Abstract
Background/Aims
A/H1N1/09 influenza is associated with a high risk of complications in patients with chronic diseases. In view of patients with cirrhosis being recognized as another high-risk group for influenza morbidity and mortality, we report a cluster of suspected A/H1N1/09 infection in 110 patients admitted to a hepatology intensive care unit.
Methods
The pattern of spread, clinical outcome, and respiratory parameters of A/H1N1/09 of 22 positive cirrhotic patients were compared with those from a control group of 88 patients with chronic liver disease (CLD) with influenza-like pneumonia who tested negative for A/H1N1/09.
Results
A/H1N1/09 infection was confirmed in 22 (20%) patients. Eighteen of 22 (81.8%) CLD patients with A/H1N1/09 died of pneumonia and acute respiratory distress syndrome despite timely antiviral treatment. In contrast, only 35 (40%)of the control group of cirrhotic patients without A/H1N1/09 died. On univariate analysis, age > 45 years [OR 1.3; 95% CI 1.1–5.7, (P = 0.054)], encephalopathy > grade 2 [OR 5.4; 95% CI 2.8–12.3, (P = 0.042)], serum bilirubin >8 mg/dl [OR 2.1; 95% CI 1.8–12.3, (P = 0.052)], serum creatinine >1.8 mg/dl [OR 2.8; 95% CI 1.9–9.2, (P = 0.042)], PaO2/FiO2 ratio <200 [OR 4.5; 95% CI 3.1–18.5, (P = 0.026)] and INR > 2.5 [OR 2.2; 95% CI 1.8–6.7, (P = 0.032)] were risk factors for mortality at presentation. However, on multivariate analysis only PaO2/FiO2 ratio <200 and serum creatinine >1.8 mg/dl remained predictors of mortality. Secondary infections, whether fungal or bacterial, were noted to be independent risk factors for disease severity in patients with cirrhosis.
Conclusion
Early detection and referral, and early antiviral treatment with a strict control of nosocomial spread is essential in patients with cirrhosis during epidemic influenza.
Abbreviations: AKI, Acute Kidney Injury; APACHE II, Acute Physiologic Assessment and Chronic Health Evaluation II; ARDS, Acute Respiratory Distress Syndrome; CLD, Chronic Liver Disease; CTP, Child Turcotte Pugh Score; CXR, Chest Radiograph; ICU, Intensive Care Unit; ILI, Influenza Like Illness; INR, International Normalized Ratio; MELD, Model for End-Stage Liver Disease; NASH, Non Alcoholic Steatohepatitis; PCR, Polymerase Chain Reaction; SOFA, Sequential Organ Failure Score
Keywords: H1N1 influenza, critical care in liver disease, pneumonia, ventilatory support
A/H1N1/09 influenza and its mortality often occurs in individuals with underlying comorbidities. Advance age, chronic renal, hepatic and cardiopulmonary diseases, hematologic disturbances, metabolic diseases such as diabetes mellitus, immune defects, HIV infection, and pregnancy are the underlying problems leading to more frequent complications and high mortality in influenza-afflicted patients.1 In this study, we have tried to extrapolate our previous experience with A/H1N1/09 infection, to try and ascertain how these cases may be better managed.2 Obesity and diabetes mellitus are two of the most common risk factors for fatality in the new pandemic flu in those aged 20 years and older.13 In a recent series, the rate of obesity was 50% in hospitalized patients with the new H1N1 pandemic influenza (2009). In one-third of hospitalized patients, obesity was the only risk factor, which highlights again that immune mechanisms are involved in influenza mortality risks.14 The association of obesity with NASH, has brought obese patients under purview of hepatologists, especially if they have underlying steatohepatitis or cirrhosis and get severe acute bacterial or viral infections.
