Abstract
Tropical botfly infection is well described, though endemic botfly myiasis in humans is rare in temperate regions. Reported is a case of myiasis from Cuterebra botfly larvae in a man from northern New York with no tropical travel. The authors discuss the epidemiology, life-cycle, and diagnosis of non-tropical botfly infection.
Keywords: Myiasis, Ectoparasite, Bot fly
Introduction
Myiasis is infestation with the larvae (maggots) of flies. Most human cases of myiasis are caused the families Oestridae (bot flies), Sarcophagidae (flesh flies), Calliphoridae (blow flies), and Muscidae (house flies). Cases are categorized as obligatory (flies require healthy host tissue) or facultative (flies colonize preexisting wounds). Human myiasis from obligatorily species is most commonly from Dermatobia hominis (American tropics) or Cordylobia species (African tropics). Myiasis caused by endemic Cuterebra species in the US and Canada is rare, with approximately 60 cases reported in the past 70 years [1]. Most Cuterebra infections manifest as furuncular myiasis with second instar larvae [1] or respiratory infection from mature third-instar larvae [2]. We report a case of respiratory myiasis diagnosed by the finding of a second-instar larva expelled from the respiratory tract of a 28-year-old man in New York State, discuss Cuterebra biology in relation to human disease, and discuss the diagnostic issues with identifying second-instar larvae.
Case report
A 28-year-old man presented in August 2017 with cough and chest tightness. This persisted for two days, whereupon he expectorated what he believed to be a parasite. His medical history was notable for prior intravenous drug use, although he had been abstinent since 2010, and was otherwise healthy. Serology for human immunodeficiency virus was negative in 2013. He lived in northern New York, in the Adirondack region, and had no travel outside of the state. He worked on an organic vegetable farm, without livestock, although frequently used horse manure and fishmeal as fertilizer. He had a pet cat which recently had killed a mouse and left the carcass in his bed, which was partially decomposed at the time of discovery. The family history was unremarkable and he took no medications.
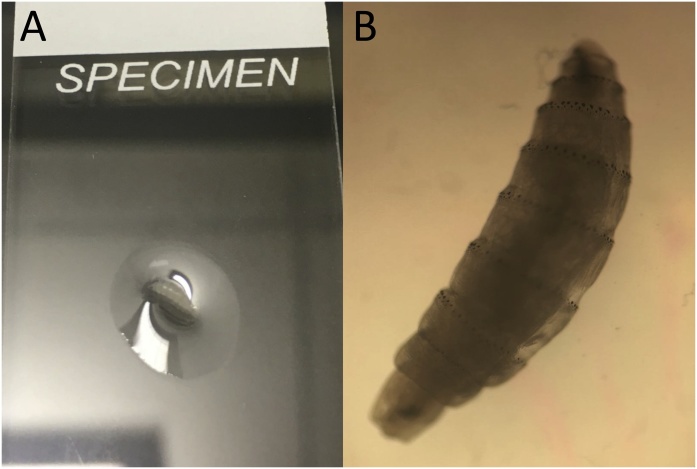
On presentation, he provided the expectorated parasitic form (Fig. 1). Vital signs were normal. The oropharnyx and nasopharnyx were thoroughly examined, without specific abnormalities or parasites identified. Lungs were clear to auscultation. A head-to-toe skin exam revealed no lesions or papules. Physical exam was otherwise unremarkable.
Fig. 1.
Panel A: Initial macroscopic view of the parasite. The specimen is on a standard-size microscope slide. Panel B: Microscopic view of the parasite on 10x magnification.
The parasite was preliminarily identified as a bot fly larva. Images of the larva were sent to two of the authors (BSP, BAM) who gave a presumptive identification of a second-instar larva of Cuterebra. The specimen was sent to Mayo Clinic Parasitology Laboratory, where dissection of the specimen revealed spiracular plates consistent with second instars. Because of concern for visceral bot fly larval infection, the patient was offered therapy with ivermectin. However, he declined and was then lost to follow-up.
Discussion
Cuterebra is a genus of oestrid flies (botflies) endemic to the New World. There are roughly 70 species, with approximately 40 species in North America. The natural hosts are rodents, rabbits, and hares but many other mammals, such as domestic cats, can serve as adequate hosts [3].
