Abstract
Background
The normal range for Aspartate and Alanine Aminotransferases (AST and ALT) levels (<40 IU/L) were set in 1950s. Recent data from certain countries suggest lower levels of AST and ALT. Aim of the study was to redefine the normal values of aminotransferases in healthy Indian adults.
Methodology
In a cross sectional prospective study, 1002 blood donors were evaluated to isolate a healthy cohort. Four and 9 subjects positive for HBsAg and anti-Hepatitis C Virus (HCV) respectively and three females were excluded. 986 male subjects were evaluated for levels of serum aminotransferases.
Results
Of total 986 subjects (Group I), 543 (55.1%) had fatty liver on ultrasound [15 (1.5%) alcoholic fatty liver and 528 (53.5%) Nonalcoholic Fatty Liver Disease (NAFLD)]. Median AST and ALT in total group (Group I) were 27.69 (Interquartile Range (IQR) 22.33–37.04) and 34.19 IU/L (IQR 23.12–54.87) and in NAFLD (Group II) were 35.67 (IQR −27.49–47.43) and 50.36 (IQR 37.70–76.58) IU/L. Of remaining 443 subjects without fatty liver, 288 had one or more components of metabolic syndrome. Out of 155 patients with no fatty liver and no component of metabolic syndrome (Group III), 103 subjects had normal Body Mass Index (BMI) and normal cholesterol and Low Density Lipoprotein (LDL) (Group IIIB). Median AST and ALT in Group IIIB were 22.56 (IQR 20.23–26.91) and 21.36 (IQR 17.49–27.21) U/L respectively with a 95th percentile of 34.28 and 36.57 U/L for AST and ALT, respectively.
Conclusion
Levels of AST and ALT in healthy men are lower than the conventional values in India.
Abbreviations: LFT, Liver Function Test; TC, Total Cholesterol; TG, Triglyceride
Keywords: ALT, transaminases, NAFLD, fatty liver, nonalcoholic steatohepatitis, metabolic syndrome
Introduction
The serum Aspartate and Alanine Aminotransferases (AST and ALT) are sensitive indicators of liver cell injury and are helpful in recognizing both acute and chronic hepatocellular diseases.1 In addition to differentiating acute from Chronic Liver Diseases (CLDs), the aminotransferases elevations are helpful in determining the disease severity.
The normal range for AST and ALT levels (<40 IU/L) were set in the 1950s and has changed little since then.1 The healthy reference populations included men and women, often medical students, blood donors, and laboratory technicians. This concentration was determined principally from studies performed before the introduction of anti-Hepatitis C Virus (HCV) testing, and prior to recognition of the concept of Nonalcoholic Fatty Liver Disease (NAFLD).1,2 Several studies have questioned whether previously established values to define the aminotransferases were accurate, and have suggested that the upper limit of normal aminotransferases should be revised.3, 4, 5, 6, 7, 8, 9 However, only few studies to redefine upper limit of normal for aminotransferases have been done in a large-scale population-based in an East Asian population, who have different body shape, dietary behaviour, and liver related disease patterns compared with Western people.5, 6, 7, 8, 9 There is also paucity of data regarding the normal level of aminotransferases in the Indian population.10, 11 With the assumption that the current reference range for aminotransferases may be inaccurate, we conducted this study to redefine the normal values of aminotransferases in healthy Indian adults.
Methods
Over a period of one and a half years, the study included prospective blood donors presenting for blood donation in the institute. The study was approved by the institute’s ethics committee and written informed consent was obtained from all participants.
Inclusion Criteria
-
1
Prospective blood donors fulfilling the requisite blood donation criteria as per Drugs and Cosmetics Act in India.12
-
2
Age ≥18 years and ≤65 years.
Exclusion Criteria
-
1
Donors with history of overt liver disease.
-
2
Donors testing positive for HBsAg, anti-HCV or HIV.
-
3
Donors with history of medication or substance abuse likely to affect AST/ALT levels.
History of alcohol intake and any medical illness including liver disease, hypertension, diabetes and cardiovascular disease, medication use or substance abuse was taken from all the participants. Total abstainers and subjects consuming less than 20 g/day of alcohol were defined as non-alcoholics.13 All subjects were subjected to an anthropometric examination and overweight, obesity and central obesity were defined as per the Asia Pacific criteria.14 (overweight—Body Mass Index (BMI) ≥23 kg/m2 but <25 kg/m2, obesity—BMI >25 kg/m2, central obesity ≥90 cm for males and ≥80 cm for females).
Laboratory Assessment
Analyses of serum biochemistry [fasting serum Total Cholesterol (TC), serum Triglycerides (TG), Low Density Lipoproteins (LDL), High Density Lipoproteins (HDL), Fasting Plasma Glucose (FPG) levels and Liver Function Tests (LFT)], were performed using Olympus AU2700 Plus analyser (Beckman—Coulter, ISO certified) after proper quality control and calibration.
