Abstract
Background/Aims
Direct-Acting Antivirals (DAAs) are now the standard of care for management of Chronic Hepatitis C (CHC) infection. The aim of this study was to evaluate change in Liver Stiffness Measurement (LSM) and Controlled Attenuation Parameter (CAP) by transient elastography (FibroScan®) after completion of DAA therapy.
Methods
LSM and CAP were measured serially (baseline pre-treatment, at 12 weeks post therapy, and one year after completion of therapy) in a prospective cohort of 372 CHC patients treated with DAAs. Patients with at least two FibroScan measurements were included.
Results
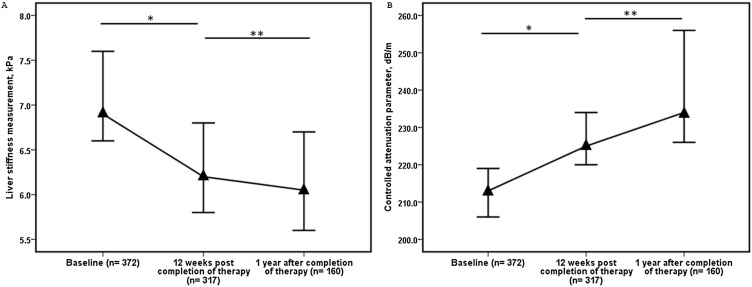
The mean age was 38.1 ± 12.6 years; 58.3% males. Cirrhosis as defined by biopsy or fibroscan measurement (≥12.5) kPa was found in 25.5%. On paired analysis (n = 317), LSM (IQR) decreased from a baseline value 7.1 (5.3–13.8) kPa to 6.2 (4.8–11.2) kPa 12 weeks post therapy with a median decline 0.7 (−0.6–2.6) kPa, P < 0.001. Similarly, on paired analysis (n = 160), LSM decreased from baseline 6.9 (5.1–12.7) kPa to 6.1 (4.8–9.4) kPa after one year of treatment with median decline 0.9 (−0.6–3.2) kPa, P < 0.001. In contrast, on paired analysis (n = 317), CAP increased from baseline of 213.0 (180.0–254.5) dB/m to 225.0 (190.0–269.0) dB/m at 12 weeks post therapy with median increase 7.0 (−23.5–45.5), P = 0.001. Similarly, on paired analysis (n = 160), CAP increased from baseline of 210.0 (180.3–260.8) dB/m to 234.0 (204.0–282.0) dB/m at one year post therapy with median increase 25.0 (−12.5–61.5) dB/m, P < 0.001. On multivariate linear regression analysis, low baseline CAP value and low albumin were significantly associated with increase in CAP values.
Conclusion
Treatment with DAAs reduces liver stiffness, but is associated with increase in hepatic steatosis.
Abbreviations: AASLD, American Association for the Study of Liver Diseases; ALT, Alanine Aminotransferase; AST, Aspartate Aminotransferase; BMI, Body Mass Index; CAP, Controlled Attenuation Parameter; CHC, Chronic Hepatitis C; DAAs, Directly-Acting Antivirals; F0-4, METAVIR Fibrosis Stage 0 to 4; HCV, Hepatitis C Virus; HIV, Human Immunodeficiency Virus; IFN, Interferon; IQR, Interquartile Range; LSM, Liver Stiffness Measurement; NAFLD, Non-alcoholic Fatty Liver Disease; SD, Standard Deviation; SVR12, Sustained Viral Response at 12 Weeks; TE, Transient Elastography
Keywords: liver stiffness measurement, controlled attenuation parameter, SVR12, Fibroscan
Introduction
Hepatitis C Virus (HCV) infection is an important cause of liver cirrhosis, decompensated liver disease requiring liver transplant, and liver-related mortality.1 Novel Direct-Acting Antivirals (DAAs) are now the standard of care for the management of HCV infection. DAAs are associated with high Sustained Virological Response at 12 Weeks (SVR12).2,3 Recent data suggests that effective DAA therapy is associated with short-term reduction of fibrosis, with unclear long-term effects.4,5
Multiple studies have shown an association of Chronic Hepatitis C (CHC) infection with the development of metabolic syndrome with insulin resistance, diabetes mellitus, and hepatic steatosis.6, 7, 8 Treatment of HCV with DAAs presumably may be associated with improvement of these associated complications. FibroScan® (vibration-controlled transient elastography) is a novel, non-invasive technique to assess hepatic fibrosis (Liver Stiffness Measurement—LSM) and steatosis (Controlled Attenuation Parameter—CAP) that has been validated in CHC.9, 10, 11, 12 There is paucity of data regarding the effect of oral DAA therapy on hepatic steatosis. A recent Japanese study reported an increase in CAP after therapy with DAAs.13 The aim of the present study was to assess the changes in hepatic fibrosis and hepatic steatosis, detected by FibroScan® (LSM and CAP) in patients treated with DAAs for CHC.
Patients and methods
Patients
CHC and compensated cirrhosis patients with detectable HCV RNA at baseline who were treated with DAAs and underwent successful baseline pretreatment transient elastography with LSM and CAP measurement along with at least one measurement at 3 months after completion of therapy or one year after completion of antiviral therapy were included in this study.