In the natural cycle, female flies lay eggs near the entrance to host burrows or along host animal trails. The eggs hatch due to warmth, either from environmental stimuli or the body heat of the host. When first-instar larvae come within physical contact of an appropriate host, they attach near an entry site (e.g. nose, mouth) or penetrate skin directly. The larvae penetrate the respiratory mucosa, migrate to the trachea, and then enter the pleural cavity. The larvae migrate through the diaphragm and abdominal cavity, eventually making their way to the subdermis. In the skin, they molt twice to become second and then third-instar larvae. Mature larvae drop from their host to the ground and find a secluded place to pupate [4]. The route of infection for humans is not well-understood, but is believed to be initiated when people come into contact with infected soil or other environmental sources, such as when gardening or removing rodent nests. In our patient’s case, the dead mouse or soil/compost exposures were the likely causative events.
The diagnosis of myiasis usually involves the identification of larvae expelled or surgically removed from the body. Identification to the genus or species level is not needed for patient management but is recommended to determine whether the causal agent is a cause of obligatory or facultative myiasis, or for epidemiologic studies [5]. Identification of myiasis-causing fly larvae can be inherently challenging. Several keys exist [[5], [6], [7], [8]], but they tend to target third-instar larvae, and the morphological differences between second and third instar larvae can be striking, especially within members of the family Oestridae.
In this case, a diagnosis was made based on morphologic features, including a habitus where the body tapers both anteriorly and posteriorly; the presence of strong cuticular spines on each body segment; and the presence of two sinuous slits on the spiracular plate, which was lacking a defined peritreme. Additionally, the patient had not traveled outside the United States, ruling-out the more commonly-encountered agents of furuncular myiasis, Dermatobia hominis and Cordylobia species.
Conclusion
In summary, we present a case of respiratory zoonotic myiasis in a North American gardener due to a Cuterebra botfly species. This rare condition may pose a diagnostic challenge for both clinicians and laboratorians due to lack of familiarity with the condition and the immature (second instar) stage of the retrieved larva. The case highlights that visceral botfly myiasis can occur in unexpected, non-tropical settings.
Conflict of interest
None of the authors report any conflicts of interest.
Sources of funding
No sources of funding to report.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contribution
Hale: manuscript creation.
Mathison: identification of parasite, manuscript creation and editing.
Pritt: identification of parasite, manuscript creation and editing.
Collins: direct clinical care of the patient; editing of the manuscript.
Authorship verification
All co-authors have seen and agree with the contents of the manuscript and have contributed significantly to the work.
Acknowledgements
None.
References
- 1.Safdar N., Young D.K., Andes D. Autochthonous furuncular myiasis in the United States: case Report and literature review. Clin Infect Dis. 2003;36:73–80. doi: 10.1086/368183. [DOI] [PubMed] [Google Scholar]
- 2.Cornet M., Florent M., Lefebvre A., Wertheimer C., Perez-Eid C., Bangs M., Bouvet A. Thraceopulmonary myiasis caused by a mature third-instar cuterebra larva: case report and review. J Clin Microbiol. 2003;41:5810–5812. doi: 10.1128/JCM.41.12.5810-5812.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sabrosky C.W. Vol. 11. Entomological Society of America; Hyattsville, MD: 1986. North American species of Cuterebra, the rabbit and rodent bot flies (Diptera: Cuterebridae) (The Thomas say monograph series). [Google Scholar]
- 4.Baird J.K., Baird C.R., Sabrosky C.W. North American cuterebrid myiasis: report of 17 new infections of human beings and review of the disease. J Am Acad Dematol. 1989;21:763–772. [PubMed] [Google Scholar]
- 5.Mathison B.A., Pritt B.S. Laboratory identification of arthropod ectoparasites. Clin Microbiol Rev. 2014;27:48–67. doi: 10.1128/CMR.00008-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zumpt F. Butterworths; London, United Kingdom: 1965. Myiasis in man and animals in the Old World. [Google Scholar]
- 7.Centers for Disease Control and Prevention . Centers for Disease Control and Prevention; Atlanta, GA: 1969. Pictorial keys to arthropods, reptiles, birds, and mammals of public health importance. [Google Scholar]
- 8.Mathison B.A., Pritt B.S. College of American Pathologists; Northfield, IL: 2015. Arthropod benchtop reference guide. [Google Scholar]