Abstract
Background/aims
Alcoholic hepatitis (AH) is an acute hepatic inflammation associated with high morbidity and mortality. Treatment with steroids is known to decrease short-term mortality in severe AH patients. Hence, we hypothesize that adrenal insufficiency can be associated with severe AH and affects prognosis. The aim of this study was (1) to evaluate relative adrenal insufficiency (RAI) in patients with AH and (2) to Compare RAI with the severity of AH.
Methods
Newly diagnosed cases of AH hospitalized in SMS Medical College and Hospital, Department of Gastroenterology were, enrolled. All patients of AH were classified as mild and severe AH on the basis of Maddrey discriminant function (DF). After baseline serum cortisol, 25 IU ACTH (Adreno Corticotrophic Hormone) was injected intramuscularly and blood sample was collected after 1 h and assessed for serum cortisol. RAI was defined as <7 μg increase in the cortisol level from baseline. RAI was compared with severity of AH.
Results
Of 120 patients of AH, 58 patients fulfilled the inclusion criteria, in which 48 patients were diagnosed as severe AH and 10 patients were diagnosed as mild AH. In patients with severe AH, the baseline mean serum cortisol level was significantly high as compared with mild AH; 26 patients (54.16 %) of 48 patients with severe AH showed RAI (P ≤ 0.001).Whereas in patients with mild AH, none of patients showed RAI. RAI also showed negative correlation with DF. There was no difference in RAI with respect to acute kidney injury (AKI).
Conclusion
RAI is a common entity in patients with severe AH, and it is related with the severity of disease.
Keywords: relative adrenal insufficiency, alcoholic hepatitis, CIRCI (Critical Illness Related Corticosteroid Insufficiency)
Abbreviations: ACTH, Adreno Corticotrophic Hormone; AH, Alcoholic Hepatitis; AI, Adrenal Insuffiency; AKI, Acute Kidney Injury; ALD, Alcoholic Liver Disease; ALT, Alanine Aminotranferase; AST, Aspartate Aminotransferase; CIRCI, Critical Illness Related Corticosteroid Insufficiency; DF, Discriminant Function; HPA, Hypothalamic Pituitary Adrenal; INR, International Normalised Ratio; MELD, Model for End-stage Liver Disease; PT, Prothrombin Time; RAI, Relative Adrenal Insuffiency; TLC, Total Leucocyte Count
Alcohol-related disease is the third most common cause of morbidity.1 Alcoholic liver disease (ALD) represents a spectrum of conditions ranging from reversible fatty liver to alcoholic hepatitis (AH) and cirrhosis. AH is characterized by rapid onset of jaundice along with fever and/or ascites. The prevalence of AH is 20% among the hospitalized alcoholic patients.2, 3, 4 About 50% patients of AH have underlying cirrhosis. These patients generally have poor prognosis and are associated with 28-day mortality as high as 50% in severe cases.5
Adrenal dysfunction/adrenal insufficiency (AI) in cirrhosis, also described as hepatoadrenal syndrome, is a well-recognized entity. AI is defined as an inadequate glucocorticoid activity with respect to the severity of illness in a patient with liver disease.6, 7, 8 This is also termed as critical illness–related corticosteroid insufficiency (CIRCI) and occurs because of either reduced adrenal steroid production or tissue resistance to glucocorticoids in patients with systemic inflammation.9 AI is frequent in compensated (31–60%)10, 11, 12, 13 and decompensated cirrhosis (26%–64%).14, 15.This hypoadrenalism is transient and is not caused by a structural lesion but could increase the risk of circulatory failure, infections, and further decompensation. It substantially increases the risk of death during acute illness as an increase of the cortisol level in acute illness is an important protective response.16, 17
Severe AH is associated with an increased level of endotoxin and proinflammatory mediators, which is quite similar to that observed in sepsis. Steroids (produced by adequate adrenal function) are associated with improved short-term mortality in patients with severe AH.18, 19 Hence, we hypothesize that AI is associated with severe AH and affects its prognosis. The aim and objective of this study were to assess the prevalence of relative AI in patients with AH and to see its correlation with the severity of AH.
