Abstract
Retinal cell therapy can have the objectives of rescue (i.e., modulation of metabolic abnormalities primarily for sight preservation) as well as replacement (i.e., replace cells lost due to injury or disease for sight restoration as well as preservation). The first clinical trials of retinal pigment epithelium (RPE) transplantation for vision‐threatening complications of age‐related macular degeneration (AMD) have begun with some preliminary signs of success (e.g., improvement in vision in some patients, anatomic evidence of transplant‐host integration with some evidence of host photoreceptor recovery, long‐term survival of autologous induced pluripotent stem cell‐derived RPE transplants without immune suppression) as well as limitations (e.g., limited RPE suspension survival in the AMD eye, limited tolerance for long‐term systemic immune suppression in elderly patients, suggestion of uncontrolled cell proliferation in the vitreous cavity). RPE survival on aged and AMD Bruch's membrane can be improved with chemical treatment, which may enhance the efficacy of RPE suspension transplants in AMD patients. Retinal detachment, currently used to deliver transplanted RPE cells to the subretinal space, induces disjunction of the first synapse in the visual pathway: the photoreceptor‐bipolar synapse. This synaptic change occurs even in areas of attached retina near the locus of detachment. Synaptic disjunction and photoreceptor apoptosis associated with retinal detachment can be reduced with Rho kinase inhibitors. Addition of Rho kinase inhibitors may improve retinal function and photoreceptor survival after subretinal delivery of cells either in suspension or on scaffolds.
Keywords: Autologous stem cell transplantation, Cell transplantation, Clinical trials, Experimental models, Induced pluripotent stem cells, Embryonic stem cells, Retina
Significance Statement.
Cell‐based therapy can be effective in chronic degenerative retinal disease, but the following obstacles must be addressed to optimize therapeutic efficacy: the abnormal microenvironment of the host, surgery‐induced changes in the host retina, and the need for immune suppressive therapy for subretinal allogeneic retinal pigment epithelium (RPE) transplants.
Introduction
Age‐related macular degeneration (AMD) is the leading cause of blindness among persons older than age 55 years in the industrialized world 1. Patients can experience profound loss of central vision, which compromises their ability to read, recognize faces, drive an automobile, and live independently. Severe central visual loss can occur as a result of pathological capillary growth from the choroidal circulation into the subretinal pigment epithelium and subretinal spaces. These vessels, termed choroidal new vessels (CNVs), bleed and fibrose, which results in photoreceptor atrophy. Because the CNVs grow primarily under the macula, which is the part of the retina that supports high acuity vision, they cause severe central vision loss. The other mechanism of central vision loss in AMD patients is geographic atrophy (GA). This process is characterized by photoreceptor apoptosis, retinal pigment epithelium (RPE) atrophy, and choriocapillaris atrophy 2. Like CNVs, GA involves primarily the macula of AMD patients. Both CNV and GA are preceded by inflammation involving the RPE, choriocapillaris, Bruch's membrane complex 3. Bruch's membrane is largely acellular tissue consisting mainly of collagen and elastin interposed between the RPE and choriocapillaris and on which the RPE reside in situ. Both forms of late AMD can exist in a single eye 4. Factors that drive progression to CNV formation versus GA are unknown, and their identification is an area of intense investigation 5, 6, 7.
Antivascular endothelial growth factor antibodies are highly effective in preventing visual loss due to CNVs 8, 9, 10, 11, 12. There is no established treatment, however, for patients with GA. The landscape of options under study includes complement pathway inhibitors (which block complement activation and are intended to reduce the inflammatory response in the RPE‐choriocapillaris that is believed to underlie AMD progression), visual cycle inhibitors (which block cis‐retinal synthesis and are intended to reduce lipofuscin formation in the RPE, thus reducing oxidative damage), intravitreal neurotrophic factors (intended to reduce photoreceptor apoptosis in GA), lipid metabolism modulators (intended to modulate lipid deposition in Bruch's membrane, thus reducing progression of AMD to development of GA), and cell‐based therapy (intended to replace damaged RPE and/or photoreceptors in patients with advanced CNVs or GA).
The purpose of this review is to provide an update on the use of cell‐based therapy to treat vision‐threatening complications of AMD. A number of cell types are under clinical study including RPE cells, neural stem cells, and bone‐marrow derived stem cells (Table 1). Cell transplants can be used as “rescue therapy.” Rescue involves preservation and, in some cases, restoration of function in tissue that is destined to die or malfunction due to an underlying disease. Rescue therapy may be sight‐restoring to the degree that dying cells, which cannot support vision, can return to normal physiological function. Cell transplants can also be used as “replacement therapy.” Replacement involves providing healthy cells (e.g., RPE) to replace those that are dead or dying with the goal of restoring physiological function to a tissue or organ. In the case of RPE transplants for GA, one attempts to replace the RPE cells lost due to GA with the goal of preventing the spread of atrophy and additional visual loss. Areas of GA may harbor some residual photoreceptor nuclei 4, 13, 14, so some recovery of visual function is also possible depending on the extent of damage prior to the transplant. As discussed elsewhere, the eye is a particularly suitable target for cell therapy in the central nervous system 15. This review will focus on results with RPE cells, as currently the earliest phase clinical trial data are available primarily for this cell type in the treatment of AMD.
Table 1.
Human cell therapy trials for late‐stage age‐related macular degeneration
| Disease (ClinicalTrials.gov identifier) | Phase | Cell type transplanted | Center (PI) | Sponsor |
|---|---|---|---|---|
| AMD‐GA (NCT01344993, NCT02563782, NCT02463344) | I/II | ESC‐RPE (MA09‐hRPE) | Jules Stein‐UCLA (Schwartz) Wills Eye Hospital (Regillo) Mass. Eye & Ear Infirmary (Eliott) Bascom Palmer Eye Institute (Rosenfeld) |
Astellas Pharma |
| AMD‐GA (NCT03178149) | Ib/II | ASP7317 (MA09‐hRPE) | Jules Stein‐UCLA Retina Specialty institute New Jersey Retina Mid‐Atlantic Retina |
Astellas Institute for Regenerative Medicine |
| AMD‐GA (NCT01674829) | I/II | ESC‐RPE (MA09‐hRPE) | CHA Bundang Medical Center (Song) | CHA Bio & Diostech |
| AMD‐GA (NCT03305029) | Interventional | SCNT‐ESC‐RPE | CHA Bundang Medical Center (Song) | CHA University |
| AMD‐CNV (NCT01691261, NCT03102138) | I | PF‐05206388 (ESC‐RPE on a polyester membrane) | University College London (Pfizer) | Pfizer |
| AMD‐GA or CNV (NCT02464956) | Observational | Autologous iPSC‐RPE | Moorfields Eye Hospital | Moorfields Eye Hospital NHS Foundation Trust |
| AMD‐GA (NCT02590692) | I/II | ESC‐RPE on a polymeric substrate (CPCB‐RPE1) | Retina Vitreous Associates Medical Group (Rahhal) USC Keck School of Medicine (Kashani) Southern California Desert Retina Consultants Orange County Retina Medical Group California Retina Consultants |
Regenerative Patch Technologies, LLC |
| AMD‐CNV | Interventional | Autologous iPSC‐RPE | Riken Institute for Developmental Biology (Takahashi) | Riken Institute for Developmental Biology |
| AMD (NCT00874783) | Observational | iPSCs | Hadassah Medical Organization (Reubinoff) | Hadassah Medical Organization |
| AMD‐GA (NCT02286089) | I/II | ESC‐RPE | Hadassah Ein Kerem University Hospital (Jaouni) Rabin Medical Center (Erlich) Kaplan Medical Center (Morori‐Katz) Tel Aviv Souraski Medical Center (Barak) Retina Vitreous Associates (Boyer) Byers Eye Institute (Do) Retina Consultants Medical Group (Telander) West Coast Retina Medical Group (McDonald) |
Cell Cure Neurosciences, Ltd. |
| AMD‐GA (NCT02749734) | I/II | ESC‐RPE | Southwest Hospital (yin) | Southwest Hospital, Chongqing, China |
| AMD‐GA (NCT03046407) | I/II | ESC‐RPE | First Affiliated Hospital of Zhengzhou University (Qi) | Chinese Academy of Sciences |
| AMD‐GA (NCT02755428) | I/II | ESC‐RPE | Beijing Tongren Hospital (qi) | Chinese Academy of Sciences |
| AMD‐GA (NCT02868424) | I | Fetal human RPE | First Affiliated Hospital with Nanjing Medical University (Liu) | First Affiliated Hospital with Nanjing Medical University, Nanjing, China |
| AMD‐GA (NCT02016508) | I/II | Bone marrow‐derived SCs | Al‐Azhar University (Safwat) | Al‐Azhar University |
| AMD (NNCT01920867) | Interventional | Bone marrow‐derived SCs | Retina Associates of South Florida (Weiss) | Retina Associates of South Florida |
| AMD‐GA (NCT01736059) | I | Bone marrow‐derived CD34+ SCs | University of California, Davis (Park) | University of California, Davis |
| AMD‐GA or CNV (NCT01518127) | I/II | Autologous bone marrow‐derived SCs | University of Sao Paulo, Brazil (Siqueira) | University of Sao Paulo |
| AMD‐GA (NCT01632527) | I/II | HuCNS‐SC | Retina Foundation of the Southwest Retina Vitreous Associates Medical Group Byers Eye Institute New York Eye and Ear Infirmary Retina Research Institute of Texas |
StemCells, Inc. |
| AMD‐GA (NCT01226628) | I | CNTO 2476 (umbilical tissue‐derived cells) | Wills Eye Hospital (Ho) | Janssen Research & Development, LLC |
| AMD‐GA (NCT00447954) | II | NT‐501 implant | Retina‐Vitreous Associates Medical Group Retina Group of Florida Bascom Palmer Eye Institute Ophthalmic Consultants of Boston Beaumont Eye Institute Retina Foundation of Southwest Vitreoretinal Consultants University of Utah |
Neurotech Pharmaceuticals |
Abbreviations: AMD, age‐related macular degeneration; CNV, choroidal neovascularization; ESC‐RPE, human embryonic stem cell‐derived RPE; iPSC‐RPE, human induced pluripotent stem cell‐derived RPE; GA, geographic atrophy; RPE, retinal pigment epithelium; SCNT‐ESC‐RPE, somatic cell nuclear transfer human embryonic stem cell derived‐RPE; NT‐501 implant, transformed RPE that overexpress ciliary neurotrophic factor.
