Abstract
Animal models show that systemically administered bone marrow‐derived mesenchymal stem cells (MSCs) home to sites of primary and metastatic prostate cancer (PC)—making them candidates to selectively deliver cytotoxic agents. To further assess this potential as a cell‐based therapeutic vehicle, a phase I study testing homing of systemically infused allogeneic MSCs preprostatectomy was conducted. The primary objective was to assess safety and feasibility and to determine if MSCs accumulate within primary PC tissue. MSCs were quantified using beads, emulsion, amplification, magnetics digital polymerase chain reaction (limit of detection: ≥0.01% MSCs) to measure allogeneic MSC DNA relative to recipient DNA. MSCs were harvested from healthy donors and expanded ex vivo using standard protocols by the Johns Hopkins Cell Therapy Laboratory. PC patients planning to undergo prostatectomy were eligible for MSC infusion. Enrolled subjects received a single intravenous infusion 4–6 days prior to prostatectomy. The first three subjects received 1 x 106 cells per kilogram (maximum 1 x 108 cells), and subsequent four patients received 2 x 106 cells per kilogram (maximum 2 x 108 cells). No dose‐limiting toxicities were observed and all patients underwent prostatectomy without delay. Pathologic assessment of prostate cores revealed ≥70% tumor involvement in cores from four subjects, with benign tissue in the others. MSCs were undetectable in all subjects, and the study was stopped early for futility. MSC infusions appear safe in PC patients. Although intended for eventual use in metastatic PC patients, in this study, MSCs did not home primary tumors in sufficient levels to warrant further development as a cell‐based therapeutic delivery strategy using standard ex vivo expansion protocols. stem cells translational medicine 2019;8:441–449
Keywords: Cellular therapy, Chemotaxis, Clinical trials, Mesenchymal stem cells
Significance Statement.
Preclinical studies have demonstrated that mesenchymal stem cells (MSCs) possess an innate tropism for cancer sites, and it has been hypothesized that they would make an ideal cell‐based therapeutic vector. A phase I study was launched to determine the safety and feasibility of allogeneic MSC infusion preradical prostatectomy and to quantify MSC accumulation within foci of primary prostate cancer tissue. It was found that systemically infused allogeneic MSCs were safe in men with localized prostate cancer; however, they did not accumulate within prostate cancer foci. To the authors' knowledge, this is the first study to test the homing efficiency of MSC in solid tumor patients.
Introduction
Although prostate cancer (PC) is a highly curable disease when detected at an early stage, men who develop distant metastases remain at high risk for death because of their cancer. Overall, >26,000 American men are estimated to have died from PC in 2017 1. Although intensive research efforts have yielded several new agents with modest effects on survival, the standard approach of inhibiting the androgen/androgen receptor‐signaling pathway has remained the cornerstone of treating metastatic disease since first described by Charles Huggins in the 1940s 2. Traditionally, this pathway has been targeted through either surgical or medical castration (i.e., androgen deprivation therapy [ADT]), most often in the form of the luteinizing hormone‐releasing hormone agonists/antagonists (e.g., leuprolide, goserelin, and degarelix). Unfortunately, after a variable period of symptom relief, ADT invariably ceases to suppress PC growth, and patients eventually succumb to their disease. The final, lethal stage of the disease is defined by progression in spite of a castrate serum testosterone level and has been termed castration‐resistant PC.
Although nontargeted cytotoxic chemotherapeutics do provide small survival gains, these treatment strategies carry substantial toxicity 3, 4, 5. A more targeted method for delivering therapeutic agents selectively to sites of PC would be highly advantageous as this would avoid toxic side effects to normal tissue, while producing higher intratumoral concentrations of drug, thereby enhancing cancer cell kill. There is strong evidence from animal models that systemically administered human bone marrow‐derived mesenchymal stem cells (MSCs) home to sites of primary and metastatic cancers, including those of the prostate 6, 7, 8, 9, 10, 11, 12, 13, 14. MSCs play a key role in tissue repair and maintenance, and this tumor homing is likely driven by the inflammatory microenvironment characteristically present within the cancer stromal compartment 10, 11, 15, 16. MSCs would therefore make a potentially ideal candidate vehicle to selectively deliver (i.e., home) therapeutic agents to sites of PC.
Strategies to exploit the natural homing ability of MSCs being explored by our group include loading them with microparticles encapsulating prodrugs activated by prostate‐specific antigen (PSA) or genetically engineering them to express a modified form of the potent bacterial protoxin, proaerolysin, that has also been engineered for selective activation by the proteolytic activity of PSA 17, 18, 19. This protoxin is inactive in the absence of enzymatically active PSA but is a converted into a potent pore‐forming toxin with picomolar potency following proteolysis of the inhibitory domain (i.e., activation) by PSA. These approaches limit systemic toxicities as PSA is uniquely expressed by prostate epithelial cells, including cancer, and is only enzymatically active within the PC microenvironment, whereas inactive in the blood circulation because of the presence of high concentrations of serum protease inhibitors (e.g., α‐1‐chymotrypsin and α‐2‐macroglobulin) 20. On this basis, delivery of PSA‐activated prodrugs or protoxins by MSCs that home to sites of PC would be highly cytotoxic, whereas MSCs accumulating in off target sites, such as the lung, would deliver an inactive payload and reduce peripheral toxicity.