Patients were excluded if they had: (1) co-infection with other hepatotropic viruses or HIV, (2) bilirubin ≥5 mg/dl or Aspartate Aminotransferase (AST)/Alanine Aminotransferase (ALT) levels ≥10 times the upper limit of normal, (3) ascites, (4) alcohol consumption >20 g/day, (5) other co-existing causes of liver disease, and (6) decompensated cirrhosis. The study was performed in accordance with the principles of the Declaration of Helsinki. The study was approved by our institutition’s ethics committee and informed consent was obtained from all patients.
Clinical and Laboratory Assessment
The following details were noted in each case: age, sex, alcohol intake, the presence of diabetes, Body Mass Index (BMI), along with various laboratory tests including hemoglobin, platelet count, AST and ALT. Tests for hepatitis B surface antigen and Human Immunodeficiency Virus (HIV) were done using standard commercially available enzyme immunoassays. SVR12 was defined as undetectable HCV-RNA at 12 weeks after the completion of treatment. HCV RNA quantification was done at baseline, end of therapy, and 12 weeks post treatment (SVR12). Transaminitis was defined as AST values >40 IU/L. All data were retrieved from a prospectively maintained data base.
Management Protocol
The management protocol of CHC patients was as per the American Association for the Study of Liver Diseases (AASLD) practice guidelines for management of HCV infection.14
Liver Stiffness Measurement and Determination of Controlled Attenuation Parameter
LSM and CAP measurements were performed on a FibroScan® touch 502 (Echosens, Paris, France). All measurements were done as per manufacturer’s recommendations. The examination was performed on the right lobe of the liver through the intercostal space by a single experienced technician. Ten successful acquisitions were performed on each patient. The Interquartile Range (IQR) was defined as the index of the intrinsic variability of LSM and CAP values corresponding to the interval containing 50% of the valid measurements between the 25th and 75th percentiles. As an indicator of variability, the ratio of the IQR of LSM value to the median value (IQR/M) was calculated. The measurements were considered reliable if 10 valid acquisitions with IQR/M of LSM less than 0.3 were obtained. Failure of transient elastography was defined as the inability to obtain valid LSM and/or CAP values. We categorized various stages of hepatic fibrosis according to the LSM values—F0–F1: 2.5–6.9 kPa; F2: 7.0–9.4 kPa; F3: 9.5–12.4 kPa and F4: ≥12.5 kPa.15
Follow-up Measurements of LSM and CAP
FibroScan® assessment was repeated at 12 weeks after completion of therapy and at the end of 1-year of follow-up. Any drop in LSM/CAP was considered as reduction in fibrosis and steatosis. Any rise in LSM/CAP was considered as increase in fibrosis and steatosis.
Body weight was recorded at 12 weeks after completion of therapy and at the end of 1-year of follow-up.
Statistical Analysis
Continuous variables were expressed as mean ± SD or median (interquartile range), as appropriate. Categorical variables were depicted as absolute numbers and percentages. Continuous variables were compared using paired t-test or Mann–Whitney tests. Categorical variables were compared by chi-square test. Linear regression was used to identify the independent variables associated with LSM and CAP as continuous variables. Statistical significance was taken as P < 0.05. Statistical analysis was performed using SPSS (SPSS version 20.0; SPSS Inc., Chicago, IL, USA).
Results
Patients Demographic Profile
A total of 408 CHC patients satisfying the inclusion criteria were evaluated between January 1, 2015 and August 31, 2017. 372 patients were included in the analysis with 36 patients excluded—four had coinfection with hepatitis B virus, two had coinfection with HIV, 22 patients had bilirubin ≥5 mg/dl or AST/ALT levels 10 times the upper limit of normal, five patients due to failure of successful fibroscan measurements, and three patients due to missing data. Of the 372 patients included in the study the mean age was 38.1 ± 12.6 years and 58.3% were males. The mean BMI was 23.4 ± 4.6 kg/m2. HCV genotype 3 was the most common (76.6%), followed by genotype 1 (18.0%).
The overall pretreatment median (IQR) LSM and CAP were 6.9 (5.2–13.0) kPa and 213 (178–254) dB/m, respectively. Predicted METAVIR stages according to baseline LSM values were as follows- F0/F1 in 50.3% of patients, F2 in 16.1%, F3 in 8.1%, and F4 in 25.5%.
There were 339 (91.1%) treatment naïve patients and 33 (9.9 %) treatment-experienced (defined as previous treatment with peginterferon and ribavirin) patients. The clinical and demographic details of the patients included in the study are shown in Table 1. Sofosbuvir based combinations included- ribavirin alone in 123 (33.1%) patients, ribavirin and peginterferon in 62 (16.7%), daclatasvir alone in 122 (32.8%), daclatasvir in combination with ribavirin in 24 (6.5%), ledipasvir alone in 39 (10.5%), ledipasvir in combination with ribavirin in two (0.5%) patients. The overall SVR12 was achieved in 365 patients (98.1%).
Table 1.