Clinical Studies
RPE Transplantation for Choroidal Neovascularization in AMD
Tezel et al. transplanted sheets of adult allogeneic RPE in conjunction with surgical removal of subfoveal CNVs in AMD patients 16. Different cadavers were used to provide RPE for different patients. Tissue was prepared according to a previously established protocol 17, and the method has been described in detail elsewhere 18. Briefly, before RPE harvesting, gelatin blocks were cut into triangular pieces and mounted on a vibratome. Gelatin sheets 100‐μm thick were cut from the blocks and kept in CO2‐free medium at 4°C. Allogeneic adult human RPE cells were harvested as intact sheets from cadaver eyes obtained within 24 hours of death. Specifically, the sclera was peeled away from the choroid, and the globe was incubated with 25 U/ml Dispase (Gibco, Grand Island, NY) for 30 minutes. Eyes were rinsed with CO2‐free medium, and a circumferential incision was made into the subretinal space along the ora serrata. Loosened RPE sheets were removed from the rest of the ocular tissue and placed on a slice of 50% gelatin containing 300 mM sucrose with the apical surface of the RPE facing upward. The gelatin film with the adult human RPE sheet was then incubated in a humidified atmosphere of 5% CO2 and 95% air at 37°C for 5 minutes to allow the gelatin to melt and encase the adult RPE sheet. The specimen was kept at 4°C for 5 minutes to solidify the gelatin and stored in CO2‐free medium at 4°C for up to 24 hours before transplantation to the subretinal space. Positive identification of the cells using immunohistochemistry confirmed their epithelial origin, and viability of the RPE sheet was assessed using standard methods. All 12 patients were treated with triple immune suppression (corticosteroids, azathioprine, cyclophosphamide) preoperatively and postoperatively. One year after surgery, there was no significant change in best‐corrected visual acuity, contrast sensitivity, or reading speed. Although there was no sign of transplant rejection among patients who were able to continue immunosuppressants for 6 months, six patients did not tolerate the regimen, and apparent rejection (i.e., graft fibrosis) was observed soon after the immune suppressive regimen was stopped in three patients. Relevant complications included migration of the graft to an extrafoveal location (one patient), retinal detachment with proliferative vitreoretinopathy (one patient), and epiretinal membrane formation distorting the macula and requiring additional surgery (two patients).
Binder et al. reported the results of RPE transplants for AMD patients with subfoveal CNVs in a prospective controlled trial 19. Patients underwent CNV excision followed by subretinal injection of autologous RPE in suspension. As described previously, the RPE cells were harvested subretinally from the nasal side of the optic disc and given to a pathologist, who centrifuged the freshly harvested RPE cells, counted them in a hemocytometer, and evaluated them under a microscope 19, 20. If there were at least 1,500 RPE cells, then they were diluted in 0.1–0.2 ml physiologic saline and returned to the surgeon for transplantation. Controls underwent subretinal CNV excision without transplantation. Reading speed was superior in the transplant group by 12 months follow‐up, and there was a trend for better visual acuity using Early Treatment Diabetic Retinopathy Study (ETDRS) visual acuity charts 21 in the transplant group.
Lu et al. reported the results of autologous RPE sheet transplants in AMD patients undergoing CNV excision 22. RPE harvesting was as follows 22. After the submacular CNV was excised, the detached RPE layer around the CNV was exposed. If the initial area of RPE detachment was not wide enough to harvest a free graft, balanced salt solution was injected into the subRPE space using a 39‐gauge needle (E7365; Bausch & Lomb, Tampa, FL) to expand the area for harvesting a graft of adequate size. The separated RPE monolayer was cut with microscissors. The free graft was gently transposed to the presumed submacular site and was fixed into position by gently pressing the edge of the graft with the smooth back surface of the microforceps as well as by using a heavier‐than‐water perfluorocarbon liquid. The retina flap, used to create access to the subretinal space and CNV, was then flipped back into position after the perfluorocarbon was removed. Vision improved from 16.61 ± 27.98 ETDRS letters to 29.16 ± 23.80 letters (p = .02) during a mean follow‐up period of 21.72 ± 11.09 months, but the series was retrospective with the potential for confounding by a number of factors including case selection and concomitant cataract surgery. Fifteen ETDRS letter improvement corresponds to doubling of the visual angle, which is generally accepted as a clinically significant degree of change in vision 23.
Mandai et al. transplanted a sheet of autologous induced pluripotent stem cell (iPSC)‐derived RPE into the subretinal space of an AMD patient who also underwent surgical excision of a subfoveal CNV 24. iPSCs were generated from skin fibroblasts using nonintegrating episomal vectors carrying GLIS1, L‐MYC, SOX2, KLF4, OCT3/4 and differentiated into RPE as described previously 25, 26. Pigmented colonies of RPE were picked manually and cultured to confluence. The pigmented cells were verified as RPE based on their ultrastructural appearance and based on biochemical features (e.g., presence of retinoid cycle enzymes [RPE65], cellular retinaldehyde binding protein [CRALBP], phagocytosis proteins [MERTK], chloride channels [BEST1], and tight junction proteins [ZO‐1] as determined by reverse transcription polymerase chain reaction and immunohistochemistry). In addition, iPSC‐derived RPE transepithelial resistance was measured as was the ability of the RPE to phagocytose porcine rod photoreceptor outer segments. The autologous iPSC‐derived RPE cells were assessed for quality and safety before transplantation, and whole‐genome sequencing, whole genome methylation profiling, and expression analyses were also performed. To generate RPE sheets without a scaffold, iPSC‐RPE were seeded on collagen gel and cultured in RPE cell sheet medium. After reaching confluence, the iPSC‐RPE was cultured in serum‐free retinal medium supplemented with basic fibroblast growth factor and SB431542 (0.5 mM) for at least 4 weeks. The medium was changed every 2–3 days. To prepare iPSC‐RPE cell sheets without any artificial scaffold, the insert membrane was removed and collagenase I was applied at 37°C for 30 minutes to dissolve the collagen gel. The iPSC‐RPE sheet was then cut at the margin to release it from the insert as an intact cell sheet. The iPSC‐RPE cell sheets were washed in phosphate‐buffered saline and transferred to a dish. These sheets were kept moist with Dulbecco's modified Eagle's medium/F12 (200 ml) until they were cut using laser microdissection. The RPE sheets were prepared for transplantation on the day of surgery. The RPE sheet was cut in one corner so that the apical surface could be identified intraoperatively. The 1.3 mm × 3 mm RPE sheet was delivered to the subretinal space using a modified 20‐gauge cannula. One year after surgery, the sheet seemed to be intact; however, there was no improvement in the patient's vision (stable at 20/200). Given the degree of foveal atrophy evident before surgery, this result is not surprising. There was no clinical or angiographic evidence of graft rejection in this patient, who was not immune suppressed.