Although in vitro and in vivo evidence supports this strategy, it has yet to be validated in humans that MSCs home to PC sites in substantial enough numbers to be considered for practical use as targeting vehicles 6. Before further development of MSCs as a cell‐based therapeutic vehicle can occur, the magnitude of MSC homing to sites of PC needs to be verified. Although the therapeutic potential for MSCs loaded with a cytotoxic agent is greatest in men with metastatic PC, tissue is more easily obtained in those undergoing surgical resection. Therefore, we performed a phase I study to determine the safety and feasibility of allogeneic MSC infusion prior to radical prostatectomy and to quantify the number of systemically administered MSCs that preferentially accumulate within malignant foci of primary PC tissue.
Materials and Methods
Patients
Patients with histologically confirmed Gleason score ≥6 PC who were scheduled for radical prostatectomy at the Johns Hopkins Hospital were eligible for the study. In order to ensure adequate tissue for analysis, patients had to have ≥3 positive cores with at least one core containing ≥30% PC. Patients had to have Sexual Health Inventory for Men (SHIM) score ≥17 and no prior radiation or systemic ADT for PC. Patients with history of penicillin or streptomycin allergy, active autoimmune disease requiring treatment, or symptomatic pulmonary dysfunction were excluded. Adequate marrow and organ function was mandated (i.e., creatinine <2 × upper limit of normal (ULN); bilirubin, aspartate transaminase, and alanine transaminase <3 × ULN; absolute neutrophil count ≥1,500 per millimeter cube, platelets ≥100,000 per millimeter cube, and hemoglobin ≥9 g/l).
Bone Marrow Donor Selection
Donors had to be male, between the ages of 18 and 30, and meet the selection and eligibility criteria as defined by the Foundation for the Accreditation of Hematopoietic Cell Therapy (FACT) and Food and Drug Administration (FDA) 21 Code of Federal Regulations Part 1271. Donors had to provide informed consent and were paid $500.00 for the time spent in the screening and donation process. In addition to routine laboratory studies, donors underwent screening for HIV 1 and 2, Hepatitis B and C, human T‐lymphotropic virus (HTLV)‐1 and HTLV‐2, cytomegalovirus, syphilis, and west nile virus.
MSC Preparation
Approximately 60 ml of bone marrow was aspirated from individual donors under sterile conditions. Bone marrow specimen was then sent to the Cell Therapy Laboratory (CTL) within The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, which is registered with the FDA as a cell and tissue facility and is accredited by FACT and the College of American Pathologists. Bone marrow was processed using lymphocyte separation medium (LSM; specific gravity 1.077) to prepare the density‐enriched, mononuclear cells (MNCs). The cells were diluted with Plasmalyte A or phosphate‐buffered saline (PBS) and layered onto LSM using conical tubes to isolate MNCs following established procedures. The MNCs were washed with Plasmalyte A or PBS containing 1% human serum albumin (HSA). The washed cells were sampled to determine the total number of viable nucleated cells.
To expand MSCs, MNCs were initially cultured in “Complete Media with Antibiotics” consisting of alpha minimum essential media media supplemented with 2 mM l‐glutamine, 10% fetal bovine serum, 100 units per milliliter penicillin and 100 μg/ml streptomycin. Subsequent passages used “Complete Media without Antibiotics.” The expansion was performed in culture vessels using a 37°C, 5% CO2‐humidified incubator. The MSCs were detached from the culture vessels using trypsin exposure. The MSCs were formulated in multiple cryopreservation bags. Each bag contained 20 ml aliquot of MSCs formulated in a cryoprotectant consisting of 6% hetastarch in 0.9% sodium chloride injection supplemented with 2% HSA and 5% dimethylsulfoxide. The MSCs were frozen in a controlled‐rate freezer until the product's temperature reaches −80°C. The MSCs were then be stored in the vapor phase of a liquid nitrogen freezer at less than −135°C. CTL quality assurance and the Laboratory Director reviewed the production records and quality control testing results prior to release the product.
Study Design
This was a single‐site, multiarm phase I study designed to determine if systemically infused MSC will home to sites of PC. The trial was supervised by the Institutional Review Board at Johns Hopkins and registered with clinicaltrials.gov (NCT01983709). After verification of eligibility and informed consent procedures, patients were treated with intravenous infusion of MSCs. The first three subjects (i.e., initial cohort) enrolled received a dose of 1 × 106 cells per kilogram up to a maximum dose of 1 × 108 total cells. Men enrolled to the initial cohort received their infusion 4 days prior to undergoing a planned prostatectomy. Each subject enrolled to the initial cohort was observed for 28 days following the MSC infusion prior to enrolling subsequent patients. After no dose‐limiting toxicities (DLTs) or other serious safety concerns were observed in the initial cohort, the full dose cohort was opened. Men in the full‐dose cohort received a dose of 2 × 106 cells per kilogram up to a maximum dose of 2 × 108 total cells. Men in the full‐dose cohort received an infusion at either 4 or 6 days prior to the planned prostatectomy.
The primary objective of the study was to quantify donor MSC DNA relative to recipient DNA at sites of PC. This outcome is measured as the relative amount of donor DNA versus recipient DNA in the prostate specimen via beads, emulsion, amplification, magnetics (BEAMing) digital polymerase chain reaction (PCR) 21, 22, 23. Secondary endpoints included: assessing the feasibility of infusing MSCs into men with localized PC who plan to undergo a prostatectomy; determining the proportion of MSC to recipient DNA in the peripheral blood at serial time points; evaluating the effects of MSCs on inflammatory markers (i.e., C‐reactive protein and erythrocyte sedimentation rate); and assessing for safety. In addition, MSCs have regenerative properties, play a role in promoting tissue healing, and have been shown to improve erectile function in animal models 24, 25, 26. Therefore, we hypothesized that allogeneic MSCs could promote healing postprostatectomy and associate with improved recovery of erectile function postprostatectomy 27, 28, 29, 30. To evaluate these effects, we had participants complete the SHIM and Expanded Prostate Cancer Index Composite (EPIC) questionnaires at serial time points postprostatectomy 31, 32, 33.
