M, Martinez F, et al. Adenovirus-mediated suppression of hypothalamic glucokinase affects feeding behavior. Sci Rep. (2017) 7:3697. 10.1038/s41598-017-03928-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barahona MJ, Llanos P, Recabal A, Escobar-Acuna K, Elizondo-Vega R, Salgado M, et al. Glial hypothalamic inhibition of GLUT2 expression alters satiety, impacting eating behavior. Glia. (2018) 66:592–605. 10.1002/glia.23267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nilaweera K, Herwig A, Bolborea M, Campbell G, Mayer CD, Morgan PJ, et al. Photoperiodic regulation of glycogen metabolism, glycolysis, and glutamine synthesis in tanycytes of the Siberian hamster suggests novel roles of tanycytes in hypothalamic function. Glia. (2011) 59:1695–705. 10.1002/glia.21216 [DOI] [PubMed] [Google Scholar]
- 37.Hawkins RA, O'Kane RL, Simpson IA, Vina JR. Structure of the blood-brain barrier and its role in the transport of amino acids. J Nutr. (2006) 136(Suppl. 1):218S−26S. 10.1093/jn/136.1.218S [DOI] [PubMed] [Google Scholar]
- 38.Heeley N, Blouet C. Central amino acid sensing in the control of feeding behavior. Front Endocrinol. (2016) 7:148. 10.3389/fendo.2016.00148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Panksepp J, Booth DA. Decreased feeding after injections of amino-acids into the hypothalamus. Nature. (1971) 233:341–2. 10.1038/233341a0 [DOI] [PubMed] [Google Scholar]
- 40.Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, et al. Hypothalamic mTOR signaling regulates food intake. Science. (2006) 312:927–30. 10.1126/science.1124147 [DOI] [PubMed] [Google Scholar]
- 41.Morrison CD, Xi X, White CL, Ye J, Martin RJ. Amino acids inhibit Agrp gene expression via an mTOR-dependent mechanism. Am J Physiol Endocrinol Metab. (2007) 293:E165–171. 10.1152/ajpendo.00675.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blouet C, Jo YH, Li X, Schwartz GJ. Mediobasal hypothalamic leucine sensing regulates food intake through activation of a hypothalamus-brainstem circuit. J Neurosci. (2009) 29:8302–11. 10.1523/JNEUROSCI.1668-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laeger T, Reed SD, Henagan TM, Fernandez DH, Taghavi M, Addington A, et al. Leucine acts in the brain to suppress food intake but does not function as a physiological signal of low dietary protein. Am J Physiol Regul Integr Comp Physiol. (2014) 307:R310–320. 10.1152/ajpregu.00116.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krauss RM, Mayer J. Influence of protein and amino acids on food intake in the rat. Am J Physiol. (1965) 209:479–83. 10.1152/ajplegacy.1965.209.3.479 [DOI] [PubMed] [Google Scholar]
- 45.Rogers QR, Tannous RI, Harper AE. Effects of excess leucine on growth and food selection. J Nutr. (1967) 91:561–72. 10.1093/jn/91.4.561 [DOI] [PubMed] [Google Scholar]
- 46.Brosnan JT, Brosnan ME. Branched-chain amino acids: enzyme and substrate regulation. J Nutr. (2006) 136(Suppl. 1):207S−11S. 10.1093/jn/136.1.207S [DOI] [PubMed] [Google Scholar]
- 47.Kanamori K, Ross BD, Kondrat RW. Rate of glutamate synthesis from leucine in rat brain measured in vivo by 15N NMR. J Neurochem. (1998) 70:1304–15. 10.1046/j.1471-4159.1998.70031304.x [DOI] [PubMed] [Google Scholar]
- 48.Sanchez del Pino MM, Peterson DR, Hawkins RA. Neutral amino acid transport characterization of isolated luminal and abluminal membranes of the blood-brain barrier. J Biol Chem. (1995) 270:14913–8. 10.1074/jbc.270.25.14913 [DOI] [PubMed] [Google Scholar]