All subjects were screened for the transfusion transmitted infections including hepatitis B virus (Advanced HBsAg ELISA, InTec Products Inc., Hialang, Xiamen), HCV (SD HCV ELISA 3.0, Standard Diagnostics, Inc., Kyonggi-do, Korea), human immune deficiency virus (SD HIV1/2 ELISA 3.0, Standard Diagnostics, Inc., Kyonggi-do, Korea), Syphilis and Malaria. Donors testing positive for any of the above infections were excluded from further analysis and referred to the relevant clinics for further management.
Presence of metabolic syndrome was defined as per the Adult Treatment Panel III (ATP III) criteria with modified waist for the Indians as defined earlier.15
Imaging
All donors were also subjected to a hepatobiliary ultrasound examination by a single observer to specifically assess the presence of fatty liver (Philips, HDI-3500 Diagnostic Ultrasound System). Hepatic steatosis was graded into mild, moderate and severe steatosis as per Saverymuttu et al.16 Subjects with presence of fatty liver on ultrasonography were categorised as alcoholic fatty liver (alcohol consumption >20 g/day) NAFLD (alcohol abstinence or consumption <20 g/day).13
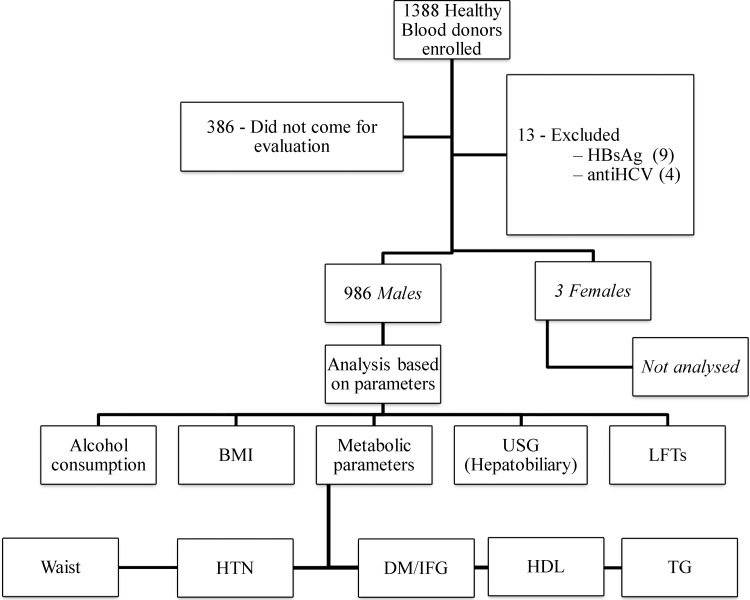
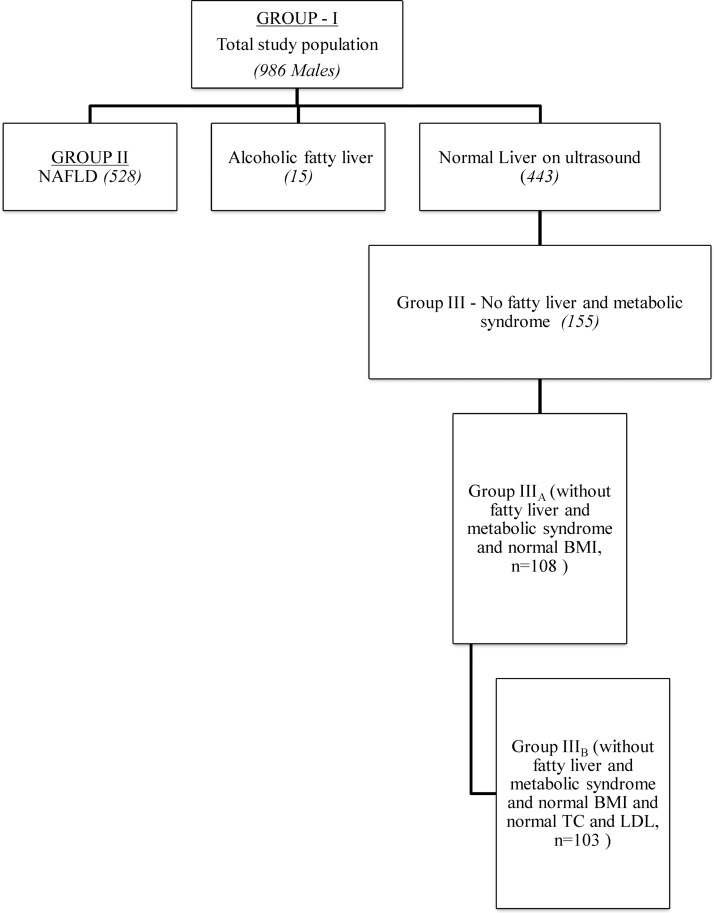
Based on the history, clinical examination, lab parameters and imaging, all subjects were divided into three groups and AST and ALT levels were evaluated in all the groups (Figure 1, Figure 2).
Figure 1.
Flow diagram showing the inclusion and work up of the subjects.
Figure 2.