BEAMing Digital PCR Assay
Ex vivo punch biopsies (diameter: 6–8 mm, N = 4‐6 per prostate) were performed on the prostate glands following prostatectomy from areas with and without documented PC (based on diagnostic needle biopsy). Frozen H&E sections were generated and reviewed by a genitourinary pathologist to select tissue samples to be sent for analysis. Tissue was stored at −70°C until ready for batch shipment to Sysmex‐Inostics GmbH (Hamburg, Germany) for analysis via BEAMing digital PCR according to standard protocols 21, 22, 23. A panel of six single nucleotide polymorphisms (SNPs) (rs560681, rs10488710, rs576261, rs6811238, rs279844, and rs6955448) from stable genomic regions in control PC tissue were selected to differentiate between donor and recipient DNA on the basis that an identical SNP profile between donor and recipient DNA was extremely unlikely to occur by chance (estimated probability of identical SNP profile: 1 in 4,049) 34, 35.
Statistical Plan
This was a phase I study with the primary goal of quantifying MSC homing efficiency to sites of PC. The analysis was primarily exploratory and descriptive. MSC homing, defined as the percentage of donor DNA among total DNA that home to sites of PC by cohort and donor, was presented for each patient. Safety and feasibility was reported using descriptive statistics. Changes from preprostatectomy to postprostatectomy in the total SHIM and EPIC survey scores were assessed using paired‐sample t tests or Wilcoxon‐signed rank tests as appropriate.
Results
BEAMing Assay Validation
Our panel of six SNPs was validated using three PC epithelial cell lines (LNCaP, LAPC‐4, and VCaP), three bone marrow‐derived primary MSC cultures (BM‐MSC‐1, BM‐MSC‐2, and BM‐MSC‐3), and a primary prostatectomy sample. We demonstrated that there were nonoverlapping SNP profiles within these samples, thus enabling us to unambiguously differentiate the origin of donor DNA (i.e., PC cell line vs. MSC culture DNA vs. primary PC DNA; Fig. 1). This SNP panel was then used to generate an “MSC standard curve” for determination of the assay‐specific limit of detection. This “MSC standard curve” was constructed through serial dilution of MSCs spiked into a suspension of prostate epithelial cells. The sensitivity of the assay allowed us to detect MSCs in suspension with PC epithelial cells at a concentration as low as 0.01% of the sample (Fig. 2).
Figure 1.
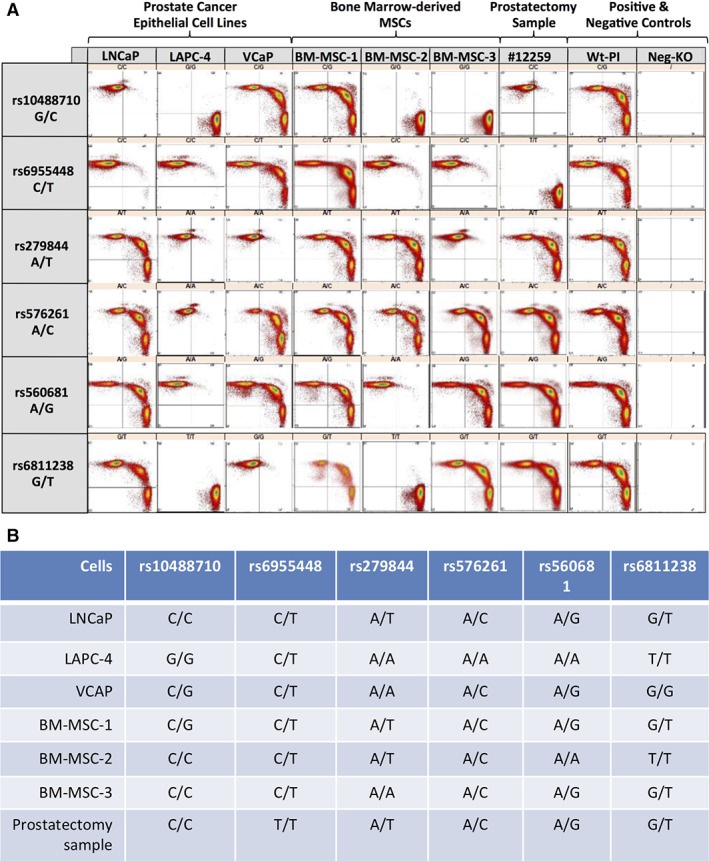
(A): Flow cytometry scatter plot of beads, emulsion, amplification, magnetics polymerase chain reaction products. (B): Summary of SNP alleles in select prostate cancer cell lines (LNCaP, LAPC‐4, and VCAP), in MSC cultures (BM‐MSC‐1, BM‐MSC‐2, and BM‐MSC‐3), and in a primary prostatectomy sample. Abbreviations: BM, bone marrow; MSC, mesenchymal stem cell.
Figure 2.