Patients and Methods
Between 1st February 2015 and 31st March 2016, 1086 patients with cirrhosis and respiratory complications were admitted to the medical ICU of the Institute of Liver and Biliary Sciences (ILBS), New Delhi. Of these patients, only 110 were suspected to have influenza like illness as they presented with sudden onset of respiratory symptoms including cough and dyspnea, minimal sputum production, hypoxia, and diffuse interstitial infiltrates on chest radiographs, and no other obvious cause of clinical deterioration. Written informed consent was taken from these subjects for participation in this observational study, and approval was taken from the Institutional Ethics Committee. Evaluation for the common causes of clinical deterioration included history of over the counter medication use, over-diuresis, alcohol intake, use of complementary and alternate medications, and serology for hepatotropic virus infections. At the time of ICU admission, Arterial Blood Gas Analysis (ABG), Chest Roentgenogram (CXR), Acute Physiology and Chronic Health Evaluation (APACHE-II score), Sequential Organ Failure Assessment (SOFA) and Clinical Pulmonary Infection Score (CPIS) were calculated. If the patient was on Mechanical Ventilation (MV), ABG, CPIS parameter, days of MV, length of stay in the ICU and outcome were documented. The study was cleared by the institutional Ethics Committee and informed consent was taken from the subjects prior to inclusion. Throat swabs were collected from all patients with acute respiratory distress, and laboratory diagnosis of influenza was made by Real-Time Reverse Transcriptase Polymerase Chain Reaction (rRT-PCR) as per CDC guidelines 2009. The samples were tested for A/H1N1/09 influenza A virus using universal primers and probes for influenza A as well as for influenza A subtype H1N1 2009 strain using specific primers and probes with Roche LightCycler 2.0 rRT-PCR system.3 All the samples were also tested for humanRNAse P gene to rule out false negativity. All patients were started on oseltamivir 150 mg twice daily within 24 h of admission into the ICU, along with broad-spectrum antibiotics covering the prevalent bacterial respiratory pathogens, as per our hospital antibiotic protocols.
Indications for Mechanical Ventilation
-
1.
Severe respiratory failure
-
2.
Failure to achieve oxygen saturation of >90% or >60 mmHg on an FIO2 <0.6.
Ventilator Settings
-
•
Pressure controlled ventilation
-
•
Low tidal volume ventilator support
-
•
Tidal volume – 6 ml/kg ideal body weight (to a maximum of 30–35 per minute)
-
•
Open lung strategy of ventilation with PEEP titration to keep the lung recruited to achieve an FIO2 of <0.5 and saturation approaching normal or PaO2 >60 mm Hg.
-
•
Plateau pressure measurement not to exceed of >30–35 mmHg.
-
•
Alternative modes of ventilation APRV (Airway pressure ventilation), IRV (Inverse Ratio ventilation) in patients with hypoxemia with persistent (SpO2 of <90% with high PEEP &FiO2 >0.8).
-
•
Rescue therapy – recruitment maneuvers, appropriate sedation, use of neuromuscular blockade and prone ventilation if the target oxygen goals are not attained.
In patients with atypical features, computed tomography was performed to better manage the cases. Sputum was induced in non-ventilated patients. For ventilated patients, a tracheal aspirate was sent every 2 days for microbiological cultures. Daily hematological and biochemical monitoring and microbiological surveillance cultures were done. Antibiotics were modified according to the culture results. Post-mortem liver and lung biopsies were performed in fatal cases.
Results
A/H1N1/09 infection was confirmed in 22 (20%) patients, of whom 18 (80%) were male. Eighteen (81.8%) of these patients with A/H1N1/09 infection and cirrhosis died of pneumonia and acute respiratory distress syndrome, with fungal or bacterial super-infection in two cases, despite antiviral treatment. In striking contrast, only 35/88 (40%) of the control group of cirrhotic patients without A/H1N1/09 died. All subjects presented with an influenza like illness (ILI) to the ICU, and received anti viral therapy pending the viral RT-PCR report. The underlying etiologies of liver disease were predominantly ethanol (76%) and Non-Alcoholic Steatohepatitis (NASH; 22%). In the H1N1 group (N = 22), the median age was 43 (25–56) years, median Child Pugh Score (CTP) 11 (9–15) and MELD 28 (18–40). The control group (N = 88) had a median age of 49 (38–55) years, median CTP 10 (9–14) and MELD 21 (14–34). In 8 patients with compensated cirrhosis with a median MELD score of 12 (range 9–16), who were on regular follow up, the H1N1 infection resulted in a percentage increase in bilirubin (45%), INR (55%) and creatinine (22%). This showed that infection with H1N1 alone led to rapid clinical deterioration in an otherwise stable patient, in absence of other precipitants of organ failure like variceal bleeding, spontaneous bacterial peritonitis, urosepsis, etc. However, 14 patients with H1N1 presented to us for the first time with respiratory compromise, and the role played by the severity of underlying liver disease as a predictor of outcomes cannot be ascertained. As demonstrated in Table 1, these patients had higher AST, ALT and bilirubin at presentation as compared with cirrhotic controls with influenza like illness. They also had a tendency to develop acute kidney injury with rapidly deteriorating creatinine often leading to volume overload, worsening ventilator requirements and electrolyte imbalances. This was reflected in the lower PaO2/FiO2 ratio in the H1N1 and control cohorts respectively (136 ± 51.3 vs. 221 ± 76.2; P = 0.002). The radiographic characteristics included bilateral chest infiltrates, pulmonary edema, and alveolar opacities, which were fluffy or nodular. In the setting of frequent superadded bacterial infections, these were by no means characteristic of viral pneumonia. Figure 1a–c shows progression of CXR findings in our index patient. We also report that these patients were at highest risk of developing Acute Respiratory Distress Syndrome (ARDS) and were difficult to ventilate (see Figure 2). In our series, asymmetric consolidation, alveolar hemorrhage and development of effusions were also noted. Thus atypical imaging does not exclude the possibility of H1 N1 infection (see Figure 3). Many subjects required non-invasive ventilation in the H1N1 group. They required high FiO2 settings in spite of relatively clear lung fields on CXR. We also utilized the use of Airway Pressure Release Ventilation (APRV) mode and recruitment strategies for ventilating these patients.