Different groups of patients included in the study.
Statistical Analysis
For quantitative data, the data was presented as mean ± SD or median and Interquartile Range (IQR), as appropriate. Correlation between various anthropometric and biochemical parameters was evaluated using Pearson’s correlation coefficient and Spearman’s correlation. For comparing the difference in various groups, one-way analysis of variance (ANOVA) followed by post hoc multiple comparisons, Kruskal—Wallis test and Mann–Whitney U test was applied. Stepwise multiple linear regression analysis was used to determine the predictors for aminotransferase levels. We set healthy aminotransferase threshold at 95th percentile. A P value of <0.05 was considered to indicate statistical significance. All calculations were two sided and performed using SPSS version 17.
Results
Of the 1388 subjects initially screened in the study, 386 did not come for evaluation. Of 1002 subjects evaluated, 999 were males (mean age 30.7 ± 8.2 years) and 3 females (mean age 44.7 ± 8.1 years). Because of small number, 3 females were excluded and further analysis was done only in 999 males (Figure 1). Nine (0.9%) and 4 (0.4%) male subjects respectively were excluded on account of HBsAg and anti-HCV reactivity. None of the participants was reactive for HIV. Final analysis was done in 986 male subjects comprising of three groups, Group I (total study population, n = 986), Group II (NAFLD, n = 528), Group III (healthy group without evidence of fatty liver and metabolic syndrome, n = 155), Group IIIA (without fatty liver and metabolic syndrome and normal BMI, n = 108) and Group IIIB (without fatty liver and metabolic syndrome and normal BMI and normal TC and LDL, n = 103) (Figure 1, Figure 2).
Total Study Population (Group I)
The median AST and ALT levels in the whole study group (Group I) were 27.69 (IQR 22.33–37.04) and 34.19 IU/L (IQR 23.12–54.87) respectively. The 95th percentile for AST and ALT were 63.51 and 111.09 U/L, respectively (Table 1).
Table 1.
Showing the Serum Aminotransferase Levels Among Three Different Study Groups.
| No. (%) | Median (IQR) |
95th % |
|||
|---|---|---|---|---|---|
| AST | ALT | AST | ALT | ||
| I | 986 | 27.69 (22.33–37.04) | 34.19 (23.12–54.87) | 63.51 | 111.09 |
| II | 543 | 35.67 (27.49–47.43) | 50.36 (37.70–76.58) | 79.59 | 132.86 |
| III | 155 | 22.50 (19.23–26.50) | 22.10 (17.49–27.60) | 34.23 | 37.66 |
| IIIA | 108 | 22.56 (20.23–26.91) | 21.66 (17.51–27.50) | 34.26 | 36.87 |
| IIIB | 103 | 22.50 (20.16–26.32) | 21.36 (17.49–27.21) | 34.28 | 36.57 |
Table 2 shows the aminotransferase levels among the various strata in the total study population (Group I). The aminotransferase levels increased with increasing age till 50 years, after which there was a significant decrease in the aminotransferase levels (Table 2). Total abstainers from alcohol had a lower AST and ALT levels in comparison to those consuming <20 g of alcohol per day and those who consumed >20 g alcohol per day had a significantly higher AST and ALT levels when compared to those consuming <20 g alcohol per day (Table 2). Subjects who were overweight and obese had a significantly higher AST and ALT levels in comparison to those who were normal weight. However there was no significant difference in AST between class I and class II obese subjects. Similarly AST and ALT levels were higher in subjects with central obesity when compared to those without central obesity. (Table 2) Subjects with hypertension, diabetes mellitus/impaired fasting glucose, waist circumference ≥90 cm, hypertriglyceridemia and low HDL also had higher AST and ALT levels in comparison to normotensive subjects, those with euglycemia and normal serum TG and normal HDL respectively. Overall, the AST and ALT levels among the subjects with metabolic syndrome were higher in comparison to those subjects without metabolic syndrome (Table 2).
Table 2.
Showing the Affect of Various Parameters on Aminotransferase Levels Among Group I Subjects (Total Study Population, n = 986).
