Abstract
Hepatocellular carcinoma (HCC) is swiftly increasing in prevalence globally with a high mortality rate. The progression of HCC in patients is induced with advanced fibrosis, mainly cirrhosis, and hepatitis. The absence of proper preventive or curative treatment methods encouraged extensive research against HCC to develop new therapeutic strategies. The Food and Drug Administration–approved Nexavar (sorafenib) is used in the treatment of patients with unresectable HCC. In 2017, Stivarga (regorafenib) and Opdivo (nivolumab) got approved for patients with HCC after being treated with sorafenib, and in 2018, Lenvima (lenvatinib) got approved for patients with unresectable HCC. But, owing to the rapid drug resistance development and toxicities, these treatment options are not completely satisfactory. Therefore, there is an urgent need for new systemic combination therapies that target different signaling mechanisms, thereby decreasing the prospect of cancer cells developing resistance to treatment. In this review, HCC etiology and new therapeutic strategies that include currently approved drugs and other potential candidates of HCC such as Milciclib, palbociclib, galunisertib, ipafricept, and ramucirumab are evaluated.
Keywords: hepatocellular carcinoma, tyrosine kinase inhibitors, cyclin-dependent kinase inhibitors, combination therapy, hepatology
Abbreviations: FDA, Food and Drug Administration; HCC, Hepatocellular carcinoma; HBV, hepatitis B virus; HCV, hepatitis B virus; HDV, hepatitis D virus; Rb, retinoblastoma protein; HBsAg, HBV surface antigen; HIV, human immunodeficiency virus; HBcAg, hepatitis B core antibody; PDGFR, platelet-derived growth factor receptor; CTGF, connective tissue growth factor; EMT, Epithelial–mesenchymal transition; ECM, extracellular matrix; TGF 1, transforming growth factor-1; CTL, cytotoxic T lymphocyte; Treg, regulatory T cells; NK, natural killer; NKT, natural killer T cell; MDSC, myeloid-derived suppressor cell; BMI, body mass index; NASH, nonalcoholic steatohepatitis; PAPSS1, 3′-phosphoadenosine 5′-phosphosulfate synthase 1; TK, tyrosine kinase; CDK, cyclin-dependent kinase; TKI, Tyrosine kinase inhibitor; ATP, adenosine 5′-triphosphate; IGFR, insulin-like growth factor; SHP1, src homology 2 domain–containing phosphatase 1; PD-L1, programmed death ligand1; GFG, fibroblast growth factor; SCF, stem cell factor; OS, overall survival; PFS, progression-free survival; TACE, transarterial chemoembolization; TRKA, tropomyosin receptor kinase A; AMPK, AMP-activated protein kinase; RFA, radiofrequency ablation; ORR, objective response rate; PD1, programmed cell death protein 1; EGFR, epidermal growth factor receptor; EFGR, endothelial growth factor receptor; PI3K, phosphoinositide 3-kinases; STAT3, signal transducer and activator of transcription 3; VEGF, vascular endothelial growth factor; PEDF, pigment epithelium-derived factor; HIF, hypoxia-inducible factor; VEGFR, vascular endothelial growth factor receptor; bFGF, basic fibroblast growth factor; MAPK, mitogen-activated protein kinase; ERK, extracellular signal-regulated kinase; JAK, janus kinase; BMF, Bcl2 modifying factor; PUMA, p53 upregulated modulator of apoptosis; PTEN, phosphatase and tensin homolog; AMP, adenosine monophosphate; CTLA, cytotoxic T-lymphocyte-associated protein
Prevalence
Hepatocellular carcinoma (HCC) is the most prevalent malignancy of the liver, which is considered the second leading cause of cancer death in the US. It is considered as the fifth most detected cancer in men and the seventh most detected cancer in women in the USA and the third most leading cause of cancer death worldwide.1 In many parts of the world including Europe, North America, and Latin America, the rate of liver cancer is increasing by 3.1% each year from 2008 to 2012, as reported by the database of the National Cancer Institute in the US.2 According to recent reports, the highest incidence rate of liver cancer in the world occurs in Africa and Asia. Hepatitis B virus (HBV) and hepatitis C virus (HCV) account for approximately 80% of HCC cases, of which HBV accounts for 50–80% of cases of virus-associated HCC, whereas HCV is known to be responsible for 10–25%.3, 4
HCC is diagnosed more commonly in men than in women possibly due to a higher prevalence of HBV, HCV, and alcohol consumption in males that translate into increased carcinogenicity.1 Other factors that contribute to the escalation of HCC include increased occurrence of obesity, aflatoxin exposure, and nonalcoholic fatty liver disease around the world.1
Etiology
Several factors associated with the etiology of HCC have a direct influence on disease progression and on the characteristic of patients.4 The greatest global HCC incidence has been reported from sub-Saharan Eastern and Western Africa, Mongolia, China, and Asia-pacific regions.5 However, the pervasiveness of HCC is lower in developed countries excluding France, Japan, and Italy. HBV, HCV, and hepatitis D virus (HDV) have a strong link with the progression of HCC; therefore, the global incidence of HCC mirrors the occurrence of these infectious viral diseases. Liver cirrhosis progresses to HCC in ∼80–90% of the cases.6 The paramount risk element for HCC to flourish is cirrhosis, and HCV and HBV are regarded as the crucial causes of cirrhosis. As a matter of fact, cirrhosis happens when hepatocytes undergo necrosis resulting in fibrosis-forming scar tissue in cirrhosis.7 It is well known that HCC is the mainspring of HBV infection.8 To a large extent, HCC linked to HBV occurs in patients who are suffering from HBV infection nearly all their lives, namely chronic hepatitis B.8
Hepatitis B virus
HBV infections may develop into HCC in the presence or absence of cirrhosis due to the genetic mutation induced by HBV.9 The HBV genetic material infiltrates normal hepatocytes and interrupts their function, which result in cancerous cells. Fragments of the HBV genome are often detected in these hepatocarcinoma cells.10 HBV has a partly double-stranded DNA that incorporates virus associated to the Hepadnaviridae family.11 HCC is induced by this virus through both direct and indirect pathways. HBV infection in the liver is the cause of hepatocyte lesion and chronic necroinflammation ensuing hepatocyte proliferation, fibrosis, and cirrhosis.12, 13, 14 The unceasing regeneration in cirrhosis persuades hepatocyte multiplication, turnover, and buildup of mutations in the host genome, resulting in genetic changes, chromosomal rearrangements, inactivation of tumor suppressor genes, and activation of oncogenes.14 Nevertheless, in the absence of cirrhosis, HBV can also induce HCC.4 However, HBV may behave as a mutagenic factor by resulting in secondary chromosomal rearrangement and escalate genomic mutability via integrating its DNA into host cells.4
While most HCC cases evolve in cirrhotic livers, a crucial fragment of HBV-associated HCC may take place in the setting of chronic hepatitis B in the lack of liver cirrhosis. The fact that a lower rate of cirrhosis in HBV-associated HCC is analogous to other etiologies, thus argues for a more direct role of HBV in the tumor formation process. Furthermore, different gene expression profiles have been diagnosed in the nontumoral livers of chronic HBV carriers. For instance, activating expression of genes linked in proapoptotic, inflammatory, and DNA repair responses indicate definite pathways activated by chronic hepatitis B.15, 16 Hepatitis B virus X (HBx) gene plays an essential role in HBV-associated HCC development. In addition, HBx functions as an activator for an enormous variety of viral promoters. Hence, HBx gene expression is of foremost significance for viral reproduction within living cells.17, 18, 19, 20, 21, 22 HBx protein of HBV or NS5A protein of HCV can cause chronic infections that trigger the PI3K/Akt/STAT3 pathway in tumor cells.23
Hepatitis C virus
HCV is associated with the Hepacivirus genus of Flaviviridae descent, and it infects approximately 170 million people globally per year.24 As compared to uninfected subjects, a 15- to 20-fold increased threat for HCC exists in HCV-infected individuals.24 Throughout the extent of 30 years of persistent infection, the momentum of HCC in cohort studies of HCV-affected persons extends from 1% to 3%. After HCV-associated cirrhosis is confirmed, HCC evolves at a yearly rate from 1% to 8% at an average of 3.5%.24, 25 Unlike HBV that can integrate into the host genome resulting in the direct carcinogenic activity, HCV is known to be an RNA virus with the restricted incorporation of its genetic information into the host genome.26 Consequently, the carcinogenic prospective of HCV is linked to indirect mechanisms.26 Although HCV elimination can play a role in preventing the progression of HCC, other factors that play a major role in HCC progression are iron overload, oxidative stress, endoplasmic reticulum stress, steatosis in hepatocytes, and inflammation.27
Nevertheless, HCV may also directly progress to HCC by amending various host regulatory pathways that are required in epithelial–mesenchymal transition, angiogenesis, apoptosis, proliferation, and DNA repair. Recent studies have identified direct targets of HCV proteins such as retinoblastoma protein (Rb) that is responsible to restrain cell proliferation primarily by suppressing the activation of E2F, a transcription factor required for S-phase ingression in the cell cycle.28, 29, 30, 31, 32
Dual infection
There are several salient similarities shared by HBV and HCV such as the modes of transmission, large diffusion globally, and the ability to trigger a chronic infection that may progress to cirrhosis and hepatocellular carcinoma.33 Gathered epidemiological data suggest that coinfection with HBV and HCV escalates the risk for the progression of HCC. A massive body of data revealed that the pervasiveness of esoteric HBV infection that is the enduring persistence of HBV genomes in person negative for HBV surface antigen (HBsAg) is specifically raised in HCV individuals.34, 35, 36 Recent studies have demonstrated that coinfection has long-term acute evolution as compared to HBV or HCV monoinfection. Furthermore, dual infection is linked with an elevated risk of development of fibrosis and the progression of cirrhosis and is a discrete predictor of HCC progression.37, 38 Thus, coinfection with HBV or HCV is an intricate clinical/virological form39 that seems to be linked with the various manifestation of chronic liver disease, and it is a major risk factor for HCC progression.40, 41
The human immunodeficiency virus (HIV) is considered as another major modulator of HCC. Studies have revealed that HIV coinfection can hasten the clinical progression of chronic HBV or HCV infection and enlarge the risk of liver cirrhosis and HCC.42, 43 The impact of HBV or HCV on HIV are, however, contentious, and some studies have described that HIV-positive patients coinfected with HCV and/or HBV have the more swift development of AIDS and associated death than patients without coinfection.44 Furthermore, HIV and HBV share a similar course of transmission as the prevalence of anti–hepatitis B core antibody (HBcAb) and HBsAg in HIV-positive patients are exceptionally elevated. Discrete, usually vital, virological profiles may be perceived that is particularly associated with the proceedings of either one or both the viruses over time.45 For the accurate diagnosis and therapeutic approach, it is obligatory to perform a cautious longitudinal assessment of the HBV and HCV titers.