Baseline Demographic and Clinical Characteristics of the Patients.
| Variable | Overall (n = 372) |
|---|---|
| Age | 38.1 ± 12.6 |
| Sex (male:female), n (%) | 217 (58.3%): 155 (41.7%) |
| Body mass index (kg/m2) | 23.4 ± 4.6 |
| History of smoking | 28 (7.5%) |
| History of alcohol | 27 (7.3%) |
| Diabetes (present) | 52 (14.0%) |
| Hemoglobin (g/dl) | 13.2 ± 2.1 |
| Total leucocyte count (per mm3) | 6979.7 ± 2110.3 |
| Platelet count (×103/mm3) | 179.0 (129.5–228.0) |
| Bilirubin (mg/dl) | 0.6 (0.5–0.9) |
| Aspartate aminotransferase (IU/L) | 56.0 (38.0–88.0) |
| Alanine aminotransferase (IU/L) | 62.0 (40.0–108.8) |
| Alkaline phosphatase (IU/L) | 206.0 (153.0–265.0) |
| Total protein (g/dl) | 7.4 ± 0.6 |
| Serum albumin (g/dl) | 4.5 ± 0.7 |
| International normalized ratio | 1.1 ± 0.1 |
| Blood urea (mg/dl) | 23.0 (19.0–29.0) |
| Serum creatinine (mg/dl) | 0.8 (0.6–0.9) |
| Genotype | |
| 1 | 67 (18.0%) |
| 2 | 3 (0.8%) |
| 3 | 285 (76.6%) |
| 4 | 10 (2.7%) |
| 5 | 1 (0.3%) |
| Not otherwise specified (NOS) | 6 (1.6%) |
| HCV RNA | |
| High viral load (≥600,000) | 181 (48.7%) |
| Low viral load (<600,000) | 191 (51.3%) |
| SVR 12 | 365 (98.1%) |
| Baseline fibroscan | |
| F0–F1 | 187 (50.3%) |
| F2 | 60 (16.1%) |
| F3 | 30 (8.1%) |
| F4 | 95 (25.5%) |
| Baseline LSM (kPa) | 6.9 (5.2–13.0) |
| Baseline LSM IQR (kPa) | 1.1 (0.6–1.9) |
| Baseline CAP (dB/m) | 213.0 (178.0–253.8) |
| Baseline CAP IQR (dB/m) | 38.0 (26.0–53.0) |
| Presence of varices | 41 (11.0%) |
Note: All values are expressed as median (IQR), mean ± SD or n (%), unless otherwise specified.
HCV, Hepatitis C Virus; SVR12, Sustained Viral Response at 12 Weeks; LSM, Liver Stiffness Measurement; CAP, Controlled Attenuation Parameter; IQR, Interquartile Range; SD, Standard Deviation.
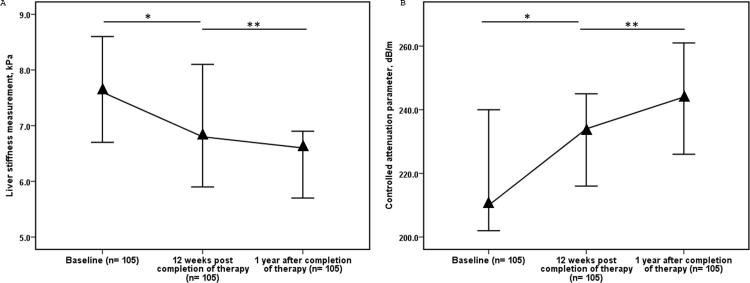
Paired fibroscan (LSM and CAP) values were available as follows: 317 patients had both baseline and 12 weeks after completion of therapy; 160 patients had baseline and 1 year post completion of therapy, and 105 patients had 12 weeks after treatment, along with 1 year post completion of therapy.
Serial LSM Values Among Patients Treated with DAAs
Overall, on paired analysis (n = 317), the LSM (IQR) decreased from baseline value of 7.1 (5.3–13.8) kPa to 6.2(4.8–11.2) kPa after 12 weeks of therapy. The overall median decline was 0.7 (−0.6–2.6) kPa, P < 0.001. On paired analysis (n = 105), the LSM decreased from 6.8 (4.9–11.8) kPa 12 weeks after completion of therapy to 6.6 (4.9–8.7) kPa after one year post therapy. The overall median decline was 0.6 (−0.5–2.6) kPa, P = 0.002 (Table 2, Figure 1). Similarly, on paired analysis (n = 160), the LSM decreased from a baseline value of 6.9 (5.1–12.7) kPa to 6.1 (4.8–9.4) kPa after one year of therapy. The overall median decline was 0.9 (−0.6–3.2) kPa, P < 0.001 (Table 2, Figure 1).
Table 2.
Change in LSM Values at Different Time Intervals After Start of Oral Directly Acting Agents.
| LSM median (IQR) | LSM median (IQR) | Number of cases | Change in LSM | P value |
|---|---|---|---|---|
| Baseline 7.1 (5.3–13.8) |
12 weeks post therapy 6.2 (4.8–11.2) |
317 | 0.7 (−0.6–2.6) | <0.001 |
| Baseline 6.9 (5.1–12.7) |
One year after completion of therapy 6.1 (4.8–9.4) |
160 | 0.9 (−0.6–3.2) | <0.001 |
| 12 weeks post therapy 6.8 (4.9–11.8) |
One year after completion of therapy 6.6 (4.9–8.7) |
105 | 0.6 (−0.5–2.6) | 0.002 |
Note: LSM, Liver Stiffness Measurement; IQR, Interquartile Range.