da Cruz et al. reported the use of human embryonic stem cell (hESC)‐derived RPE transplants to treat two AMD patients with subfoveal CNVs associated with significant subretinal hemorrhage 27. The hESCs were expanded on vitronectin‐coated culture dishes and spontaneously differentiated into pigmented RPE cells that were manually isolated and passaged. With immunohistochemistry and transmission electron microscopy, these cells exhibited typical features of mature RPE such as expression of CRALBP, BEST1, ZO‐1, pigment epithelium‐derived factor, premelanosomes, and apical‐basal polarization. In addition, they phagocytosed photoreceptor outer segments. A 6 mm × 3 mm patch of a well differentiated RPE monolayer resting on a vitronectin‐coated polyester membrane was transplanted into the subretinal space and positioned under the macula. Patients were immune suppressed with perioperative oral prednisone and intravitreal implants providing sustained delivery of fluocinolone acetonide. One patient developed a severe retinal detachment after the transplant procedure and underwent successful retinal reattachment surgery. In the patient with the least foveal atrophy before surgery, vision improved 29 letters on the ETDRS vision chart, from 20/640 to 20/160 (normal = 20/20), and reading speed improved from 0 words per minute to ∼80 words per minute (normal = 200 words per minute) by 12 months after surgery. In the patient with the postoperative retinal detachment, who had more profound foveal atrophy before the transplant procedure, vision improved 21 ETDRS letters, from 20/800 to 20/150, and reading speed improved from 0 words per minute to ∼50 words per minute by 12 months after surgery. Because vision can improve after subretinal surgery alone in this setting, with approximately 25% of eyes improving 10 or more ETDRS letters, and because there were no control surgeries in this series, one cannot ascribe these improvements to the transplants with complete certainty 19, 22, 28, 29. There was, however, anatomic evidence of integration of the RPE transplant with host retina and focal improvement in photoreceptor anatomy over the transplant in both patients.
RPE Transplantation for GA in AMD
Schwartz et al. reported the use of ESC‐derived RPE suspensions to treat nine AMD patients with GA 30, 31, 32. A hESC line (MA09) was used to generate a master cell bank that was thawed and expanded on mitomycin‐C‐treated mouse embryonic fibroblasts for three passages. After embryoid body formation and cellular outgrowth, pigmented RPE patches were isolated with collagenase. The RPE cells were isolated, purified, expanded, and cryopreserved at passage‐2 for clinical use. RPE suspensions were delivered to the subretinal space via a small retinotomy (38‐gauge) and were directed to the border area of GA. Patients were immunosuppressed with low dose tacrolimus and mycophenolyate mofetil before surgery and for 6 weeks after surgery. At week‐6, tacrolimus was to be discontinued with continuation of mycophenolate for an additional 6 weeks. Complications were associated with this regimen in the AMD patients indicating that older patients probably will not tolerate sustained systemic immune suppression 33. After surgery, the transplants seemed to expand with increased pigmentation in the subretinal space. There was no evidence, however, of significant expansion of pigmented tissue into the area of GA. Pigmented cells seemed to expand away from and surrounding the area of GA but not into it. Two of nine patients developed pigmented epiretinal membranes (i.e., growth of pigmented cells in a sheet on the surface of the retina) that were not clinically significant. Among the eight eyes that did not develop cataract after surgery, median improvement in best‐corrected visual acuity was 14 letters by month 12 (interquartile range [IQR] 3.0–19.0 letters). Fellow eyes lost a median of one letter by month 12 (IQR −5.0 to +6.1 letters). Potential confounders in the interpretation of these results include: lack a of control group receiving subretinal fluid injection without cells (sham surgery can have a rescue effect), bias (neither the examiner nor the patient were masked regarding the treatment received) 34, and improvement due to repeat testing 35. There was no correlation between the presence of postoperative pigmentation and postoperative visual improvement, nor did the absence of hyperpigmentation preclude visual improvement.
Kashani et al. transplanted 3.5 mm × 6.25 mm sheets of hESC‐derived RPE monolayers into areas of GA in five AMD patients 36. Since RPE cells are lacking in areas of GA, these experiments explicitly replace lost native RPE in the area of GA. The RPE spontaneously differentiated from an ESC line (NIH‐H9) through removal of soluble growth factors. Second‐passage hESC‐RPE was cryopreserved as an intermediate cell bank 37, 38. The authors nanoengineered a parylene C scaffold for RPE sheet delivery 39. The scaffolds are 6 μm thick to provide mechanical support with 0.3 μm thick, 40 μm diameter diffusion zones to facilitate nutrient transfer from the choriocapillaris. The diffusion zones occupy ∼60% of the scaffold surface area. Passage‐3 RPE were seeded onto one of these vitronectin‐coated parylene‐C membranes and grown to confluence for ∼4 weeks, achieving a density of 105 cells per scaffold. Before transplantation, the scaffolds were checked for confluence, pigmentation, and cobblestone morphology. These cells demonstrate RPE markers (e.g., RPE65) as well as phagocytosis of photoreceptor outer segments 38. The scaffold could not be implanted in one patient due to the presence of fibrinoid debris in the subretinal space. Surgery for the other four patients was uneventful with delivery of the RPE‐scaffold into the area of GA. In these latter patients, the appearance, location, and size of the implants did not change during follow‐up, which ranged from 120 to 365 days (mean 260 days) after surgery. Four of the five subjects showed no substantial change in vision from baseline. One transplant recipient, however, improved by 17 ETDRS letters, and this improvement was maintained at last follow‐up (day 120 after surgery). Contralateral fellow eyes showed no significant change in vision or worsened during follow‐up. This degree of visual improvement is almost never observed in GA patients 40, so it seems likely that the improvement was related to the surgical procedure. Normally, the eye fixates on an object by moving so that the image of regard falls on the fovea. When the fovea is damaged, fixation becomes unsteady and involves extrafoveal fixation points 41. Two subjects developed stable fixation over the implant.
Optical coherence tomography (OCT) provides 3‐μm resolution images of the retina and adjacent structures (i.e., RPE, Bruch's membrane, choriocapillaris) 42, 43. Four highly reflective bands in OCT images of the outer retina have been identified as the external limiting membrane (ELM; comprising heterotypic adherens junctions between photoreceptor inner segments and the Muller cell apical processes), the ellipsoid zone (comprising the outer portion of photoreceptor inner segments enriched in mitochondria), the interdigitation zone (corresponding to the contact cylinder, a structure defined by ensheathment of the cone outer segments by the apical processes of the RPE), and the RPE band 44, 45. Changes in outer retinal OCT images associated with different types of photoreceptor pathology are consistent with this classification 46, 47. Recovery of visual acuity following successful macular hole surgery is associated with restoration of the ELM 48. Visual recovery following antivascular endothelial growth factor therapy for CNVs in AMD patients is associated with restoration of the ellipsoid zone, which occurs where the ELM is retained 49. Kashani et al. noted that OCT images demonstrated formation of an ELM over the transplant in four patients (including the patient with marked visual improvement), which may mean that the transplanted RPE cells were integrating with the overlying host photoreceptors 14, 36, 47. One may question whether visual improvement is possible in areas of GA, but, as noted above, histopathological studies have demonstrated that some photoreceptor cells lacking inner and outer segments persist in these areas 4, 13, 14. Some studies indicate that relatively few photoreceptors are needed to support functional vision 50. Thus, a rescue effect may underlie the visual improvements noted in this study.