| No. (%) | Median (IQR) |
P-value |
||||
|---|---|---|---|---|---|---|
| AST | ALT | AST | ALT | |||
| Age | 18–30 | 570 (57.8) | 26.10 (21.64–34.33) | 29.08 (21.48–48.50) | 0.000 0.451 0.005 |
0.000 0.360 0.000 |
| 30–40 | 279 (28.3) | 31.03 (23.58–40.91) | 40.50 (27.47–65.60) | |||
| 40–50 | 113 (11.5) | 30.67 (24.87–43.57) | 42.98 (30.07–65.88) | |||
| 50–65 | 24 (2.4) | 24.89 (21.27–31.85) | 30.02 (20.17–40.10) | |||
| Alcohol consumption | Abstainer | 733 (74.3) | 26.56 (22.02–34.19) | 30.29 (21.97–47.05) | 0.000 0.000 |
0.000 0.000 |
| <20 g/d | 238 (24.1) | 34.04 (24.30–49.45) | 46.36 (30.63–79.18) | |||
| >20 g/d | 15 (1.5) | 41.47 (22.3–45.91) | 49.52 (25.18–64.90) | |||
| BMI | <23 | 322 (32.7) | 23.72 (20.65–28.01) | 24.71 (18.98–30.98) | 0.000 0.000 0.481 |
0.000 0.000 0.280 |
| 23–24.99 | 205 (20.8) | 25.97 (21.82–32.67) | 29.57 (22.13–43.95) | |||
| 25–29.99 | 382 (38.7) | 34.08 (26.10–46.63) | 48.35 (34.23–75.08) | |||
| >30 | 77 (7.8) | 36.31 (26.58–52.97) | 59.23 (38.00–85.23) | |||
| Waist | <90 cm | 585 (59.3) | 24.87 (21.23–30.88) | 26.88 (20.33–39.41) | 0.000 | 0.000 |
| ≥90 cm | 401 (40.7) | 34.42 (26.01–46.18) | 48.79 (34.31–74.54) | |||
| HTN | Absent | 734 (74.4) | 26.01 (21.73–33.59) | 29.35 (21.52–44.79) | 0.036 | 0.000 |
| Present | 252 (25.6) | 36.00 (26.85–50.34) | 50.90 (34.66–78.65) | |||
| DM/IFG | Absent | 818 (83.0) | 26.56 (21.76–34.56) | 30.87 (21.92–48.11) | 0.000 | 0.000 |
| Present | 168 (17.0) | 36.85 (26.80–49.71) | 56.79 (36.59–80.64) | |||
| TG | <150 | 468 (47.5) | 26.05 (21.17–34.61) | 28.64 (20.61–44.19) | 0.000 | 0.000 |
| ≥150 | 518 (52.5) | 29.47 (23.71–40.35) | 40.02 (26.58–64.41) | |||
| HDL | ≥40 | 477 (48.4) | 26.81 (21.76–36.51) | 31.85 (21.93–49.94) | 0.036 | 0.000 |
| <40 | 509 (52.5) | 28.78 (23.27–37.91) | 37.48 (25.02–59.27) | |||
| Metabolic syndrome criteria fulfilled | 0 | 177 (18.0) | 23.28 (20.09–28.30) | 23.70 (18.52–32.35) | 0 | 0 |
| 1 | 253 (26.5) | 24.71 (21.19–31.12) | 27.53 (20.36–39.92) | 0.000 | 0.000 | |
| 2 | 243 (24.6) | 28.14 (23.56–38.12) | 35.65 (25.68–59.50) | 0.000 | 0.000 | |
| 3 | 181 (18.4) | 33.09 (24.70–47.31) | 47.94 (33.05–78.43) | 0.000 | 0.000 | |
| 4 | 92 (9.3) | 36.89 (29.59–47.53) | 51.62 (39.53–72.84) | 0.507 | 0.051 | |
| 5 | 40 (4.1) | 38.24 (26.11–49.56) | 62.64 (42.53–83.13) | 0.230 | 0.270 | |
| Metabolic syndrome | Present | 313 (31.3) | 35.40 (26.30–47.48) | 50.30 (35.43–77.45) | <0.000 | <0.000 |
| Absent | 673 (68.3) | 25.61 (21.42–32.88) | 28.38 (21.02–42.26) | |||
On Pearson’s/Spearman’s coefficient correlation (Table 3), age, body weight, BMI, waist circumference, blood pressure, FPG, TC, LDL, number of metabolic syndrome parameters and NAFLD were found to have positive correlation with both AST and ALT levels, while serum TG levels had a positive correlation with only ALT level. On stepwise multiple linear regression analysis, only age, systolic blood pressure and NAFLD were found to be independent predictors of both AST and ALT levels (Table 4). Age had a negative correlation with both AST and ALT levels. DBP independently affected AST levels while BMI and LDL levels independently affected ALT levels.
Table 3.
Showing the Correlation of AST/ALT Levels With Various Parameters in Group I Subjects (Total Study Population, n = 986).
| Factor | AST |
ALT |
||
|---|---|---|---|---|
| Correlation coefficient | P-value | Correlation coefficient | P-value | |
| Age | .108** | 0.001 | .122** | 0.000 |
| Weight | .267** | 0.000 | .357** | 0.000 |
| BMI | .330** | 0.000 | .435** | 0.000 |
| Waist | .299** | 0.000 | .383** | 0.000 |
| SBP | .300** | 0.000 | .323** | 0.000 |
| DBP | .280** | 0.000 | .275** | 0.000 |
| FPG | .192** | 0.000 | .226** | 0.000 |
| HDL | −.037 | 0.250 | −.036 | 0.263 |
| TG | .037 | 0.247 | .096** | 0.003 |
| TC | .171** | 0.000 | .284** | 0.000 |
| LDL | .178** | 0.000 | .299** | 0.000 |
| NAFLD | .402** | 0.000 | .519** | 0.000 |
| Number of metabolic syndrome parameters | .171** | 0.000 | .228** | 0.000 |
| AST | – | – | .743** | 0.000 |
| ALT | .743** | 0.000 | – | – |
Bold values are statistically signifiant.
