Abstract
Background
Heart failure (HF) is one of the world leading causes of hospitalization and rehospitalization. Cognitive impairment has been identified as a risk factor for rehospitalization in patients with heart failure. However, previous studies reported mixed results. Therefore, we conducted a systematic review and meta-analysis to assess the association between cognitive impairment and 30-day rehospitalization in patients with HF.
Method
We performed a comprehensive literature search through July 2018 in the databases of MEDLINE and EMBASE. Included studies were cohort studies, case-control studies, cross-sectional studies or randomized controlled trials that compared the risk of 30-day rehospitalization in HF patients with cognitive impairment and those without. We calculated pooled relative risk (RR) with 95% confidence intervals (CI) and I2 statistic using the random-effects model.
Results
Five studies with a total of 2,342 participants (1,004 participants had cognitive impairment) were included for meta-analysis. In random-effect model, cognitive impairment significantly increased the risk of 30-day rehospitalization in HF participants (pooled RR=1.63, 95%CI: 1.19-2.24], I2=64.2%, p=0.002). Subgroup analysis was performed on the studies that excluded patients with dementia. The results also showed that cognitive impairment significantly increased the risk of 30-day rehospitalization in participants with HF (pooled RR=1.29, 95%CI: 1.05–1.59, I2=0.0%, p=0.016), which was consistent with our overall analysis.
Conclusion
Our meta-analysis demonstrated that the presence of cognitive impairment is associated with 30-day rehospitalization in patients with HF.
Keywords: heart failure, cognitive impairment, cognitive dysfunction, rehospitalization
Abbreviations
- CI
Confidence interval
- HF
Heart failure
- HR
Hazard ratio
- MoCA
Montreal Cognitive Assessment
- NOS
Newcastle–Ottawa scale
- OR
Odds ratio
- RR
Relative risk
- SPMSQ
Short Portable Mental Status Questionnaire
1. Introduction
Heart failure (HF) is one of the leading causes of both hospitalization and rehospitalization.1, 2 Hospitalization with HF is not only associated with higher moribund risks but also exhausted more healthcare cost projecting from $24.7 billion in 2010 to $ 77.7 billion in 2030 in the United States,3 resulting in more economic burden. Accordingly, the preventive protocol, especially risk factors control, is essential to decrease the hospitalization rate of HF. One study demonstrated that several risk factors, including race, Medicare coverage, myocardial infarction, renal disease, longer length of stay, hospitalization from another facility, and emergent rehospitalization, were linked to HF rehospitalization .4 Recently, several studies suggested that cognitive impairment might be another factor that also increases the risk of rehospitalization.5, 6, 7, 8, 9
Cognitive function can be characterized into five domains including learning and memory, language, visuospatial, executive, and psychomotor. Cognitive impairment is defined by declined or loss of at least one of the five domains of the cognitive function.10 The impairment of cognition is not only associated with poor medication adherence, poor self-care, and impaired functional activities of daily life, but also might be related to rehospitalization.11, 12, 13 Currently, studies evaluating the relationship between cognitive impairment and HF rehospitalization have shown inconsistent results.5, 6, 7, 8, 9, 14, 15 Thus, our objective is to determine the association between cognitive impairment on 30-day rehospitalization among HF patients.
2. Methods
2.1. Search strategy
Two investigators (J.K. and C.K.) independently searched for published studies indexed in MEDLINE (from 1946 to July 2018), Cochrane, and Embase databases (from 1980 to July 2018) using a search strategy that included the terms “cognitive impairment,” “cognitive dysfunction,” “acute heart failure,” “heart failure,” readmission,” and “rehospitalization.” Only English language publications were included. A manual search for additional pertinent studies and review articles using references from retrieved articles was also completed.
2.2. Inclusion criteria
The eligibility criteria included the following:
-
(1)
Cohort studies (prospective or retrospective), case-control studies, cross-sectional studies or randomized controlled trials that compared the risk of rehospiralization in HF patients with cognitive impairment and those without.
-
(2)
Relative risk, hazard ratio, odds ratio, incidence ratio, or standardized incidence ratio with 95% confidence intervals (CIs) or sufficient raw data for these calculations had to be provided.
The study eligibility was independently determined by two investigators (P.R. and R.M.), and differences were resolved by mutual consensus. Newcastle-Ottawa quality assessment scale, ranging from 0 to 9, was used to evaluate each study in three domains: recruitment and selection of the participants, similarity and comparability between the groups, and ascertainment of the outcome of interest among cohort studies.16
2.3. Data extraction
A standardized data collection form was used to obtain the following information from each study: the title of study, name of the first author, year of publication, country of origin, number of participants, demographic data of participants, method used to identify cognitive impairment, definitions of outcomes of interest (30-day rehospitalization), and average duration of follow-up. If available, confounders would also be assessed, and adjusted effect estimates with 95% CI would be included in the meta-analysis.