Preclinical Studies
The results of the studies cited above are consistent with a modest treatment benefit for patients with the exudative and atrophic complications of late AMD. As these studies are early phase clinical trials, the subjects enrolled tend to have advanced disease with limited potential for visual improvement. Nonetheless, the results available indicate several potential obstacles to clinical translation on a large scale. Potential obstacles include: (a) RPE survival on Bruch's membrane in AMD eyes; (b) cell delivery to the subretinal space; (c) physiological cell behavior following transplantation (e.g., integration with host retina, prevention of retinal detachment‐induced synaptic disjunction between host photoreceptors and bipolar cells; (d) host immune response to allogeneic transplants (including stem cell‐derived transplants); and (e) cancer risk. In this review, the focus will be on: (a) the impact of the retinal and subretinal microenvironment in AMD eyes and (b) the impact of iatrogenic retinal detachment required for cell delivery.
Retinal and Subretinal Microenvironment
Bruch's membrane exhibits a number of abnormalities with aging and in AMD, including thickening and accumulation of abnormal extracellular material, protein crosslinking and noncollagenous protein deposition, lipidization, and deposition of inflammatory mediators 2, 3, 51, 52, 53. In addition, RPE exhibit signs of senescence, and areas of choriocapillaris dropout are present 54. These changes may prevent transplanted RPE from surviving and differentiating in AMD eyes 55.
As there are no animal models that faithfully reproduce all aspects of AMD pathology 56, we have studied RPE transplant survival in organ culture using post‐mortem human AMD eyes to analyze RPE‐Bruch's membrane interactions that may occur during RPE transplants in AMD patients. Data from post‐mortem human eyes indicate that RPE transplant survival and differentiation on untreated aged and AMD Bruch's membrane is poor 57. The results observed in organ culture mimic closely those observed in transplants of RPE suspensions in humans with GA 33, 58. Furthermore, we found that in organ culture, survival and differentiation of human RPE suspensions in areas of GA could be improved substantially by incubating the cells in bovine corneal endothelial cell‐conditioned medium 58. Human ESC‐RPE survival as well as fetal human RPE survival increased 400% on aged and AMD Bruch's membrane in the presence of this conditioned medium (Figs. 1, 2). In these experiments, the organ culture medium is changed three times per week as the volume of conditioned medium is low (200 μl), and it tends to evaporate. In addition, the conditioned medium vehicle for these experiments is Madin‐Darby Bovine Kidney Maintenance Medium (MDBK‐MM; Sigma–Aldrich, St. Louis, MO) supplemented with 2.5 μg/ml amphotericin B and 50 μg/ml gentamicin. MDBK‐MM is a serum‐ and protein‐free, defined medium designed for maintaining high‐density cultures of MDBK cells. Providing a serum‐containing vehicle does not materially improve RPE survival on aged human Bruch's membrane 58. We have found that unless the cells are exposed to the conditioned medium throughout 21 days in organ culture, RPE survival is not optimal 58. This result may mean that continuous delivery of the bioactive components may be needed for an extended period of time in situ. All the RPE survival‐related biological activity of this conditioned medium is present in a fraction that contains components filtered through a 50 kDa molecular weight (MW) cutoff filter 59. In fact, two MW filtrates (a low MW fraction collected from a 3 kDa cutoff filter and a 10–50 kDa filtrate collected from 50 kDa filtrates further purified with a 10 kDa cutoff filter) when combined are sufficient for the full biological effect of the conditioned medium. The identity of the bioactive components is under investigation, but the important point is that one may be able to improve the survival and differentiation of RPE suspensions in areas of GA without using a scaffold. Scaffolds have a number of potential advantages: (a) one can deliver a monolayer of differentiated RPE, which may promote more rapid and effective photoreceptor rescue than a cell solution; (b) the scaffold prevents transplanted RPE from interacting with aged/damaged Bruch's membrane, which may contain death signals (or their equivalent) 60; and (c) RPE on scaffolds may be more resistant to oxidative damage than RPE suspensions 61, 62. Scaffolds are associated, however, with some limitations. First, the size of the retinotomy required to deliver the cells is considerably greater (10–20×) than with cell suspensions, which creates a greater risk for epiretinal membrane formation and postoperative retinal detachment; 38‐ to 41‐gauge retinotomies are essentially self‐sealing whereas the retinotomies used to deliver scaffolds currently require a retinal incision of a size that is best treated with laser photocoagulation to prevent postoperative retinal detachment. Second, the surgical procedure for subretinal scaffold delivery is more complex than injection of a cell suspension; it requires special instruments and involves creating a larger area of retinal detachment to deliver the scaffold to the subretinal space. Considering the number of patients that would require cell therapy, if it were highly effective, the greater ease of skill acquisition associated with delivery of cell suspensions may present a considerable advantage with regard to adoption of treatment by practicing retina surgeons. Finally, molecules that improve RPE survival as a differentiated monolayer on AMD Bruch's membrane can also be incorporated into scaffold technology, which might improve long‐term RPE survival in situ when using this approach to cell delivery.
Figure 1.
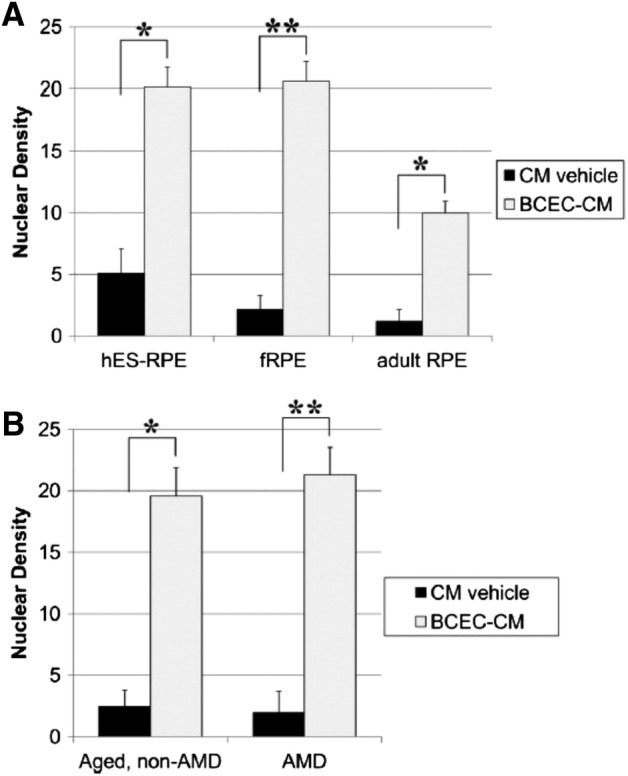
Nuclear densities of cells seeded on aged submacular Bruch's membrane explants after 21‐day culture in conditioned media (CM) vehicle or bovine corneal endothelial cell (BCEC)‐CM (paired explants from the same donor). (A): Nuclear density comparison of retinal pigment epithelium (RPE) cells derived from hESC‐RPE (n = 6), cultured human fetal RPE (fRPE, n = 22), and cultured human adult RPE (donor ages 58, 71, and 78 years; n = 7). Within each group, significant differences were observed between cells cultured in CM vehicle and cells cultured in BCEC‐CM. The nuclear density of cells cultured in CM vehicle was not statistically different between groups. The nuclear densities of hES‐RPE and fRPE were not significantly different from each other but were significantly higher than the nuclear density of adult RPE cells after culture in BCEC‐CM. (B): Comparison of nuclear densities of fRPE on age‐matched, non‐AMD versus AMD Bruch's membrane at day 21. Explants seeded with fRPE on aged Bruch's membrane (n = 9) were compared with explants seeded on age‐related macular degeneration (AMD) submacular Bruch's membrane (n = 13). No significant differences were observed in the nuclear densities of fRPE on non‐AMD versus AMD explants for a given medium, although the nuclear density was significantly higher in the presence of BCEC‐CM versus CM vehicle. Nuclear density values are counts of nuclei of cells directly in contact with Bruch's membrane, expressed as mean nuclear density per millimeter Bruch's membrane. Bars indicate mean ± SE nuclear density. *, p < .05; **, p < .001. Reproduced with permission from Sugino et al. 55.