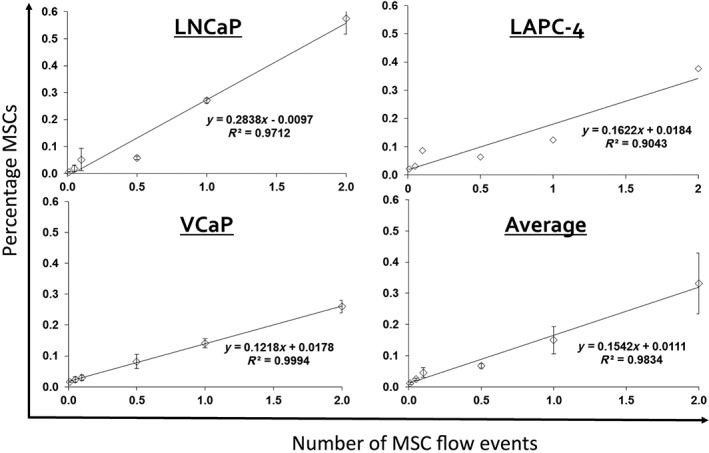
MSC standard curves. Assay‐specific limit of detection = 0.01%. Note: Beads, emulsion, amplification, magnetics assay was performed using the following SNPs: rs10488710 (LNCaP), rs6811238 (LAPC‐4), and rs279844 (VCaP). Abbreviation: MSC, mesenchymal stem cell.
Clinical Trial Clinical Endpoints
Seven eligible patients were accrued from March 2014 to May 2016. All patients were clinical stage T1c with baseline PSA <10 ng/ml (range: 0.2–8.2 ng/ml; Table 1 and Supporting Information Table S1). The first three patients (recipients 1–3) were treated at a dose of 1 × 106 MSCs per kilogram (up to a maximum of 1 × 08 cells) and received dosing 4 days prior to planned radical prostatectomy. Subsequently, the next four patients (recipients 4–7) received a dose of 2 × 106 MSCs per kilogram (up to a maximum of 2 × 108 cells). Two of these patients (recipients 4 and 5) received dosing at 4 days and two of these (recipients 6 and 7) at 6 days prior to prostatectomy. No DLTs were observed, and all patients underwent prostatectomy with no delays. Pathologic assessment of ex vivo prostate tissue cores revealed ≥70% tumor involvement in the cores from four subjects, with benign tissue in the other three subjects' cores. All patients achieved an undetectable PSA (i.e., <0.1 ng/ml) at 30 days postsurgery, and there were no PC recurrences out to 2 years postprostatectomy. The study was initially designed to treat a total of 12 men (6 per time point); however, the trial was stopped early for futility related to inability to detect MSCs above the limit of detection of the assay.
Table 1.
Demographics
| Characteristic | Overall (n = 7) |
|---|---|
| Mean age at surgery ± SD | 54.1 ± 6.9 |
| Race, n (%) | |
| White | 5 (71.4%) |
| Black | 2 (28.6%) |
| History of hypertension, n (%) | 2 (28.6%) |
| History of diabetes, n (%) | 0 (0%) |
| Smoking status, n (%) | |
| Never smoker | 5 (71.4%) |
| Former smoker | 2 (28.6%) |
| Charlson age‐comorbidity index, n (%) | |
| 2 | 2 (28.6%) |
| 3 | 3 (42.9%) |
| 4 | 1 (14.3%) |
| 8 | 1 (14.3%) |
| PSA (ng/ml) | |
| Median (IQR) | 6.8 (4.4–7.9) |
| Mean ± SD | 5.8 ± 2.7 |
| Clinical stage, n (%) | |
| cT1c | 5 (71.4%) |
| cT2a | 2 (28.6%) |
| Biopsy grade group, n (%) | |
| 1 | 1 (14.3%) |
| 2 | 3 (42.9%) |
| 3 | 2 (28.6%) |
| 4 | 1 (14.3%) |
Abbreviation: IQR, interquartile range; PSA, prostate‐specific antigen; SD, standard deviation.
BEAMing Results
Donor and recipient SNP profiles were determined at baseline (i.e., prior to MSC infusion) to select one informative SNP to use for the BEAMing digital PCR prostate tissue analyses. Table 2 provides the recipient SNP profile and the profile for the MSC donor used for each subject. There was high background noise in pretreatment samples from the recipients, limiting our ability to detect intraprostatic MSC DNA fraction at very low levels. Overall, there was no evidence of MSC homing on the basis of BEAMing digital PCR as our assay failed to identify differential SNP profiles within PC tissue at a level sufficient to rule out false positive results (Table 3).
Table 2.
Donor and recipient SNP profiles
| SNP | ||||||
|---|---|---|---|---|---|---|
| rs10488710 (G/C) | rs6955448 (C/T) | rs1279844 (A/T) | rs576261 (A/C) | rs560681 (A/G) | rs6811238 (G/T) | |
| Donor 1 | G/C | C/C | T/T | A/A | A/G | G/T |
| Recipient 1 | G/G | C/T | T/T | A/T | A/G | G/T |
| Donor 1 | G/C | C/C | T/T | A/A | A/G | G/T |
| Recipient 2 | C/C | C/C | A/T | A/C | A/A | G/T |
| Donor 1 | G/C | C/C | T/T | A/A | A/G | G/T |
| Recipient 3 | G/G | C/C | T/T | A/C | A/A | G/T |
| Donor 1 | G/C | C/C | T/T | A/A | A/G | G/T |
| Donor 2 | G/C | T/T | A/A | A/A | A/A | G/T |
| Recipient 4a | G/G | C/T | A/A | A/A | A/A | G/T |
| Donor 2 | G/C | T/T | A/A | A/A | A/A | G/T |
| Recipient 5 | C/C | C/C | A/A | A/C | A/G | T/T |
| Donor 2 | G/C | T/T | A/A | A/A | A/A | G/T |
| Recipient 6 | G/C | C/C | A/A | A/C | A/A | G/T |
| Donor 2b | G/C | T/T | A/A | A/A | A/A | G/T |
| Recipient 7a | G/C | C/C | A/T | A/C | A/A | T/G |
Six SNPs were chosen based on their predicted ability to differentiate between MSC donor (donor) and MSC recipient (recipient) DNA. One SNP, which was different for each donor‐recipient pair, was chosen for the tissue‐based DNA analysis (highlighted in bold).