Table 1.
Comparison Between H1N1 Patients and the Control Group With Influenza Like Illness at Presentation.
| Variable | Case: CLD with H1N1 (N = 22) | Control: CLD with influenza like illness (N = 88) | P |
|---|---|---|---|
| Age (years) | 44.5 ± 11.6 | 48.5 ± 13.7 | 0.198 |
| Males | 80% | 66% | |
| Weight (kg) | 68.5 ± 14.2 | 65.4 ± 10.8 | 0.044 |
| Body mass index (BMI) | 26.56 | 25.54 | 0.062 |
| CTP | 11.9 ± 2.1 | 10.2 ± 1.9 | 0.183 |
| MELD Na | 28 ± 3.3 | 21 ± 4.2 | 0.024 |
| Hemoglobin g/l | 10.5 ± 2.8 | 9.4 ± 2.2 | 0.132 |
| Total leukocyte count ×109/l | 11.8 ± 4.8 | 12.5 ± 7.7 | 0.178 |
| Platelet ×109/l | 152.5 ± 78.3 | 119.3 ± 58.6 | 0.164 |
| Total bilirubin mg/dl | 9.1 ± 5.6 | 7.7 ± 6.6 | 0.041* |
| Aspartate transaminase (AST; IU/l) | 149.7 ± 73.4 | 121.1 ± 42.3 | 0.006* |
| Alanine transaminase (ALT; IU/l) | 66 ± 25.5 | 43.3 ± 23.6 | 0.03* |
| Alkaline phosphatase (SAP; IU/l) | 126.8 ± 54.6 | 133 ± 59.1 | 0.074 |
| INR | 1.8 ± 0.74 | 1.56 ± 0.49 | 0.326 |
| Albumin (g/l) | 1.9 ± 0.69 | 2.12 ± 0.54 | 0.02 |
| Creatinine (mg/dl) | 1.49 ± 0.9 | 1.2 ± 0.48 | 0.429 |
| PaO2/FiO2 ratio | 136 ± 51.3 | 221 ± 76.2 | 0.002* |
| Duration of ICU stay (days) | 14.6 ± 5.6 days | 12.4 ± 7.2 days | 0.476 |
| Hepatic encephalopathy (N, %) | 17(77%) | 50(56%) | 0.029* |
| Acute kidney injury (N, %) | 15(67%) | 32(36%) | 0.036* |
| SOFA score | 7.9 ± 3.6 | 7.1 ± 3.4 | 0.078 |
| APACHE II score | 11.8 ± 4.3 | 9.4 ± 3.6 | 0.046 |
| CPIS score | 8(4–9) | 6(3–9) | 0.234 |
Abbreviations: CTP, Child Turcotte Pugh Score; CPIS, Clinical Pulmonary Infection Score; APACHE II, Acute Physiologic Assessment and Chronic Health Evaluation II; MELD, Model for End-Stage Liver Disease; AKI, Acute Kidney injury; ICU, Intensive Care Unit; INR, International Normalized Ratio; CLD, Chronic Liver Disease; SOFA, Sequential Organ Failure Score; PaO2/FiO2 ratio, Arterial Oxygen Tension (PaO2)/Fractional Inspired Oxygen (FiO2).
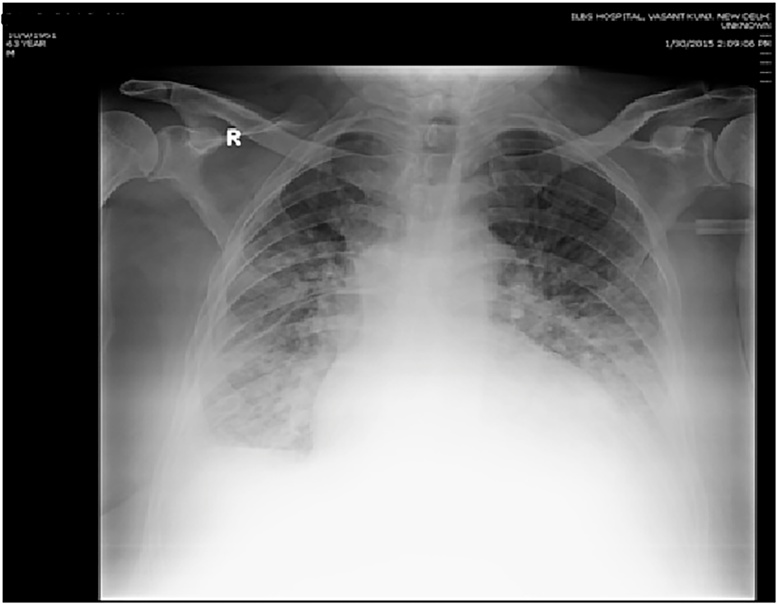
Figure 1.