Patient heterogeneity
Patient heterogeneity is a part of the natural alterations that can be assigned to the attributes of those patients.46, 47 Interpatient heterogeneity is described by the discrepancy of tumor cell populations within patients.48, 49 Hepatocellular carcinoma has diverse modifications that rely on tumor size and histological grade. Recent studies demonstrated that HCCs approximately 1.0 cm in size have artery-like vessels that are not properly grown with vague capillarization of the blood expanse and the main portal supply within cancerous nodules.48 At distinct phases of tumor development, angiogenic shifts come from the balance between proangiogenic and antiangiogenic elements. Hence, angiogenic heterogeneity is related to angiogenic molecules such as VEGF, PEDF, and HIF-1 alpha. Therapy against angiogenic elements is crucial in restraining the recurrence in patients with HCC.50 There are various antiangiogenesis targets such as VEGF, VEGFR, bFGF, platelet-derived growth factor receptor (PDGFR), and angiopoietin Ang-1 and Ang-2. A Food and Drug Administration (FDA)–approved kinase inhibitor, Sorafenib, could lessen the expression of VEGFR-2, VEGFR-3, and PDGFR.51 The angiogenic heterogeneity of HCC is required to be taken into deliberation because angiogenic elements could be distinct among different tumor sizes and time span throughout the course of hepatocarcinogenesis.50
The connective tissue growth factor (CTGF) is overexpressed in individuals with HCC, but it is also known that the downregulation of CTGF could hamper HCC development.52 It could potentially be a future therapeutic target for HCC treatment. Epithelial–mesenchymal transition (EMT) is a vital course of action in hepatocarcinogenesis, which includes the association between cells and extracellular matrix (ECM) mediated by transforming growth factor-1 (TGF 1) or PDGFR signaling. It is an onerous point to inhibit ECM proteins due to several ECM proteins and intricate mechanisms, and thus, it is important to consider it for target therapy.53 The immune microenvironment in HCC also appears to be heterogeneous. Cell types enclosed within surrounding tumors incorporate CD8+ cytotoxic T lymphocytes (CTLs), regulatory T cells (Treg), natural killer (NK) cells, natural killer T (NKT) cells, and myeloid-derived suppressor cells (MDSCs).54 The cells can be involved in developing or inhibiting HCC development.55 The development of immunotherapy requires a comprehension of the heterogeneous microenvironment, regulation of cytokines at various stages of HCC, the functional activity of CTLs and NK cells, etc.54 The heterogeneity of HCC cells comes from distinctive gene expression and genetic variations that modify signaling pathways and protein function.49 In one study, it was found that intratumor heterogeneity was detectable in 87% of HCC cases. The same study also suggested that on the basis of tumor morphology alone, 26% of the cases were heterogenic.56
Various kinase inhibitors have been under development or reviewed for their clinical significance. Besides, it is important to develop a novel therapy averse to drug-resistant cancer stem cells. For subsequent clinical research design, it is obligatory to think about how to remove the confounding effects from genetic interpatient and intratumor heterogeneity. Targeting the heterogeneity of cancer cells/cancer stem cells, angiogenesis, invasion, and immune reactions might be a promising strategy for individual personal treatment options.
Aflatoxin
Aflatoxins are naturally prevailing subsidiary metabolites of the fungi Aspergillus flavus and Aspergillus parasiticus.57 It is known to be a food contaminant and a well-known human hepatocarcinogen which is a prime agent in the pathologic process of HCC.58 Aflatoxins may be present in a broad range of food items that include peanuts, meat, milk, oilseeds, corn, and dried fruits. There are numerous factors that influence the development of Aspergillus and the amount of aflatoxin contamination in foods. One of the factors that increase the susceptibility of plants to Aspergillus resulting in aflatoxin contamination is drought stress.58 Once consumed, AFB1 is metabolized to a functional transitional compound, AFB1-exo-8,9-epoxide, that can attach to DNA.59 AFB1 generates a mutation at serine 249 in the tumor suppressor p53 that was diagnosed in 30–60% of HCC samples collected from people in the aflatoxin-prevalent region, most of whom were suffering from HBV disease.60 Assays have been established to diagnose specific aflatoxin-related DNA mutations in tissues and to calculate metabolites in urine and AFB1–albumin adducts in serum.59
Metabolic syndrome
As compared to patients without metabolic syndrome, it is reported that those with metabolic syndrome such as obesity and diabetes have a higher incidence of HCC.1, 61
Obesity
A study of liver cancer manifestation indicated that a body mass index (BMI) ≥25 kg/m2 is implicated with a higher risk of developing primary liver cancer.62 The connection between liver cancer and BMI is independent of alcohol ingestion, geographic location, and diabetic history. Nevertheless, the link between BMI and HCC is much greater in individuals with HCV infection than in individuals with HBV infection.63 Obese males had a higher risk of primary liver cancer than obese females.64 Moreover, people with nonalcoholic steatohepatitis (NASH) often have metabolic syndromes such as obesity, insulin resistance, and hyperlipidemia. The widespread presence of obesity and the series of hepatic histological damage are considered to be related with NASH.65 However, obesity increases liver inflammation that leads to fibrosis and further progress toward NASH-related cirrhosis.65
Genetic factors
In addition to the well-established role of hepatitis virus infections and consumption of alcohol in the progression of HCC, various genetic aspects or syndromes also play a vital role.66 Family-based studies proposed that a genetic locus on a 4th chromosome, encoding the candidate 3′-phosphoadenosine 5′-phosphosulfate synthase 1 (PAPSS1) gene, can regulate the risk of HCC in HBV-positive individuals in the Chinese community.66 It has been established that the yearly prevalence of HCC is 4% in patients with hereditary hemochromatosis.67 Moreover, it appears that concomitance of these genetic conditions with known HCC risk components such as viral hepatitis and alcoholism would increase their oncogenic potential. Thus, patients with familial genetic disorders of the liver should be frequently counseled to elude toxic and environmental damage to the liver.67
Regulation of kinases in HCC
Two major kinase types are dysregulated in HCC, namely the tyrosine kinases (TKs) and cyclin-dependent kinases (CDKs). These groups of kinases play a crucial role in cell growth and metabolism and are emerging targets for the treatment of HCC.
Tyrosine kinases
TKs are phosphorylating enzymes that take part in various cellular pathways. In general, TKs play a vital role in regulating growth factor signaling. The activation of TKs results in increased tumor cell proliferation and growth, has antiapoptotic effects, and stimulates angiogenesis. Protein kinases are involved in commencing tumorigenesis when they are activated by somatic mutations. Tyrosine kinase inhibitors (TKIs), as their name entails, bind TKs or suppress adenosine 5′-triphosphate (ATP) binding to interfere with signal transduction.68, 69, 70
TKIs are an essential class of target-defined therapy for a diverse range of malignancies in addition to HCC. TKIs possess antitumor activity and share a common mechanism of action by competitively inhibiting ATP binding at the catalytic domain of various oncogenic TKs. Although the inhibitors such as sorafenib, sunitinib, regorafenib, lenvatinib, palbociclib, and abemacicilib vary by their pharmacokinetic effects, subject-defined harmful outcomes and diversity of targeted kinases are considered as important factors that define the effectiveness of TKIs.71 TKIs are categorized into three major categories. Nearly all the current TKIs are ATP-competitive inhibitors and are categorized as type I inhibitors. There are various problems that hinder the development of specific/particular TKIs of type 1 because of the immensely conservative ATP-binding sites in TK domains and an elevated rate of competition with intracellular ATP. Hence, TKIs might target other kinases, thus indicating that the antitumor effects are possible because of other signaling molecules. Moreover, TKIs that are non-ATP competitors are known as types II and III. These non-ATP competitors have conformation shifts causing structural changes in receptor TKs that result in the modification of TK domain in such a way that the TK domain reduces its kinase activity. In addition, these inhibitors can bind to the remaining part within the TK domain and impede tyrosine phosphorylation.72, 73 Numerous cellular functions that include differentiation, cell growth and survival, transduction of signals from membrane-bound tyrosine kinase receptors including vascular epidermal growth factor receptor (EGFR), insulin-like growth factor (IGFR), endothelial growth factor receptor (EFGR), and PDGFR to the cell nucleus via Ras/MAPK signaling pathway. Dysregulation of the Ras/MAPK pathway leads to improper cellular activities such as increased cell growth and differentiation, and eventually to cancer.