To ensure accuracy, two investigators (J.K. and A.T.) independently performed this data extraction process. Should there be any data discrepancy, we referred back to the original articles.
2.4. Definition of HF
HF was defined differently based on each recruited study (Table 1). There were insufficient data regarding the characterization of HF; thus, we were not able to further characterize HF group in the study.
Table 1.
Study characteristics.
| First author | Arslanian-Engoren16 | Patel15 | Agarwal6 | Huynh10 | Sterling9 |
| Year | 2014 | 2015 | 2016 | 2016 | 2018 |
| Country | USA | USA | USA | Australia | USA |
| Study type | Cross-sectional | Prospective cohort | Prospective cohort | Prospective Longitudinal study | Prospective cohort |
| Participant description | Patients ≥65 years who were hospitalized for acute HF | Patients ≥65 years who were hospitalized for acute HF | Individuals aged 70 years and older screened before home discharge | Patients with HF from MARATHON study | Hospitalized patients diagnosis of acute decompensated HF |
| Exclusion criteria | -Prisoners -Treated in the ICU -Underlying condition of psychosis, dementia, or encephalopathy |
-Mini-Cog incomplete -Intrahospital death -Discharged to hospice |
-On cardiac transplant or LVAD list -ESRD undergoing dialysis -Discharged to hospice or nursing home. |
-Age <18 years -Valvular disease -Underlying CAD -Patients with LVAD -Potentially reversible LVD -Terminal disease prognosis<12 months |
-Age <18 years of age -Unstable psychiatric illness -On hospice -Incomplete screening -Severe cognitive impairment or delirium |
| Dementia excluded | Yes | No | No | Yes | Yes |
| Participants, N - Total - With CI - Non-CI |
53 36 (68%) 17 (32%) |
720 169 (23%) 551 (77%) |
121 82 (67.7%) 39 (32.3) |
565 255 (45%) 310 (55%) |
883 462 (53%) 416 (47%) |
| Mean age ± SD (years) | 72 ± 5 | 76 ± N/A | 78.9 ± 4.8 | 74 ± N/A | 60 ± N/A |
| Gender (male), N (%) | 35 (66%) | 408 (56%) | 63 (52%) | 344 (61%) | 473 (54%) |
| CI assessment tool | Cogstate test during hospitalization | Mini-Cog test | Mini-Cog test | MoCA | SPMSQ |
| HF definition | Defined by New York Heart Association class IV | Diagnosed by treating physician | Diagnosed by treating physician | Diagnosed by treating physician | Diagnosed by treating physician |
| Participants with rehospitalization, N (%) - Total - With CI - Non-CI |
12 (22.6%) 9 (25%) 3 (17.6%) |
199 (27.6%) 78 (46%) 121 (22%) |
27 (22.3%) 22 (26.8%) 5 (12.8%) |
122 (21%) N/A N/A |
210 (23.8%) 120 (25.9%) 90 (21.63%) |
| Odd/hazard ratio (95% CI) | N/A | 1.90 (1.47–2.44) | N/A | 1.60 (1.02–2.54) | 1.12 (0.99–1.27) |
| Conclusion by author | The CI status did not differ between patients who were readmitted and not readmitted | Poor performance on the Mini-Cog was related to poor posthospitalization outcomes, most commonly hospital rehospitalization. | CI may indicate greater risk of rehospitalization for individuals with HF than those without. | Mild CI predicts short-term outcomes in HF, independent of clinical and nonclinical factors. | Numeracy, health literacy, and cognition were not associated with 30-day rehospitalization among this sample of patients hospitalized with acute decompensated HF |
HF, heart failure; MARATHON, Multicentre Australian Risk Algorithm to predict Heart failure readmission; ICU, intensive care unit; LVAD, left ventricular–assisted device; LVD, left ventricular dysfunction; ESRD, end-stage renal disease; CAD, coronary artery disease; SPMSQ, 10-item Short Portable Mental Status Questionnaire; MoCA, Montreal Cognitive Assessment; CI, cognitive impairment; HF, heart failure.
2.5. Definition of cognitive impairment
Cognitive impairment was defined as an abnormality of at least one of the five domains of the cognitive function or as defined in each study.10 The screening tools, including the 10-item Short Portable Mental Status Questionnaire (SPMSQ), Montreal Cognitive Assessment (MoCA), Cogstate test, or Mini-Cog test,17, 18, 19, 20 were used.