(a–c) Progressive CXR changes in the index patient showing progressive alveolar opacities and interstitial thickening.
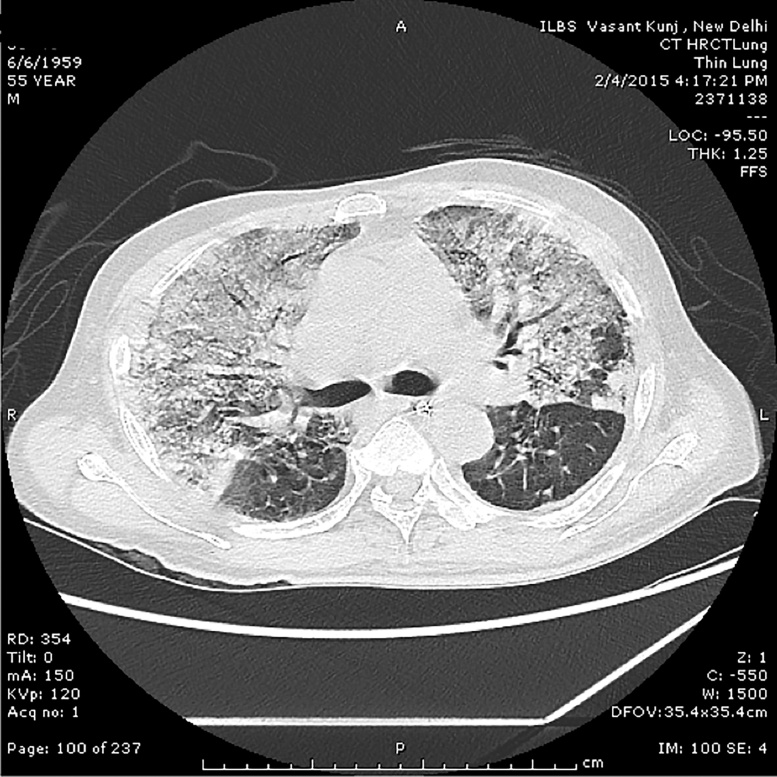
Figure 2.
High resolution contrast CT image of the chest showing dense consolidation in lung parenchyma merging into a background of widespread ground-glass attenuation. There is mild interstitial edema, more prominent at the lung bases.
Figure 3.
Atypical imaging – new onset pleural effusion noted in addition to areas of reticular and ground-glass opacities in the lung parenchyma.
Post-mortem liver biopsies revealed liver cirrhosis with evidence of cholestasis, necrosis and mixed inflammation (see Figure 4). One patient with ACLF showed bridging necrosis and marked cholestasis. However no characteristic changes were noted. Lung biopsies showed evidence of interstitial pneumonia (see Figure 5). Most of these patients also suffered from a secondary bacterial infection as evidenced by positive tracheal cultures. These were typically multi drug resistant pathogens like Klebsiella pneumoniae, Acinetobacter baumanni or E. coli. The other 88 patients admitted during the same time period with influenza like illness, and, who tested negative for H1N1 infection, were compared for disease pattern, severity and outcome in Table 1. There was no difference in clinical presentation in these two groups but the H1N1 cohort progressed rapidly.
Figure 4.
Liver biopsy of a patient with parenchymal disarray, sinusoidal congestion and multiacinar inflammation. There is portal inflammation without duct damage with evidence of bilirubinostasis as a poor prognostic marker.
Figure 5.
Lung biopsy of a patient showing interstitial edema, alveolar thickening with fluid accumulation. A predominantly mononuclear inflammation is noted.