Cyclin-dependent kinases
A cell cycle is comprised of a series of interconnected biochemical pathways that are tightly regulated via checkpoints to ensure the passing of intact genetic material from one parent cell to the newly divided cells. Overall, a cell cycle has four phases, G1 or gap phase (cell growth), S or synthesis phase (DNA synthesis), G2 or second gap phase (prepares to divide), and M or mitosis phase (cell division). The cell cycle progression is driven by the centrally placed CDKs, which are serine/threonine protein kinases that phosphorylate key substrates to promote DNA synthesis and mitotic progression. CDKs are normally present in molar excess but are inactive until bound by their cognate cyclin subunits. These subunits are tightly regulated at both the levels of synthesis and ubiquitin-dependent proteolysis.74 CDKs are essential for the control of the cell cycle and to regulate apoptosis. Moreover, CDKs are found to be deregulated in most cancer cells. In HCC, there is an upregulation of CDKs via inactivation of CDK inhibitory proteins and through increasing levels of cyclins.75, 76, 77, 78, 79 Studies demonstrate that CDKs are considered to have various functions as they are included in glucose homeostasis, mRNA regulation, and nerve cells differentiation.79
The CDKs consist of a family of about 20 members, and among them, the CDK1–CDK6 have been related to control of cell cycle, development, and homeostasis. To form an activated complex, CDKs get associated with a cyclin. The activity of CDKs is closely regulated throughout the various phases by different mechanisms.80, 81, 82 CDK7 and CDK20 play a crucial role in transcription and cell cycle control. For therapeutic drugs, the suppression of CDKs via transcription and regulators of the cell cycle is regarded as a good strategy.83, 84, 85, 86, 87, 88, 89
Deregulation of CDK activity has also been detected in other cancers. Various studies have been performed to identify small molecules that target the CDKs, and numerous clinical trials have been conducted with pan-CDKIs.90
Current drugs for HCC
The development of HCC involves dysregulation of the cell cycle, apoptosis, and many other cellular pathways. Tumor cell progression involves mutations in various proteins responsible for the regulation of cell cycle.80 Hence, the recent advances in the treatment of HCC include molecules that target proteins such as CDKs or growth factors to suppress the tumor development.91
Currently approved drugs
Sorafenib
The first orally administered drug approved to target multiple kinases was Bayer's Nexavar (sorafenib), and it is currently the standard of care for systemic treatment of patients with advanced HCC who are not potential for curative options such as surgical resection, liver transplantation, and pharmaceutical interventions. Sorafenib inhibits PDGF-α and PDGF-β, VEGFR-1, VEGFR-2, and VEGFR-3, c-kit, and various proteins of the kinase cascade, Ras, C-Raf, B-Raf, ERK, as well as MAPK.91, 92, 93 Recent studies have shown that inhibition of this kinase cascade by sorafenib has the ability to correct abnormal glycosylation in HCC cells by a reduction in expression of the protein Ets-1, which provided a significant improvement in the overall survival (OS).94 In spite of the proven efficacy of sorafenib to significantly increase the OS in patients with advanced HCC, it was unable to stop the disease progression because of development of resistance to antiproliferative therapies.95, 96 It also failed to be an economical treatment method for patients with advanced HCC, and there is no ultimate treatment for second-line therapy in patients who fail to respond to sorafenib treatment.
Regorafenib
Regorafenib (Stivarga) developed by Bayer received approval in June 2017 as a second-line oral drug for unresectable HCC.97 It has similar targets and structure as sorafenib but is a more effective inhibitor of STAT3 signaling. It does so by inducing src homology 2 domain–containing phosphatase 1 (SHP1).98 In addition, it inhibits various oncogenic factors such as V600-mutated BRAF and Tie2.97 Sorafenib-refractory patients who received regorafenib as a second-line therapy were shown to provide a survival benefit. Furthermore, regorafenib has shown more potency in inhibiting TKs and phosphatases than sorafenib and a better drug tolerance profile in patients with HCC.99 In patients treated with regorafenib, the median survival was observed to be 10.6 months as compared to 7.8 months in placebo. As regorafenib offers this survival benefit in patients with HCC, it can be considered in combination with other drugs in patients where regorafenib cannot be tolerated in high doses or after sorafenib. At present, regorafenib is the standard second-line chemotherapy for patients refractory to sorafenib. However, only a few patients were eligible for regorafenib treatment due to their intolerance to sorafenib and deterioration of liver function.
Nivolumab
Human anti–programmed cell death protein 1 (PD1) (nivolumab) was approved by the FDA for advanced HCC after the molecular targeted therapy with sorafenib. Anti-PD1, with the brand name Opdivo, was developed by Bristol-Myers Squibb to prevent the immune tolerance in tumors. This human monoclonal antibody prevents the association of PD1 with its ligands, programmed death ligand1 (PD-L1) and 2 (PD-L2) as this binding otherwise leads to a poor T-cell response toward HCC tumors.100 Nivolumab is anticipated to be a promising immune checkpoint inhibitor to treat HCC with a good safety profile.101 New strategies such as combining sunitinib with other immunotherapeutic drugs such as anti-PD1 to inhibit tumor growth and to develop more economical combination therapies are currently in consideration. In this direction, Opdivo from Bristol-Myers Squibb Company is indicated for the treatment of patients with HCC. However, treatment with Opdivo resulted in some serious adverse reactions such as fatigue, abdominal pain, rash, cough, and decreased appetite in patients with HCC. Hence, Opdivo has been discontinued due to adverse reactions in 11% of patients, and 32% of patients had a dose delay due to an adverse reaction.101
Lenvatinib
Lenvatinib (Lenvima by Eisai) is more effective than sorafenib in targeting angiogenesis in hepatocarcinoma cell lines.102 Lenvatinib has also proven to be superior to sorafenib in increasing the OS in patients with HCC where tumors cannot be surgically removed. It targets VEGFA, VEGFC, fibroblast growth factor (FGF-1-4), and stem cell factor (SCF) to reduce angiogenesis and lymphoangiogenesis.102 Its higher potency than sorafenib in targeting FGF-1– and FGF-2–orchestrated angiogenesis makes it a promising candidate for HCC.102 Other targets of lenvatinib include VEGFR3, KIT, RET and PDGF receptor α.103 To overcome the problem of acquired resistance to lenvatinib, it is being tested in combination with golvatinib.104 The half inhibitory concentrations of different inhibitors on tumor cell lines, as published in the literature, are listed in table 1.
Table 1.
IC50 Values of Drug in Different HCC Cell Lines.
| Drugs for hepatocellular carcinoma | HCC cell lines | IC50 |
|---|---|---|
| Sorafenib105, 106, 107, 108, 109 | HepG2 MHCC97H HepG2.215 |
8–9.9 μM 12–31 μM 5–7 μM |
| Sunitinib110, 111 | HepG2 | 32–49 μM |
| Lenvatinib112 | HepG2 | 0.25 μM |
| Regorafenib113 | HepG2 Hep3B |
1 μM 5 μM |
HCC, hepatocellular carcinoma.
Cabozantinib
Cabozantinib (Cabometyx by Exelixis), an orally bioavailable multikinase inhibitor targeting MET, RET, VEGFR, and AXL, was approved by the European Commission in patients with HCC who have been previously treated with sorafenib. The approval is based on the results from a phase III CELESTIAL trial that showed significant improvement in OS in patients with advanced HCC. It has also been approved by the FDA for the treatment of advanced renal cell carcinoma and medullary thyroid cancer. This dual VEGFR/MET blockade is important as the therapies that are resistant to VEGFR are considered to be arising from the upregulation of proangiogenic pathways including the MET pathway.114 Moreover, results from a subgroup analysis of patients with advanced HCC who received sorafenib showed that the median OS was 11.3 months versus 7.2 months for placebo and median progressive-free survival (PFS) was 5.5 months versus 1.9 months with placebo. However, the noted adverse effects were fatigue (12.4%), diarrhea (17%), and palmar-plantar erythrodysesthesia (8.7%).115
Pembrolizumab
Pembrolizumab (Keytruda) developed by Merck is a recombinant humanized IgG4 monoclonal antibody against PD1, which was approved by the FDA for patients with advanced HCC pretreated with sorafenib. The FDA approval was based on findings from a phase II KEYNOTE-224 trial of 104 patients with pretreated liver cancer who received pembrolizumab. It has also been approved by the FDA in 2014 to treat patients with advanced melanoma and non–small-cell lung cancer who do not respond to other treatments. The most common treatment-related adverse events are fatigue, increased aspartate aminotransferase (AST) and alanine aminotransferase (ALT), and hypothyroidism. Results from phase II KEYNOTE-224 trial showed a median PFS of 4.9 months and OS of 12.9 months.116 A list of currently approved drugs by the FDA for the treatment of HCC is given in table 2.
Table 2.