Figure 1.
(A) Serial Liver Stiffness Measurements (LSM) at baseline pre-treatment, 12 weeks post therapy, and one year after completion of therapy in chronic hepatitis C patients treated with directly acting antivirals (*P < 0.001, **P = 0.002); (B) serial controlled attenuation parameters (CAP) at baseline pre-treatment, 12 weeks post therapy, and one year after completion of therapy in chronic hepatitis C patients treated with directly acting antivirals (*P = 0.001, **P = 0.003). Data are medians, and error bars represent 95% confidence interval.
The reduction of LSM values over time were significantly higher in the patients with significant fibrosis (≥F2). The details of changes in LSM are shown in Supplementary Tables 1, 2 and 3. Similarly, reduction in LSM values over time was higher in patients with cirrhosis (F4) as compared to those with F0–F3 fibrosis (Supplementary Table 4).
On analysis of 105 patients with LSM values available at baseline, 12 weeks after completion of therapy and one year post therapy, there was a significant serial decline in the LSM values (Supplementary Table 5 and Supplementary Figure 1).
Changes in the Fibrosis Stages After DAA Therapy as per Baseline LSM Values
There was an increase in the percentage of patients in predicted stage F0/F1 and decline in percentage of patients with significant fibrosis (≥F2) at the end of 1 year. The percentage of patients with cirrhosis (25.5%) at baseline declined to (18.1%) at 1 year of follow-up (Supplementary Figure 2).
Changes in the LSM Values After DAA Therapy According to Baseline Transaminase Levels
There were significant differences in the median change of LSM (between 12 weeks after therapy and 1 year of follow up, and baseline and at 1 year of follow up) values among patients with transaminitis than those without transaminitis (Supplementary Table 6). There was no significant difference in the median change of LSM values among patients with or without transaminitis between baseline and 12 weeks after therapy.
Factors Predicting Decline in LSM Values from Baseline to One Year Post Treatment
In univariate regression model, increased age, increased baseline LSM value, low platelet count and hemoglobin levels, increased AST levels and bilirubin, longer duration of therapy (24 weeks vs 12 weeks), low BMI and low albumin level were significantly associated with higher reduction in LSM values between baseline and one year post treatment. On multivariate linear regression analysis, increased baseline LSM value, low platelet count and low BMI were independently associated with reduction of LSM (Table 3).
Table 3.
Univariate and Multivariate Analysis of Factors Associated with Reduction of LSM.
| Change LSM | Univariate | R2 | P value | Multivariate | R2 | P value |
|---|---|---|---|---|---|---|
| Genotype | 0.995 (−0.954–2.943) | 0.007 | 0.315 | |||
| Treatment type | 0.164 (−0.993–1.322) | 0.000 | 0.779 | |||
| Sex (female) | 0.167 (−1.474–1.808) | 0.000 | 0.841 | |||
| Diabetes (present) | −1.043 (−3.430–1.344) | 0.005 | 0.389 | |||
| Smoking | −0.586 (−4.292–3.119) | 0.001 | 0.755 | |||
| Age | 0.088 (0.021–0.156) | 0.041 | 0.016 | |||
| Duration of therapy (24 weeks vs 12 weeks) | 1.653 (0.033–3.273) | 0.023 | 0.010 | |||
| Baseline LSM | 0.279 (0.216–0.341) | 0.330 | <0.001 | 0.260 (0.189–0.331) | 0.376 | <0.001 |
| Baseline HCV RNA | 0.022 (−1.598–1.643) | 0.000 | 0.978 | |||
| Hemoglobin | −0.473 [−0.850–(−0.095)] | 0.037 | 0.014 | |||
| Platelet count | −0.023 [−0.033–(−0.014)] | 0.122 | <0.001 | −0.013 [−0.023–(−0.003)] | 0.376 | 0.014 |
| Bilirubin | 3.165 (1.400–4.930) | 0.001 | 0.074 | |||
| Aspartate aminotransferase | 0.019 (0.003–0.036) | 0.032 | 0.024 | |||
| Alanine aminotransferase | 0.002 (−0.009–0.013) | 0.001 | 0.738 | |||
| Alkaline phosphatase | 0.005 (−003–0.014) | 0.004 | 0.398 | |||
| Albumin | −2.633 [−3.745–(−1.522)] | 0.122 | <0.001 | |||
| INR | −0.482 (−7.791–6.827) | 0.000 | 0.896 | |||
| Body mass index | −0.153 (−0.327–0.021) | 0.021 | 0.085 | −0.182 [−0.328–(−0.036)] | 0.376 | 0.015 |
| Baseline CAP | −0.003 (−0.018–0.011) | 0.001 | 0.668 |
Note: LSM, Liver Stiffness Measurement; HCV, Hepatitis C Virus, INR, International Normalized Ratio, CAP, Controlled Attenuation Parameter.