In our series, 18 (81.8%)patients with H1N1 and cirrhosis died of pneumonia and acute respiratory distress syndrome despite timely antiviral treatment. One subject developed a secondary aspergillus infection after a prolonged ICU stay. In contrast, only 35 (40%)of the control group of cirrhotic patients without H1N1 died. The 4 surviving H1N1 patients were clinically well, with lower mean MELD (16.4 ± 3.4) and SOFA scores (7.5 ± 2.8). They did not have organ failures like shock, AKI and encephalopathy. Only one required invasive MV for a low PaO2/FiO2 ratio but was weaned early; the rest were successfully managed with Non-Invasive Ventilation (NIV) only. Table 2 shows the dynamic changes in number of organ failures in either group. The mean MELDNa in the H1 N1 group vs control group remained higher at 28 ± 3.3 vs 21 ± 4.2 at Day 0 (P-0.026) and 29.6 ± 4.5 vs 18.6 ± 4.1 at Day 7 (P = 0.014) respectively. Similarly, the median CPIS remained higher in the H1N1 group (P = 0.052) over the first 7 days of hospital stay.
Table 2.
Evolution in Organ Failures in Influenza Like Illness in Chronic Liver Disease in the H1N1 and Control Cohort.
| Parameter | Case: CLD with H1N1 (N = 22) |
P value | Control: CLD with influenza like illness (N = 88) |
P value | P value | ||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 1vs 2 | 3 | 4 | 3 vs 4 | 1 vs 3 | |
| Day 0 | Day 7 | Day 0 | Day 7 | ||||
| Organ failurea | N (%) | N (%) | |||||
| 1. Renal | 15 (68.1) | 18 (81.8) | 0.063 | 32 (36.3) | 12 (13.6) | 0.044 | 0.041 |
| 2. Coagulation | 8 (36.3) | 12 (54.5) | 0.082 | 12 (13.6) | 24 (27.2) | 0.032 | 0.042 |
| 3. Circulation | 4 (18.1) | 10 (45.4) | 0.042 | 8 (9.0) | 12 (13.6) | 0.553 | 0.021 |
| 4. Respiratory | 16 (72.7) | 18 (81.8) | 0.145 | 32(36.3) | 22 (25) | 0.041 | 0.032 |
| 5. Cerebral | 2 (9.0) | 8 (36.3) | 0.039 | 6 (6.8) | 14 (15.9) | 0.566 | 0.445 |
| Severity scoresb | |||||||
| MELD Na | 28 ± 3.3 | 29.6 ± 4.5 | 0.582 | 21 ± 4.2 | 18.6 ± 4.1 | 0.766 | 0.332 |
| APACHE II | 11.8 ± 4.3 | 22.5 ± 9.2 | 0.087 | 9.4 ± 3.6 | 12.6 ± 5.6 | 0.055 | 0.375 |
| CPIS | 8 (4–9) | 9 (4–11) | 0.766 | 6 (3–9) | 4 (3–6) | 0.164 | 0.072 |
Organ failures were defined as follows; renal failure, serum creatinine > 1.5 mg/dl; coagulation failure, INR > 2.5; circulation failure, presence of mean arterial pressure < 70 mmHg or need for ionotropes to maintain MAP ≥ 70 mmHg; respiratory failure, PaO2/FiO2 ratio < 200; cerebral failure ≥ Grade 2 hepatic encephalopathy by West-Haven Criteria.
Abbreviations: APACHE II, Acute Physiologic Assessment and Chronic Health Evaluation II; MELD, Model for End-Stage Liver Disease; CPIS, Clinical Pulmonary Infection Score.
Risk Factors for Mortality at Presentation
Numerous factors other than H1N1 infection, such as severity of underlying liver disease, severity of lung injury, development of organ failures like AKI, shock, secondary infections, may have influenced the outcome. However, the higher mortality in the H1N1 study group (82% vs. 35%) does suggest that the patients were at increased risk of secondary sepsis, organ failures and poor outcomes. Table 3 shows the risk factors for mortality at presentation in the H1N1 cohort. On univariate analysis, age > 45 years [OR 1.3; 95% CI 1.1–5.7, (P = 0.054)], encephalopathy > grade 2 [OR 5.4; 95%CI 2.8–12.3, (P = 0.042)], serum bilirubin >8 mg/dl [OR 2.1; 95% CI 1.8–12.3, (P = 0.052)], serum creatinine >1.8 mg/dl [OR 2.8; 95% CI 1.9–9.2, (P = 0.042)], PaO2/FiO2 ratio <200 [OR 4.5; 95% CI 3.1–18.5, (P = 0.026)] and INR > 2.5 [OR 2.2; 95% CI 1.8–6.7, (P = 0.032)] were risk factors for mortality at presentation. However, on multivariate analysis only PaO2/FiO2 ratio <200 and serum creatinine> 1.8 mg/dl remained predictors of mortality.
Table 3.