Currently Approved Drugs for HCC.
| Drugs | Developed by | Target | Therapeutic line | Trial | Approved for HCC (Year) |
|---|---|---|---|---|---|
| Sorafenib (Nexavar) | Bayer and Onyx | Multitargeted TKI | 1 | SHARP | 2007 in the US |
| Regorafenib (Stivarga) | Bayer | Multitargeted TKI | 2 | RESORCE | 2017 in the US |
| Nivolumab (Opdivo) | Bristol-Myers Squibb | PD1 immune checkpoint inhibitor | 2 | CheckMate-040 | 2017 in the US |
| Lenvatinib (Lenvima) | Eisai Co. | Multitargeted TKI | 1 | REFLECT | 2018 in the US |
| Cabozantinib (Cabometyx) | Exelixis Inc. | Multitargeted TKI | 2 | CELESTIAL | 2018 in Europe |
| Pembrolizumab (Keytruda) | Merck | PD1 immune checkpoint inhibitor | 2 | KEYNOTE-224 | 2018 in the US |
HCC, hepatocellular carcinoma; PD1, programmed cell death protein 1; TKI, tyrosine kinase inhibitor.
Combination therapy
Treating patients with HCC has become very challenging because of the heterogeneity in the patient population and the variation in the risk factors involved in each patient. Moreover, many molecular pathways are involved in the development of drug resistance in HCC cells. Therefore, understanding these molecular mechanisms and combining drugs with other molecular or immune therapies to overcome these drawbacks has become an area of utmost importance.117 Many studies were conducted to understand the characteristics of sorafenib-resistant HCC cells and to determine the mechanisms responsible for acquired resistance to sorafenib. It was observed that the resistant HCC cells highly expressed activated STAT3, JAK1, JAK2, AKT, and p85.95 Recently, miRNAs highly expressed in HCC have been found to be good therapeutic targets to overcome resistance to sorafenib. One of the strategies was to suppress miR-222/221 as it induces sorafenib resistance via activation of the PI3K/AKT pathway.118 Moreover, miR-181a plays a major role in inducing sorafenib resistance by inhibiting the protein RASSF1.119 It has also been shown that downregulation of proapoptotic factors such as BMF, BIM, PUMA and PTEN by miR-221 promotes drug resistance and tumor survival.120 Interestingly, miR-122 targets the IGF-1 and can be used in combination with sorafenib to overcome the problem of acquired drug resistance.121 In some in vivo studies, when sorafenib was combined with vinorelbine, there was no increase in the toxicity levels or any reduction in the efficacy, but it was accompanied by slowing down the tumor growth compared with the individual monotherapies.122 In phase III clinical studies, comparing sorafenib to placebo in differentiated thyroid cancer, the median PFS time was 10.8 months in the sorafenib group compared to 5.8 months in the placebo group.123 Studies are going on for sorafenib in combination with transarterial chemoembolization (TACE) for the treatment of HCC as chemoembolization triggers are associated with metastasis and the spread of cancer cells around the body. Hence, using sorafenib with the antiangiogenic mechanism of action could potentiate the effects of TACE.110 In another phase II study, the efficacy of sorafenib–gemcitabine was evaluated in patients with advanced HCC, and it was considered to be an unsafe therapy as gemcitabine-related thrombocytopenia was followed by sorafenib-related hand-foot skin reaction and anorexia was observed in patients with this combination therapy.124 Because of these findings, there is an urgent need in clinical trials for sorafenib to be used in combination with different anticancer drug candidates.
Tiziana Lifesciences has recently licensed out a new form of CDK inhibitor called as Milciclib. Milciclib maleate is in phase II clinical development for the treatment of patients with cancer and is identified as a potent CDK2 inhibitor and has activity toward closely related CDKs, that is CDK1, CDK4, and CDK5 and tropomyosin receptor kinase A (TRKA). The postulated mechanism of action of the compound, as determined in biochemical assays, is that it blocks the G1 phase of the cell cycle. Milciclib has shown to lower tumor growth through downregulation of miR-221 and miR-222 in an animal model of HCC. Phase I clinical studies of Milciclib have shown improvement in patients with advanced solid tumors of colon, thymus, and pancreas. Therefore, Milciclib can be a potential candidate alone or in combination for the treatment of patients with HCC. In combination studies, Milciclib exhibited synergistic or more than additive activity when administered with gemcitabine.125 In clinical phase II, this combination regimen has shown a promising advantage in about 36% of patients which also includes gemcitabine-resistant patients.125 Also, Milciclib (a CDK inhibitor) has shown to reduce the expression of miR-221 and miR-222 in many in vitro models and can be tested in combination with sorafenib to eliminate the problem of miRNA-induced sorafenib resistance in HCC tumors, as described in Figure 1. Hence, Milciclib seems to be a promising candidate for combination therapies in patients with cancer.
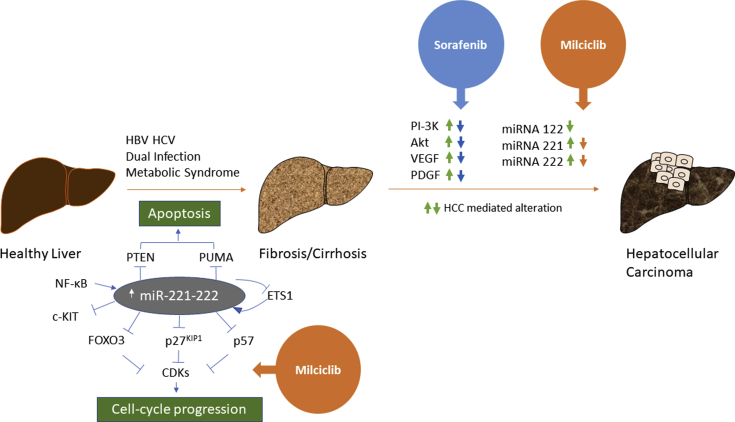
Figure 1.
Schematic representation of combination therapy for HCC. The figure shows the factors that are responsible for the progression from a healthy liver to fibrosis and eventually hepatocellular carcinoma. Individuals with HBV and HCV infection and metabolic syndromes such as obesity or diabetes have a higher incidence of HCC. The most important players in HCC progression are miRNA 221 and 222 that inhibit major tumor suppressors such as PTEN, CDKN1B/p27kip, CDKN1C/p57kip, and PUMA. PTEN and PUMA induce apoptosis, whereas p27 and p57 keep a check on the CDKs which otherwise lead to uncontrolled cell cycle progression. The TKI sorafenib targets PI3K, AKT, VEGF, and PDGF but is unable to inhibit the miRNAs which lead to sorafenib resistance in patients with HCC. Hence, we need a drug that can overcome sorafenib resistance, and this can be achieved by combining sorafenib with milciclib as it downregulates miRNA 221 and 222. This combination therapy will improve the OS as it will take care of a multitude of antiapoptotic and carcinogenic factors. HCC, hepatocellular carcinoma; OS, overall survival; HBV, hepatitis B virus; HCV, hepatitis C virus; TKI, tyrosine kinase inhibitor.
To overcome the problem of acquired resistance to lenvatinib, it is being tested in combination with golvatinib.104 Golvatinib (developed by Eisai) is an inhibitor of c-MET, vegfr-2, c-KIT, RON, Tie2, and EphB4. EphB4 and angiopoietin 2–activated Tie2 are responsible for acquired resistance against lenvatinib, and hence, lenvatinib was combined with golvatinib in preclinical studies to produce more effective results in HCC.104 Moreover, regorafenib is being tested in combination with erlotinib, which inhibits platelet-derived EGFR signaling to increase its potency to inhibit proliferation of HCC cell lines. This combination was proposed as platelets have the ability to reverse the antitumor effects of regorafenib and sorafenib in vitro.126
Pfizer's palbociclib (PD0332991, ibrance) is a specific CDK4/6 inhibitor that induces the tumor suppressor AMP-activated protein kinase (AMPK) to downregulate the expression of protein phosphatase 5. This mechanism promotes apoptosis and autophagy in HCC cells.127 Palbociclib arrests the cell cycle irreversibly by inhibiting phosphorylation of retinoblastoma (RB1) by CDK4/6, and the treatment also results in accumulation of cyclin D1. RB1 needs to be in its intact form for palbociclib to induce senescence of cancer cells in patients with HCC, and a mutation in the gene encoding this protein can lead to the development of resistance to this drug.128 Palbociclib is being considered in a combination regimen with other drugs such as sorafenib to reduce adverse events and increase the OS in patients with HCC.128 The schematic representation of combination therapy is displayed in Figure 1.
Owing to the late diagnosis and patient heterogeneity involved in HCC, therapies such as neoadjuvant or locoregional therapies have been widely used for tumor growth inhibition in patients with HCC. These therapies refer to a variety of treatment including systemic chemotherapy, TACE, radiofrequency ablation (RFA), hepatic resection, and transarterial radioembolization (TARE) that decrease the risk of dropout and improve the OS of patients with HCC.129, 130 However, various studies to examine the role of adjuvant or neoadjuvant or locoregional therapy alone in HCC are still under active investigation, but none of them have provided strong evidence in patients with HCC. Hence, treatment strategies continue to make progress to obtain better survival in patients with HCC. In this direction, the combination of molecular targeted therapies with locoregional therapies may have a beneficial effect on patient prognosis. Other than having promising results with TACE, sorafenib is also being evaluated in the neoadjuvant setting to prevent tumor progression and the risk of dropout of patients undergoing surgical resection and transplant. Another clinical trial of nivolumab in combination with ipilimumab as neoadjuvant therapy is ongoing beginning from April 2018. Hence, future trials will able to address the synergistic effect of combining molecular targeted therapies with neoadjuvant or locoregional therapy in patients with intermediate-HCC.