*Correlation is significant at the 0.05 level (2-tailed).
Correlation is significant at the 0.01 level (2-tailed).
Table 4.
Showing the Multivariate Analysis by Regression Analysis of Association Between AST/ALT and Various Factors in Group I (Total Study Population, n = 986).
| Variable | AST |
ALT |
||||||
|---|---|---|---|---|---|---|---|---|
| Regression coefficient | SE | T value | Significance | Regression coefficient | SE | T value | Significance | |
| Age | −0.149 | 0.085 | −4.376 | 0.000 | −0.175 | 0.135 | −5.523 | 0.000 |
| BMI | 0.346 | 0.942 | 0.03 | 0.496 | 0.096 | 0.368 | 2.58 | 0.010 |
| Waist | 0.886 | 0.143 | 0.005 | 0.516 | 0.498 | −0.677 | −0.022 | 0.336 |
| SBP | 0.107 | 0.118 | 2.491 | 0.013 | 0.125 | 0.161 | 3.654 | 0.000 |
| DBP | 0.135 | 0.172 | 3.576 | 0.000 | 0.054 | 1.928 | 0.062 | 0.560 |
| FBS | 0.239 | 1.178 | 0.038 | 0.760 | 0.132 | 1.509 | 0.048 | 0.749 |
| Ch | 0.624 | 0.491 | 0.016 | 0.842 | 0.617 | −0.500 | −0.016 | 0.247 |
| TG | 0.093 | −1.681 | −0.054 | 0.957 | 0.6 | −0.525 | −0.017 | 0.947 |
| HDL | 0.945 | −0.069 | −0.002 | 0.988 | 0.941 | −0.075 | −0.002 | 0.983 |
| LDL | 0.261 | 1.124 | 0.036 | 0.871 | 0.138 | 0.035 | 4.879 | 0.000 |
| MS criteria | 0.967 | 0.041 | 0.001 | 0.816 | 0.745 | 0.326 | 0.01 | 0.807 |
| NAFLD | 0.33 | 1.455 | 9.798 | 0.000 | 0.395 | 2.812 | 10.338 | 0.000 |
Bold values are statistically signifiant.
Nonalcoholic Fatty Liver Disease (Group II)
Out of the 986 males included in the study, 543 (55.1%) had evidence of fatty liver on ultrasound. Of these, 15 subjects (1.5%) had alcoholic fatty liver (alcohol consumption >20 g/day), 528 (53.5%) subjects had NAFLD (alcohol abstinence or consumption <20 g/day). Majority (469, 88.8%) of subjects with NAFLD had mild hepatic steatosis whereas 54 (10.2%) and 5 (0.9%) subjects respectively had moderate and severe hepatic steatosis on ultrasound. NAFLD subjects had a median age of 32 years (IQR 27–39) with a median BMI of 26.79 (IQR 25.11–28.67) kg m−2. Thirty six (6.8%) of them had normal BMI (<23 kg m2) where as 492 subjects (93.2%) were either overweight (85, 16.1%) or obese (407, 77.1%). Among the obese group, 332 subjects (62.9%) had class I obesity (BMI 25–30 kg/m2) while 75 subjects (14.2%) had class II obesity (BMI >30 kg/m2). The median waist circumference of the subjects was 93 cm (IQR 87–100) with 357 subjects (67.7%) having a waist circumference ≥90 cm (central obesity). Two hundred and fifteen subjects (40.7%) had hypertension and 145 (27.5%) of them either had diabetes or impaired fasting glucose. Three hundred and forty two of them (64.8%) had abnormal serum TG (≥150 mg/dl) and 315 subjects (69.7%) had abnormal HDL (<40 mg/dl). Overall, 280 subjects (54.2%) among the NAFLD subjects had presence of metabolic syndrome (ATP III criteria ≥3 components).
Median AST and ALT levels amongst 528 subjects with NAFLD were 35.67 IU/L (IQR −27.49–47.43) and 50.36 IU/L (IQR 37.70–76.58) IU/L with 95th percentile for AST and ALT being 79.59 and 132.86 U/L respectively (Table 1). As in Group I, NAFLD subjects who were obese had a significantly higher AST and ALT levels in comparison to those who were normal (median 35.54 vs. 35.84 IU/L, P < 0.050 AST and median 51.23 vs. 39.08 IU/L, P < 0.001 for ALT). However AST and ALT levels were not significantly different among NAFLD subjects with central obesity when compared to those without central obesity (median 36.01 vs. 35.19 IU/L, P = 0.742 AST and mean 51.03 vs. 48.79 IU/L, P = 0.552 for ALT). Overall, the AST and ALT levels among the subjects with metabolic syndrome were higher in comparison to those subjects without metabolic syndrome (median 36.24 vs. 34.87 IU/L, P = 0.409 for AST and median 48.78 vs. 53.26 IU/L, P = 0.189 for ALT). On Pearson’s/Spearman’s coefficient correlation, age was found to have a negative correlation with the ALT levels among the NAFLD subjects whereas BMI and blood pressure were found to have positive correlation with both AST and ALT levels and FPG, TC and LDL had positive correlation with only ALT levels. On stepwise multiple linear regression analysis age, BMI, waist circumference, SBP, total cholesterol and number of metabolic syndrome parameters were found to be independent predictors of NAFLD.