Figure 2.
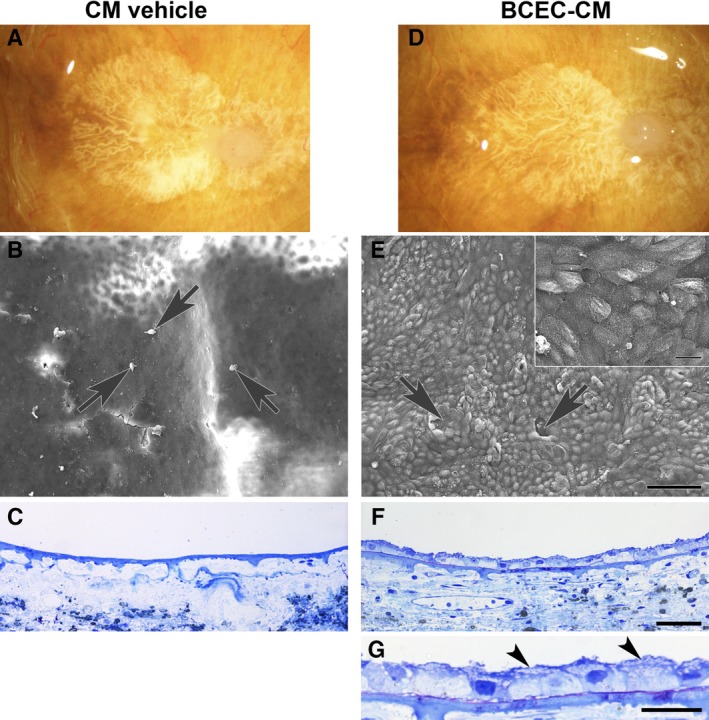
Paired explants from an 82‐year‐old woman with geographic atrophy, seeded with fetal retinal pigment epithelium (RPE) cells. The patient's clinical history noted age‐related macular degeneration (AMD) for 20 years. (A, D): Post‐mortem clinical photographs showing subfoveal geographic atrophy before RPE cell seeding. In conditioned media (CM) vehicle, (B) only a few dead cells (arrows) and cellular debris are present on the explant surface. (C): No cells are present on Bruch's membrane surface. In bovine corneal endothelial cell (BCEC)‐CM, (E) RPE cells fully resurface Bruch's membrane in the area of geographic atrophy with a few very small defects (arrows). Localized areas of multilayering are present. Cell surfaces show abundant apical processes (inset). (F): In this field, cells resurfacing the BCEC‐CM explant are predominantly bilayered. Cells directly on Bruch's membrane are small and tightly packed; flat cells appear to overlie the cells in contact with Bruch's membrane. (G): Flattened cell processes overlying cells on top of Bruch's membrane are indicated by arrowheads. The cell processes contain vesicles. CM vehicle nuclear density (ND), 0; BCEC‐CM ND, 19.61 ± 0.43. Scale bars: 100 μm (E); 20 μm (E, inset); 50 μm (F); 20 μm (G). Toluidine blue staining. Reproduced with permission from Sugino et al. 58.
Cell Delivery Via Retinal Detachment
All methods of RPE transplantation require creation of a retinal detachment so that cells can be delivered to the space between Bruch's membrane and the neural retina (Fig. 3). Retinal detachment affects synapses in the outer plexiform layer 63, 64. Synaptic injury begins with retraction of rod presynaptic terminals toward their cell bodies (Fig. 4). Axonal retraction results in disjunction of the first synapse in the visual pathway as the rod presynaptic terminal disconnects from the postsynaptic bipolar cell dendrite 65. Retinal detachment also disrupts cone terminals, which lose their synaptic invaginations and normal connections with bipolar cells 64, 66. Shortly after rod terminal retraction, bipolar cell dendrites extend into the outer nuclear layer, and horizontal cells exhibit sprouting and sometimes extend into the subretinal space 64. Rod axon retraction and bipolar and horizontal cell sprouting occur in humans after retinal detachment 66, 67. Retinal detachment is also associated with some degree of neuronal apoptosis even if the detachment is relatively brief. Retinal reattachment allows photoreceptor outer segments to regrow but does not restore retinal synaptic structure completely 68, 69.
Figure 3.
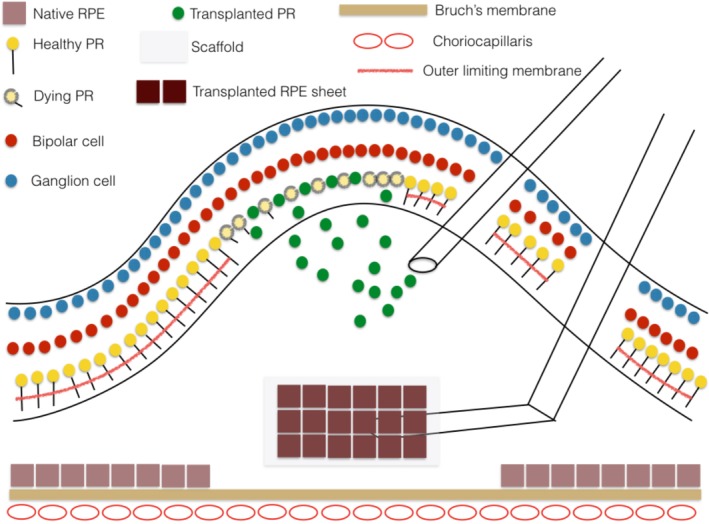
Schematic drawing illustrating subretinal injection of a suspension of rod photoreceptor precursor cells as might be done for a patient with photoreceptor degeneration due to a retinal dystrophy. The cells integrate into the retina preferentially in areas of external limiting membrane breakdown. Also shown is subretinal delivery of a retinal pigment epithelium (RPE) sheet on a scaffold to replace a localized RPE defect on Bruch's membrane as could occur in patients with geographic atrophy. Cell delivery to the subretinal space requires creating a localized retinal detachment. Reproduced with permission from Zarbin 15.
Figure 4.
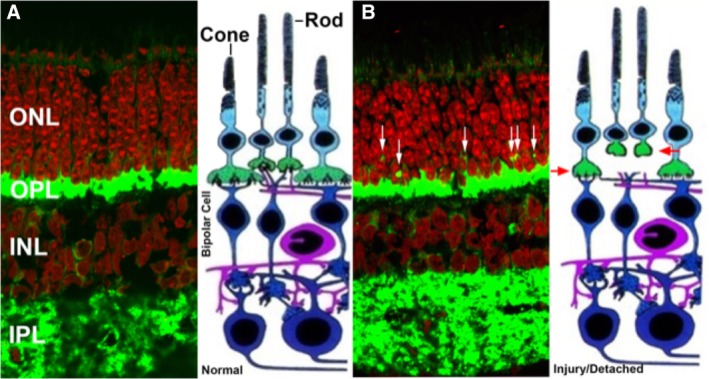
Injury‐induced synaptic disjunction. (A): Normal retina labeled for synaptic protein (SV2, green) and nuclei (red). (B): After detachment, rod terminals retract from the outer plexiform layer into the outer nuclear layer (white arrows) and cone terminals either round up or flatten due to reduction in invaginations in the cone terminals (red arrows, injured cone and rod terminals). Cone terminals are not readily evident in the photomicrograph (visualized as a reduction in invaginations in the cone terminals) due to the paucity of cones in this region but are illustrated in the adjacent schematic drawing. Abbreviations: INL, inner nuclear layer; IPL inner plexiform layer.
Fontainhas and Townes‐Anderson showed that synaptic retraction is associated with a significant increase in RhoA‐GTP formation, and this biochemical change begins within minutes after retinal injury or detachment 70. Synaptic retraction can be blocked using Rho A antagonists 70. RhoA activates Rho‐associated protein kinase (ROCK). ROCK belongs to the AGC (PKA/PKG/PKC) family of serine‐threonine kinases. ROCK is involved mainly in regulating the shape and movement of cells by acting on the cytoskeleton. Through its actions on LIM kinase and cofilin, ROCK increases actin depolymerization. In addition, by phosphorylating myosin light chain, ROCK induces actin binding to myosin II and contractility increases. Human ROCK1 is a major downstream effector of the small GTPase RhoA. Thus, one can block the effects of RhoA with ROCK inhibitors.