- 49.Lee WJ, Hawkins RA, Vina JR, Peterson DR. Glutamine transport by the blood-brain barrier: a possible mechanism for nitrogen removal. Am J Physiol. (1998) 274:C1101–7. 10.1152/ajpcell.1998.274.4.C1101 [DOI] [PubMed] [Google Scholar]
- 50.O'Kane RL, Hawkins RA. Na+-dependent transport of large neutral amino acids occurs at the abluminal membrane of the blood-brain barrier. Am J Physiol Endocrinol Metab. (2003) 285:E1167–73. 10.1152/ajpendo.00193.2003 [DOI] [PubMed] [Google Scholar]
- 51.Oldendorf WH, Brown WJ. Greater number of capillary endothelial cell mitochondria in brain than in muscle. Proc Soc Exp Biol Med. (1975) 149:736–8. 10.3181/00379727-149-38889 [DOI] [PubMed] [Google Scholar]
- 52.Sershen H, Lajtha A. Capillary transport of amino acids in the developing brain. Exp Neurol. (1976) 53:465–74. 10.1016/0014-4886(76)90086-8 [DOI] [PubMed] [Google Scholar]
- 53.Christensen HN. Developments in amino acid transport, illustrated for the blood-brain barrier. Biochem Pharmacol. (1979) 28:1989–92. 10.1016/0006-2952(79)90213-2 [DOI] [PubMed] [Google Scholar]
- 54.Oxender DL, Christensen HN. Distinct mediating systems for the transport of neutral amino acids by the ehrlich cell. J Biol Chem. (1963) 238:3686–99. [PubMed] [Google Scholar]
- 55.Betz AL, Goldstein GW. Polarity of the blood-brain barrier: neutral amino acid transport into isolated brain capillaries. Science. (1978) 202:225–7. 10.1126/science.211586 [DOI] [PubMed] [Google Scholar]
- 56.Sanchez del Pino MM, Hawkins RA, Peterson DR. Neutral amino acid transport by the blood-brain barrier. Membrane vesicle studies. J Biol Chem. (1992) 267:25951–7. [PubMed] [Google Scholar]
- 57.Tayarani I, Lefauconnier JM, Roux F, Bourre JM. Evidence for an alanine, serine, and cysteine system of transport in isolated brain capillaries. J Cereb Blood Flow Metab. (1987) 7:585–91. 10.1038/jcbfm.1987.109 [DOI] [PubMed] [Google Scholar]
- 58.Hargreaves KM, Pardridge WM. Neutral amino acid transport at the human blood-brain barrier. J Biol Chem. (1988) 263:19392–7. [PubMed] [Google Scholar]
- 59.Tovar A, Tews JK, Torres N, Harper AE. Some characteristics of threonine transport across the blood-brain barrier of the rat. J Neurochem. (1988) 51:1285–93. 10.1111/j.1471-4159.1988.tb03098.x [DOI] [PubMed] [Google Scholar]
- 60.Kilberg MS, Handlogten ME, Christensen HN. Characteristics of an amino acid transport system in rat liver for glutamine, asparagine, histidine, and closely related analogs. J Biol Chem. (1980) 255:4011–9. [PubMed] [Google Scholar]
- 61.Hutchison HT, Eisenberg HM, Haber B. High-affinity transport of glutamate in rat brain microvessels. Exp Neurol. (1985) 87:260–9. 10.1016/0014-4886(85)90216-X [DOI] [PubMed] [Google Scholar]
- 62.O'Kane RL, Martinez-Lopez I, DeJoseph MR, Vina JR, Hawkins RA. Na(+)-dependent glutamate transporters (EAAT1, EAAT2, and EAAT3) of the blood-brain barrier. A mechanism for glutamate removal. J Biol Chem. (1999) 274:31891–5. 10.1074/jbc.274.45.31891 [DOI] [PubMed] [Google Scholar]
- 63.Blouet C, Schwartz GJ. Brainstem nutrient sensing in the nucleus of the solitary tract inhibits feeding. Cell Metab. (2012) 16:579–87. 10.1016/j.cmet.2012.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Karnani MM, Apergis-Schoute J, Adamantidis A, Jensen LT, de Lecea L, Fugger L, et al. Activation of central orexin/hypocretin neurons by dietary amino acids. Neuron. (2011) 72:616–29. 10.1016/j.neuron.2011.08.027 [DOI] [PubMed] [Google Scholar]
- 65.Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. Mammalian sweet taste receptors. Cell. (2001) 106:381–90. 10.1016/S0092-8674(01)00451-2 [DOI] [PubMed] [Google Scholar]