Healthy Population (Group III)
As mentioned earlier, 543 (55.1%) out of total of 986 subject had evidence of fatty liver on ultrasound. Of the remaining 443 subjects without fatty liver, 288 subjects did have one or the other component of metabolic syndrome (central obesity, hypertension, diabetes mellitus, dyslipidemia) and thus there were only 155 subjects with no evidence of fatty liver and without any component of metabolic syndrome and were grouped as healthy group (Group III). The baseline characteristics of Group III are shown in Table 5. The median AST and ALT amongst Group III were 22.50 (IQR 19.23–26.50) and 22.10 (IQR 17.49–27.60) respectively, with a 95th percentile of 34.23 and 37.66 IU/L for AST and ALT, respectively with no significant difference between Groups IIIA and IIIB (Tables 1 and Table 5). The Group IIIB with minimal risk of liver disease in the present study had a median of 22.56 (IQR 20.23–26.91) and 21.36 (IQR 17.49–27.21) U/L for AST and ALT respectively, with a 95th percentile of 34.28 and 36.57 U/L for AST and ALT, respectively (Table 1). The median and 95th percentile of AST and ALT levels among the healthy population (Group III) was significantly lower than that among the total population study (Group I) and NAFLD subjects (Group II) (all P-values <0.001).Various parameters were correlated with AST and ALT levels in the normal population by Pearson’s/Spearman’s correlation coefficient. None of the parameters studied (age, weight, BMI, blood pressure, FPG) affected the AST and ALT levels among the healthy subjects (Group III) (Table 5).
Table 5.
Showing the Baseline Characteristics of the Group IIIA and IIIB.
| Group IIIA |
Group IIIB |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Percentiles |
Mean | Percentiles |
|||||||
| 25 | 50 | 75 | 95 | 25 | 50 | 75 | 95 | |||
| Age | 25.5 | 21 | 24.5 | 28 | 36 | 25.1 | 21 | 24 | 28 | 35 |
| Weight | 59.92 | 55 | 60 | 65 | 70 | 59.97 | 55 | 60 | 65 | 70 |
| BMI | 20.55 | 19.40 | 20.68 | 21.79 | 22.83 | 20.53 | 19.36 | 20.68 | 21.61 | 22.83 |
| Waist | 75.6 | 71 | 75 | 80 | 85 | 75.44 | 71 | 75 | 79 | 85 |
| SBP | 115.89 | 110 | 120 | 120 | 120 | 115.69 | 110 | 120 | 120 | 120 |
| DBP | 76.15 | 70 | 80 | 80 | 80 | 75.92 | 70 | 80 | 80 | 80 |
| FPG | 84.9 | 79.3 | 85.1 | 91.4 | 96.3 | 84.7 | 79.3 | 84.7 | 90.4 | 96.3 |
| TG | 101.48 | 76.83 | 98.28 | 125.93 | 144.77 | 101.16 | 76.17 | 98.28 | 125.94 | 145.00 |
| HDL | 52.04 | 43.76 | 46.00 | 50.92 | 58.39 | 52.21 | 43.72 | 45.88 | 51.13 | 58.40 |
| Ch | 150.66 | 134.29 | 152.61 | 169.77 | 194.91 | 148.71 | 134.00 | 152.43 | 167.34 | 191.92 |
| LDL | 89.90 | 76.81 | 89.02 | 99.69 | 130.20 | 87.06 | 76.33 | 88.19 | 98.56 | 114.85 |
| AST | 23.45 | 20.22 | 22.56 | 26.91 | 34.26 | 23.32 | 20.16 | 22.50 | 26.32 | 34.28 |
| ALT | 22.98 | 17.51 | 21.66 | 27.50 | 36.87 | 22.79 | 17.49 | 21.36 | 27.21 | 36.57 |
Discussion
Serum aminotransferases AST and ALT are routinely measured to assess the liver function in patients with suspected liver disease. The normal range for AST and ALT levels (<40 IU/L) have not changed for the last more than 65 years.1 The healthy reference values at that time were determined principally from studies performed before the introduction of anti-HCV testing, and prior to the recognition of high prevalence of NAFLD in the general population.1,2 The upper limits of normal thus set at that time may have included many patients with chronic hepatitis C (CHC) and NAFLD thereby falsely raising the upper limits. Lately, several studies which looked at the upper limit of normal aminotransferases in different populations have suggested that the levels may be lowered.3, 4, 5, 6, 7, 8, 9 Our study by including 3 different groups of subjects also support the concept that the upper limits of AST and ALT should be revised in normal healthy adults and presence of NAFLD should be taken into consideration while assessing the normal levels of AST and ALT.