Material and methods
This study was conducted in SMS Medical College and Hospital, Department of Gastroenterology over a period of 1 year (March 2016–March 2017). Newly diagnosed cases of AH who were hospitalized in our ward were enrolled after written informed consent. Diagnosis of AH was made by the clinical criteria that included resent onset (<3 months) of jaundice (bilirubin > 3 mg/dl) associated with the intake of heavy alcohol <3 week before jaundice, elevated AST up to 500 IU/L, aspartate aminotransferase (AST) to alanine aminotransferase (ALT) ratio >2 and international normalised ratio (INR) >1.5.20
All patients underwent routine investigations and were classified as mild or severe hepatitis on the basis of Maddrey discriminant function (DF). A baseline 8 AM sample for estimation of the serum cortisol was collected and relative adrenal insufficiency (RAI) was assessed. Afterwards, an injection of ACTH (Acton Prolongatum—Ferring Pharmaceuticals) 25 IU was injected intramuscularly, and blood sample was again collected after 1 h for estimation of the serum cortisol. The serum cortisol level was assessed via Architect chemiluminescent microparticle immunoassay method. Delta cortisol was defined as difference between the baseline and post-ACTH cortisol level. RAI was defined as delta cortisol < 7 μgm/dl. RAI was compared with the severity of AH by using Maddrey DF (4.6× patients' prothrombin time−control prothrombin time) + serum bilirubin). The study was approved by the institutional ethical committee.
Patients were excluded on the basis of the following exclusion criteria: (1) patients who had obvious evidence of infection (such as on chest X-ray, blood culture, urine examination), (2) patients with positive serology of hepatitis B/C, (3) patients with hemodynamic instability (mean arterial blood pressure <80 mmHg), (4) The current or recent history of corticosteroid use in the past 3 months, (5) high baseline serum cortisol level (>33 μgm/dl), and (6) patients who did not give consent.
Statistical analysis
The quantitative data were analyzed by using Z-test, and qualitative data were analyzed by using chi-square test. Correlation between the two data was measured by a scatter plot graph.
Results
We studied totally 120 newly diagnosed patients of AH. On the basis of the exclusion criteria, 62 patients were excluded. Among 58 patients included, 10 were diagnosed as having mild AH and 48 were diagnosed as severe AH. Basic parameters of all the patients are given in Table 1. All the patients were men.
Table 1.
Characteristics of the Study Population According to Maddrey Discriminant function (DF).
| Parameters | Mean ± SD |
P value | Significance | |
|---|---|---|---|---|
| DF < 32 (n = 10) | DF ≥ 32 (n = 48) | |||
| Age (years) | 43.10 ± 8.02 | 37.35 ± 9.80 | <0.001 | HS |
| Hb (gm/dl) | 8.89 ± 2.11 | 9.22 ± 2.40 | >0.05 | NS |