Risk Factors for Mortality in the H1N1 Cohort of Patients.
| Univariate analysis |
Multivariate analysis | ||||
|---|---|---|---|---|---|
| Cut off | Odds ratio (OR) | 95% CI | P value | ||
| Age | >45 years | 1.3 | 1.1–5.7 | 0.054 | PaO2/FiO2 ratio < 200 |
| Presence of encephalopathy | >grade 2 | 5.4 | 2.8–12.3 | 0.042 | Serum creatinine > 1.8 mg/dl |
| Total leukocyte count | >15.5 × 109/l | 1.5 | 1.2–5.5 | 0.057 | |
| Serum bilirubin | >8 mg/dl | 2.1 | 1.8–12.3 | 0.052 | |
| Serum creatinine | >1.8 mg/dl | 2.8 | 1.9–9.2 | 0.042 | |
| PaO2/FiO2 | <200 | 4.5 | 3.1–18.5 | 0.026 | |
| INR | >2.5 | 2.2 | 1.8–6.7 | 0.032 | |
Discussion
Viral influenza may increase the risk of decompensation in cirrhotic patients.4 This could be attributed to direct hepatic damage by the influenza virus or due to immune mediated tissue damage during systemic infection.5 Many these cases suffer from ‘bystander hepatitis’, with secondary immune damage triggered by primed T cells in the liver. These T cells had been pre-sensitized by prior episodes of viral influenza, and on infection with the H1N1 strain, these T cells then initiate immune mediated hepatocyte damage.6 Polakos et al. reported that in the absence of viral antigen in the liver, virus specific CD8+ T cells generated in the lungs could damage hepatocytes. In their series of 15 volunteers infected with H1N1 influenza virus, 4 patients developed transaminitis up to three times the upper limit of normal. This happened even in the absence of virus isolation from the liver in animal models.7 Circulating cytokines and chemokines further contribute to hepatocyte and endothelial damage. Moreover, concomitant pleural effusion, decreased lung compliance, hepato-pulmonary syndrome and porto-pulmonary hypertension may also contribute to make the disease more severe. In our study, we evaluated matched patients who presented with respiratory distress, comparable disease severity and rapidly progressive course which appeared to be viral influenza like. Hence all patients, who were suspected cases of viral influenza, received oseltamivir for the first two days till tissue H1N1 RT-PCR reports were available to confirm or refute the diagnosis. Hence it would appear that the only difference in the patient subgroup with H1N1 and the ILI reflected by worsening clinical course, and poor outcome. It is not possible to attribute extra pulmonary organ failures to H1 N1 infection per se. In our patients, the cause of organ failures is multifactorial with the presence of co morbidities like diabetes, presence of secondary bacterial infections, etc. For instance, in our patients with acute kidney injury, it is likely that sepsis, shock, and hepato-renal syndrome could all have contributed to renal dysfunction. This disease may have a higher mortality rate in immunosuppressed patients.8 The conventional list of such cases includes those with chronic kidney disease, heart failure or chronic obstructive pulmonary disease. Similarly, those with Chronic Liver Disease (CLD), especially those suffering from autoimmune liver disease and liver transplant recipients who use immunosuppressive drugs are also at high risk. Infection in patients on immunosuppression has a longer duration with more viral shedding and more chance of antiviral resistance.9, 10, 11 It may also be noted that influenza infection per se has been reported to provoke rejection in kidney transplant patients. However, this observation has not been substantiated in post-liver transplant patients.12 The higher complication rate of the new pandemic flu should alert physicians taking care for such patients and any sign of ILI should be taken seriously and appropriate referral should be done in a timely manner.
In our study, age > 45 years (P = 0.054), encephalopathy > grade 2 (P = 0.042), serum bilirubin > 8 mg/dl (P = 0.052), serum creatinine > 1.8 mg/dl (P = 0.042), PaO2/FiO2 ratio < 200 (P = 0.026) and INR > 2.5 (P = 0.032) were risk factors for mortality.
We face a challenge when managing subjects with H1N1 and liver disease, and these factors are enumerated in Table 4.
Table 4.
Unique Associations of H1N1 Pneumonia and Chronic Liver Disease.
| Known unknowns in cirrhosis and viral influenza | Antigenic type and phenotype |
| Susceptibility/resistance to antivirals | |
| Age-groups and clinical groups most affected | |
| Age-groups with most transmission | |
| Clinical attack rates | |
| Pathogenicity (case-fatality rates) | |
| Precise parameters needed for modeling and forecasting (serial intervals) | |
| Severity of the epidemic | |
| Precise clinical case definition | |
| The effectiveness of interventions and counter-measures including pharmaceuticals | |
| The safety of pharmaceutical interventions |
Current treatment guidelines recommend the patients be classified into three categories15, 16:
Category-A
-
•
Patients with mild fever plus cough/sore throat with or without body ache, headache, diarrhea and vomiting will be categorized as Category-A. They do not require Oseltamivir and should be treated for the symptoms mentioned above. The patients should be monitored for their progress and reassessed at 24–48 h by the doctor.