Recipient 4 received MSCs from two donors because MSC quantities were limited. Note that the rs10488710 SNP allowed us to differentiate between the two donors (both with the C allele) and the recipient (G allele).
Haplotyping was used to discriminate between differences in donor 2 and recipient 7 DNA.
Abbreviations: MSC, mesenchymal stem cell.
Table 3.
Beads, emulsion, amplification, magnetics (BEAMing) digital polymerase chain reaction (PCR) results
| Recipient 1 | Recipient 2 | Recipient 3 | Recipient 4 | Recipient 5 | Recipient 6 | |
|---|---|---|---|---|---|---|
| Tumor involvementa | 0% | 0% | 70%–80% | 0% | 70%–80% | 70%–80% |
| Informative SNP selected | rs10488710 | rs560681 | rs560681 | rs10488710 | rs6955448 | rs6955448 |
| Donor allele tested | Allele C | Allele G | Allele G | Allele C | Allele T | Allele T |
| Fraction donor allelea | 0.0050 | 0.0010 | 0.0000 | 0.0030 | 0.0235 | 0.0179 |
| Background allele fraction | 0.0110 | 0.0021 | 0.0010 | 0.0000 | 0.0347 | 0.0237 |
| Recipient HLA‐A haplotype | Aa02:01:01 | |||||
| Donor HLA‐A haplotype | Aa11:01:01 | |||||
| Fraction donor haplotype | 0 | |||||
| Background haplotype fraction | 1 × 105 |
Prostate tissue was tested for the presence of donor mesenchymal stem cell (MSC) DNA using digital PCR at one informative SNP (see Table 2). The fraction of the donor allele is reported. Note: all samples were tested in triplicate. Peripheral blood mononuclear cells were obtained pre‐MSC infusion, and BEAMing digital PCR was used to evaluate for the presence of the donor allele fraction in order to obtain an estimate for “background noise” using our assay. Donor allelic fractions in prostate tissue that fell below the background allele fraction level likely represented false positives. In addition to BEAMing digital PCR, recipient 6 also underwent haplotyping to evaluate for evidence of allogeneic donor MSC DNA within the prostate.
Tumor involvement in core used for MSC DNA quantification studies.
Abbreviation: HLA, human leukocyte antigen.
Because our BEAMing assay was unable to accurately detect low quantities of donor DNA (i.e., allogeneic MSC DNA) within prostate tissue, we explored more sensitive means to differentiate between donor and recipient DNA. The human leukocyte antigen (HLA)‐A locus is highly polymorphic, and work by Eshleman et al. has identified regions in this locus containing 18 closely spaced SNPs, which can be used to differentiate between donor‐recipient DNA 36. Two human bone marrow samples (BM‐MSC‐4 and BM‐MSC‐5) and the LNCaP human PC cell line were haplotyped to identify an informative pair of samples. BM‐MSC‐4 and LNCaP were identified as the most informative. Next, a “standard curve” was generated by spiking BM‐MSC‐4 into a suspension of LNCaP cells followed by making a dilution series ranging from 0% to 1%. Genomic DNA was then extracted from each dilution and sequencing of the HLA‐A locus performed to confirm a level of detection of 0.01% MSC in suspension with LNCaP cells (data not shown). There was also no evidence of MSC accumulation within the prostate using this haplotyping method (Table 3). Tissue from recipient 7 was not tested given that there was no evidence of MSC homing in the previously analyzed samples from recipients 1–6, in addition to the high background previously detected for the informative SNP identified in this donor‐recipient pair.
Safety and Quality of Life
Overall, the infusion of MSCs was well‐tolerated and had no discernible effect on prostatectomy in terms of operative procedure, and no postoperative complication such as increased bleeding, delayed wound healing, or increased length of hospital stay were observed. No adverse events attributable to MSC infusion were reported throughout the study. No serious adverse events occurred. The most common adverse events were those attributable to the prostatectomy and included urinary incontinence (grade 1), erectile dysfunction (grades 1–2), and pelvic pain (grades 1–2) that were reported at the day 30 visit. As expected, after prostatectomy, there were significant declines in quality of life as determined by the SHIM and EPIC surveys (Fig. 3). It is notable that the urinary function EPIC subscale did not significantly change during the course of the study, whereas the sexual function EPIC subscale did.
Figure 3.