Using an in vivo model of retinal detachment in pigs, Wang et al. found that rod‐bipolar synaptic disjunction not only occurs in the area of the detachment, it also occurs in attached retina millimeters away from the area of detachment (Fig. 5) 71. The implication of this finding is that even if surgeons attempt to spare the fovea from detachment when delivering drugs, cells, or genes to the subretinal space, an extrafoveal detachment in the macular area is likely to induce synaptic changes in the fovea that may compromise the patient's final vision. In addition, Wang et al. found that rod‐bipolar synaptic disjunction could be reduced with subretinal administration of a ROCK inhibitor (Fig. 5) 71. Townes‐Anderson et al. reported that subretinal injection of fasudil, a ROCK inhibitor that is approved for clinical use for a different indication, also reduces rod‐bipolar synaptic disjunction 72. Intravitreal injection is also effective, which may facilitate clinical use, as intravitreal injections are done routinely in an outpatient setting in most retina clinics. Another benefit of ROCK inhibition is that it reduces photoreceptor apoptosis induced by retinal detachment 72.
Figure 5.
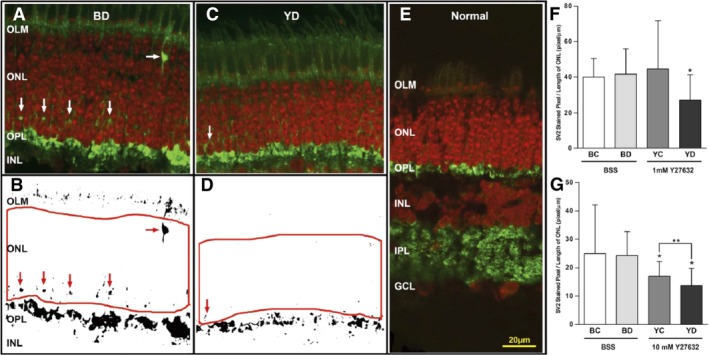
Effect of the ROCK inhibitor, Y27632. On axonal retraction by photoreceptors after retinal detachment in vivo. (A, C): Representative confocal images of control detached retina (BD) and detached retina treated with 10 mM Y27632 (YD) labeled for SV2 (green) and nuclei (red). SV2 labels rod presynaptic terminals. SV2‐labeled spots (white arrows) in the outer nuclear layer (ONL) indicate axonal retraction. (B, D): Binary images created from (A) and (C). SV2‐labeled spots are indicated with red arrows. The number of labeled pixels in the ONL delimited by the red borders was determined and divided by the length of examined ONL. (E): Normal retina without detachment is shown for comparison. (F, G): Treatment with 1 mM and 10 mM Y27632. Comparison of SV2‐labeled pixels per unit retinal length in different retinal areas (BC and BD, attached and detached areas, respectively, in the eye using balanced salt solution [BSS] for detachment; YC and YD, attached and detached areas, respectively, in the eye using Y27632 for detachment). In both treatment groups, there are no significant differences between BC and BD in SV2‐labeled pixels. For 1 mM Y27632, labeled pixels in YD were 34.5% less than labeled pixels in BD (*, p = .02; n = 48 retinal sections; 16 samples; four pigs). There was also a reduction in pixel labeling to 38.7% in YD compared with that in YC (p = .06; n = 48 retinal sections; 16 samples; four pigs). For 10 mM Y27632, labeled pixels in YD and YC were 43.7% (*, p = .02) and 29.9% (*, p = .04), respectively, less than labeled pixels in BD. Thus, only with 10 mM Y27632 was there a reduction in pixel labeling in the YC area compared with that in BD. Finally, there was also a significant reduction in labeled pixels in YD compared with that in YC by 24.4% (**, p = .009; n = 48 retinal sections; 16 samples; four pigs). Reproduced with permission from Wang et al. 71.
Conclusion
Cell therapy can have the objectives of rescue (i.e., modulation of metabolic abnormalities primarily for sight preservation) as well as replacement (i.e., replace cells lost due to injury or disease with the goal of sight restoration as well as preservation). The first clinical trials of RPE transplantation for the late complications of AMD have begun with some preliminary signs of success (e.g., improvement in vision in some patients, anatomic evidence of transplant‐host integration with some evidence of host photoreceptor recovery, long‐term survival of autologous iPSC transplants without immune suppression) as well as limitations (e.g., limited RPE suspension survival in the AMD eye, limited tolerance for long‐term systemic immune suppression in elderly patients, suggestion of uncontrolled cell proliferation in the vitreous cavity). RPE survival on aged and AMD Bruch's membrane can be improved with chemical treatment. This finding establishes the possibility that RPE transplants may survive in AMD eyes without the use of a scaffold, which, if true, may enhance the efficacy of transplants of RPE suspensions in AMD eyes. Delivery of cell suspensions is technically easier and possibly safer than delivery of cells on a scaffold. Nonetheless, these bioactive moieties might also be integrated into scaffolds used to deliver cells to the subretinal space. Retinal detachment, currently used to deliver transplanted RPE cells, induces disjunction of the first synapse in the visual pathway: the photoreceptor‐bipolar synapse. This synaptic change occurs even in areas of attached retina near the locus of detachment. Synaptic disjunction and photoreceptor apoptosis associated with retinal detachment can be reduced with Rho kinase inhibitors. Addition of Rho kinase inhibitors may improve retinal function and photoreceptor survival after subretinal delivery of cells either in suspension or on scaffolds.
Author Contributions
M.Z.: conception, preparation of manuscript, generation of some data presented in manuscript; I.S., E.T.‐A.: revision of manuscript, generation of some data presented in manuscript.
Disclosure of Potential Conflicts of Interest
Dr. Zarbin is a paid consultant for Cell Cure, Chengdu Kanghong Biotechnology Co., Coherus Biosciences, Inc., Daiichi Sankyo, Frequency Therapeutics, Genentech/Roche, Healios KK, Inc., Iridex, Isarna Therapeutics, Makindus, Novartis Pharma AG, Ophthotech Corp., and Percept Corp. Work on improving RPE survival on AMD Bruch's membrane is covered in U.S. Patent no. 9598672, issued March 21, 2017 (“Production of extracellular matrix, conditioned media and uses thereof.” Inventors: Sugino I (Madison, NJ), Zarbin, M. (Chatham, NJ), Sun Q. (West Orange, NJ), Birge R. (New York, NY). The other authors indicated no potential conflicts of interest.
Acknowledgments
This work was supported in part by the New Jersey Lions Eye Research Foundation (M.Z., I.S.), the Joseph J. and Marguerite DiSepio Retina Research Fund (M.Z., I.S.), the Edward N. & Della L. Thome Memorial Foundation Awards Program in Age‐Related Macular Degeneration Research (M.Z., I.S.), the Eng Family Foundation (M.Z., I.S.), the F.M. Kirby Foundation (E.T.‐A.), and NIH grant EY021542 (E.T.‐A., M.Z., I.S.).