2.6. Definition of rehospitalization
Rehospitalization was defined by all-cause rehospitalization after the initial discharge, which was due to acute HF.
2.7. Statistical analysis
The meta-analysis was performed by using a random-effects model and fixed effect model. The extracted studies were excluded from the analysis if they did not justify an outcome in each cohort. We pooled the point estimates from each study using the generic inverse-variance method of Der Simonian and Laird.21 The heterogeneity of effect size estimates across these studies was measured using the I2. The I2 statistic ranges in the value from 0 to 100% (I2 < 25%, low heterogeneity; I2 = 25%–50%, moderate heterogeneity; and I2 > 50%, substantial heterogeneity). A sensitivity analysis was also conducted. Publication bias was assessed using a funnel plot and Egger's regression test (p < 0.05 was considered significant). All data analyses were performed using the Stata SE 14.1 software from StataCorp LP.
3. Results
3.1. Study inclusion and characteristic
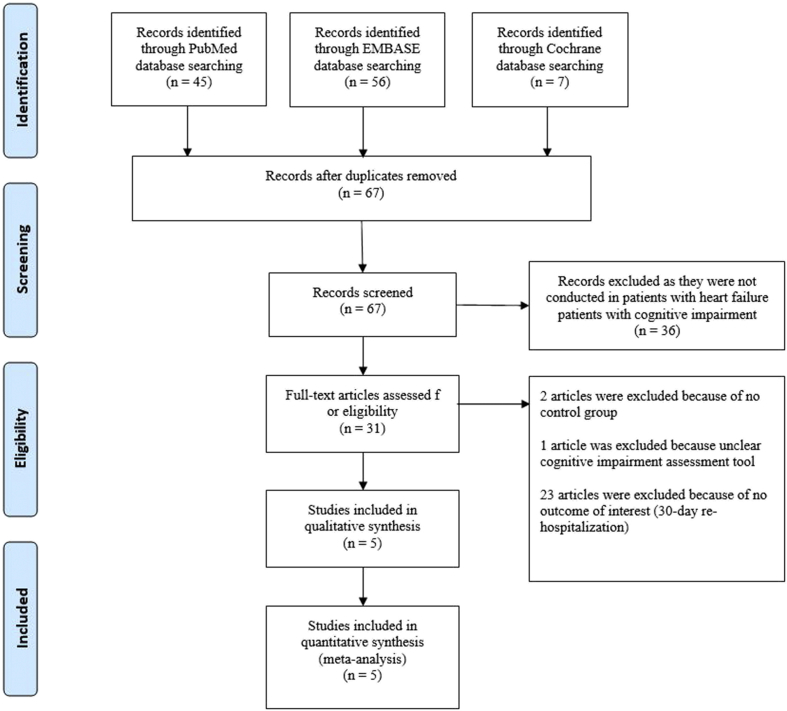
A total number of 108 potentially relevant studies (full article) conducted were identified (56 studies from Embase, 45 studies from PubMed, and seven from Cochrane). After exclusion of 41 duplicate studies, 67 studies underwent title and abstract review. Thirty-six studies were excluded as they were not conducted in HF patients with cognitive impairment. Therefore, 31 studies underwent full-article reviewed. Further 26 studies were excluded as they did not report 30-day rehospitalization rate and/or no control group and/or unclear cognitive impairment assessment tools. Therefore, five eligible studies (five prospective cohorts) with the total number of 2,342 participants (1,004 with cognitive impairment) were included for analysis. The PRISMA flow diagram is shown in Fig. 1. The rehospitalization rate within 30 days after discharge was compared between HF patient with and without cognitive impairment. Cognitive impairment was assessed by the Cogstate test, Mini-Cog test, MoCA, or SPMSQ, and prevalence of cognitive impairment is ranging from 23% to 67.7% among studies. Main characteristics of the included studies in the meta-analysis are summarized in Table 1.
Fig. 1.
PRISMA 2009 flow diagram.
3.2. Quality of eligible studies
Newcastle–Ottawa scale (NOS) was used to evaluate the quality of studies included in the analysis. Higher scores represent higher quality of the study. All the studies were considered high-quality according to NOS (score ≥ 8) (Table 2).
Table 2.