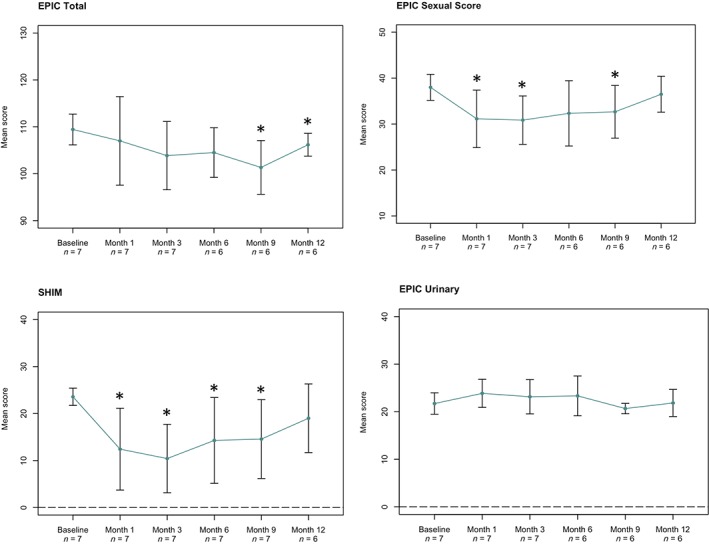
Change in quality of life survey score. Higher scores indicate improvement in quality of life. *, significant change in score at a given time point (p ≤ .05) based on paired t test. Abbreviations: EPIC, Expanded Prostate Cancer Index Composite; SHIM, Sexual Health Inventory for Men.
Discussion
MSCs have previously been shown to display a tropism for sites of cancer in preclinical models, and it has been hypothesized that this would make them an ideal cell‐based therapeutic vector 6, 7, 8, 9, 18. In addition, because these cells are immune evasive, allogeneic MSCs need not be HLA matched between donor and recipient 37. Clinically, MSCs have been studied as a way to mitigate inflammation and to augment tissue healing in diseases ranging from graft versus host disease in posthematopoietic stem cell transplant to patients recovering from a myocardial infarction 27, 28, 30, 37. On this basis, we sought to determine if allogeneic MSCs home to sites of primary human PC, thus supporting the further development of MSCs as a cell‐based therapeutic vector. Although systemically infused allogeneic MSCs were well tolerated, we were unable to demonstrate that these cells accumulated at levels sufficient to warrant their further development at least for this clinical indication using allogeneic MSCs prepared according to the standard methods described here.
Prior studies have shown that MSCs accumulate within the PC microenvironment, which is likely related to the role that MSCs play in wound healing 6, 8. Cancer has been described as an unhealing wound, characterized by an inflammatory milieu similar to other sites of tissue damage 38. MSCs display a tropism for sites of inflammation—whether as a result of malignancy or a wound—and play a role in tissue maintenance and regeneration 10. Several explanations could account for the lack of observable MSCs within prostatectomy specimens from men enrolled to this study.
It is possible that digital BEAMing PCR was not sufficiently sensitive to detect MSCs within the inflammatory PC microenvironment. Although our baseline “standard curve,” which was generated by spiking MSCs into PC cell cultures, demonstrated a limit of detection of 0.01%, in practice, we found baseline “noise” made the BEAMing assay unable to rule out false positive MSC homing at low levels. Because of these limitations, we evaluated sequencing SNPs within the HLA‐A locus as a means to quantify low‐level donor MSC DNA within the tumor microenvironment. This assay was similarly not sensitive enough to detect MSC homing to the prostate at the levels achieved, if any occurred. Overall, these two approaches indicate that MSCs expanded under standard culture conditions do not home at levels sufficient to warrant their further development as part of a PC therapeutic strategy. Preclinical studies using MSCs to deliver a PSA‐activated prodrug encapsulated in internalized microparticles have demonstrated that MSCs need to reach ≥1% of total tumor cells to achieve a therapeutic effect at the reported drug loading level, although higher drug loading or a more potent drug could potentially produce antitumor effects in the 0.1%–1.0% range 18.
Another possibility that may account for the lack of clinically significant MSC homing observed is related to patient selection issues. in vivo models have consistently shown that systemically infused MSCs accumulate within tumor sites; however, the efficiency of homing is often not robustly quantified, and more recent evidence suggests that this is a very inefficient process, likely as a result of mechanical barriers 39. In addition, the PC immune microenvironment is relatively noninflammatory, with a low number of cytotoxic T‐cells and an abundance of immunosuppressive infiltrates (e.g., regulatory T‐cells, myeloid‐derived suppressor cells, and endogenously recruited MSCs already present within the tumor), which could further impair homing of systemically infused exogenous MSCs as performed in this study 6, 8, 40, 41, 42, 43. As such, it is not clear that preclinical models are representative of the low to intermediate risk cancers included in this study 6, 7, 8, 9, 10, 11, 12, 13, 14. Furthermore, it is worth noting that many tissue samples analyzed as part of this trial had minimal to no tumor content because of the low‐grade nature of many of these cases, which could further impact the degree of inflammation present in the samples that were analyzed 44, 45. Interestingly, our group has previously shown that there are significantly more tumor‐infiltrating lymphocytes (TIL) in the small subset of PC cases with mismatch repair deficiency, with a linear relationship between TIL density and mutational load 46. It is possible that had we included patients with more inflammatory tumors, such as those with mismatch repair deficiency and a high mutational load, we would have observed more efficient MSC homing.
Conclusion
Moving forward, it does not seem that unmodified MSCs are a viable means to deliver a therapeutic antitumor payload to primary PC. However, it is possible that through genetic engineering, cell surface modifications, or preconditioning regimens, MSCs could be modified to enhance their homing efficiency to sites of PC in order to make this a practical strategy 39. Additional preclinical work aimed at modifying MSCs to improve their PC tropism is necessary before revisiting their therapeutic potential in the clinic.
Author Contributions
M.T.S., T.J.B., A.W.P., J.T.I., W.N.B., S.R.D.: conception/design, provisions of study material or patients; H.W.: conception/design; J.M.K., J.T.I., S.R.D.: financial support; S.R.D.: administrative support; M.T.S., T.J.B., J.T.I., W.N.B., S.R.D.: collection and/or assembly of data; M.T.S., H.W., T.J.B., A.W.P., S.J.L., C.C., R.A., O.L., N.A.B., J.M.K., A.D.M., J.T.I., W.N.B., S.R.D.: data analysis and interpretation, final approval of manuscript; M.T.S., W.N.B., S.R.D.: manuscript writing.