References
- 1. Wong WL, Su X, Li X et al. Global prevalence of age‐related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta‐analysis. Lancet Glob Health 2014;2:e106–e116. [DOI] [PubMed] [Google Scholar]
- 2. Zarbin MA. Current concepts in the pathogenesis of age‐related macular degeneration. Arch Ophthalmol 2004;122:598–614. [DOI] [PubMed] [Google Scholar]
- 3. Gehrs KM, Jackson JR, Brown EN et al. Complement, age‐related macular degeneration and a vision of the future. Arch Ophthalmol 2010;128:349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Green WR, Enger C. Age‐related macular degeneration histopathologic studies. The 1992 Lorenz E. Zimmerman Lecture. Ophthalmology 1993;100:1519–1535. [DOI] [PubMed] [Google Scholar]
- 5. Ambati J, Atkinson JP, Gelfand BD. Immunology of age‐related macular degeneration. Nat Rev Immunol 2013;13:438–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fowler BJ, Gelfand BD, Kim Y et al. Nucleoside reverse transcriptase inhibitors possess intrinsic anti‐inflammatory activity. Science 2014;346:1000–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim Y, Tarallo V, Kerur N et al. DICER1/Alu RNA dysmetabolism induces Caspase‐8‐mediated cell death in age‐related macular degeneration. Proc Natl Acad Sci USA 2014;111:16082–16087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rosenfeld PJ, Brown DM, Heier JS et al. Ranibizumab for neovascular age‐related macular degeneration. N Engl J Med 2006;355:1419–1431. [DOI] [PubMed] [Google Scholar]
- 9. Brown DM, Kaiser PK, Michels M et al. Ranibizumab versus verteporfin for neovascular age‐related macular degeneration. N Engl J Med 2006;355:1432–1444. [DOI] [PubMed] [Google Scholar]
- 10. Comparison of Age‐related Macular Degeneration Treatments Trials Research G , Martin DF, Maguire MG et al. Ranibizumab and bevacizumab for treatment of neovascular age‐related macular degeneration: Two‐year results. Ophthalmology 2012;119:1388–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Heier JS, Brown DM, Chong V et al. Intravitreal aflibercept (VEGF trap‐eye) in wet age‐related macular degeneration. Ophthalmology 2012;119:2537–2548. [DOI] [PubMed] [Google Scholar]
- 12. Chakravarthy U, Harding SP, Rogers CA et al. Alternative treatments to inhibit VEGF in age‐related choroidal neovascularisation: 2‐Year findings of the IVAN randomised controlled trial. Lancet 2013;382:1258–1267. [DOI] [PubMed] [Google Scholar]
- 13. Bird AC, Phillips RL, Hageman GS. Geographic atrophy: A histopathological assessment. JAMA Ophthalmol 2014;132:338–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sarks JP, Sarks SH, Killingsworth MC. Evolution of geographic atrophy of the retinal pigment epithelium. Eye 1988;2:552–577. [DOI] [PubMed] [Google Scholar]
- 15. Zarbin M. Cell‐based therapy for degenerative retinal disease. Trends Mol Med 2016;22:115–134. [DOI] [PubMed] [Google Scholar]
- 16. Tezel TH, Del Priore LV, Berger AS et al. Adult retinal pigment epithelial transplantation in exudative age‐related macular degeneration. Am J Ophthalmol 2007;143:584–595. [DOI] [PubMed] [Google Scholar]
- 17. Tezel TH, Del Priore LV, Kaplan HJ. Harvest and storage of adult human retinal pigment epithelial sheets. Curr Eye Res 1997;16:802–809. [DOI] [PubMed] [Google Scholar]
- 18. Del Priore LV, Kaplan HJ, Tezel TH et al. Retinal pigment epithelial cell transplantation after subfoveal membranectomy in age‐related macular degeneration: Clinicopathologic correlation. Am J Ophthalmol 2001;131:472–480. [DOI] [PubMed] [Google Scholar]
- 19. Binder S, Krebs I, Hilgers RD et al. Outcome of transplantation of autologous retinal pigment epithelium in age‐related macular degeneration: A prospective trial. Invest Ophthalmol Vis Sci 2004;45:4151–4160. [DOI] [PubMed] [Google Scholar]
- 20. Binder S, Stolba U, Krebs I et al. Transplantation of autologous retinal pigment epithelium in eyes with foveal neovascularization resulting from age‐related macular degeneration: A pilot study. Am J Ophthalmol 2002;133:215–225. [DOI] [PubMed] [Google Scholar]
- 21. Kaiser PK. Prospective evaluation of visual acuity assessment: A comparison of snellen versus ETDRS charts in clinical practice (an AOS thesis). Trans Am Ophthalmol Soc 2009;107:311–324. [PMC free article] [PubMed] [Google Scholar]
- 22. Lu Y, Han L, Wang C et al. A comparison of autologous transplantation of retinal pigment epithelium (RPE) monolayer sheet graft with RPE‐Bruch's membrane complex graft in neovascular age‐related macular degeneration. Acta Ophthalmol 2017;95:e443–e452. [DOI] [PubMed] [Google Scholar]
- 23. Lindblad AS, Clemons TE. Responsiveness of the National Eye Institute Visual Function Questionnaire to progression to advanced age‐related macular degeneration, vision loss, and lens opacity: AREDS report no. 14. Arch Ophthalmol 2005;123:1207–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mandai M, Watanabe A, Kurimoto Y et al. Autologous induced stem‐cell‐derived retinal cells for macular degeneration. N Engl J Med 2017;376:1038–1046. [DOI] [PubMed] [Google Scholar]
- 25. Kamao H, Mandai M, Okamoto S et al. Characterization of human induced pluripotent stem cell‐derived retinal pigment epithelium cell sheets aiming for clinical application. Stem Cell Rep 2014;2:205–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nakagawa M, Koyanagi M, Tanabe K et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol 2008;26:101–106. [DOI] [PubMed] [Google Scholar]
- 27. da Cruz L, Fynes K, Georgiadis O et al. Phase 1 clinical study of an embryonic stem cell‐derived retinal pigment epithelium patch in age‐related macular degeneration. Nat Biotechnol 2018;36:328–337. [DOI] [PubMed] [Google Scholar]
- 28. Bressler NM, Bressler SB, Childs AL et al. Surgery for hemorrhagic choroidal neovascular lesions of age‐related macular degeneration: Ophthalmic findings: SST report no. 13. Ophthalmology 2004;111:1993–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Falkner CI, Leitich H, Frommlet F et al. The end of submacular surgery for age‐related macular degeneration? A meta‐analysis. Graefes Arch Clin Exp Ophthalmol 2007;245:490–501. [DOI] [PubMed] [Google Scholar]
- 30. Schwartz SD, Regillo CD, Lam BL et al. Human embryonic stem cell‐derived retinal pigment epithelium in patients with age‐related macular degeneration and Stargardt's macular dystrophy: Follow‐up of two open‐label phase 1/2 studies. Lancet 2015;385:509–516. [DOI] [PubMed] [Google Scholar]
- 31. Schwartz SD, Tan G, Hosseini H et al. Subretinal transplantation of embryonic stem cell‐derived retinal pigment epithelium for the treatment of macular degeneration: An assessment at 4 years. Invest Ophthalmol Vis Sci 2016;57:ORSFc1–ORSFc9. [DOI] [PubMed] [Google Scholar]
- 32. Schwartz SD, Hubschman JP, Heilwell G et al. Embryonic stem cell trials for macular degeneration: A preliminary report. Lancet 2012;379:713–720. [DOI] [PubMed] [Google Scholar]
- 33. Schwartz SD, Regillo CD, Lam BL et al. Human embryonic stem cell‐derived retinal pigment epithelium in patients with age‐related macular degeneration and Stargardt's macular dystrophy: Follow‐up of two open‐label phase 1/2 studies. Lancet 2015;385:509–516. [DOI] [PubMed] [Google Scholar]
- 34. Schulz KF, Chalmers I, Hayes RJ et al. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408–412. [DOI] [PubMed] [Google Scholar]
- 35. Sunness JS. Stem cells in age‐related macular degeneration and Stargardt's macular dystrophy. Lancet 2015;386:29. [DOI] [PubMed] [Google Scholar]
- 36. Kashani AH, Lebkowski JS, Rahhal FM et al. A bioengineered retinal pigment epithelial monolayer for advanced, dry age‐related macular degeneration. Sci Transl Med 2018;10:eaao4097. [DOI] [PubMed] [Google Scholar]
- 37. Koss MJ, Falabella P, Stefanini FR et al. Subretinal implantation of a monolayer of human embryonic stem cell‐derived retinal pigment epithelium: A feasibility and safety study in Yucatan minipigs. Graefes Arch Clin Exp Ophthalmol 2016;254:1553–1565. [DOI] [PubMed] [Google Scholar]
- 38. Thomas BB, Zhu D, Zhang L et al. Survival and functionality of hESC‐derived retinal pigment epithelium cells cultured as a monolayer on polymer substrates transplanted in RCS rats. Invest Ophthalmol Vis Sci 2016;57:2877–2887. [DOI] [PubMed] [Google Scholar]