| TLC (cumm) | 9119.00 ± 3583.15 | 17237.08 ± 10335.81 | >0.05 | NS |
| Platelets (lakh/ml) | 1.30 ± 0.81 | 1.38 ± 0.76 | >0.05 | NS |
| PT (sec) | 16.20 ± 1.54 | 28.32 ± 11.48 | <0.001 | HS |
| INR (%) | 1.28 ± 0.17 | 2.54 ± 1.01 | <0.001 | HS |
| Protein (gm/dl) | 6.47 ± 0.66 | 6.14 ± 0.90 | >0.05 | NS |
| Albumin (gm/dl) | 2.84 ± 0.44 | 2.72 ± 0.48 | >0.05 | NS |
| Globulin (gm/dl) | 3.53 ± 0.62 | 3.43 ± 0.88 | >0.05 | NS |
| Bilirubin (mg/dl) | 8.75 ± 7.31 | 18.83 ± 9.11 | <0.001 | HS |
| Direct B (mg/dl) | 4.34 ± 4.45 | 8.87 ± 4.40 | <0.001 | HS |
| AST (U/L) | 136.50 ± 63.26 | 157.31 ± 78.19 | >0.05 | NS |
| ALT (U/L) | 57.20 ± 45.25 | 55.17 ± 35.29 | >0.05 | NS |
| Urea (mg/dl) | 36.62 ± 12.43 | 70.53 ± 63.40 | <0.001 | HS |
| Creatinine (mg/dl) | 0.95 ± 0.50 | 2.14 ± 1.87 | <0.001 | HS |
| DF | 21.31 ± 6.25 | 87.71 ± 55.91 | <0.001 | HS |
| Cortisol1 (microgram/dl) | 11.87 ± 3.73 | 17.06 ± 5.99 | <0.001 | HS |
| Cortisol2 (microgram/dl) | 31.94 ± 6.07 | 25.44 ± 10.69 | <0.001 | HS |
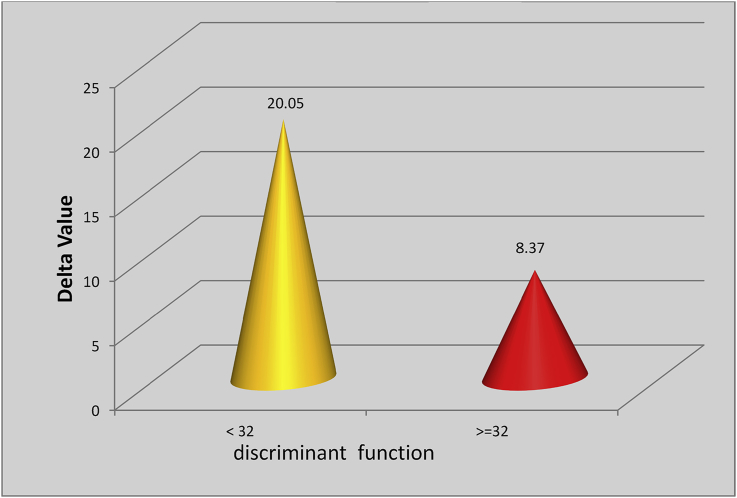
| Delta value | 20.05 ± 4.69 | 8.37 ± 7.23 | <0.001 | HS |
SD, Standard Deviation; HS, Highly Significant; NS, Not Significant; TLC, Total Leucocyte Count.
P value < 0.05 significant; P value > 0.05 not significant.
Some baseline parameters were significantly different among mild and severe AH group (age [37.35 ± 9.80 years V/S 43.10 ± 8.02 years], DF [87.71 ± 55.91 V/S 21.31 ± 6.25]), while other parameters including hemoglobin, total leukocyte count,platelet counts, total serum protein, serum albumin, serum globulin, AST and ALT were not showing any significant difference (P > 0.05).
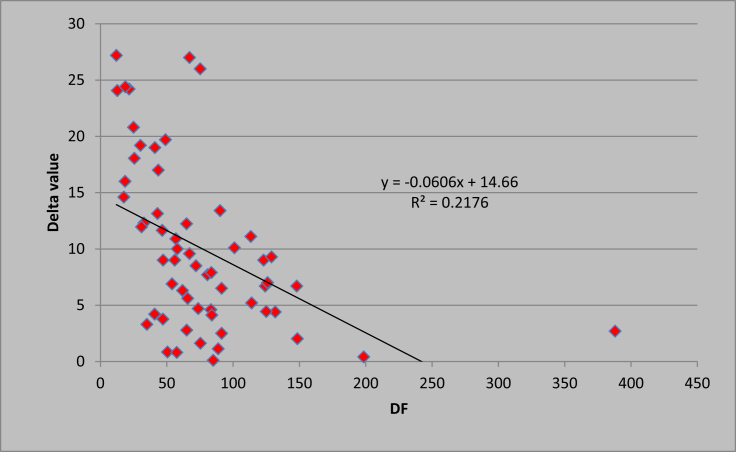
Patients with mild AH had a baseline mean serum cortisol level of 11.87 ± 3.73 μgm/dl and had a post-ACTH mean cortisol level of 31.94 ± 6.07 μgm/dl. Patients with severe AH had a baseline mean serum cortisol level of 17.06 ± 5.99 μgm/dl and post-ACTH mean cortisol level of 25.44 ± 10.69 μgm/dl (P < 0.001). None of the patients with mild AH had an evidence of RAI, while 26/48 patients (54.16%) with severe AH had RAI (P < 0.001). In patients with mild AH, the mean delta cortisol level was 20.05 ± 4.69 μgm/dl, and in patients with severe AH, the mean delta serum cortisol was 8.37 ± 7.23 μgm/dl (P < 0.001) Figure 1). Baseline clinical parameters in the two groups were compared with respect to the presence or absence of RAI. There was a statistically significant difference in prothrobin time (PT)/INR and DF. The difference in other variables was not clinically significant (Table 2). There was a significant negative correlation (Figure 2) of delta cortisol, while correlating with DF as r −0.466 (P < 0.05). When RAI was compared according to renal function, there was no significant correlation between RAI and acute kidney injury (Table 3).
