Abstract
Objective
We aimed to assess the efficacy and safety of ticagrelor compared to clopidogrel in Asian patients with acute coronary syndrome (ACS) in real-world practice.
Methods
PubMed, Web of Science and Scopus databases were searched systematically to obtain relevant Asian observational studies.
Results
The meta-analysis included six studies with 27959 participants. Compared with clopidogrel, ticagrelor was significantly beneficial in prevention of major adverse cardiac events (MACCEs) (OR=0.62; 95% CI: 0.46-0.83, I2=69%, p=0.001) mainly driven by reducing stroke (OR=0.62; 95% CI: 0.49-0.78, I2=0%, p<0.001). No differences were found between ticagrelor and clopidogrel in the risk of cardiovascular mortality (OR=0.66; 95% CI: 0.41-1.06, I2=0%, p=0.09), target vessel revascularization (OR=0.53; 95% CI: 0.21-1.35, I2=82%, p=0.18), major bleeding (OR=1.11; 95% CI: 0.62-2.00, I2=75%, p=0.73), and net adverse clinical and cerebral events (OR=0.76; 95% CI: 0.55-1.04, I2=78%, p=0.09). However, ticagrelor significantly increased the incidence of major/minor (OR=1.73; 95% CI: 1.36-2.21, I2=0%, p<0.001) and minor bleeding (OR=1.73; 95% CI: 1.29-2.32, I2=0%, p<0.001). Sensitivity analyses did not find consistent effect of ticagrelor on prevention of all-cause death and myocardial infarction.
Conclusion
This meta-analysis suggested that ticagrelor might reduce the risk of MACCEs mainly by reducing stroke in Asian patients with ACS without increasing the rates of major bleeding. Ticagrelor did not show a significant effect on other parts of MACCEs. Considerable increase in the risk of major/minor and minor bleeding was observed in ticagrelor compared with clopidogrel users. Further high-quality studies are required to support these findings.
Keywords: Acute coronary syndrome, Asia, Clopidogrel, Mortality, Ticagrelor
1. Introduction
Ticagrelor is a reversible, fast-acting P2Y12 antagonist with a considerably greater platelet inhibition effect than clopidogrel.1, 2 The clinical superiority of ticagrelor over clopidogrel was proved in the multinational, randomized, double-blinded Platelet Inhibition and Patient Outcomes (PLATO) trials.3 The PLATO results allow the international cardiac societies to recommend using ticagrelor as first-line P2Y12 inhibitors in patients with acute coronary syndrome (ACS) as opposed to clopidogrel.4, 5 However, ticagrelor provides ischaemic benefits at the cost of a significant increase in haemorrhagic events,3 which is vitally important for bleeding-prone Asian patients.6 Recent meta-analyses of randomized controlled trials (RCTs) reported that ticagrelor numerically increased bleeding risk among Asian individuals and did not provided proper thrombotic benefits.7, 8 Nevertheless, the existing evidence from RCTs is somewhat insufficient to draw clear conclusions. Goto et al's trial, with an equivalent study design, was underpowered to detect a benefit–risk ratio for ticagrelor in Asian population.9 Other RCTs had serious limitations in randomization process, which could bias their findings.10, 11 As a consequence, we aimed to explore systematically and synthesize statistically the evidence from observational studies on the efficacy and safety of ticagrelor compared with clopidogrel in Asian patients with ACS.
2. Methods
2.1. Search strategy
The review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement, the Cochrane Handbook for Systematic Reviews of Interventions.12, 13 We searched systematically Asian studies in English language in PubMed (2010–2 October 2018), Web of Science and Scopus databases (2010–4 September 2018). The following keywords were typed in different combinations: “ticagrelor”, AZD6140, “clopidogrel”, “platelet aggregation inhibitors”, “P2Y12 inhibitor”, “acute coronary syndrome” and “myocardial infarction”. In PubMed, these search terms were combined with a Boolean operator “AND” and keywords depicting the Asian origin of studies. Medical Subject Headings synonyms of the most terms were also applied in the search strategy. In Scopus and Web of Science, we used filters for countries of origin to select only Asian trials. Reference lists of obtained articles and conference meeting proceedings were also checked to retrieve further trials.
2.2. Inclusion and exclusion criteria
Studies were selected if they fulfil the following criteria: (1) studies included Asian patients with ACS older than 18 years without a specific upper age limit; (2) where patients in an experimental group received ticagrelor (a loading dose of 180 mg and a maintenance dose of 90 mg twice daily); (3) where patients in a control group received clopidogrel (a loading dose of 600/300 mg and a maintenance dose of 75 mg once daily); (4) studies with clinical endpoints and (5) observational studies with a follow-up period of 6 months or more.