Disclosure of Potential Conflicts of Interest
M.T.S. declared advisory role with Janssen and research funding from Zenith Pharmaceuticals, Janssen, AstraZeneca, and Hoffmann La Roce. J.M.K. declared patent holder in regenerative medicine including one that focuses on engineered mesenchymal stem cells; consultant to multiple companies including Frequency Therapeutics, Celltex, Landsdowne Labs, Gecko Biomedical, Alivio Therapeutics, Molecular Infusions, and Takeda; received honoraria for multiple speaking events and ownership interests for multiple companies with equity including Frequency Therapeutics, Landsdowne Labs, Gecko Biomedical, Alivio Therapeutics, Molecular Infusions, and Skintifique; and consultant to LifeVaultBio and GyroGear. A.D.M. declared consultant/advisory role with Cepheid Inc. and research funding from Myriad Genetics and Janssen R&D. The other authors indicated no potential conflicts of interest.
Supporting information
Table S1 Supporting Information.
Acknowledgments
We thank James Eshleman and Marija Debeljak for helpful discussions. This study was supported by Prostate Cancer Foundation Challenge Award (J.M.K., J.T.I., and S.R.D.), Prostate Cancer Foundation Young Investigator Award (M.T.S. and W.N.B.), NIH‐Prostate SPORE (P50 CA058236; J.T.I. and S.R.D.), NIH Grant R01 HL095722 (J.M.K) and a Department of Defense Prostate Cancer Research Program Synergy Award (W81XWH‐13‐1‐0304; J.M.K., J.T.I., and S.R.D.).
Contributor Information
Michael T. Schweizer, Email: schweize@uw.edu.
Samuel R. Denmeade, Email: denmesa@jhmi.edu
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- 2. Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. 1941. J Urol 2002;167:948–951. discussion 952. [PubMed] [Google Scholar]
- 3. de Bono JS, Oudard S, Ozguroglu M et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration‐resistant prostate cancer progressing after docetaxel treatment: A randomised open‐label trial. Lancet 2010;376:1147–1154. [DOI] [PubMed] [Google Scholar]
- 4. Petrylak DP, Tangen CM, Hussain MH et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 2004;351:1513–1520. [DOI] [PubMed] [Google Scholar]
- 5. Tannock IF, de Wit R, Berry WR et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004;351:1502–1512. [DOI] [PubMed] [Google Scholar]
- 6. Brennen WN, Chen S, Denmeade SR et al. Quantification of mesenchymal stem cells (MSCs) at sites of human prostate cancer. Oncotarget 2013;4:106–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brennen WN, Denmeade SR, Isaacs JT. Mesenchymal stem cells as a vector for the inflammatory prostate microenvironment. Endocr Relat Cancer 2013;20:R269–R290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brennen WN, Zhang B, Kulac I et al. Mesenchymal stem cell infiltration during neoplastic transformation of the human prostate. Oncotarget 2017;8:46710–46727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kidd S, Spaeth E, Dembinski JL et al. Direct evidence of mesenchymal stem cell tropism for tumor and wounding microenvironments using in vivo bioluminescent imaging. Stem Cells 2009;27:2614–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kidd S, Spaeth E, Klopp A et al. The (in) auspicious role of mesenchymal stromal cells in cancer: Be it friend or foe. Cytotherapy 2008;10:657–667. [DOI] [PubMed] [Google Scholar]
- 11. Spaeth E, Klopp A, Dembinski J et al. Inflammation and tumor microenvironments: Defining the migratory itinerary of mesenchymal stem cells. Gene Ther 2008;15:730–738. [DOI] [PubMed] [Google Scholar]
- 12. Sarkar D, Spencer JA, Phillips JA et al. Engineered cell homing. Blood 2011;118:e184–e191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Levy O, Mortensen LJ, Boquet G et al. A small‐molecule screen for enhanced homing of systemically infused cells. Cell Rep 2015;10:1261–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sarkar D, Vemula PK, Zhao W et al. Engineered mesenchymal stem cells with self‐assembled vesicles for systemic cell targeting. Biomaterials 2010;31:5266–5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pittenger MF, Mackay AM, Beck SC et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999;284:143–147. [DOI] [PubMed] [Google Scholar]
- 16. Myers TJ, Granero‐Molto F, Longobardi L et al. Mesenchymal stem cells at the intersection of cell and gene therapy. Expert Opin Biol Ther 2010;10:1663–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Williams SA, Merchant RF, Garrett‐Mayer E et al. A prostate‐specific antigen‐activated channel‐forming toxin as therapy for prostatic disease. J Natl Cancer Inst 2007;99:376–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Levy O, Brennen WN, Han E et al. A prodrug‐doped cellular Trojan Horse for the potential treatment of prostate cancer. Biomaterials 2016;91:140–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ankrum JA, Miranda OR, Ng KS et al. Engineering cells with intracellular agent‐loaded microparticles to control cell phenotype. Nat Protoc 2014;9:233–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Denmeade SR, Sokoll LJ, Chan DW et al. Concentration of enzymatically active prostate‐specific antigen (PSA) in the extracellular fluid of primary human prostate cancers and human prostate cancer xenograft models. Prostate 2001;48:1–6. [DOI] [PubMed] [Google Scholar]
- 21. Li M, Diehl F, Dressman D et al. BEAMing up for detection and quantification of rare sequence variants. Nat Methods 2006;3:95–97. [DOI] [PubMed] [Google Scholar]