Newcastle-Ottawa quality assessment scale of included studies in meta-analysis.
| Study | Representativeness | Selection of the nonexposed cohort | Ascertainment | Endpoint not present at start | Comparability |
Assessment of outcomes | Follow-up duration | Adequacy follow-up | Score |
|---|---|---|---|---|---|---|---|---|---|
| (Confounding) | |||||||||
| Arslanian-Engoren 2014 | * | * | * | * | * | * | * | * | 8 |
| Patel 2014 | * | * | * | * | * | * | * | * | 8 |
| Agarwal 2016 | * | * | * | * | ** | * | * | * | 9 |
| Huynh 2016 | * | * | * | * | * | * | * | * | 8 |
| Sterling 2017 | * | * | * | * | * | * | * | * | 8 |
The Newcastle–Ottawa scale uses a star system (0–9) to evaluate the included studies on three domains: selection, comparability, and outcomes. Star (*) = item presents. Maximum one star (*) for selection and outcome components and two stars (**) for comparability components. Higher scores represent higher study quality.
3.3. Quantitative analyses
Study heterogeneity analysis evaluated by I2 tests showed substantial heterogeneity among studies (I2 = 64.2%). Therefore, both random and fixed-effects models were used to assess the pooled risk ratio (RR).
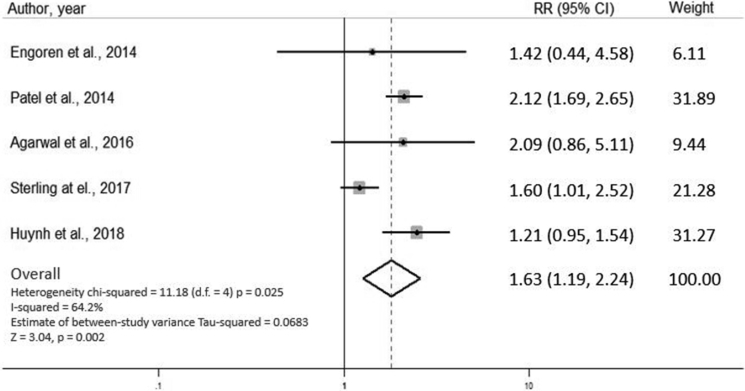
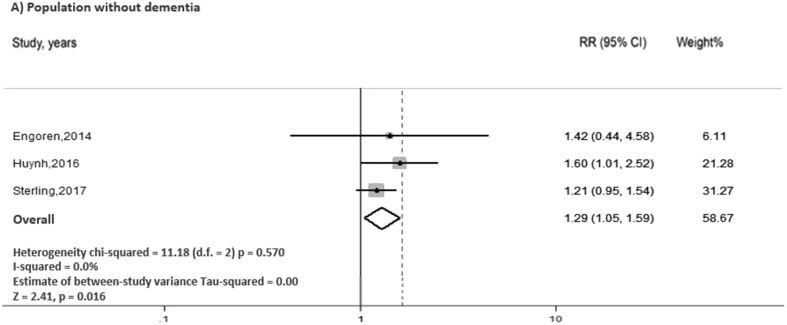
In random effect model, our analysis showed that cognitive impairment significantly increased the risk of 30-day rehospitalization in HF participants (pooled RR = 1.63, 95% CI: 1.19–2.24, I2 = 64.2%, p = 0.002) (Fig. 2). Subgroup analysis was performed on the studies that excluded patients with dementia (n = 1501). The results from the subgroup analysis also showed that cognitive impairment significantly increased the risk of 30-day rehospitalization in participants with HF (pooled RR = 1.29, 95% CI: 1.05–1.59, I2 = 0.0%, p = 0.016), which was consistent with our overall analysis (Fig. 3).
Fig. 2.
The forest plot of the included studies assessing the association between cognitive impairment and 30-day rehospitalization in patients with acute heart failure. CI, confidence interval.
Fig. 3.
Forest plot of the subgroup analysis of studies that excluded patients with dementia, assessing the association between cognitive impairment and 30-day rehospitalization in patients with acute heart failure. CI, confidence interval.
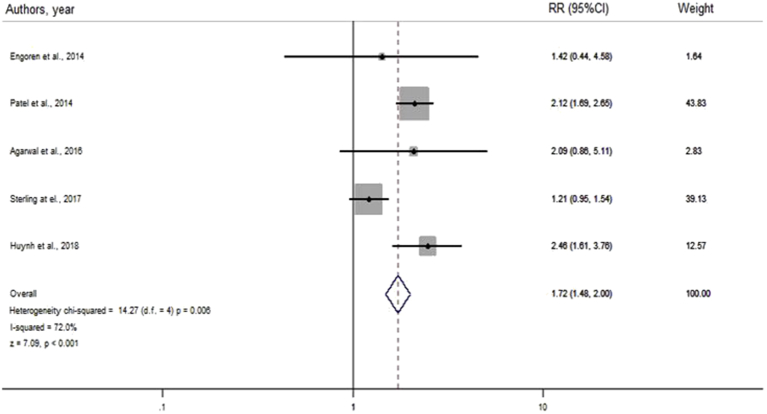
In fixed-effects model, the result also showed that the presence of cognitive impairment is significantly associated with 30-day rehospitalization (RR = 1.72, 95% CI = 1.48–2.00, I2 = 72.0%, p < 0.001), similar to the results from the random effect model (Supplementary file 3).