The first study on the subject came from Italy which included 3927 blood donors (1995 men, 1932 women) with normal BMI, normal lipids and glucose levels and found the upper limit of ALT to be 30 IU/L for men and 19 IU/L for women.3 But unlike our study, detailed evaluation for metabolic syndrome and NAFLD was not done in that study. Piton et al.17 from France while evaluating blood donors suggested that definitions of normal ALT values should be adjusted for gender and BMI to reduce the artificial heterogeneity. Another study carried out in Spain18 with 1036 consecutive blood donors found upper normal value for ALT in men to be 56 IU/L and 34 IU/L for women. Comparatively high values in the study were attributed to drugs, exercise, or infections.
The first study in Asia to define upper lower normal for serum ALT and factors associated with serum ALT activity was conducted by Mohamadnejad et al.5 in Iran with 1939 blood donors. Upper limit of normal for non-overweight women (BMI of <25 kg/m2) was 34 U/L, and for non-overweight men was 40 U/L. The higher ULN in their study is likely the result of higher BMI cut-off of 25 kg/m2 for overweight in comparison to 23 kg/m2 taken in our study. In a large retrospective study from Israel,4 in the healthy population of 17,496 subjects the new ULN for ALT was 44.9 U/L for males, higher than that observed in our study. Potential limitations to their study were the retrospective design of the study, lack of tests for anti-HCV or HBsAg in many subjects, and no BMI data, which is the single most determinant of ALT as been observed in many studies.5,17 A study from Korea in 1105 potential liver donors with biopsy-proven normal livers found 35 IU/L as the threshold for ALT in men which are similar to that observed in our study.6 In another recent study with 175 potential living liver donors with biopsy proven normal liver on histology, from Saudi Arabia, showed healthy ALT values of 33 IU/L for men and 22 IU/L for women.19 Slightly lower values in their study in comparison to ours could be due to undiagnosed NAFLD which might not have been picked up on ultrasound in some of our subjects. The most recent Asian study was done in China with 2137 apparently healthy subjects excluding NAFLD and metabolic parameters that affect AST and ALT.9 The upper cut-off values of serum AST and ALT were 25.35 U/L and 22.15 U/L for men and 24.25 U/L and 22.40 U/L for women, respectively. Similar to our results, they also found that alcohol consumption, serum cholesterol and TG levels, and BMI had significant impact on AST and ALT levels.
There is also paucity of data regarding the normal level of aminotransferases in the Indian population and the current reference range for aminotransferases may underestimate the prevalence of CLD. Dietary and behavioural risk for liver disease is widespread in India; hence definition for healthy range of aminotransferase levels is warranted. A study from Western India with 4917 individuals (4643 males, 94.4% and 274 females, 5.6%) reported mean AST and ALT levels in males as 23.4 ± 9.9 IU/L and 27.0 ± 17.3 IU/L and in females 19.1 ± 9.8 IU/L and 17.7 ± 11.2 IU/L, respectively.10 With increase in BMI and waist hip ratio, there was a significant increase in AST and ALT levels. Another study from North India with 67 male and 97 female liver donors with normal histology, 95th percentile for AST and ALT were 33.8 IU/L and 38.6 IU/L for males and 31 IU/L and 35.2 IU/L for females, respectively.11
In our study, the median AST and ALT in the total study population were 27.69 (IQR 22.33–37.04) and 34.19 IU/L (IQR 23.12–54.87), respectively. The 95th percentile for AST and ALT in the total study population group (Group I) were 63.51 and 111.09 U/L, respectively. Since the subjects in total study population had overweight, obesity, multiple metabolic parameters, metabolic syndrome and NAFLD, these figures above could not be taken to define normal values of aminotransferase levels. Since majority of the subjects (93%) in the NAFLD group had overweight or obesity or central obesity and 242 (54.2%) had metabolic syndrome, AST and ALT levels in this group may not be the true representatives of healthy population. The median AST and ALT levels among Group III subjects (155) with no evidence of NAFLD and no metabolic syndrome parameter was 22.50 (IQR 19.23–26.50) and 22.10 (IQR 17.49–27.60) respectively, with a 95th percentile of 34.23 and 37.66 U/L for AST and ALT, respectively. Further exclusion of subjects with abnormal TC and LDL also did not result in any significant difference in AST/ALT levels between Groups IIIA and IIIB. The Group IIIB with minimal risk of liver disease in the present study had a median of 22.56 (IQR 20.23–26.91) and 21.36 (IQR 17.49–27.21) U/L for AST and ALT respectively, with a 95th percentile of 34.28 and 36.57 U/L for AST and ALT, respectively. The median and 95th percentile of AST and ALT levels among the healthy population (Group III) was significantly lower than that among the total population study (Group I) and NAFLD subjects (Group II) (all P-values <0.001).