Serial CAP Values Among Patients Treated with DAAs
On paired analysis (n = 317), the CAP value increased from a baseline of 213.0 (180.0–254.5) dB/m to 225.0 (190.0–269.0) dB/m 12 weeks post therapy. The overall median increase was 7.0 (−23.5–45.5) dB/m, P = 0.001. On paired analysis (n = 105), the CAP value increased from 234.0 (182.5–268.5) dB/m at 12 weeks post therapy value to 244.0 (206.0–286.0) dB/m at 1 year after completion of therapy. The overall median increase was 15.0 (−17.0–43.5) dB/m, P = 0.003 (Figure 1). Similarly, on paired analysis (n = 160), the CAP value increased from 210.0 (180.3–260.8) dB/m at baseline to 234.0 (204.0–282.0) dB/m one year post therapy. The overall median increase was 25.0 (−12.5–61.5) dB/m, P < 0.001 (Table 4, Figure 1). When comparing the baseline CAP value to the one year post treatment CAP value in the 160 patients there was increase in CAP value in 106 (66.2 %) patients, decrease in CAP value in 51 (32.2%) and no change in 3 (1.9%) (Supplementary Figure 3). In patients in whom there was increase in CAP value (n = 106) at the 1 year of follow up, there was no significant change in mean body weight (61.4 kg vs 62.2 kg, P = 0.147) or BMI (23.5 kg/m2 vs 23.8 kg/m2, P = 0.147) during the same time period.
Table 4.
Change in CAP Values at Different Time Intervals After Start of Oral Directly Acting Agents.
| CAP scores (IQR) | CAP scores (IQR) | Number of cases | Change in CAP | P value |
|---|---|---|---|---|
| Baseline 213.0 (180.0–254.5) |
12 weeks post therapy 225.0 (190.0–269.0) |
317 | 7.0 (−23.5–45.5) | 0.001 |
| Baseline 210.0 (180.3–260.8) |
One year after completion of therapy 234.0 (204.0–282.0) |
160 | 25.0 (−12.5–61.5) | <0.001 |
| 12 weeks post therapy 234.0 (182.5–268.5) |
One year after completion of therapy 244.0 (206.0–286.0) |
105 | 15.0 (−17.0–43.5) | 0.003 |
Note: CAP, Controlled Attenuation Parameter; IQR, Interquartile Range.
On analysis of 105 patients with all available CAP values at baseline, 12 weeks after completion of therapy and one year post therapy, there was a serial increase in the CAP values. (Supplementary Table 7 and Supplementary Figure 1). The rise in CAP was significant between baseline and 1 year after completion of therapy and 12 weeks post therapy and 1 year after completion of therapy.
The increase in CAP values with time was seen in patients with F0–3 as well as F4 (Supplementary Table 8).
Changes in the CAP Values After DAA Therapy According to Baseline Transaminase Levels
There were no significant differences in the median change of CAP values (between baseline and 12 weeks after therapy, 12 weeks after therapy and 1 year of follow up, and baseline and at 1 year of follow up) among patients with transaminitis and those without transaminitis (Supplementary Table 6).
Factors Predicting Increase in CAP Values from Baseline to One Year Post Treatment
In a univariate regression model, low baseline CAP value, low albumin, and low alkaline phosphatase levels were associated with increase in CAP values from baseline to 1 year post therapy. HCV genotype and baseline BMI were not significantly different between those who had a increase in CAP score as compared to those who did not have an increase in CAP score.
On multivariate linear regression analysis, only low baseline CAP value and low albumin were associated significantly with increase in CAP values (Table 5). There was no significant effect of the different DAA regimens on the increase in CAP score.
Table 5.
Univariate and Multivariate Analysis of Factors Associated with Increase in CAP Score.
| Variables | Univariate | R2 | P value | Multivariate | R2 | P value |
|---|---|---|---|---|---|---|
| Genotype | −6.005 (−29.292–17.281) | 0.002 | 0.611 | |||
| Treatment type | −5.988 (−19.594–7.617) | 0.005 | 0.386 | |||
| Sex (female) | −5.583 (−24.900–13.733) | 0.002 | 0.569 | |||
| Diabetes (present) | 9.524 (−18.626–37.674) | 0.003 | 0.505 | |||
| Smoking | −10.658 (−54.301–32.985) | 0.001 | 0.630 | |||
| Age | −0.150 (−0.958–0.657) | 0.001 | 0.713 | |||
| Duration | 4.825 (−13.358–23.009) | 0.002 | 0.601 | |||
| Baseline LSM | 0.221 (−0.677–1.120) | 0.001 | 0.627 | |||
| Baseline HCV RNA | 6.600 (−12.464–25.663) | 0.003 | 0.495 | |||
| Hemoglobin | 2.933 (−1.575–7.440) | 0.010 | 0.201 | |||
| Platelet count | 0.012 (−0.113–0.137) | 0.000 | 0.851 | |||
| Bilirubin | −12.806 (−34.312–8.700) | 0.009 | 0.241 | |||
| Aspartate aminotransferase | 0.138 (−0.059–0.335) | 0.012 | 0.168 | |||
| Alanine aminotransferase | 0.111 (−0.022–0.244) | 0.017 | 0.102 | |||
| Alkaline phosphatase | −0.113 [−0.211–(−0.015)] | 0.032 | 0.024 | |||
| Albumin | −13.060 (−26.882–0.763) | 0.022 | 0.064 | −12.757 [−24.210–(−1.303)] | 0.337 | 0.029 |
| INR | −52.701 (−137.839–32.436) | 0.010 | 0.223 | |||
| Body mass index | 0.366 (−1.664–2.395) | 0.001 | 0.722 | |||
| Baseline CAP | −0.609 [−0.750–(−0.468)] | 0.316 | <0.001 | −0.608 [−0.748–(−0.469)] | 0.337 | <0.001 |
Note: LSM, Liver Stiffness Measurement; HCV, Hepatitis C Virus; INR, International Normalized Ratio; CAP, Controlled Attenuation Parameter.