3.4. Sensitivity analysis
We assessed the stability of the results of the meta-analysis. We conducted a sensitivity analysis by excluding one study at a time. None of the results were significantly altered; the results after removing one study at a time were similar to those of the main meta-analysis. This indicated that our results were robust.
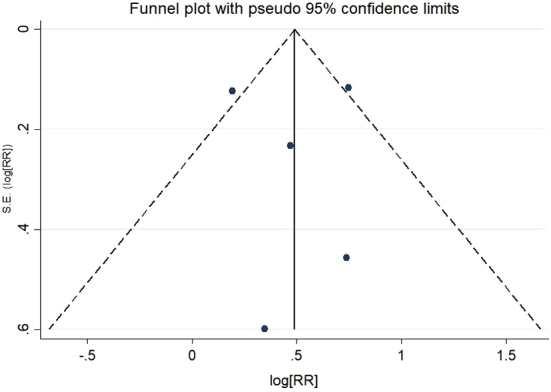
3.5. Publication bias
Funnel plot and Egger's test were performed to confirm the absence of publication bias. The funnel plot was symmetric indicating no publication bias, and Egger's test reports no small studies effect [95% CI (-5.41, 5.47), p = 0.987].
4. Discussion
Studies reported the prevalence of cognitive impairment in patients with HF, acute or chronic, ranging from 23% to 80%.22 Recent systematic review and meta-analysis found a strong association between cognitive impairment and HF.23 However, factors such as the type and timing of the cognitive screening, severity of HF, and the presence of other comorbidities may result in a variety of cognitive impairment prevalences. There are multiple suggested pathophysiologies behind this association. Systemic hypotension, which was found to be significantly associated with the presence of cognitive impairment in patients with HF,24 leading to chronic poor perfusion of the brain, together with occult cardiogenic emboli, is believed to be the main mechanism.25 Other known factors found to be related to cognitive impairment in patients with HF include anemia, CKD, older age, and lower LVEF.26, 27, 28 In modern practice, at least eight methods, such as MoCA, Mini-Cog, and Mini-Mental Status Examination, have been introduced for the measurement of cognitive function. Of the five domains, the most common deficit aspect of the cognition is learning and memory.29 Patients with cognitive impairment are different from patients with dementia. While patients with dementia have significant difficulties in their daily life, patients with cognitive impairment usually are independent and do not require assistance for their daily activities.10 Because of this, many patients with cognitive impairment were undiagnosed and would remain unnoticed until their cognition has already severely declined.
Although screening for a change in cognition is recommended in the current practice of HF management, there is still not enough evidence regarding the most sensitive or the most specific method and the optimal timing for the test.30 In addition, the true consequences and appropriate management of cognitive impairment among this population are still in debate. Several studies have reported mixed results of outcomes regarding the effect of cognitive impairment on mortality and rehospitalization rate in patients with HF.
Owing to the high economic and social burden of HF, attempts to decrease the hospitalization rate and comorbidity have always been a compelling topic. In addition to implementing self-care intervention and appropriate medical adjustment, identifying risk factors for outcomes in this population is also essential. To the best of author's knowledge, this is the first meta-analysis to examine the association between cognitive impairment and rehospitalization in this population.
The prevalence of cognitive impairment among the five studies ranged from 23% to 68%. Patel et al14 reported the lowest prevalence, whereas studies by Agarwal et al5 and Arslanian-Engoren et al15 showed a high prevalence of 67.7% and 68%, respectively. This might due to the difference of the screening tools in each study that led to a difference of cognitive impairment prevalence. However, the study by Patel et al. and Agarwal et al. still reported the major difference of prevalence of cognitive impairment (23% vs 67.7%), even though the two studies both used the same screening tool. Despite the lowest prevalence of cognitive impairment reported in the study, the study by Patel et al. showed the highest risk of 30-day rehospitalization (RR = 2.12, 95% CI: 1.69–2.65). The study by Sterling et al8 had the highest number of participants (n = 883) and showed the lowest risk of 30-day rehospitalization (RR = 1.21, 95% CI: 0.95–1.54).