Figure 1.
Graph showing the mean value of delta cortisol according to DF (P < 0.001 [HS]). DF, Discriminant Function; HS, Highly Significant.
Table 2.
Characteristics of the Study Population According to RAI in Severe Alcoholic Hepatitis.
| Parameters | Mean ± SD |
P value | Significance | |
|---|---|---|---|---|
| RAI present (n = 26) | No RAI (n = 22) | |||
| Age (years) | 35.61 ± 8.11 | 39.23 ± 10.90 | >0.05 | NS |
| Hb (gm/dl) | 8.92 ± 2.34 | 9.41 ± 2.37 | >0.05 | NS |
| TLC (cumm) | 18241.54 ± 8402.03 | 16448.18 ± 12120.89 | >0.05 | NS |
| Platelets (lakh/ml) | 1.38 ± 0.65 | 1.53 ± 1.12 | >0.05 | NS |
| PT (sec) | 31.10 ± 14.20 | 24.75 ± 5.71 | <0.05 | Sig |
| INR (%) | 2.80 ± 1.17 | 2.21 ± 0.68 | <0.05 | Sig |
| Protein (gm/dl) | 6.11 ± 0.98 | 6.17 ± 0.79 | >0.05 | NS |
| Albumin (gm/dl) | 2.68 ± 0.58 | 2.77 ± 0.31 | >0.05 | NS |
| Globulin (gm/dl) | 3.42 ± 0.96 | 3.45 ± 0.78 | >0.05 | NS |
| Bilirubin (mg/dl) | 19.83 ± 9.70 | 18.30 ± 8.40 | >0.05 | NS |
| Direct B (mg/dl) | 8.87 ± 4.74 | 9.00 ± 4.05 | >0.05 | NS |
| AST (U/L) | 151.88 ± 69.94 | 162.77 ± 87.18 | >0.05 | NS |
| ALT (U/L) | 56.77 ± 28.86 | 51.20 ± 41.57 | >0.05 | NS |
| Urea (mg/dl) | 66.54 ± 63.95 | 72.34 ± 62.13 | >0.05 | NS |
| Creatinine (mg/dl) | 2.11 ± 1.81 | 2.21 ± 1.92 | >0.05 | NS |
| DF | 102.11 ± 68.88 | 70.07 ± 26.61 | <0.05 | Sig |
| MELD (unit) | 33.33 ± 9.42 | 31.16 ± 9.07 | >0.05 | NS |
SD, Standard Deviation; RAI, Relative Adrenal Insufficiency; NS, Not Significant; Sig, Significant; Model for End-stage Liver Disease(MELD); TLC, Total Leucocyte Count.
Figure 2.
Scatter plot graph showing the correlation between the discriminant factor (DF, X axis) and delta cortisol (Y axis), r −0.466 (P < 0.05). DF, Discriminant Function.
Table 3.
Correlation of RAI and AKI in Patients With Severe Alcoholic Hepatitis.
| Creatinine | RAI |
Total | |
|---|---|---|---|
| Present | Absent | ||
| ≤1.5 | 14 | 10 | 24 |
| >1.5 | 12 | 12 | 24 |
| Total | 26 | 22 | 48 |
RAI, Relative Adrenal Insufficiency; NS, Not Significant; AKI, Acute Kidney Injury.
X2 = 0.336, d.f. = 1, P > 0.05 (NS).
Discussion
AH is a frequent life-threatening cause of liver failure, particularly in its severe form. In our study, the mean age of presentation of AH was 38.5 years, which was younger than that documented in the previous studies.21 The mean age of patients with severe AH was less than that with mild AH. Our findings are consistent with previous studies, which showed that younger people are at higher risk to develop AH.22, 23 The reason behind this observation might be a genetic predisposition or binge drinking behavior of younger patients.