- 66.Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, Ryba NJ, et al. An amino-acid taste receptor. Nature. (2002) 416:199–202. 10.1038/nature726 [DOI] [PubMed] [Google Scholar]
- 67.Lazutkaite G, Solda A, Lossow K, Meyerhof W, Dale N. Amino acid sensing in hypothalamic tanycytes via umami taste receptors. Mol Metab. (2017) 6:1480–92. 10.1016/j.molmet.2017.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, et al. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest. (2012) 122:153–62. 10.1172/JCI59660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Valdearcos M, Robblee MM, Benjamin DI, Nomura DK, Xu AW, Koliwad SK. Microglia dictate the impact of saturated fat consumption on hypothalamic inflammation and neuronal function. Cell Rep. (2014) 9:2124–38. 10.1016/j.celrep.2014.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Valdearcos M, Douglass JD, Robblee MM, Dorfman MD, Stifler DR, Bennett ML, et al. Microglial inflammatory signaling orchestrates the hypothalamic immune response to dietary excess and mediates obesity susceptibility. Cell Metab. (2017) 26:185–97 e183. 10.1016/j.cmet.2017.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee DA, Bedont JL, Pak T, Wang H, Song J, Miranda-Angulo A, et al. Tanycytes of the hypothalamic median eminence form a diet-responsive neurogenic niche. Nat Neurosci. (2012) 15:700–2. 10.1038/nn.3079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Recabal A, Caprile T, Garcia-Robles MLA. Hypothalamic neurogenesis as an adaptive metabolic mechanism. Front Neurosci. (2017) 11:190. 10.3389/fnins.2017.00190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Spector R. Fatty acid transport through the blood-brain barrier. J Neurochem. (1988) 50:639–43. 10.1111/j.1471-4159.1988.tb02958.x [DOI] [PubMed] [Google Scholar]
- 74.Edmond J. Essential polyunsaturated fatty acids and the barrier to the brain: the components of a model for transport. J Mol Neurosci. (2001) 16:181–93; discussion 215–121. 10.1385/JMN:16:2-3:181 [DOI] [PubMed] [Google Scholar]
- 75.Hofmann K, Lamberz C, Piotrowitz K, Offermann N, But D, Scheller A, et al. Tanycytes and a differential fatty acid metabolism in the hypothalamus. Glia. (2017) 65:231–49. 10.1002/glia.23088 [DOI] [PubMed] [Google Scholar]
- 76.Rodriguez E, Guerra M, Peruzzo B, Blazquez JL. Tanycytes: a rich morphological history to underpin future molecular and physiological investigations. J Neuroendocrinol. (2019) 31:e12690. 10.1111/jne.12690 [DOI] [PubMed] [Google Scholar]
- 77.Clasadonte J, Prevot V. The special relationship: glia-neuron interactions in the neuroendocrine hypothalamus. Nat Rev Endocrinol. (2018) 14:25–44. 10.1038/nrendo.2017.124 [DOI] [PubMed] [Google Scholar]
- 78.Oyarce K, Bongarzone ER, Nualart F. Unconventional neurogenic niches and neurogenesis modulation by vitamins. J Stem Cell Res Ther. (2014) 4:184. 10.4172/2157-7633.1000184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kawaguchi R, Yu J, Honda J, Hu J, Whitelegge J, Ping P, et al. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science. (2007) 315:820–5. 10.1126/science.1136244 [DOI] [PubMed] [Google Scholar]
- 80.Ross SA, McCaffery PJ, Drager UC, De Luca LM. Retinoids in embryonal development. Physiol Rev. (2000) 80:1021–54. 10.1152/physrev.2000.80.3.1021 [DOI] [PubMed] [Google Scholar]
- 81.Napoli JL. Physiological insights into all-trans-retinoic acid biosynthesis. Biochim Biophys Acta. (2012) 1821:152–67. 10.1016/j.bbalip.2011.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J. (1996) 10:940–54. 10.1096/fasebj.10.9.8801176 [DOI] [PubMed] [Google Scholar]