Results of 30-day rehospitalization were available in five studies. Only studies by Patel et al. and Huynh et al9 reported the significant association of cognitive impairment and rehospitalization in patients with HF. The other three studies5, 8, 15 also showed an increased risk of 30-day rehospitalization in HF patients with cognitive impairment, although not statistically significant. Two of the three studies, the study by Arslanian-Engoren et al. and Agarwal et al., had a lower number of patients enrolled (n = 53 and 131, respectively). Sterling et al. had the highest number of participants; however, the SMSQ was used as a screening tool in their study. The SMSQ is a well-known and well-validated test but does not assess an executive function, which is the most commonly affected aspect of cognitive function in the HF population.31 Nevertheless, from our meta-analysis, we found that overall cognitive impairment is significantly associated with 30-day rehospitalization.
4.1. Subgroup analysis
Two studies by Patel et al. and Agarwal et al. did not exclude patients with dementia from the analysis. The studies by Patel et al. and Agarwal et al. reported relatively higher RR of rehospitalization than the other three studies, although the results from Agarwal's trial were not statistically significant. Thus, we performed a subgroup analysis of only studies that exclude patients with dementia (Fig. 3). The results showed that even in the absence of dementia, mild cognitive impairment was still found to be a predictor of 30-day rehospitalization in patients with acute HF.
4.2. Heterogeneity
The random effect models showed substantial heterogeneity (I2 = 64.2%). Meta-regression analysis of dementia patient population reported almost statistically significant results, and the heterogeneity on our subgroup analysis of patient population without dementia showed a greatly diminished I2 from 64.2% to 0.0% (Fig. 3). We suggested that the heterogeneity was due to the presence of dementia in the studies reported by Patel et al. and Agarwal et al.
4.3. Clinical implications
It is still unclear how cognitive impairment leads to the increased risk of rehospitalization in patients with HF. Studies showed that patients with cognitive impairment were prone to have less effective self-care and medical adherence, despite proper knowledge about their condition.12, 32, 33 Another possible cause may be owing to a more severe extent of HF that was more commonly found among patients with cognitive impairment.27 In addition, other chronic conditions, such as renal failure and anemia, were reported to be more common in patients with declined cognition.26 Management of cognitive impairment in HF is not yet standard. Current evidence is guiding toward slowing down the progression of cognitive impairment by controlling other related comorbidities, such as hypertension, atrial fibrillation, and depression.29 Other behavior-related changes such as exercise, diet control, or weight loss are also still in consensus.
Currently, screening for cognitive impairment in this HF population receives little emphasis on the current practice.34 Dodson et al.7 reported that only 23% of HF patients with cognitive impairment had it documented by the time of discharge. The results of this study could further emphasize the need to detect patients with cognitive impairment in HF management. Patients with objective evidence of cognitive impairment should be closely observed regarding their medication compliant or change in weight and symptoms. In short, cognitive impairment should be addressed, in addition to other comorbidities, in attempt to decrease the rehospitalization rate and reduce both economic and social burden of HF.
Bases from the limited published data, there is no consensus evidence regarding the routine screening method or intervention to reduce rehospitalization in this patient population. Agarwal et al. demonstrated that additional education on the family and caregivers was shown to reduce the rehospitalization rate but was concluded that it may due to the availability of engaged family rather than the effect of the education. A previous randomized control trial showed that a targeted self-care intervention can improve HF knowledge but was unable to significantly reduce the 30-day rehospitalization rate in this population.33 However, the study had a relatively small number of participants and thus may fail to detect the difference in the rehospitalization rate. Further studies may have more patients enrolled or may focus on whether any intervention can decrease the rehospitalization rate in this population.
Although our analysis demonstrated the association between cognitive impairment and the increased 30-day rehospitalization, data regarding long-term rehospitalization and mortality are currently limited and unclear. Published studies reported different outcomes with a varied follow-up time, which lead to heterogeneity among studies. This could be the area of focus for further study or systematic review.
4.4. Strengths and limitations
The strength of this meta-analysis is that most studies were prospective studies. The limitation of our analysis includes a small number of studies and participants. Second, the different screening tools may affect the heterogeneity of our analysis. Although Patel et al. and Agarwal et al. did not exclude patients with dementia from their studies, the analysis without the two studies still showed a significant association between cognitive impairment and 30-day rehospitalization rate. Third, none of the included studies statistically evaluate the cause of the association of cognitive impairment and rehospitalization. Moreover, the included studies did not clearly state whether the patients with HF had a first onset of acute HF or acute decompensated HF on top of the underlying chronic HF, as the prognosis and rehospitalization rate may differ between the two populations. Finally, the extracted data regarding the outcome of 30-day rehospitalization were not adjusted for confounders, such as age, LVEF, in most of the included studies. Thus, the association we reported must be interpreted carefully as it may be affected by such confounders and other mentioned limitations.