Among the factors influencing the outcome in patients of ALD, RAI was demonstrated to be associated with the severity of liver disease.24 A possible explanation of this could be an increased concentration of circulating cytokines, which may interfere with appropriate activation of the hypothalamic pituitary adrenal (HPA) axis.25
AI has been a well-known phenomenon in liver disease patients,26, 27 but it has only recently been addressed with concern when investigators found relatively insufficient response to adrenal gland stimulation12 and favorable response to addition of steroids in certain group of patients (e.g. sepsis).28
O' Beirne J et al reported AI in 33% patients with acute liver insufficiency, in 65% patients with chronic liver disease and sepsis, and in 92% patients who have undergone transplantation and received a steroid-free treatment protocol.29
Previously published studies have shown a very high prevalence of AI in cirrhosis patients with sepsis12, 20, 28, 30 and without sepsis.6, 10 AI correlates with severity of hepatic dysfunction and poor prognosis and has an inverse correlation between the peak cortisol response and severity of hepatic dysfunction.12
Till date, there are no data available on RAI in patients with AH. Steroid treatment in severe AH improves short-term survival.31 This effect could be due to the correction of RAI. In our study, 26/48 patients (54.16%) with severe AH had an evidence of RAI compared with none in mild AH group (P < 0.001). RAI depended on the severity of hepatic dysfunction as documented in the previous published data on adrenal dysfunction in hepatic diseases.12
Baseline serum cortisol level was higher in patients with severe AH compared with mild AH, which is suggestive of a state of stress in the severe AH group. In patients with severe illness, activation of the HPA axis is an important feature in host adaptation to acute illness. Activation of this axis initiated by the action of cytokines on the hypothalamus promotes the release of corticotrophin-releasing hormone, corticotrophin, which acts on the pituitary gland, in turn increasing cortisol secretion by the adrenal glands. During an acute illness, negative feedback of cortisol on corticotrophin-releasing hormone and corticotrophin is depressed, which keeps a sustained activation of the HPA axis. Consequently, in well-compensated adaptation response to optimize the effect, the cortisol level in the circulation and in the tissue is increased.32, 33
Normal plasma ACTH values do not rule out the mild secondary AI particularly in patients with critical illness.34 Therefore, dynamic testing is required to establish the diagnosis of RAI.35 Most appropriate form of stimulation test for critically ill patients with/without liver disease is measuring the peak and the change in cortisol concentration (delta cortisol). It is likely that patients with AH also come under the previously mentioned category, and hence, their adrenal function should be assessed according to the CIRCI criteria. The majority of these patients have an evidence of systemic inflammatory response syndrome.
We used an intramuscular ACTH test, and it has been found to be effective in evaluation of adrenal function in all suspected cases of the primary or secondary AI.36
This is the first study that has demonstrated RAI in patients with AH, and it provokes a new thought for treatment with steroid.
However, our study has a few limitations, such as we have included very few mild AH patients; steroid was not given to any severe AH patients; and it was a single-point cross-sectional study as no follow-up and mortality data were collected. So, the future study should be conducted with larger number and to see the effect of steroid on severe AH patients with RAI to assess the survival benefit.
Conclusion
RAI seems to be common in patients with liver failure including AH, irrespective of the presence of sepsis. RAI correlates with the severity of AH. However, further prospective, randomized clinical trials are necessary to assess RAI in AH and the effect of corticosteroid therapy on AH patients with RAI.
Conflicts of interest
The authors have none to declare.