- 39. Lu B, Tai YC, Humayun MS. Microdevice‐based cell therapy for age‐related macular degeneration. Dev Ophthalmol 2014;53:155–166. [DOI] [PubMed] [Google Scholar]
- 40. Sunness JS, Margalit E, Srikumaran D et al. The long‐term natural history of geographic atrophy from age‐related macular degeneration: Enlargement of atrophy and implications for interventional clinical trials. Ophthalmology 2007;114:271–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sunness JS, Applegate CA. Long‐term follow‐up of fixation patterns in eyes with central scotomas from geographic atrophy that is associated with age‐related macular degeneration. Am J Ophthalmol 2005;140:1085–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Menghini M, Duncan JL. Diagnosis and complementary examinations. Dev Ophthalmol 2014;53:53–69. [DOI] [PubMed] [Google Scholar]
- 43. Menghini M, Lujan BJ, Zayit‐Soudry S et al. Correlation of outer nuclear layer thickness with cone density values in patients with retinitis pigmentosa and healthy subjects. Invest Ophthalmol Vis Sci 2015;56:372–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Spaide RF, Curcio CA. Anatomical correlates to the bands seen in the outer retina by optical coherence tomography: Literature review and model. Retina 2011;31:1609–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Staurenghi G, Sadda S, Chakravarthy U et al. Proposed lexicon for anatomic landmarks in normal posterior segment spectral‐domain optical coherence tomography: The IN*OCT consensus. Ophthalmology 2014;121:1572–1578. [DOI] [PubMed] [Google Scholar]
- 46. Mitamura Y, Mitamura‐Aizawa S, Katome T et al. Photoreceptor impairment and restoration on optical coherence tomographic image. J Ophthalmol 2013;2013:518170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schmitz‐Valckenberg S, Fleckenstein M, Gobel AP et al. Optical coherence tomography and autofluorescence findings in areas with geographic atrophy due to age‐related macular degeneration. Invest Ophthalmol Vis Sci 2011;52:1–6. [DOI] [PubMed] [Google Scholar]
- 48. Bottoni F, De Angelis S, Luccarelli S et al. The dynamic healing process of idiopathic macular holes after surgical repair: A spectral‐domain optical coherence tomography study. Invest Ophthalmol Vis Sci 2011;52:4439–4446. [DOI] [PubMed] [Google Scholar]
- 49. Oishi A, Shimozono M, Mandai M et al. Recovery of photoreceptor outer segments after anti‐VEGF therapy for age‐related macular degeneration. Graefes Arch Clin Exp Ophthalmol 2013;251:435–440. [DOI] [PubMed] [Google Scholar]
- 50. Geller AMSP. How many cones are required to "see?": Lessons from Stargardt's macular dystrophy and from modeling with degenerate photoreceptor arrays In: al JHe , ed. Retinal Degeneration. New York: Plenum Press, 1993:25–34. [Google Scholar]
- 51. Nozaki M, Raisler BJ, Sakurai E et al. Drusen complement components C3a and C5a promote choroidal neovascularization. Proc Natl Acad Sci USA 2006;103:2328–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Karwatowski WS, Jeffries TE, Duance VC et al. Preparation of bruch's membrane and analysis of the age‐related changes in the structural collagens. Br J Ophthalmol 1995;79:944–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Haimovici R, Gantz DL, Rumelt S et al. The lipid composition of drusen, Bruch's membrane, and sclera by hot stage polarizing light microscopy. Invest Ophthalmol Vis Sci 2001;42:1592–1599. [PubMed] [Google Scholar]
- 54. Seddon JM, McLeod DS, Bhutto IA et al. Histopathological insights into choroidal vascular loss in clinically documented cases of age‐related macular degeneration. JAMA Ophthalmol 2016;134:1272–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sugino IK, Sun Q, Wang J et al. Comparison of FRPE and human embryonic stem cell‐derived RPE behavior on aged human Bruch's membrane. Invest Ophthalmol Vis Sci 2011;52:4979–4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zarbin MA, Casaroli‐Marano RP, Rosenfeld PJ. Age‐related macular degeneration: Clinical findings, histopathology, imaging techniques In: Casaroli‐Marano RP, Zarbin MA, eds. Cell‐Based Therapy for Retinal Degenerative Disease. Basel, Switzerland: Karger Medical and Scientific Publishers, 2014:1–32. [Google Scholar]
- 57. Gullapalli VK, Sugino IK, Van Patten Y et al. Retinal pigment epithelium resurfacing of aged submacular human Bruch's membrane. Trans Am Ophthalmol Soc 2004;102:123–137. discussion 37‐8. [PMC free article] [PubMed] [Google Scholar]
- 58. Sugino IK, Rapista A, Sun Q et al. A method to enhance cell survival on Bruch's membrane in eyes affected by age and age‐related macular degeneration. Invest Ophthalmol Vis Sci 2011;52:9598–9609. [DOI] [PubMed] [Google Scholar]
- 59. Sugino IK, Sun Q, Springer C et al. Two bioactive molecular weight fractions of a conditioned medium enhance RPE cell survival on age‐related macular degeneration and aged Bruch's membrane. Transl Vis Sci Technol 2016;5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Handa JT, Verzijl N, Matsunaga H et al. Increase in the advanced glycation end product pentosidine in Bruch's membrane with age. Invest Ophthalmol Vis Sci 1999;40:775–779. [PubMed] [Google Scholar]
- 61. Diniz B, Thomas P, Thomas B et al. Subretinal implantation of retinal pigment epithelial cells derived from human embryonic stem cells: Improved survival when implanted as a monolayer. Invest Ophthalmol Vis Sci 2013;54:5087–5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hsiung J, Zhu D, Hinton DR. Polarized human embryonic stem cell‐derived retinal pigment epithelial cell monolayers have higher resistance to oxidative stress‐induced cell death than nonpolarized cultures. Stem Cells Translational Medicine 2015;4:10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Erickson PA, Fisher SK, Anderson DH et al. Retinal detachment in the cat: The outer nuclear and outer plexiform layers. Invest Ophthalmol Vis Sci 1983;24:927–942. [PubMed] [Google Scholar]
- 64. Lewis GP, Linberg KA, Fisher SK. Neurite outgrowth from bipolar and horizontal cells after experimental retinal detachment. Invest Ophthalmol Vis Sci 1998;39:424–434. [PubMed] [Google Scholar]
- 65. Linberg KA, Lewis GP, Fisher SK. Retraction and remodeling of rod spherules are early events following experimental retinal detachment: An ultrastructural study using serial sections. Mol Vis 2009;15:10–25. [PMC free article] [PubMed] [Google Scholar]
- 66. Fisher SK, Lewis GP. Muller cell and neuronal remodeling in retinal detachment and reattachment and their potential consequences for visual recovery: A review and reconsideration of recent data. Vision Res 2003;43:887–897. [DOI] [PubMed] [Google Scholar]
- 67. Sethi CS, Lewis GP, Fisher SK et al. Glial remodeling and neural plasticity in human retinal detachment with proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci 2005;46:329–342. [DOI] [PubMed] [Google Scholar]
- 68. Guerin CJLG, Fisher SK, Anderson DH. Recovery of photoreceptor outer segment length and analysis of memrbane assembly rates in regenerating primate photoreceptor outer segments. Invest Ophthalmol Vis Sci 1993;34:175–183. [PubMed] [Google Scholar]
- 69. Fisher SK, Lewis GP, Linberg KA et al. Cellular remodeling in mammalian retina: Results from studies of experimental retinal detachment. Prog Retin Eye Res 2005;24:395–431. [DOI] [PubMed] [Google Scholar]
- 70. Fontainhas AM, Townes‐Anderson E. RhoA inactivation prevents photoreceptor axon retraction in an in vitro model of acute retinal detachment. Invest Ophthalmol Vis Sci 2011;52:579–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wang J, Zarbin M, Sugino I et al. RhoA signaling and synaptic damage occur within hours in a live pig model of CNS injury, retinal detachment. Invest Ophthalmol Vis Sci 2016;57:3892–3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Townes‐Anderson E, Wang J, Halasz E et al. Fasudil, a clinically used ROCK inhibitor, stabilizes rod photoreceptor synapses after retinal detachment. Transl Vis Sci Technol 2017;6:22. [DOI] [PMC free article] [PubMed] [Google Scholar]