Since our study was limited to only male subjects, the effect of gender on AST/ALT activity could not be analyzed. The reason for the absence of females in our study was related to the differential gender distribution among the donors in our institute. Even though we screened a large number of subjects, the numbers of healthy subjects were reduced due to the presence of fatty liver and metabolic derangements in many subjects. In conclusion, our data suggests that upper limit of normal (ULN = 95th percentile) of AST and ALT in subjects without fatty liver, no metabolic risk factors with normal BMI and lipids should be 34.28 U/L and 36.57 U/L in the healthy Indian males. Metabolic risk factors and NAFLD are important parameters which affect the AST and ALT levels in healthy blood donors.
Conflicts of interest
The authors have none to declare.
References
- 1.Kaplan M. Alanine aminotransferase levels: what’s normal? Ann Intern Med. 2002;137:49. doi: 10.7326/0003-4819-137-1-200207020-00012. [DOI] [PubMed] [Google Scholar]
- 2.Akkaya O., Kiyici M., Yilmaz Y. Clinical significance of activity of ALT enzyme in patients with hepatitis C virus. World J Gastroenterol. 2007;13:5481–5487. doi: 10.3748/wjg.v13.i41.5481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prati D., Taioli E., Zanella A. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002;137:1. doi: 10.7326/0003-4819-137-1-200207020-00006. [DOI] [PubMed] [Google Scholar]
- 4.Kariv R., Leshno M., Beth-Or A. Re-evaluation of serum alanine aminotransferase upper normal limit and its modulating factors in a large-scale population study. Liver Int. 2006;26:445–450. doi: 10.1111/j.1478-3231.2006.01197.x. [DOI] [PubMed] [Google Scholar]
- 5.Mohamadnejad M., Pourshams A., Malekzadeh R. Healthy ranges of serum alanine aminotransferase levels in Iranian blood donors. World J Gastroenterol. 2003;9:2322–2324. doi: 10.3748/wjg.v9.i10.2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee J., Shim J., Lee H. Estimation of the healthy upper limits for serum alanine aminotransferase in Asian populations with normal liver histology. Hepatology. 2009;51:1577–1583. doi: 10.1002/hep.23505. [DOI] [PubMed] [Google Scholar]
- 7.Al-hamoudi W., Ali S., Hegab B. Revising the upper limit of normal for levels of serum alanine aminotransferase in a middle eastern population with normal liver histology. Dig Dis Sci. 2013;58:2369–2375. doi: 10.1007/s10620-013-2659-0. [DOI] [PubMed] [Google Scholar]
- 8.Sohn W., Jun D., Kwak M. Upper limit of normal serum alanine and aspartate aminotransferase levels in Korea. J Gastroenterol Hepatol. 2013;28:522–529. doi: 10.1111/j.1440-1746.2012.07143.x. [DOI] [PubMed] [Google Scholar]
- 9.Zhang P., Wang C.Y., Li Y.X., Pan Y., Niu J.Q., He S.M. Determination of the upper cut-off values of serum alanine aminotransferase and aspartate aminotransferase in Chinese. World J Gastroenterol. 2015;21:2419–2424. doi: 10.3748/wjg.v21.i8.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amarapurkar D., Kamani P., Patel N. Prevalence of non-alcoholic fatty liver disease: population based study. Ann Hepatol. 2007;6:161–163. [PubMed] [Google Scholar]
- 11.Choudhary N., Saraf N., Saigal S., Gautam D., Lipi L., Soin A. Estimation of normal values of serum transaminases based on liver histology in healthy Asian Indians. J Gastroenterol Hepatol. 2015;30:763–766. doi: 10.1111/jgh.12836. [DOI] [PubMed] [Google Scholar]
- 12.Malik V. 16th ed. Eastern Book Company; Lucknow: 1940. Drugs and Cosmetics Act; pp. 279–303. [Google Scholar]
- 13.Chalasani N., Younossi Z., Lavine J. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592–1609. doi: 10.1053/j.gastro.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 15.Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 16.Saverymuttu S., Joseph A., Maxwell J. Ultrasound scanning in the detection of hepatic fibrosis and steatosis. BMJ. 1986;(292):13–15. doi: 10.1136/bmj.292.6512.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piton A., Poynard T., Imbert-Bismut F. Factors associated with serum alanine transaminase activity in healthy subjects: consequences for the definition of normal values, for selection of blood donors, and for patients with chronic hepatitis C. Hepatology. 1998;27:1213–1219. doi: 10.1002/hep.510270505. [DOI] [PubMed] [Google Scholar]
- 18.Lozano M., Cid J., Bedini J.L. Study of serum alanine-aminotransferase levels in blood donors in Spain. Haematologica. 1998;83:237–239. [PubMed] [Google Scholar]
- 19.Al-hamoudi W., Ali S., Hegab B. Revising the upper limit of normal for levels of serum alanine aminotransferase in a middle eastern population with normal liver histology. Dig Dis Sci. 2013;58:2369–2375. doi: 10.1007/s10620-013-2659-0. [DOI] [PubMed] [Google Scholar]