Reduction of LSM was seen in all drug regimens (Supplementary Table 9). Similarly, increase in CAP score was also seen across all drug regimens (Supplementary Table 10).
Discussion
This study demonstrated that in a large number of CHC patients—predominantly genotype 3 treated with generic sofosbuvir-based regimens, there was an observed decline in LSM values post DAA therapy, which reflects a decrease in hepatic fibrosis. In contrast, there was an increase in CAP values, representing hepatic steatosis after therapy.
The management of HCV has undergone a paradigm shift from interferon based therapies that were associated with low SVR rates to oral DAAs, which are associated with very high rates of SVR12. Although considered to be safe in the short term, there is no available data regarding the long term effects of oral DAAs. Recent guidelines recommend oral DAAs for all HCV positive patients, irrespective of the underlying fibrosis.14,16 Furthermore, the World Health Organization has recently launched a drive to eliminate viral hepatitis by 2030.17
Our study supports previous claims that treatment with oral DAAs is associated with decline in LSM values.5,18 LSM measurement is affected by presence of transaminitis.19 We observed a significant decrease in in LSM values over time among patients with transaminitis than those without transaminitis. We found that high baseline LSM values, low platelet count, and low BMI were independently associated with reduction of LSM values after one year of therapy. The levels of transaminases were not significantly associated with reduction of LSM on multivariate analysis. This suggests that there was a more dramatic reduction in LSM values in patients with higher grades of fibrosis (as suggested by high LSM and low platelet counts). Moreover, the decrease in LSM values was seen in both genotypes 1 and 3, irrespective of the type of regimen used.
An interesting finding in the study was a gradual increase in CAP scores during therapy and one year post therapy, a finding that correlates with an increase in hepatic steatosis.20 An increase in CAP score was seen in 66.3% of our patients, whereas 31.9% had a decline in CAP score, as compared with baseline values. This increase in scores was independent of the genotype and type of sofosbuvir regimen used. Genotype 3 HCV infection is known to be associated with metabolic syndrome and hepatic steatosis, the mechanism of which is not clear at this time.21,22 In a previous study, treatment with peginterferon and ribavirin was associated with a reduction of hepatic steatosis, especially in genotype 3 as compared with other genotypes.23 In contrast, we found that therapy with DAAs was associated with an increase in CAP scores. A recent study from Japan, which included elderly patients with a median age of 71 years, also reported an increase in CAP after therapy with DAAs.13
Why CAP score (steatosis) tends to increase, along with the reduction of LSM (fibrosis), in CHC patients treated with oral DAAs is unclear. The HALT-C trial reported that, in CHC patients, steatosis decreases as the disease progresses from advanced fibrosis to cirrhosis.24 Our results suggest that as the fibrosis reduces, there in an increase in steatosis.
Multiple factors have been associated with development of non-alcoholic fatty liver (NAFLD)—importantly: obesity, dyslipidemia and insulin resistance; and also drugs, alcohol and viral infections (HCV). The increasing incidence and prevalence of NAFLD has been linked to the rise in obesity and diabetes.25 Recent data among predominantly genotype 1 HCV-infected, or HCV-HIV coinfected patients, suggests an effect of DAAs on lipid profile.26,27 Interferon (IFN)-free DAA regimens lead to an increase in cholesterol levels, with no effect on triglycerides.26 On the other hand, IFN-based therapy leads to a decrease in cholesterol and increase in triglycerides, both during and after achieving SVR.26 Whether this increase in triglycerides can explain the increase in steatosis needs to be explored. We cannot speculate on this association as we did not assess the changes in lipid profile in our cohort. Moreover, our cohort had predominantly genotype 3 patients and 16% had received peginterferon based therapy. Furthermore, a redistribution of fat and insulin resistance could also lead to increase in hepatic steatosis; our study was not designed to assess these factors and future studies need to evaluate these associations. All patients who were consuming alcohol prior to therapy had stopped alcohol intake and none continued drinking. We documented no significant change in the body weight pre- and post DAA therapy and these facts suggest that factors other than excessive calorie consumption may be responsible for the increase in steatosis. We observed an increase in CAP scores after starting DAAs, the rise continued even after stopping the therapy. Whether this increase in steatosis is related to oral DAAs or other factors needs further research. In the present study, we assessed hepatic steatosis on the basis of transient elastography (TE)-CAP score, which has been validated in CHC. We excluded patients in which TE was not measurable or the results could have been fallacious especially in patients with elevated bilirubin and AST and ALT values, as per the manufacturer’s instructions. The ideal gold standard for measuring fibrosis and steatosis is liver biopsy, which may not be ethically justifiable for the management of CHC in all patients.