-
•
No testing of the patient for H1N1 is required.
-
•
Patients should confine themselves at home and avoid mixing up with public and high-risk members in the family.
Category-B
-
(i)
In addition to all the signs and symptoms mentioned under Category-A, if the patient has high fever and severe sore throat, may require home isolation and Oseltamivir.
-
(ii)In addition to all the signs and symptoms mentioned under Category-A, individuals having one or more of the following high-risk conditions shall be treated with Oseltamivir:
-
-Children with mild illness but with predisposing risk factors.
-
-Pregnant women.
-
-Persons aged 65 years or older.
-
-Patients with lung diseases, heart disease, liver disease.
-
-
Category-C
-
•
Patients with kidney disease, blood disorders, diabetes, neurological disorders, cancer and HIV/AIDS.
-
•
Patients on long-term cortisone therapy.
No tests for H1N1 are required for Category-B (i) and (ii).
All patients of Category-B (i) and (ii) should confine themselves at home and avoid mixing with public and high-risk members in the family.
All these patients mentioned above in Category-C require testing, immediate hospitalization and treatment. These patients were also difficult to ventilate. On basis of prior recommendations, and the experience at our center, we found that the patients needed isolation, and early referral to the ICU for monitoring and initiation of ventilatory support.16 Many of the patients in our subset presented late during the disease course, and thus even starting oseltamivir at the time of presentation to our center did not modify outcomes as organ failures had already begun to set in.
Available treatments for swine flu include neuraminidase inhibitors (e.g., oseltamivir and zanamivir). Whether these drugs need dose adjustment in patients with hepatic failure is not known. Prolonged virus shedding and development of viral resistance in immunosuppressed subjects receiving these antiviral agents has been described. There is concern about the efficacy of vaccines in immunosuppressed patients. There is no data on the protective efficacy of influenza vaccination in cirrhosis. Reduced immunogenicity has been reported in liver transplant recipients after receiving influenza vaccine.17 Previous recommendation advised yearly seasonal flu vaccination for all of solid organ transplant recipients six months after transplantation, but there are concerns that vaccination in these patients may evoke a T cell response leading to rejection.18 The WHO recommends annual vaccination for children aged between 6 months to 5 years, elderly individuals aged more than 65 years, individuals with chronic medical conditions, and health-care workers. The new H1N1 vaccine strain, called A/Michigan/45/2015, replaces A/California/7/2009, which has been in use as a vaccine strain since the 2009 H1N1 virus became a regularly circulating seasonal flu strain after the 2009–10 pandemic. As of now there is no definite recommendation regarding timing of influenza vaccination in cirrhosis.19 Given the high risk of influenza related mortality in subjects on immunosuppression, it may however be recommended that this subset indeed merits timely vaccination.20
Conclusion
In conclusion, as the current pandemic of new H1N1 virus is progressing, physicians and hepatologists should be aware of the potential effects of this infection on patients with CLD. These patients, especially those on immunosuppressive agents and transplantation recipients are candidates for vaccination, preferably with an inactivated vaccine. Deterioration of hepatic function in patients with stable liver disease might be caused by flu. Early diagnosis and treatment is essential to prevent liver failure and improve outcomes.
Patient Consent Statement
Written informed consent has been taken from the subjects and all images have been modified to protect their identity.
Authors’ Contributions
Madhumita Premkumar, Shivani Dudha, and Devaraja were the primary physicians and drafted the manuscript; Dr. Shrruti Grover and Dr. Chhagan Bihari were the concerned pathologists. Dr. Rakhi and Dr. Sachin were the ICU critical care consultants and Prof Sarin was responsible for drafting, critical revision of the manuscript for important intellectual content and is the article guarantor.
All the authors have read and approved the manuscript.
Conflicts of Interest
The authors have none to declare.