- 22. Diehl F, Li M, He Y et al. BEAMing: Single‐molecule PCR on microparticles in water‐in‐oil emulsions. Nat Methods 2006;3:551–559. [DOI] [PubMed] [Google Scholar]
- 23. Dressman D, Yan H, Traverso G et al. Transforming single DNA molecules into fluorescent magnetic particles for detection and enumeration of genetic variations. Proc Natl Acad Sci USA 2003;100:8817–8822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bivalacqua TJ, Deng W, Kendirci M et al. Mesenchymal stem cells alone or ex vivo gene modified with endothelial nitric oxide synthase reverse age‐associated erectile dysfunction. Am J Physiol Heart Circ Physiol 2007;292:H1278–H1290. [DOI] [PubMed] [Google Scholar]
- 25. Kendirci M, Trost L, Bakondi B et al. Transplantation of nonhematopoietic adult bone marrow stem/progenitor cells isolated by p75 nerve growth factor receptor into the penis rescues erectile function in a rat model of cavernous nerve injury. J Urol 2010;184:1560–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Strong TD, Gebska MA, Champion HC et al. Stem and endothelial progenitor cells in erection biology. Int J Impot Res 2008;20:243–254. [DOI] [PubMed] [Google Scholar]
- 27. Hare JM, Fishman JE, Gerstenblith G et al. Comparison of allogeneic vs autologous bone marrow‐derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: The POSEIDON randomized trial. JAMA 2012;308:2369–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Quarto R, Mastrogiacomo M, Cancedda R et al. Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med 2001;344:385–386. [DOI] [PubMed] [Google Scholar]
- 29. Lazarus HM, Koc ON, Devine SM et al. Cotransplantation of HLA‐identical sibling culture‐expanded mesenchymal stem cells and hematopoietic stem cells in hematologic malignancy patients. Biol Blood Marrow Transplant 2005;11:389–398. [DOI] [PubMed] [Google Scholar]
- 30. Le Blanc K, Frassoni F, Ball L et al. Mesenchymal stem cells for treatment of steroid‐resistant, severe, acute graft‐versus‐host disease: A phase II study. Lancet 2008;371:1579–1586. [DOI] [PubMed] [Google Scholar]
- 31. Rosen RC, Cappelleri JC, Smith MD et al. Development and evaluation of an abridged, 5‐item version of the International Index of Erectile Function (IIEF‐5) as a diagnostic tool for erectile dysfunction. Int J Impot Res 1999;11:319–326. [DOI] [PubMed] [Google Scholar]
- 32. Cappelleri JC, Siegel RL, Glasser DB et al. Relationship between patient self‐assessment of erectile dysfunction and the sexual health inventory for men. Clin Ther 2001;23:1707–1719. [DOI] [PubMed] [Google Scholar]
- 33. Chang P, Szymanski KM, Dunn RL et al. Expanded prostate cancer index composite for clinical practice: Development and validation of a practical health related quality of life instrument for use in the routine clinical care of patients with prostate cancer. J Urol 2011;186:865–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bova GS, Isaacs WB. Review of allelic loss and gain in prostate cancer. World J Urol 1996;14:338–346. [DOI] [PubMed] [Google Scholar]
- 35. Pakstis AJ, Speed WC, Fang R et al. SNPs for a universal individual identification panel. Hum Genet 2010;127:315–324. [DOI] [PubMed] [Google Scholar]
- 36. Debeljak M, Freed DN, Welch JA et al. Haplotype counting by next‐generation sequencing for ultrasensitive human DNA detection. J Mol Diagn 2014;16:495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: Immune evasive, not immune privileged. Nat Biotechnol 2014;32:252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dvorak HF. Tumors: Wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 1986;315:1650–1659. [DOI] [PubMed] [Google Scholar]
- 39. Krueger TEG, Thorek DLJ, Denmeade SR et al. Concise review: Mesenchymal stem cell‐based drug delivery: The good, the bad, the ugly, and the promise. 2018; 7: 651–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schweizer MT, Drake CG. Immunotherapy for prostate cancer: Recent developments and future challenges. Cancer Metastasis Rev 2014;33:641–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sfanos KS, Bruno TC, Maris CH et al. Phenotypic analysis of prostate‐infiltrating lymphocytes reveals TH17 and Treg skewing. Clin Cancer Res 2008;14:3254–3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lopez‐Bujanda Z, Drake CG. Myeloid‐derived cells in prostate cancer progression: Phenotype and prospective therapies. J Leukoc Biol 2017;102:393–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Krueger TE, Thorek DLJ, Meeker AK et al. Tumor‐infiltrating mesenchymal stem cells: Drivers of the immunosuppressive tumor microenvironment in prostate cancer? Prostate 2019;79:320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hahn E, Liu SK, Vesprini D et al. Immune infiltrates and PD‐L1 expression in treatment‐naive acinar prostatic adenocarcinoma: An exploratory analysis. J Clin Pathol 2018;71:1023–1027. [DOI] [PubMed] [Google Scholar]
- 45. Linch M, Goh G, Hiley C et al. Intratumoural evolutionary landscape of high‐risk prostate cancer: The PROGENY study of genomic and immune parameters. Ann Oncol 2017;28:2472–2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Guedes LB, Antonarakis ES, Schweizer MT et al. MSH2 loss in primary prostate cancer. Clin Cancer Res 2017;23:6863–6874. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Supporting Information.