Recent data suggests an increasing incidence of NAFLD in India.28 Although therapy with DAAs is considered safe, the findings of our study, if confirmed at other centers, will be of public importance. The long term implications of the increase is hepatic steatosis is unclear. Our results suggest that there is a need for future research to evaluate the factors responsible for increase in CAP scores in patients treated with DAAs. Also, we excluded decompensated patients. It is unclear whether DAAs lead to increase in hepatic steatosis in patients with decompensated cirrhosis.
This study had a few limitations. Several patients did not report for transient liver elastography at the prespecified time intervals. Another limitation is lack of follow-up data of features of metabolic syndrome—including lipid profiles, markers of insulin resistance, abdominal girth, and blood pressure. We did not collect data reflecting the patients’ lifestyles, such as dietary practices and levels of physical activity. We used generic sofosbuvir-based combinations; whether similar observations are seen across the branded DAAs needs evaluation. Our study cohort predominantly consisted of patients with genotype 3 infection and while we did not find any significant effect of genotype on hepatic steatosis, it is unclear whether these findings can be extrapolated to other genotypes given smaller patient populations in other genotypes.
In conclusion, LSM values (suggestive of hepatic stiffness) decrease and CAP values (suggestive of hepatis steatosis) increase with DAA therapy in CHC patients. Mechanisms of increase in hepatic steatosis are unclear. Further studies are needed, including liver histology and longer follow-up time, to fully define the impact of treatment with newer antiviral therapy on hepatic steatosis, and its long-term implications.
Authorship statement
-
1
Gyanranjan Rout, MD: acquisition of data; analysis and interpretation of data; drafting of manuscript
-
2
Baibaswata Nayak, PhD: draft writing, study concept.
-
3
Arpan H. Patel, MD: draft writing.
-
4
Deepak Gunjan, DM: data acquisition, draft writing.
-
5
Vishwajeet Singh, MSc: statistical analysis.
-
6
Saurabh Kedia, DM: data acquisition.
-
7
Shalimar, DM: draft writing, study concept, design, statistical analysis, critical revision of manuscript.
Grants and financial support
This study was supported by grant from Department of Health Research, Ministry of Health and Family Welfare, Government of India (No. R.11012/14/2017-HR).
All authors approved the final version of the manuscript.
Conflicts of interest
The authors have none to declare.
Acknowledgement
Mr Anurag and Ms Manisha for data handling.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jceh.2018.06.009.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Davila J.A., Morgan R.O., Shaib Y., McGlynn K.A., El-Serag H.B. Hepatitis C infection and the increasing incidence of hepatocellular carcinoma: a population-based study. Gastroenterology. 2004;127(5):1372–1380. doi: 10.1053/j.gastro.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 2.Falade-Nwulia O., Suarez-Cuervo C., Nelson D.R., Fried M.W., Segal J.B., Sulkowski M.S. Oral direct-acting agent therapy for hepatitis C virus infection: a systematic review. Ann Intern Med. 2017;166(9):637–648. doi: 10.7326/M16-2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta S., Rout G., Patel A.H. Efficacy of generic oral directly acting agents in patients with hepatitis C virus infection. J Viral Hepat. 2018;25(7):771–778. doi: 10.1111/jvh.12870. [DOI] [PubMed] [Google Scholar]
- 4.Poynard T., Moussalli J., Munteanu M. Slow regression of liver fibrosis presumed by repeated biomarkers after virological cure in patients with chronic hepatitis C. J Hepatol. 2013;59(4):675–683. doi: 10.1016/j.jhep.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 5.Chekuri S., Nickerson J., Bichoupan K. Liver stiffness decreases rapidly in response to successful hepatitis C treatment and then plateaus. PLOS ONE. 2016;11(7):e0159413. doi: 10.1371/journal.pone.0159413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stepanova M., Lam B., Younossi Y., Srishord M.K., Younossi Z.M. Association of hepatitis C with insulin resistance and type 2 diabetes in US general population: the impact of the epidemic of obesity. J Viral Hepat. 2012;19(5):341–345. doi: 10.1111/j.1365-2893.2011.01554.x. [DOI] [PubMed] [Google Scholar]
- 7.White D.L., Ratziu V., El-Serag H.B. Hepatitis C infection and risk of diabetes: a systematic review and meta-analysis. J Hepatol. 2008;49(5):831–844. doi: 10.1016/j.jhep.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Negro F., Sanyal A.J. Hepatitis C virus, steatosis and lipid abnormalities: clinical and pathogenic data. Liver Int. 2009;29(suppl. 2)):26–37. doi: 10.1111/j.1478-3231.2008.01950.x. [DOI] [PubMed] [Google Scholar]