Drug candidates in the pipeline
Multiple companies and academic institutions have drugs under development for the treatment of metastatic HCC. Tiziana Lifesciences plc. has obtained Milciclib as a potent CKD2 inhibitor that belongs to the pyrazolo (4,3-h) quinazoline chemical class. It shows activity also toward closely related CDKs such as CDK1, CDK4, and CDK5 and TRKA. This profile has advantages over other CDKs as it may have the potential for synergistic inhibition. Milciclib possesses an unusual kinase inhibitory profile, as well as potent activity against a limited number of other kinases, such as members of the Src tyrosine kinase and splicing kinase families. It is in phase II clinical development as an oral anticancer treatment for patients with advanced hepatic malignancies. Also, Milciclib was found to be safe in patients with thymic cancers and thymoma.
Eli Lilly and company has galunisertib under phase II clinical trials for the treatment of HCC. It is a small molecule inhibitor that blocks TGF-β signaling.131, 132, 133 Galunisertib may hinder cancer-initiating stem cells and thus prevent TGF-β–dependent tumor cell growth and migration.134, 135, 136
Sunitinib (Pfizer's Sutent), such as sorafenib, is an orally administered TKI but failed to be superior to sorafenib in clinical trials because of severe adverse events.93, 96 It targets tumor angiogenesis via VEGFR-1, VEGFR-2, VEGFR-3, PDGFR-α, PDGFR-β, c-KIT, FMS-like tyrosine kinase 3, and other kinases.93 Failure of sunitinib to treat advanced HCC may be contributed to its inability to reach the target micrometastases and islands of small tumors supported by the surrounding liver cells.137
Ipafricept is a potent antagonist of Wnt signaling, developed by OncoMed Pharmaceuticals and is currently under phase I clinical trials. Wnt signaling is implicated in strong antitumor activity in hepatocellular, breast, colorectal, and other cancers.138 Ipafricept is a recombinant fusion protein that includes extracellular ligand-binding domain of the human frizzled (FZD) 8 receptor and a human IgG1 Fc fragment.139 It promotes differentiation, inhibits metastatic growth, and reduces cancer stem cell frequency. Ipafricept works either as a single agent or in combination with other approved drugs in patient-derived cancer xenograft models.139 Bristol-Myers Squibb has developed Brivanib for the treatment of hepatocellular carcinoma, and currently, it is under phase III clinical trials. It is a selective inhibitor of fibroblast growth factor receptor and VEGFR and a type of antiangiogenesis agent.140
Ramucirumab (Cyramza, Lilly) is a recombinant IgG1 monoclonal antibody that antagonizes the VEGFR-2 activation and inhibits endothelial proliferation and migration. Recent reports suggested a survival benefit in patients with elevated alpha-fetoprotein (AFP) > 400 ng/ml at diagnosis and who progressed or were intolerant to sorafenib.141 In a phase III clinical study comparing ramucirumab to placebo in advanced HCC, the objective response rate (ORR) of 9.5%, a median PFS of 4.0 months, and a median OS of 12.0 months were reported.142 The most common adverse events were hypertension, hyponatremia, peripheral edema, and headache. It is in an ongoing phase III REACH-2 trial as a single agent in the second-line treatment (post-sorafenib) for patients with unresectable HCC and gastric cancer with elevated alpha-fetoprotein (AFP) levels. A list of future drugs in development for the treatment of HCC is given in table 3.
Table 3.
Future Drugs for HCC.
| Drugs | Developed by | Target | Phase |
|---|---|---|---|
| Milciclib (PHA-848125) | Tiziana LifeSciences | CDK2/TRKA | II |
| Palbociclib (Ibrance) | Pfizer | CDK4/CDK6 | II |
| Galunisertib (LY2157299) | Eli Lilly | TGF-beta | II |
| Ipafricept (OMP-54F28) | OncoMed | Wnt signaling | I |
| Ipilimumab (Yervoy) | Bristol-Myers Squibb | CTLA-4 checkpoint inhibitor | II |
| Ramucirumab (Cyramza) | Eli Lilly | Anti-VEGF | III |
TGF, transforming growth factor; CDK, cyclin-dependent kinase; TRKA, tropomyosin receptor kinase A; HCC, hepatocellular carcinoma.
Prospects for future research
Molecular studies of HCC have determined abnormal activation of different signaling pathways, which illustrate key targets for novel molecular therapies. Other agents such as linifanib, ramucirumab, bevacizumab, axitinib, cediranib, dovitinib, vandetanib, oratinib, nintedanib etc. have demonstrated potential results in clinical phase 1-2 trials, but further studies are required to indicate their efficacy. Overall, combination therapies that would provide a synergistic effect and reduce drug toxicity are new directions for the upcoming treatments of HCC.
Conclusion
In conclusion, genetic alteration leads to hepatocarcinogenesis that affects multiple signaling cascades and results in uncontrolled growth of the hepatocytes. There are systemic targeted therapies that focus on the critical steps of the carcinogenic pathways but limits in the widespread systemic toxicity. Hence, drugs such as Milciclib seem to be a promising candidate for combination therapies in patients with cancer. On account of the heterogeneity of HCC, proper combinative targeted therapy may improve the prognosis of advanced HCC. Therefore, combination therapy is an encouraging treatment modality for HCC.
Conflicts of interest
All authors have none to declare.
References
- 1.Mittal S., El-Serag H.B. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol. 2013;47(suppl l):S2–S6. doi: 10.1097/MCG.0b013e3182872f29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ryerson A.B. Annual Report to the Nation on the Status of Cancer, 1975-2012, featuring the increasing incidence of liver cancer. Cancer. 2016;122(9):1312–1337. doi: 10.1002/cncr.29936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGlynn K.A., London W.T. The global epidemiology of hepatocellular carcinoma: present and future. Clin Liver Dis. 2011;15(2):223–243. doi: 10.1016/j.cld.2011.03.006. [vii-x] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanyal A.J., Yoon S.K., Lencioni R. The etiology of hepatocellular carcinoma and consequences for treatment. Oncol. 2010;15(suppl 4):14–22. doi: 10.1634/theoncologist.2010-S4-14. [DOI] [PubMed] [Google Scholar]
- 5.Zamor P.J., deLemos A.S., Russo M.W. Viral hepatitis and hepatocellular carcinoma: etiology and management. J Gastrointest Oncol. 2017;8(2):229–242. doi: 10.21037/jgo.2017.03.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghouri Y.A., Mian I., Rowe J.H. Review of hepatocellular carcinoma: epidemiology, etiology, and carcinogenesis. J Carcinog. 2017;16:1. doi: 10.4103/jcar.JCar_9_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bataller R., Brenner D.A. Liver fibrosis. J Clin Invest. 2005;115(2):209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Bisceglie A.M. Hepatitis B and hepatocellular carcinoma. Hepatology. 2009;49(5 suppl l):S56–S60. doi: 10.1002/hep.22962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu S. Associations between hepatitis B virus mutations and the risk of hepatocellular carcinoma: a meta-analysis. J Natl Cancer Inst. 2009;101(15):1066–1082. doi: 10.1093/jnci/djp180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang Z. The effects of hepatitis B virus integration into the genomes of hepatocellular carcinoma patients. Genome Res. 2012;22(4):593–601. doi: 10.1101/gr.133926.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gowans E.J. Patterns of single- and double-stranded hepatitis B virus DNA and viral antigen accumulation in infected liver cells. J Gen Virol. 1983;64(Pt 6):1229–1239. doi: 10.1099/0022-1317-64-6-1229. [DOI] [PubMed] [Google Scholar]
- 12.Brown A., Goodman Z. Hepatitis B-associated fibrosis and fibrosis/cirrhosis regression with nucleoside and nucleotide analogs. Expert Rev Gastroenterol Hepatol. 2012;6(2):187–198. doi: 10.1586/egh.12.4. [DOI] [PubMed] [Google Scholar]
- 13.Arora G., Keeffe E.B. Chronic hepatitis B with advanced fibrosis or cirrhosis: impact of antiviral therapy. Rev Gastroenterol Disord. 2007;7(2):63–73. [PubMed] [Google Scholar]
- 14.Fung J. Prevalence of fibrosis and cirrhosis in chronic hepatitis B: implications for treatment and management. Am J Gastroenterol. 2008;103(6):1421–1426. doi: 10.1111/j.1572-0241.2007.01751.x. [DOI] [PubMed] [Google Scholar]
- 15.McGaughan G.W., Shackel N.A., Gorrell M.D. Discussion on differential gene expression between chronic hepatitis B and C hepatic lesion. Gastroenterology. 2001;121(5):1263–1264. doi: 10.1053/gast.2001.29470. [DOI] [PubMed] [Google Scholar]