Duplicate publications, pharmacodynamic and experimental trials, case reports, case–control studies, narrative reviews, economic evaluations and correspondences were excluded.
The primary efficacy endpoint of this review was major adverse cardiac and cerebrovascular events (MACCEs) with definitions accepted in the included studies. Considerable bleeding was chosen as a primary safety endpoint, and secondary endpoints were all-cause or cardiovascular mortality, myocardial infarction (MI), target vessel revascularization (TVR), stroke, minor bleeding, a composite of major and minor bleeding and net adverse clinical and cerebral events (NACCEs).
2.3. Quality assessment and data extraction
The included observational studies were evaluated according to the Newcastle–Ottawa quality assessment scale for cohort studies.14 The predesigned Excel form was used to extract necessary information from the full texts of the selected articles. This information included data on study characteristics (authors, publication year, country, design, follow-up period and sample size), clinical and demographic features of participants, study endpoints and main results.
The literature search, study selection, quality assessment and data extraction were performed by two authors independently, with any disagreements resolved by discussion.
2.4. Statistical analysis
We used an inverse-variance analysis method with a random-effects model (DerSimonian and Laird method) to estimate summary odds ratio (OR) with 95% confidence interval (CI).13 In cases of a low rate of events (below 1%), the Peto analysis method with a fixed effects model was used as this method was shown to be superior in such situations.13 As many of the included studies reported time-to-event outcomes, we also performed analyses with hazard ratio (HR) selected as an effect estimate to exclude the misinterpretation of the data by calculating OR.15 Several statistical methods and models were used to reveal possible discrepancies; however, no significant differences were observed. Statistical heterogeneity was calculated with both χ2 test and Higgins's I2 statistics. Statistically significant heterogeneity was considered when χ2 p-value was less than 0.05 or an I2 statistic was more than 75%.
We also performed several types of sensitivity analyses. First, we conducted a standard leave-one-out sensitivity analysis by removing the included studies one after another to validate the robustness of the results. Second, in cases of several studies based on the same database, we included all these studies one by one in the estimation of pooled OR to reveal possible discrepancies. Third, we excluded the studies without adjustment statistics from the quantitative synthesis to minimize bias from confounding factors.
Subgroup analyses according to different characteristics of participants were also undertaken. Given the small number of included investigations, the assessment of publication bias and meta-regression analyses were not conducted. All statistical procedures were performed with Review Manager (RevMan) 5.3 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). In addition, to obtain missing information for calculating HR, we used an Excel spreadsheet proposed by Tierney et al.15
3. Results
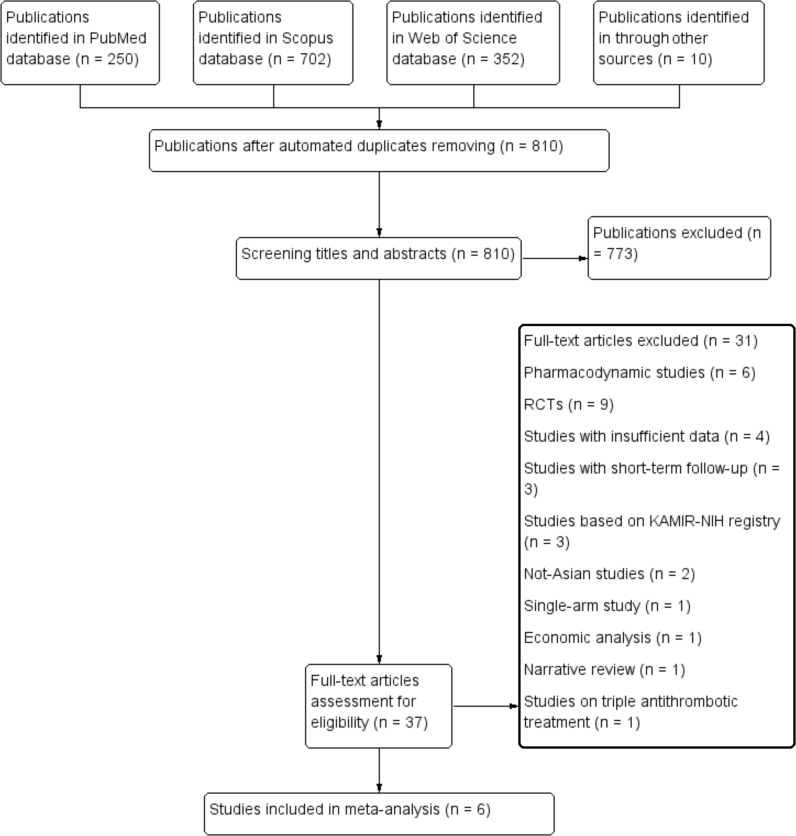
The extensive search detected 1314 potentially relevant citations, of which six studies with 27959 participants were included in the meta-analysis (Fig. 1).16, 17, 18, 19, 20, 21 Of note, several author teams analysed the data from the Korea Acute Myocardial Infarction Registry-National Institute of Health (KAMIR-NIH) database with controversial results.19, 22, 23, 24 To avoid double counting, we included only the findings provided by Sim et al as this study incorporated the most recent data from the KAMIR-NIH registry.19 Because Sim et al did not publish the data on cardiovascular mortality, this information was obtained from the work of Park et al.24
Fig. 1.