- 9.Sasso M., Tengher-Barna I., Ziol M. Novel controlled attenuation parameter for noninvasive assessment of steatosis using Fibroscan(®): validation in chronic hepatitis C. J Viral Hepat. 2012;19(4):244–253. doi: 10.1111/j.1365-2893.2011.01534.x. [DOI] [PubMed] [Google Scholar]
- 10.Karlas T., Petroff D., Sasso M. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol. 2017;66(5):1022–1030. doi: 10.1016/j.jhep.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 11.Poynard T., de Ledinghen V., Zarski J.P. Relative performances of FibroTest, Fibroscan, and biopsy for the assessment of the stage of liver fibrosis in patients with chronic hepatitis C: a step toward the truth in the absence of a gold standard. J Hepatol. 2012;56(3):541–548. doi: 10.1016/j.jhep.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Rout G., Kedia S., Nayak B. Controlled attenuation parameter for assessment of hepatic steatosis in Indian patients. J Clin Exp Hepatol. 2018 doi: 10.1016/j.jceh.2018.02.010. https://doi.org/10.1016/j.jceh.2018.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogasawara N., Kobayashi M., Akuta N. Serial changes in liver stiffness and controlled attenuation parameter following direct-acting antiviral therapy against hepatitis C virus genotype 1b. J Med Virol. 2018;90(2):313–319. doi: 10.1002/jmv.24950. [DOI] [PubMed] [Google Scholar]
- 14.AASLD/IDSA HCV Guidance Panel Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62(3):932–954. doi: 10.1002/hep.27950. [DOI] [PubMed] [Google Scholar]
- 15.Castéra L., Vergniol J., Foucher J. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128(2):343–350. doi: 10.1053/j.gastro.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 16.European Association for the Study of the Liver EASL recommendations on treatment of hepatitis C. 2016. J Hepatol. 2017;66(1):153–194. doi: 10.1016/j.jhep.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Towards elimination of viral hepatitis by 2030. Lancet. 2016;388(10042):308. doi: 10.1016/S0140-6736(16)31144-8. [DOI] [PubMed] [Google Scholar]
- 18.Fontana R.J., Bonkovsky H.L., Naishadham D. Serum fibrosis marker levels decrease after successful antiviral treatment in chronic hepatitis C patients with advanced fibrosis. Clin Gastroenterol Hepatol. 2009;7(2):219–226. doi: 10.1016/j.cgh.2008.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goyal R., Mallick S.R., Mahanta M. Fibroscan can avoid liver biopsy in Indian patients with chronic hepatitis B. J Gastroenterol Hepatol. 2013;28(11):1738–1745. doi: 10.1111/jgh.12318. [DOI] [PubMed] [Google Scholar]
- 20.Garg H., Aggarwal S., Shalimar Utility of transient elastography (fibroscan) and impact of bariatric surgery on nonalcoholic fatty liver disease (NAFLD) in morbidly obese patients. Surg Obes Relat Dis. 2018;14(1):81–91. doi: 10.1016/j.soard.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 21.Yoon E.J., Hu K.-Q. Hepatitis C virus (HCV) infection and hepatic steatosis. Int J Med Sci. 2006;3(2):53–56. doi: 10.7150/ijms.3.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Negro F. HCV infection and metabolic syndrome: which is the chicken and which is the egg? Gastroenterology. 2012;142(6):1288–1292. doi: 10.1053/j.gastro.2011.12.063. [DOI] [PubMed] [Google Scholar]
- 23.Castéra L., Hézode C., Roudot-Thoraval F. Effect of antiviral treatment on evolution of liver steatosis in patients with chronic hepatitis C: indirect evidence of a role of hepatitis C virus genotype 3 in steatosis. Gut. 2004;53(3):420–424. doi: 10.1136/gut.2002.009936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lok A.S., Everhart J.E., Chung R.T. Evolution of hepatic steatosis in patients with advanced hepatitis C: results from the hepatitis C antiviral long-term treatment against cirrhosis (HALT-C) trial. Hepatology. 2009;49(6):1828–1837. doi: 10.1002/hep.22865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loomba R., Sanyal A.J. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10(11):686–690. doi: 10.1038/nrgastro.2013.171. [DOI] [PubMed] [Google Scholar]
- 26.Mauss S., Berger F., Wehmeyer M.H. Effect of antiviral therapy for HCV on lipid levels. Antivir Ther. 2017;21(1):81–88. doi: 10.3851/IMP3094. [DOI] [PubMed] [Google Scholar]
- 27.Townsend K., Meissner E.G., Sidharthan S. Interferon-free treatment of hepatitis C Virus in HIV/hepatitis C virus-coinfected subjects results in increased serum low-density lipoprotein concentration. AIDS Res Hum Retroviruses. 2016;32(5):456–462. doi: 10.1089/aid.2015.0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duseja A. Nonalcoholic fatty liver disease in India—a lot done, yet more required! Indian J Gastroenterol. 2010;29(6):217–225. doi: 10.1007/s12664-010-0069-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.