- 16.Neuveut C., Wei Y., Buendia M.A. Mechanisms of HBV-related hepatocarcinogenesis. J Hepatol. 2010;52(4):594–604. doi: 10.1016/j.jhep.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 17.Ayub A., Ashfaq U.A., Haque A. HBV induced HCC: major risk factors from genetic to molecular level. BioMed Res Int. 2013;2013:810461. doi: 10.1155/2013/810461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang X., Zhang H., Ye L. Effects of hepatitis B virus X protein on the development of liver cancer. J Lab Clin Med. 2006;147(2):58–66. doi: 10.1016/j.lab.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Hwang G.Y. Detection of the hepatitis B virus X protein (HBx) antigen and anti-HBx antibodies in cases of human hepatocellular carcinoma. J Clin Microbiol. 2003;41(12):5598–5603. doi: 10.1128/JCM.41.12.5598-5603.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forgues M. Interaction of the hepatitis B virus X protein with the Crm1-dependent nuclear export pathway. J Biol Chem. 2001;276(25):22797–22803. doi: 10.1074/jbc.M101259200. [DOI] [PubMed] [Google Scholar]
- 21.Weil R. Direct association and nuclear import of the hepatitis B virus X protein with the NF-kappaB inhibitor IkappaBalpha. Mol Cell Biol. 1999;19(9):6345–6354. doi: 10.1128/mcb.19.9.6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sirma H. Cytosol is the prime compartment of hepatitis B virus X protein where it colocalizes with the proteasome. Oncogene. 1998;16(16):2051–2063. doi: 10.1038/sj.onc.1201737. [DOI] [PubMed] [Google Scholar]
- 23.Choudhari S.R. Deactivation of Akt and STAT3 signaling promotes apoptosis, inhibits proliferation, and enhances the sensitivity of hepatocellular carcinoma cells to an anticancer agent. Atiprimod. Mol Cancer Ther. 2007;6(1):112–121. doi: 10.1158/1535-7163.MCT-06-0561. [DOI] [PubMed] [Google Scholar]
- 24.de Oliveria Andrade L.J. Association between hepatitis C and hepatocellular carcinoma. J Global Infect Dis. 2009;1(1):33–37. doi: 10.4103/0974-777X.52979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El-Serag H.B., Kanwal F. Epidemiology of hepatocellular carcinoma in the United States: where are we? Where do we go? Hepatology. 2014;60(5):1767–1775. doi: 10.1002/hep.27222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goossens N., Hoshida Y. Hepatitis C virus-induced hepatocellular carcinoma. Clin Mol Hepatol. 2015;21(2):105–114. doi: 10.3350/cmh.2015.21.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanda T., Yokosuka O., Omata M. Hepatitis C virus and hepatocellular carcinoma. Biology. 2013;2(1):304–316. doi: 10.3390/biology2010304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vescovo T. Molecular mechanisms of hepatitis C virus-induced hepatocellular carcinoma. Clin Microbiol Infect. 2016;22(10):853–861. doi: 10.1016/j.cmi.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 29.Mitchell J.K., Lemon S.M., McGivern D.R. How do persistent infections with hepatitis C virus cause liver cancer? Curr Opin Virol. 2015;14:101–108. doi: 10.1016/j.coviro.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heim M.H., Thimme R. Innate and adaptive immune responses in HCV infections. J Hepatol. 2014;61(1 suppl l):S14–S25. doi: 10.1016/j.jhep.2014.06.035. [DOI] [PubMed] [Google Scholar]
- 31.Arzumanyan A., Reis H.M., Feitelson M.A. Pathogenic mechanisms in HBV- and HCV-associated hepatocellular carcinoma. Nat Rev Canc. 2013;13(2):123–135. doi: 10.1038/nrc3449. [DOI] [PubMed] [Google Scholar]
- 32.Bartosch B. Hepatitis C virus-induced hepatocarcinogenesis. J Hepatol. 2009;51(4):810–820. doi: 10.1016/j.jhep.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 33.Perz J.F. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45(4):529–538. doi: 10.1016/j.jhep.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 34.Raimondo G. Statements from the Taormina expert meeting on occult hepatitis B virus infection. J Hepatol. 2008;49(4):652–657. doi: 10.1016/j.jhep.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 35.Cacciola I. Occult hepatitis B virus infection in patients with chronic hepatitis C liver disease. N Engl J Med. 1999;341(1):22–26. doi: 10.1056/NEJM199907013410104. [DOI] [PubMed] [Google Scholar]
- 36.Torbenson M., Thomas D.L. Occult hepatitis B. Lancet Infect Dis. 2002;2(8):479–486. doi: 10.1016/s1473-3099(02)00345-6. [DOI] [PubMed] [Google Scholar]
- 37.Donato F., Boffetta P., Puoti M. A meta-analysis of epidemiological studies on the combined effect of hepatitis B and C virus infections in causing hepatocellular carcinoma. Int J Canc. 1998;75(3):347–354. doi: 10.1002/(sici)1097-0215(19980130)75:3<347::aid-ijc4>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 38.Cho L.Y. Coinfection of hepatitis B and C viruses and risk of hepatocellular carcinoma: systematic review and meta-analysis. Int J Canc. 2011;128(1):176–184. doi: 10.1002/ijc.25321. [DOI] [PubMed] [Google Scholar]
- 39.European Association For The Study Of The L. EASL clinical practice guidelines: management of chronic hepatitis B virus infection. J Hepatol. 2012;57(1):167–185. doi: 10.1016/j.jhep.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 40.Huang Y.T. Lifetime risk and sex difference of hepatocellular carcinoma among patients with chronic hepatitis B and C. J Clin Oncol. 2011;29(27):3643–3650. doi: 10.1200/JCO.2011.36.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weltman M.D. Coinfection with hepatitis B and C or B, C and delta viruses results in severe chronic liver disease and responds poorly to interferon-alpha treatment. J Viral Hepat. 1995;2(1):39–45. doi: 10.1111/j.1365-2893.1995.tb00070.x. [DOI] [PubMed] [Google Scholar]
- 42.Thio C.L. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS) Lancet. 2002;360(9349):1921–1926. doi: 10.1016/s0140-6736(02)11913-1. [DOI] [PubMed] [Google Scholar]
- 43.Soriano V. Viral hepatitis and HIV co-infection. Antivir Res. 2010;85(1):303–315. doi: 10.1016/j.antiviral.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 44.Joshi D. Increasing burden of liver disease in patients with HIV infection. Lancet. 2011;377(9772):1198–1209. doi: 10.1016/S0140-6736(10)62001-6. [DOI] [PubMed] [Google Scholar]
- 45.Italian Association for the Study of the, L., T.D. Italian Society of Infectious. D. Italian Society for the Study of Sexually Transmitted Practice guidelines for the treatment of hepatitis C: recommendations from an AISF/SIMIT/SIMAST Expert Opinion Meeting. Dig Liver Dis. 2010;42(2):81–91. doi: 10.1016/j.dld.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 46.Ramaekers B.L., Joore M.A., Grutters J.P. How should we deal with patient heterogeneity in economic evaluation: a systematic review of national pharmacoeconomic guidelines. Value Health. 2013;16(5):855–862. doi: 10.1016/j.jval.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 47.Grutters J.P. Acknowledging patient heterogeneity in economic evaluation : a systematic literature review. Pharmacoeconomics. 2013;31(2):111–123. doi: 10.1007/s40273-012-0015-4. [DOI] [PubMed] [Google Scholar]
- 48.Baeriswyl V., Christofori G. The angiogenic switch in carcinogenesis. Semin Canc Biol. 2009;19(5):329–337. doi: 10.1016/j.semcancer.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 49.Bedard P.L. Tumour heterogeneity in the clinic. Nature. 2013;501(7467):355–364. doi: 10.1038/nature12627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu X.Z., Xie G.R., Chen D. Hypoxia and hepatocellular carcinoma: the therapeutic target for hepatocellular carcinoma. J Gastroenterol Hepatol. 2007;22(8):1178–1182. doi: 10.1111/j.1440-1746.2007.04997.x. [DOI] [PubMed] [Google Scholar]
- 51.Chiang I.T. Sorafenib inhibits TPA-induced MMP-9 and VEGF expression via suppression of ERK/NF-kappaB pathway in hepatocellular carcinoma cells. In Vivo. 2012;26(4):671–681. [PubMed] [Google Scholar]
- 52.Jia X.Q. Inhibition of connective tissue growth factor overexpression decreases growth of hepatocellular carcinoma cells in vitro and in vivo. Chin Med J (Engl) 2011;124(22):3794–3799. [PubMed] [Google Scholar]
- 53.Riener M.O. Expression of the extracellular matrix protein periostin in liver tumours and bile duct carcinomas. Histopathology. 2010;56(5):600–606. doi: 10.1111/j.1365-2559.2010.03527.x. [DOI] [PubMed] [Google Scholar]
- 54.Junttila M.R., de Sauvage F.J. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature. 2013;501(7467):346–354. doi: 10.1038/nature12626. [DOI] [PubMed] [Google Scholar]
- 55.Jenne C.N., Kubes P. Immune surveillance by the liver. Nat Immunol. 2013;14(10):996–1006. doi: 10.1038/ni.2691. [DOI] [PubMed] [Google Scholar]
- 56.Friemel J. Intratumor heterogeneity in hepatocellular carcinoma. Clin Canc Res. 2015;21(8):1951–1961. doi: 10.1158/1078-0432.CCR-14-0122. [DOI] [PubMed] [Google Scholar]
- 57.Kew M.C. Aflatoxins as a cause of hepatocellular carcinoma. J Gastrointest Liver Dis. 2013;22(3):305–310. [PubMed] [Google Scholar]
- 58.Magnussen A., Parsi M.A. Aflatoxins, hepatocellular carcinoma and public health. World J Gastroenterol. 2013;19(10):1508–1512. doi: 10.3748/wjg.v19.i10.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.El-Serag H.B. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142(6):1264–1273 e1. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shen H.M., Ong C.N. Mutations of the p53 tumor suppressor gene and ras oncogenes in aflatoxin hepatocarcinogenesis. Mutat Res. 1996;366(1):23–44. doi: 10.1016/s0165-1110(96)90005-6. [DOI] [PubMed] [Google Scholar]
- 61.Regimbeau J.M. Obesity and diabetes as a risk factor for hepatocellular carcinoma. Liver Transplant. 2004;10(2 suppl 1):S69–S73. doi: 10.1002/lt.20033. [DOI] [PubMed] [Google Scholar]