- 83.Mangas A, Bodet D, Duleu S, Yajeya J, Geffard M, Covenas R. Direct visualization of retinoic acid in the rat hypothalamus: an immunohistochemical study. Neurosci Lett. (2012) 509:64–8. 10.1016/j.neulet.2011.12.053 [DOI] [PubMed] [Google Scholar]
- 84.Shearer KD, Goodman TH, Ross AW, Reilly L, Morgan PJ, McCaffery PJ. Photoperiodic regulation of retinoic acid signaling in the hypothalamus. J Neurochem. (2010) 112:246–57. 10.1111/j.1471-4159.2009.06455.x [DOI] [PubMed] [Google Scholar]
- 85.Helfer G, Ross AW, Russell L, Thomson LM, Shearer KD, Goodman TH, et al. Photoperiod regulates vitamin A and Wnt/beta-catenin signaling in F344 rats. Endocrinology. (2012) 153:815–24. 10.1210/en.2011-1792 [DOI] [PubMed] [Google Scholar]
- 86.Shearer KD, Stoney PN, Nanescu SE, Helfer G, Barrett P, Ross AW, et al. Photoperiodic expression of two RALDH enzymes and the regulation of cell proliferation by retinoic acid in the rat hypothalamus. J Neurochem. (2012) 122:789–99. 10.1111/j.1471-4159.2012.07824.x [DOI] [PubMed] [Google Scholar]
- 87.Ross AW, Webster CA, Mercer JG, Moar KM, Ebling FJ, Schuhler S, et al. Photoperiodic regulation of hypothalamic retinoid signaling: association of retinoid X receptor gamma with body weight. Endocrinology. (2004) 145:13–20. 10.1210/en.2003-0838 [DOI] [PubMed] [Google Scholar]
- 88.Bolborea M, Laran-Chich MP, Rasri K, Hildebrandt H, Govitrapong P, Simonneaux V, et al. Melatonin controls photoperiodic changes in tanycyte vimentin and neural cell adhesion molecule expression in the Djungarian hamster (Phodopus sungorus). Endocrinology. (2011) 152:3871–83. 10.1210/en.2011-1039 [DOI] [PubMed] [Google Scholar]
- 89.Stoney PN, Helfer G, Rodrigues D, Morgan PJ, McCaffery P. Thyroid hormone activation of retinoic acid synthesis in hypothalamic tanycytes. Glia. (2016) 64:425–39. 10.1002/glia.22938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang TW, Zhang H, Parent JM. Retinoic acid regulates postnatal neurogenesis in the murine subventricular zone-olfactory bulb pathway. Development. (2005) 132:2721–32. 10.1242/dev.01867 [DOI] [PubMed] [Google Scholar]
- 91.Jacobs S, Lie DC, DeCicco KL, Shi Y, DeLuca LM, Gage FH, et al. Retinoic acid is required early during adult neurogenesis in the dentate gyrus. Proc Natl Acad Sci USA. (2006) 103:3902–7. 10.1073/pnas.0511294103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bonnet E, Touyarot K, Alfos S, Pallet V, Higueret P, Abrous DN. Retinoic acid restores adult hippocampal neurogenesis and reverses spatial memory deficit in vitamin A deprived rats. PLoS ONE. (2008) 3:e3487. 10.1371/journal.pone.0003487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nualart F, Mack L, Garcia A, Cisternas P, Bongarzone ER, Heitzer M, et al. Vitamin C transporters, recycling and the bystander effect in the nervous system: SVCT2 versus gluts. J Stem Cell Res Ther. (2014) 4:209. 10.4172/2157-7633.1000209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pastor P, Cisternas P, Salazar K, Silva-Alvarez C, Oyarce K, Jara N, et al. SVCT2 vitamin C transporter expression in progenitor cells of the postnatal neurogenic niche. Front Cell Neurosci. (2013) 7:119. 10.3389/fncel.2013.00119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Oyarce K, Silva-Alvarez C, Ferrada L, Martinez F, Salazar K, Nualart F. SVCT2 is expressed by cerebellar precursor cells, which differentiate into neurons in response to ascorbic acid. Mol Neurobiol. (2018) 55:1136–49. 10.1007/s12035-016-0366-5 [DOI] [PubMed] [Google Scholar]