References
- 1.Marzano A., Marengo A., Ruggiero T. Clinical impact of A/H1/N1/09 influenza in patients with cirrhosis: experience from a nosocomial cluster of infection. J Med Virol. 2013;85(1):1–7. doi: 10.1002/jmv.23454. [DOI] [PubMed] [Google Scholar]
- 2.Bal C.K., Bhatia V., Kumar S. Influenza A/H1/N1/09 infection in patients with cirrhosis has a poor outcome: a case series. Indian J Gastroenterol. 2014;33:178–182. doi: 10.1007/s12664-014-0443-5. [DOI] [PubMed] [Google Scholar]
- 3.Panning M., Eickmann M., Landt O. Detection of influenza A(H1N1) virus by real-time RT-PCR. Euro Surveill. 2009;14(36):19329. [PubMed] [Google Scholar]
- 4.Stanley T.V. Acute liver failure following influenza B infection. N Z Med J. 1994;107(986 Pt 1):382. [PubMed] [Google Scholar]
- 5.Adams D.H., Hubscher S.G. Systemic viral infections and collateral damage in the liver. Am J Pathol. 2006;168(4):1057–1059. doi: 10.2353/ajpath.2006.051296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fislová T., Gocník M., Sládková T. Multiorgan distribution of human influenza A virus strains observed in a mouse model. Arch Virol. 2009;154(3):409–419. doi: 10.1007/s00705-009-0318-8. [DOI] [PubMed] [Google Scholar]
- 7.Polakos N.K., Cornejo J.C., Murray D.A. Kupffer cell dependent hepatitis occurs during influenza infection. Am J Pathol. 2006;168(4):1169–1178. doi: 10.2353/ajpath.2006.050875. quiz 1404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duchini A., Hendry R.M., Redfield D.C., Pockros P.J. Influenza infection in patients before and after liver transplantation. Liver Transpl. 2000;6(5):531–542. doi: 10.1053/jlts.2000.9738. [DOI] [PubMed] [Google Scholar]
- 9.Kunisaki K.M., Janoff E.N. Influenza in immunosuppressed populations: a review of infection frequency, morbidity, mortality, and vaccine responses. Lancet Infect Dis. 2009;9(8):493–504. doi: 10.1016/S1473-3099(09)70175-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soesman N.M., Rimmelzwaan G.F., Nieuwkoop N.J. Efficacy of influenza vaccination in adult liver transplant recipients. J Med Virol. 2000;61(1):85–93. [PubMed] [Google Scholar]
- 11.Connolly J., Douglas J., Kumar R. Influenza virus vaccination and renal transplant rejection. Br Med J. 1974;1(5908):638. doi: 10.1136/bmj.1.5908.638-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lawal A., Basler C., Branch A., Gutierrez J., Schwartz M., Schiano T.D. Influenza vaccination in orthotopic liver transplant recipients: absence of post administration ALT elevation. Am J Transplant. 2004;4(11):1805–1809. doi: 10.1111/j.1600-6143.2004.00564.x. [DOI] [PubMed] [Google Scholar]
- 13.Louie J.K., Acosta M., Winter K. Factors associated with death or hospitalization due to pandemic 2009 influenza A(H1N1) infection in California. JAMA. 2009;302(17):1896–1902. doi: 10.1001/jama.2009.1583. [DOI] [PubMed] [Google Scholar]
- 14.Shieh W.J., Blau D.M., Denison A.M. 2009 pandemic influenza A (H1N1): pathology and pathogenesis of 100 fatal cases in the United States. Am J Pathol. 2010;177(1):166–175. doi: 10.2353/ajpath.2010.100115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hellenbrand W., Jorgensen P., Schweiger B. Prospective hospital-based case–control study to assess the effectiveness of pandemic influenza A(H1N1)pdm09 vaccination and risk factors for hospitalization in 2009–2010 using matched hospital and test-negative controls. BMC Infect Dis. 2012;31(12):127. doi: 10.1186/1471-2334-12-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choudhry A., Singh S., Khare S. Emergence of pandemic 2009 influenza A H1N1, India. Indian J Med Res. 2012;135(4):534–537. [PMC free article] [PubMed] [Google Scholar]
- 17.Ríos F.G., Estenssoro E., Villarejo F. Lung function and organ dysfunctions in 178 patients requiring mechanical ventilation during the 2009 influenza A (H1N1) pandemic. Crit Care. 2011;15(4):R201. doi: 10.1186/cc10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith K.G., Isbel N.M., Catton M.G., Leydon J.A., Becker G.J., Walker R.G. Suppression of the humoral immune response by mycophenolate mofetil. Nephrol Dial Transplant. 1998;13(1):160–164. doi: 10.1093/ndt/13.1.160. [DOI] [PubMed] [Google Scholar]
- 19.Mazzone P.J., Mossad S.B., Mawhorter S.D., Mehta A.C., Mauer J.R. Cell-mediated immune response to influenza vaccination in lung transplant recipients. J Heart Lung Transplant. 2004;23(10):1175–1181. doi: 10.1016/j.healun.2003.08.033. [DOI] [PubMed] [Google Scholar]
- 20.Avery R.K., Michaels M. Update on immunizations in solid organ transplant recipients: what clinicians need to know. Am J Transplant. 2008;8(1):9–14. doi: 10.1111/j.1600-6143.2007.02051.x. [DOI] [PubMed] [Google Scholar]