- 62.Rui R. Excess body mass index and risk of liver cancer: a nonlinear dose-response meta-analysis of prospective studies. PLoS One. 2012;7(9):e44522. doi: 10.1371/journal.pone.0044522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hashimoto M. Influence of higher BMI for hepatitis B- and C-related hepatocellular carcinomas. Langenbeck's Arch Surg. 2017;402(5):745–755. doi: 10.1007/s00423-017-1589-2. [DOI] [PubMed] [Google Scholar]
- 64.Nordenstedt H., White D.L., El-Serag H.B. The changing pattern of epidemiology in hepatocellular carcinoma. Dig Liver Dis. 2010;42(suppl 3):S206–S214. doi: 10.1016/S1590-8658(10)60507-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wong R.J., Ahmed A. Obesity and non-alcoholic fatty liver disease: disparate associations among Asian populations. World J Hepatol. 2014;6(5):263–273. doi: 10.4254/wjh.v6.i5.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dragani T.A. Risk of HCC: genetic heterogeneity and complex genetics. J Hepatol. 2010;52(2):252–257. doi: 10.1016/j.jhep.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 67.Villanueva A., Newell P., Hoshida Y. Inherited hepatocellular carcinoma. Best Pract Res Clin Gastroenterol. 2010;24(5):725–734. doi: 10.1016/j.bpg.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 68.Kim S., Abou-Alfa G.K. The role of tyrosine kinase inhibitors in hepatocellular carcinoma. Clin Adv Hematol Oncol. 2014;12(1):36–41. [PubMed] [Google Scholar]
- 69.Llovet J.M. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 70.Arora A., Scholar E.M. Role of tyrosine kinase inhibitors in cancer therapy. J Pharmacol Exp Therapeut. 2005;315(3):971–979. doi: 10.1124/jpet.105.084145. [DOI] [PubMed] [Google Scholar]
- 71.Hartmann J.T. Tyrosine kinase inhibitors - a review on pharmacology, metabolism and side effects. Curr Drug Metabol. 2009;10(5):470–481. doi: 10.2174/138920009788897975. [DOI] [PubMed] [Google Scholar]
- 72.Hojjat-Farsangi M. Small-molecule inhibitors of the receptor tyrosine kinases: promising tools for targeted cancer therapies. Int J Mol Sci. 2014;15(8):13768–13801. doi: 10.3390/ijms150813768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Garuti L., Roberti M., Bottegoni G. Non-ATP competitive protein kinase inhibitors. Curr Med Chem. 2010;17(25):2804–2821. doi: 10.2174/092986710791859333. [DOI] [PubMed] [Google Scholar]
- 74.Malumbres M. Cyclin-dependent kinases. Genome Biol. 2014;15(6):122. doi: 10.1186/gb4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Haider C. Novel inhibitors of cyclin-dependent kinases combat hepatocellular carcinoma without inducing chemoresistance. Mol Canc Therapeut. 2013;12(10):1947–1957. doi: 10.1158/1535-7163.MCT-13-0263. [DOI] [PubMed] [Google Scholar]
- 76.Bloom J., Cross F.R. Multiple levels of cyclin specificity in cell-cycle control. Nat Rev Mol Cell Biol. 2007;8(2):149–160. doi: 10.1038/nrm2105. [DOI] [PubMed] [Google Scholar]
- 77.Rossi A.G. Cyclin-dependent kinase inhibitors enhance the resolution of inflammation by promoting inflammatory cell apoptosis. Nat Med. 2006;12(9):1056–1064. doi: 10.1038/nm1468. [DOI] [PubMed] [Google Scholar]
- 78.Fornari F. MiR-221 controls CDKN1C/p57 and CDKN1B/p27 expression in human hepatocellular carcinoma. Oncogene. 2008;27(43):5651–5661. doi: 10.1038/onc.2008.178. [DOI] [PubMed] [Google Scholar]
- 79.Senderowicz A.M. Targeting cell cycle and apoptosis for the treatment of human malignancies. Curr Opin Cell Biol. 2004;16(6):670–678. doi: 10.1016/j.ceb.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 80.Bisteau X., Caldez M.J., Kaldis P. The complex relationship between liver cancer and the cell cycle: a story of multiple regulations. Cancers. 2014;6(1):79–111. doi: 10.3390/cancers6010079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Malumbres M. Cyclin-dependent kinases: a family portrait. Nat Cell Biol. 2009;11(11):1275–1276. doi: 10.1038/ncb1109-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gopinathan L., Ratnacaram C.K., Kaldis P. Established and novel Cdk/cyclin complexes regulating the cell cycle and development. Results Probl Cell Differ. 2011;53:365–389. doi: 10.1007/978-3-642-19065-0_16. [DOI] [PubMed] [Google Scholar]
- 83.Paiva C. Cyclin-dependent kinase inhibitor P1446A induces apoptosis in a JNK/p38 MAPK-dependent manner in chronic lymphocytic Leukemia B-cells. PLoS One. 2015;10(11):e0143685. doi: 10.1371/journal.pone.0143685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lam F. Targeting RNA transcription and translation in ovarian cancer cells with pharmacological inhibitor CDKI-73. Oncotarget. 2014;5(17):7691–7704. doi: 10.18632/oncotarget.2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Albert T.K. Characterization of molecular and cellular functions of the cyclin-dependent kinase CDK9 using a novel specific inhibitor. Br J Pharmacol. 2014;171(1):55–68. doi: 10.1111/bph.12408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kitagawa M. Cell cycle regulation by long non-coding RNAs. Cell Mol Life Sci. 2013;70(24):4785–4794. doi: 10.1007/s00018-013-1423-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu X. CDKI-71, a novel CDK9 inhibitor, is preferentially cytotoxic to cancer cells compared to flavopiridol. Int J Canc. 2012;130(5):1216–1226. doi: 10.1002/ijc.26127. [DOI] [PubMed] [Google Scholar]
- 88.Liu X. In vitro antitumor mechanism of a novel cyclin-dependent kinase inhibitor CDKI-83. Invest N Drugs. 2012;30(3):889–897. doi: 10.1007/s10637-011-9641-5. [DOI] [PubMed] [Google Scholar]
- 89.Johnson N. Pre-clinical evaluation of cyclin-dependent kinase 2 and 1 inhibition in anti-estrogen-sensitive and resistant breast cancer cells. Br J Canc. 2010;102(2):342–350. doi: 10.1038/sj.bjc.6605479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Albanese C. Dual targeting of CDK and tropomyosin receptor kinase families by the oral inhibitor PHA-848125, an agent with broad-spectrum antitumor efficacy. Mol Canc Therapeut. 2010;9(8):2243–2254. doi: 10.1158/1535-7163.MCT-10-0190. [DOI] [PubMed] [Google Scholar]
- 91.Adnane L. Sorafenib (BAY 43-9006, Nexavar), a dual-action inhibitor that targets RAF/MEK/ERK pathway in tumor cells and tyrosine kinases VEGFR/PDGFR in tumor vasculature. Methods Enzymol. 2006;407:597–612. doi: 10.1016/S0076-6879(05)07047-3. [DOI] [PubMed] [Google Scholar]
- 92.Keating G.M., Santoro A. Sorafenib: a review of its use in advanced hepatocellular carcinoma. Drugs. 2009;69(2):223–240. doi: 10.2165/00003495-200969020-00006. [DOI] [PubMed] [Google Scholar]
- 93.Zhu A.X. Development of sunitinib in hepatocellular carcinoma: rationale, early clinical experience, and correlative studies. Cancer J. 2009;15(4):263–268. doi: 10.1097/PPO.0b013e3181af5e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu T. Sorafenib induced alteration of protein glycosylation in hepatocellular carcinoma cells. Oncol Lett. 2017;14(1):517–524. doi: 10.3892/ol.2017.6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhai B., Sun X.Y. Mechanisms of resistance to sorafenib and the corresponding strategies in hepatocellular carcinoma. World J Hepatol. 2013;5(7):345–352. doi: 10.4254/wjh.v5.i7.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cheng A.L. Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J Clin Oncol. 2013;31(32):4067–4075. doi: 10.1200/JCO.2012.45.8372. [DOI] [PubMed] [Google Scholar]
- 97.Bruix J. Regorafenib as second-line therapy for intermediate or advanced hepatocellular carcinoma: multicentre, open-label, phase II safety study. Eur J Cancer. 2013;49(16):3412–3419. doi: 10.1016/j.ejca.2013.05.028. [DOI] [PubMed] [Google Scholar]
- 98.Tai W.T. STAT3 mediates regorafenib-induced apoptosis in hepatocellular carcinoma. Clin Canc Res. 2014;20(22):5768–5776. doi: 10.1158/1078-0432.CCR-14-0725. [DOI] [PubMed] [Google Scholar]
- 99.Bruix J. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 100.Kudo M. Immune checkpoint blockade in hepatocellular carcinoma: 2017 update. Liver Cancer. 2016;6(1):1–12. doi: 10.1159/000449342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.El-Khoueiry A.B. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yamamoto Y. Lenvatinib, an angiogenesis inhibitor targeting VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft models associated with microvessel density and pericyte coverage. Vasc Cell. 2014;6:18. doi: 10.1186/2045-824X-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ikeda K. Phase 2 study of lenvatinib in patients with advanced hepatocellular carcinoma. J Gastroenterol. 2017;52(4):512–519. doi: 10.1007/s00535-016-1263-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nakazawa Y. Multitargeting strategy using lenvatinib and golvatinib: maximizing anti-angiogenesis activity in a preclinical cancer model. Cancer Sci. 2015;106(2):201–207. doi: 10.1111/cas.12581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rangwala F. Differential effects of arsenic trioxide on chemosensitization in human hepatic tumor and stellate cell lines. BMC Canc. 2012;12:402. doi: 10.1186/1471-2407-12-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Morisaki T. Combining celecoxib with sorafenib synergistically inhibits hepatocellular carcinoma cells in vitro. Anticancer Res. 2013;33(4):1387–1395. [PubMed] [Google Scholar]