5. Conclusions
In conclusion, our systematic review and meta-analysis showed that the presence of cognitive impairment is strongly associated with 30-day rehospitalization among patients with HF. The exact mechanism of this relationship is still unclear. Despite the relatively small evidence, the presence of cognitive impairment in this population should not be neglected. Further research is needed for the proper understanding of the definite pathophysiology and to correctly guide the management of cognitive impairment.
Acknowledgments
The authors would like to thank Dr. Michael Tee, MD, PhD, for language proof and editing.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ihj.2018.12.006.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interests
All authors have none to declare.
Appendix A. Supplementary data
The following are the supplementary data to this article:
figs1.
figs2.
References
- 1.Benjamin E.J., Blaha M.J., Chiuve S.E. Heart disease and stroke statistics-2017 update: a report from the American heart association. Circulation. 2017;135(10):e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jencks S.F., Williams M.V., Coleman E.A. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 3.Tung Y.C., Chou S.H., Liu K.L. Worse prognosis in heart failure patients with 30-day readmission. Acta Cardiol Sin. 2016;32(6):698–707. doi: 10.6515/ACS20151113A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krumholz H.M., Lin Z., Keenan P.S. Relationship between hospital readmission and mortality rates for patients hospitalized with acute myocardial infarction, heart failure, or pneumonia. Jama. 2013;309(6):587–593. doi: 10.1001/jama.2013.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agarwal K.S., Kazim R., Xu J., Borson S., Taffet G.E. Unrecognized cognitive impairment and its effect on heart failure readmissions of elderly adults. J Am Geriatr Soc. 2016;64(11):2296–2301. doi: 10.1111/jgs.14471. [DOI] [PubMed] [Google Scholar]
- 6.Sokoreli I., Pauws S.C., Steyerberg E.W. Prognostic value of psychosocial factors for first and recurrent hospitalizations and mortality in heart failure patients: insights from the OPERA-HF study. Eur J Heart Fail. 2018;20(4):689–696. doi: 10.1002/ejhf.1112. [DOI] [PubMed] [Google Scholar]
- 7.Dodson J.A., Truong T.T., Towle V.R., Kerins G., Chaudhry S.I. Cognitive impairment in older adults with heart failure: prevalence, documentation, and impact on outcomes. Am J Med. 2013;126(2):120–126. doi: 10.1016/j.amjmed.2012.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sterling M.R., Safford M.M., Goggins K. Numeracy, health literacy, cognition, and 30-day readmissions among patients with heart failure. J Hosp Med. 2018;13(3):145–151. doi: 10.12788/jhm.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huynh Q.L., Negishi K., Blizzard L. Mild cognitive impairment predicts death and readmission within 30days of discharge for heart failure. Int J Cardiol. 2016;221:212–217. doi: 10.1016/j.ijcard.2016.07.074. [DOI] [PubMed] [Google Scholar]
- 10.Knopman D.S., Petersen R.C. Mild cognitive impairment and mild dementia: a clinical perspective. Mayo Clin Proc. 2014;89(10):1452–1459. doi: 10.1016/j.mayocp.2014.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alkhuja S., Duffy K. Cognitive impairment and medication adherence in outpatients with heart failure. Heart Lung : J Crit Care. 2013;42(5):387. doi: 10.1016/j.hrtlng.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 12.Hawkins L.A., Kilian S., Firek A., Kashner T.M., Firek C.J., Silvet H. Cognitive impairment and medication adherence in outpatients with heart failure. Heart & lung. J Crit Care. 2012;41(6):572–582. doi: 10.1016/j.hrtlng.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Alosco M.L., Spitznagel M.B., Raz N. Executive dysfunction is independently associated with reduced functional independence in heart failure. J Clin Nurs. 2014;23(5-6):829–836. doi: 10.1111/jocn.12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel A., Parikh R., Howell E.H., Hsich E., Landers S.H., Gorodeski E.Z. Mini-cog performance: novel marker of post discharge risk among patients hospitalized for heart failure. Circulation Heart failure. 2015;8(1):8–16. doi: 10.1161/CIRCHEARTFAILURE.114.001438. [DOI] [PubMed] [Google Scholar]