References
- 1.WHO Status Report on Alcohol 2011. World Health Organization; 2011. [Google Scholar]
- 2.Christoffersen P., Nielsen K. Histological changes in human liver biopsies from chronic alcoholics. Acta Pathol Microbiol Scand A. 1972;80:557–565. doi: 10.1111/j.1699-0463.1972.tb00316.x. [DOI] [PubMed] [Google Scholar]
- 3.Mendenhall C.L. Alcoholic hepatitis. Clin Gastroenterol. 1981;10:417–441. [PubMed] [Google Scholar]
- 4.Trabut J.B., Plat A., Thepot V. Influence of liver biopsy on abstinence in alcohol-dependent patients. Alcohol. 2008;43:559–563. doi: 10.1093/alcalc/agn046. [DOI] [PubMed] [Google Scholar]
- 5.Dunn W., Jamil L.H., Brown L.S. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology. 2005;41:353–358. doi: 10.1002/hep.20503. [DOI] [PubMed] [Google Scholar]
- 6.Marik P.E., Gayowski T., Starzl T.E. The hepatoadrenal syndrome: a common yet unrecognized clinical condition. Crit Care Med. 2005;33:1254–1259. doi: 10.1097/01.ccm.0000164541.12106.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rothwell P.M., Udwadia Z.F., Lawler P.G. Cortisol response to corticotropin and survival in septic shock. Lancet. 1991;337:582–583. doi: 10.1016/0140-6736(91)91641-7. [DOI] [PubMed] [Google Scholar]
- 8.Cooper M.S., Stewart P.M. Corticosteroid insufficiency in acutely ill patients. N Engl J Med. 2003;348:727–734. doi: 10.1056/NEJMra020529. [DOI] [PubMed] [Google Scholar]
- 9.Marik P.E., Pastores S.M., Annane D., American College of Critical Care Medicine Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: consensus statements from an international task force by the American College of Critical Care Medicine. Crit Care Med. 2008;36:1937–1949. doi: 10.1097/CCM.0b013e31817603ba. [DOI] [PubMed] [Google Scholar]
- 10.G Fede, Spadaro L., Tomaselli T. Assessment of adrenocortical reserve in stable patients with cirrhosis. J Hepatol. 2011;54:243–250. doi: 10.1016/j.jhep.2010.06.034. [DOI] [PubMed] [Google Scholar]
- 11.Galbois A., Rudler M., Massard J. Assessment of adrenal function in cirrhotic patients: salivary cortisol should be preferred. J Hepatol. 2010;52:839–845. doi: 10.1016/j.jhep.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 12.McDonald J.A., Handelsman D.J., Dilworth P., Conway A.J., McCaughan G.W. Hypothalamic-pituitary adrenal function in end-stage non alcoholic liver disease. J Gastroenterol Hepatol. 1993:247–253. doi: 10.1111/j.1440-1746.1993.tb01195.x. [DOI] [PubMed] [Google Scholar]
- 13.Tan T., Chang L., Woodward A. Characterising adrenal function using directly measured plasma free cortisol in stable severe liver disease. J Hepatol. 2010;53:841–848. doi: 10.1016/j.jhep.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 14.Triantos C.K., Marzigie M., Fede G. Critical illness-related corticosteroid insufficiency in patients with cirrhosis and variceal bleeding. Clin Gastroenterol Hepatol. 2011;9:595–601. doi: 10.1016/j.cgh.2011.03.033. [DOI] [PubMed] [Google Scholar]
- 15.Risso A., Alessandria C., Elia C. Adrenal dysfunction in nonseptic cirrhotic patients with ascites: impact on survival [Abstract] Dig Liver Dis. 2011;43(suppl 2):S74–S75. [Google Scholar]
- 16.Annane D., Sébille V., Troché G., Raphaël J.C., Gajdos P., Bellissant E. A 3-level prognostic classification in septic shock based on cortisol levels and cortisol response to corticotropin. JAMA. 2000;283:1038–1045. doi: 10.1001/jama.283.8.1038. [DOI] [PubMed] [Google Scholar]
- 17.Aron D., Findling J., Tyrrell J. Glucocorticoids and adrenal androgens. In: Gardner D., Shoback D., editors. Greenspan's Basic & Clinical Endocrinology. 8th ed. McGraw-Hill; New York: 2007. pp. 356–363. [Google Scholar]
- 18.Carithers R.L., Jr., Herlong H.F., Diehl A.M. Methylprednisolone therapy in patients with severe alcoholic hepatitis. A randomized multicenter trial. Ann Intern Med. 1989;110:685–690. doi: 10.7326/0003-4819-110-9-685. [DOI] [PubMed] [Google Scholar]