- 107.Deng L. Schedule-dependent antitumor effects of 5-fluorouracil combined with sorafenib in hepatocellular carcinoma. BMC Canc. 2013;13:363. doi: 10.1186/1471-2407-13-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen W. Activation of c-Jun predicts a poor response to sorafenib in hepatocellular carcinoma: preliminary Clinical Evidence. Sci Rep. 2016;6:22976. doi: 10.1038/srep22976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.He S. Study of RNA interference targeting NET-1 combination with sorafenib for hepatocellular carcinoma therapy in vitro and in vivo. Gastroenterol Res Pract. 2013;2013:685150. doi: 10.1155/2013/685150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pascale F. Comparative chemosensitivity of VX2 and HCC cell lines to drugs used in TACE. Anticancer Res. 2015;35(12):6497–6503. [PubMed] [Google Scholar]
- 111.Zhang J. Synthesis and antitumor activity of 5-Bromo-7-azaindolin-2-one derivatives containing a 2,4-Dimethyl-1H-pyrrole-3-carboxamide moiety. Molecules. 2016;21(12) doi: 10.3390/molecules21121674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Martins P. Heterocyclic anticancer compounds: recent advances and the paradigm shift towards the use of nanomedicine's tool box. Molecules. 2015;20(9):16852–16891. doi: 10.3390/molecules200916852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Carr B.I. Fluoro-Sorafenib (Regorafenib) effects on hepatoma cells: growth inhibition, quiescence, and recovery. J Cell Physiol. 2013;228(2):292–297. doi: 10.1002/jcp.24148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cochin V. [Cabozantinib: mechanism of action, efficacy and indications] Bull Cancer. 2017;104(5):393–401. doi: 10.1016/j.bulcan.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 115.Schoffski P. Phase II randomised discontinuation trial of cabozantinib in patients with advanced solid tumours. Eur J Cancer. 2017;86:296–304. doi: 10.1016/j.ejca.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 116.Zhu A.X. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19(7):940–952. doi: 10.1016/S1470-2045(18)30351-6. [DOI] [PubMed] [Google Scholar]
- 117.Yang J., Yan L., Wang W. Current status of multimodal & combination therapy for hepatocellular carcinoma. Indian J Med Res. 2012;136(3):391–403. [PMC free article] [PubMed] [Google Scholar]
- 118.Tang S. An artificial lncRNA targeting multiple miRNAs overcomes sorafenib resistance in hepatocellular carcinoma cells. Oncotarget. 2016;7(45):73257–73269. doi: 10.18632/oncotarget.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Azumi J. miR-181a induces sorafenib resistance of hepatocellular carcinoma cells through downregulation of RASSF1 expression. Cancer Sci. 2016;107(9):1256–1262. doi: 10.1111/cas.13006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fornari F. In hepatocellular carcinoma miR-221 modulates sorafenib resistance through inhibition of Caspase-3-mediated apoptosis. Clin Canc Res. 2017;23(14):3953–3965. doi: 10.1158/1078-0432.CCR-16-1464. [DOI] [PubMed] [Google Scholar]
- 121.Basu S., Bhattacharyya S.N. Insulin-like growth factor-1 prevents miR-122 production in neighbouring cells to curtail its intercellular transfer to ensure proliferation of human hepatoma cells. Nucleic Acids Res. 2014;42(11):7170–7185. doi: 10.1093/nar/gku346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ferrario C. Phase I/II trial of sorafenib in combination with vinorelbine as first-line chemotherapy for metastatic breast cancer. PLoS One. 2016;11(12):e0167906. doi: 10.1371/journal.pone.0167906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chu Y.D. A novel thyroid function index associated with opposite therapeutic outcomes in advanced hepatocellular carcinoma patients receiving chemotherapy or sorafenib. Asia Pac J Clin Oncol. 2018;14(5):e341–e351. doi: 10.1111/ajco.12983. [DOI] [PubMed] [Google Scholar]
- 124.Naqi N. Efficacy and safety of sorafenib-gemcitabine combination therapy in advanced hepatocellular carcinoma: an open-label Phase II feasibility study. Hematol Oncol Stem Cell Ther. 2014;7(1):27–31. doi: 10.1016/j.hemonc.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 125.Aspeslagh S. Phase I dose-escalation study of milciclib in combination with gemcitabine in patients with refractory solid tumors. Cancer Chemother Pharmacol. 2017;79(6):1257–1265. doi: 10.1007/s00280-017-3303-z. [DOI] [PubMed] [Google Scholar]
- 126.D'Alessandro R. Modulation of Regorafenib effects on HCC cell lines by epidermal growth factor. Cancer Chemother Pharmacol. 2015;75(6):1237–1245. doi: 10.1007/s00280-015-2751-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hsieh F.S. Palbociclib induces activation of AMPK and inhibits hepatocellular carcinoma in a CDK4/6-independent manner. Mol Oncol. 2017;11(8):1035–1049. doi: 10.1002/1878-0261.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bollard J. Palbociclib (PD-0332991), a selective CDK4/6 inhibitor, restricts tumour growth in preclinical models of hepatocellular carcinoma. Gut. 2017;66(7):1286–1296. doi: 10.1136/gutjnl-2016-312268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Eggert T., Greten T.F. Current standard and future perspectives in non-surgical therapy for hepatocellular carcinoma. Digestion. 2017;96(1):1–4. doi: 10.1159/000464282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gabr A. Outcomes of surgical resection after radioembolization for hepatocellular carcinoma. J Vasc Intervent Radiol. 2018;29(11):1502–1510 e1. doi: 10.1016/j.jvir.2018.06.027. [DOI] [PubMed] [Google Scholar]
- 131.Herbertz S. Clinical development of galunisertib (LY2157299 monohydrate), a small molecule inhibitor of transforming growth factor-beta signaling pathway. Drug Des Dev Ther. 2015;9:4479–4499. doi: 10.2147/DDDT.S86621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rodon J. Pharmacokinetic, pharmacodynamic and biomarker evaluation of transforming growth factor-beta receptor I kinase inhibitor, galunisertib, in phase 1 study in patients with advanced cancer. Invest N Drugs. 2015;33(2):357–370. doi: 10.1007/s10637-014-0192-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Maier A. Anti-tumor activity of the TGF-beta receptor kinase inhibitor galunisertib (LY2157299 monohydrate) in patient-derived tumor xenografts. Cell Oncol. 2015;38(2):131–144. doi: 10.1007/s13402-014-0210-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Anido J. TGF-beta receptor inhibitors target the CD44(high)/Id1(high) glioma-initiating cell population in human glioblastoma. Cancer Cell. 2010;18(6):655–668. doi: 10.1016/j.ccr.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 135.Penuelas S. TGF-beta increases glioma-initiating cell self-renewal through the induction of LIF in human glioblastoma. Cancer Cell. 2009;15(4):315–327. doi: 10.1016/j.ccr.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 136.Hardee M.E. Resistance of glioblastoma-initiating cells to radiation mediated by the tumor microenvironment can be abolished by inhibiting transforming growth factor-beta. Cancer Res. 2012;72(16):4119–4129. doi: 10.1158/0008-5472.CAN-12-0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bagi C.M., Gebhard D.F., Andresen C.J. Antitumor effect of vascular endothelial growth factor inhibitor sunitinib in preclinical models of hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2012;24(5):563–574. doi: 10.1097/MEG.0b013e328350916f. [DOI] [PubMed] [Google Scholar]
- 138.Le P.N., McDermott J.D., Jimeno A. Targeting the Wnt pathway in human cancers: therapeutic targeting with a focus on OMP-54F28. Pharmacol Ther. 2015;146:1–11. doi: 10.1016/j.pharmthera.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jimeno A. A first-in-human Phase 1 study of the anti-cancer stem cell agent ipafricept (OMP-54F28), a decoy receptor for Wnt ligands, in patients with advanced solid tumors. Clin Canc Res. 2017;23(24):7490–7497. doi: 10.1158/1078-0432.CCR-17-2157. [DOI] [PubMed] [Google Scholar]
- 140.Huynh H. Brivanib alaninate, a dual inhibitor of vascular endothelial growth factor receptor and fibroblast growth factor receptor tyrosine kinases, induces growth inhibition in mouse models of human hepatocellular carcinoma. Clin Canc Res. 2008;14(19):6146–6153. doi: 10.1158/1078-0432.CCR-08-0509. [DOI] [PubMed] [Google Scholar]
- 141.Kudo M. Ramucirumab as second-line treatment in patients with advanced hepatocellular carcinoma: Japanese subgroup analysis of the REACH trial. J Gastroenterol. 2017;52(4):494–503. doi: 10.1007/s00535-016-1247-4. [DOI] [PubMed] [Google Scholar]
- 142.Zhu A.X. A phase II and biomarker study of ramucirumab, a human monoclonal antibody targeting the VEGF receptor-2, as first-line monotherapy in patients with advanced hepatocellular cancer. Clin Canc Res. 2013;19(23):6614–6623. doi: 10.1158/1078-0432.CCR-13-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]