- 15.Arslanian-Engoren C., Giordani B.J., Algase D., Schuh A., Lee C., Moser D.K. Cognitive dysfunction in older adults hospitalized for acute heart failure. J Card Fail. 2014;20(9):669–678. doi: 10.1016/j.cardfail.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 17.Scanlan J., Borson S. The Mini-Cog: receiver operating characteristics with expert and naive raters. Int J Geriatr Psychiatr. 2001;16(2):216–222. doi: 10.1002/1099-1166(200102)16:2<216::aid-gps316>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 18.Maruff P., Lim Y.Y., Darby D. Clinical utility of the cogstate brief battery in identifying cognitive impairment in mild cognitive impairment and Alzheimer's disease. BMC psychology. 2013;1(1):30. doi: 10.1186/2050-7283-1-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hawkins M.A., Gathright E.C., Gunstad J. The MoCA and MMSE as screeners for cognitive impairment in a heart failure population: a study with comprehensive neuropsychological testing. Heart Lung : J Crit Care. 2014;43(5):462–468. doi: 10.1016/j.hrtlng.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23(10):433–441. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 21.DerSimonian R., Laird N. Meta-analysis in clinical trials. Contr Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 22.Cannon J.A., McMurray J.J., Quinn T.J. 'Hearts and minds': association, causation and implication of cognitive impairment in heart failure. Alzheimer's Res Ther. 2015;7(1):22. doi: 10.1186/s13195-015-0106-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cannon J.A., Moffitt P., Perez-Moreno A.C. Cognitive impairment and heart failure: systematic review and meta-analysis. J Card Fail. 2017;23(6):464–475. doi: 10.1016/j.cardfail.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 24.Zuccala G., Onder G., Pedone C. Hypotension and cognitive impairment: selective association in patients with heart failure. Neurology. 2001;57(11):1986–1992. doi: 10.1212/wnl.57.11.1986. [DOI] [PubMed] [Google Scholar]
- 25.Georgiadis D., Sievert M., Cencetti S. Cerebrovascular reactivity is impaired in patients with cardiac failure. Eur Heart J. 2000;21(5):407–413. doi: 10.1053/euhj.1999.1742. [DOI] [PubMed] [Google Scholar]
- 26.Pulignano G., Del Sindaco D., Di Lenarda A. Chronic renal dysfunction and anaemia are associated with cognitive impairment in older patients with heart failure. J Cardiovasc Med. 2014;15(6):481–490. doi: 10.2459/JCM.0000000000000005. [DOI] [PubMed] [Google Scholar]
- 27.Jurgens C.Y., Faulkner K.M., Lee C.S. Phenotypic profiling of cognitive impairment risk among patients with heart failure: a literature review of the usefulness of cardiac-related variables. Eur J Cardiovasc Nurs. 2013;12(2):109–131. doi: 10.1177/1474515112470046. [DOI] [PubMed] [Google Scholar]
- 28.Dardiotis E., Giamouzis G., Mastrogiannis D. Cognitive impairment in heart failure. Cardiol Res Pract. 2012;2012:595821. doi: 10.1155/2012/595821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yzeiraj E., Tam D.M., Gorodeski E.Z. Management of cognitive impairment in heart failure. Curr Treat Options Cardiovasc Med. 2016;18(1):4. doi: 10.1007/s11936-015-0425-7. [DOI] [PubMed] [Google Scholar]
- 30.Cameron J., Gallagher R., Pressler S.J. Detecting and managing cognitive impairment to improve engagement in heart failure self-care. Curr Heart Fail Rep. 2017;14(1):13–22. doi: 10.1007/s11897-017-0317-0. [DOI] [PubMed] [Google Scholar]
- 31.Davis K.K., Allen J.K. Identifying cognitive impairment in heart failure: a review of screening measures. Heart Lung : J Crit Care. 2013;42(2):92–97. doi: 10.1016/j.hrtlng.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 32.Currie K., Rideout A., Lindsay G., Harkness K. The association between mild cognitive impairment and self-care in adults with chronic heart failure: a systematic review and narrative synthesis. J Cardiovasc Nurs. 2015;30(5):382–393. doi: 10.1097/JCN.0000000000000173. [DOI] [PubMed] [Google Scholar]
- 33.Davis K.K., Mintzer M., Dennison Himmelfarb C.R., Hayat M.J., Rotman S., Allen J. Targeted intervention improves knowledge but not self-care or readmissions in heart failure patients with mild cognitive impairment. Eur J Heart Fail. 2012;14(9):1041–1049. doi: 10.1093/eurjhf/hfs096. [DOI] [PubMed] [Google Scholar]
- 34.Moyer V.A. Screening for cognitive impairment in older adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(11):791–797. doi: 10.7326/M14-0496. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.