- 19.MJ Ramond, Poynard T., Rueff B. A randomized trials of prednisolone in patients with severe alcoholic hepatitis. N Engl J Med. 1992;326:507–512. doi: 10.1056/NEJM199202203260802. [DOI] [PubMed] [Google Scholar]
- 20.Crabb D.W., Bataller R., Chalasani N.P. Standard definitions and common data elements for clinical trials in patients with alcoholic hepatitis: recommendation from the NIAAA Alcoholic Hepatitis Consortia. Gastroenterology. 2016;150:785–790. doi: 10.1053/j.gastro.2016.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Menon K.V., Gores G.J., Shah V.H. Pathogenesis, diagnosis, and treatment of alcoholic liver disease. Mayo Clin Proc. 2001;76:1021–1029. doi: 10.4065/76.10.1021. [DOI] [PubMed] [Google Scholar]
- 22.Singal A.K., Kamath P.S., Gores G.J., Shah V.H. Alcoholic hepatitis: current challenges and future directions. Clin Gastroenterol Hepatol. 2014;12:555–564. doi: 10.1016/j.cgh.2013.06.013. [PubMed: 23811249] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Becker U.1, Deis A., Sørensen T.I. Prediction of risk of liver disease by alcohol intake, sex, and age: a prospective population study. Hepatology. 1996;23:1025–1029. doi: 10.1002/hep.510230513. [PubMed: 8621128] [DOI] [PubMed] [Google Scholar]
- 24.Zietz B., Lock G., Plach B. Dysfunction of the hypothalamic-pituitary-glandular axes and relation to Child-Pugh classification in male patients with alcoholic and virus-related cirrhosis. Eur J Gastroenterol Hepatol. 2003;15(5):495–501. doi: 10.1097/01.meg.0000059115.41030.e0. [DOI] [PubMed] [Google Scholar]
- 25.Marik P.E., Zaloga G.P. Adrenal insufficiency in the critically ill: a new look at an old problem. Chest. 2002;122(5):1784–1796. doi: 10.1378/chest.122.5.1784. [DOI] [PubMed] [Google Scholar]
- 26.Trifan A., Chiriac S., Stanciu C. Update on adrenal insufficiency in patients with liver cirrhosis. World J Gastroenterol. 2013;19:445–456. doi: 10.3748/wjg.v19.i4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peterson R.E. Adrenocortical steroid metabolism and adrenal cortical function in liver disease. J Clin Invest. 1960;39:320–331. doi: 10.1172/JCI104043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernández J., Escorsell A., Zabalza M. Adrenal insufficiency in patients with cirrhosis and septic shock: effect of treatment with hydrocortisone on survival. Hepatology. 2006;44:1288–1295. doi: 10.1002/hep.21352. [DOI] [PubMed] [Google Scholar]
- 29.O'Beirne J., Holmes M., Agarwal B. Adrenal insufficiency in liver disease—what is the evidence? J Hepatol. 2007;47:418–423. doi: 10.1016/j.jhep.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 30.Tsai M.H., Peng Y.S., Chen Y.C. Adrenal insufficiency in patients with cirrhosis, severe sepsis and septic shock. Hepatology. 2006;43:673–681. doi: 10.1002/hep.21101. [DOI] [PubMed] [Google Scholar]
- 31.Thursz M.R., Richardson P., Allison M. Prednisolone or pentoxifylline for alcoholic hepatitis. STOPAH Trial N Engl J Med. 2015;372(17):1619. doi: 10.1056/NEJMoa1412278. [DOI] [PubMed] [Google Scholar]
- 32.Tsai M.H.1, Peng Y.S., Chen Y.C. Adrenal insufficiency in patients with cirrhosis, severe sepsis and septic shock. Hepatology. 2006;43:673–681. doi: 10.1002/hep.21101. [DOI] [PubMed] [Google Scholar]
- 33.Schuetz P., Muller B. The hypothalamic pituitary adrenal axis in critical illness. Endocrinol Metab Clin N Am. 2006;35:823–838. doi: 10.1016/j.ecl.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 34.Oelkers W. Adrenal insufficiency. N Engl J Med. 1996;335(16):1206–1212. doi: 10.1056/NEJM199610173351607. [DOI] [PubMed] [Google Scholar]
- 35.Parker K. 1st ed. Vol. 1. 2002. pp. 837–844. (Addison's disease (adrenal insufficiency). Oxford Textbook of Endocrinology and Diabetes). [Google Scholar]
- 36.Gundgurthi A., Garg M.K., Dutta M.K., Pakhetra R. Intramuscular ACTH stimulation test for assessment of adrenal function. J Assoc Phys India. 2013 May;61(5):320–324. [PubMed] [Google Scholar]