Flowchart of search strategy. RCTs, randomized controlled trials; KAMIR-NIH, Korea Acute Myocardial Infarction Registry-National Institute of Health.
3.1. Characteristics of included studies and quality assessment
Table 1 presents the main features of the included studies. Lee et al included 22815 patients in their study, and, consequently, the weight of this study in the statistical analysis was the largest.17 Although patients' mean age and gender structure were similar, substantial differences in other characteristics were observed between the selected studies.
Table 1.
Main features of the included studies.
| Study name, year | Chen et al, 201616 | Lee et al, 201817 | Nur'amin et al, 201718 | Sim et al, 201819 | Wang et al, 201820 | Xin et al, 201821 |
|---|---|---|---|---|---|---|
| Country | Taiwan | Taiwan | Indonesia | Korea | China | China |
| Study design | RS | RS | RS | RS | RS | RS |
| Study duration, months | 19 | 24 | Unknown | 49 | 34 | 12 |
| Sample size, n | 928 | 27339 | 361 | 7791 | 20816 | 206 |
| Ticagrelor | 324 | 2844 | 111 | 1554 | 779 | 145 |
| Clopidogrel | 604 | 24495 | 250 | 6237 | 20037 | 61 |
| Mean age, years | ||||||
| Ticagrelor | 63.8 | 62.2 | 55.8 | 62.1 | 60.54 | 61.9 |
| Clopidogrel | 63.7 | 63.1 | 55.9 | 62.6 | 60.97 | 65.0 |
| Men, % | ||||||
| Ticagrelor | 79.9 | 81.9 | 92.8 | 79.4 | 71.1 | 68.3 |
| Clopidogrel | 79.5 | 78.6 | 92.8 | 77.9 | 71.7 | 73.8 |
| Type of participants | ACS | Acute MI | PCI | Acute MI | ACS | ACS |
| Smoking, % | Unknown | |||||
| Ticagrelor | 47.3 | 36.0 | 64.6 | 57.3 | 55.2 | |
| Clopidogrel | 46.0 | 43.6 | 65.2 | 57.8 | 55.7 | |
| DM, % | ||||||
| Ticagrelor | 37.1 | 35.9 | 40.5 | 24.6 | 24.6 | 37.9 |
| Clopidogrel | 42.9 | 38.2 | 41.6 | 24.5 | 23.8 | 34.4 |
| Hypertension, % | ||||||
| Ticagrelor | 55.4 | 62.2 | 58.6 | 45.9 | 57.9 | 61.4 |
| Clopidogrel | 57.6 | 64.5 | 64.8 | 47.6 | 54.7 | 57.4 |
| CKD, % | Unknown | |||||
| Ticagrelor | 39.3 | 14.6 | 6.3 | – | 2.4 | 28.9 |
| Clopidogrel | 39.3 | 16.1 | 8.8 | – | 2.4 | 40.9 |
| Dyslipidaemia, % | Unknown | |||||
| Ticagrelor | 46.0 | 42.8 | 34.2 | 11.0 | – | 19.3 |
| Clopidogrel | 44.2 | 42.6 | 33.6 | 11.3 | – | 22.9 |
| Previous MI | Unknown | Unknown | ||||
| Ticagrelor | 8.0 | – | – | 4.1 | 15.5 | 15.2 |
| Clopidogrel | 8.5 | – | – | 3.5 | 17.3 | 22.9 |
| Previous stroke, % | ||||||
| Ticagrelor | 8.0 | 6.5 | 36.0 | 3.9 | 8.9 | 22.7 |
| Clopidogrel | 9.4 | 8.1 | 43.6 | 3.5 | 8.3 | 31.1 |
| Heart failure, % | Unknown | Unknown | ||||
| Ticagrelor | 5.4 | 8.9 | 20.7 | 0.6 | ||
| Clopidogrel | 7.1 | 10.5 | 27.2 | 0.3 | ||
| ACEI/ARB use, % | Unknown | |||||
| Ticagrelor | 47.3 | 76.9 | 73.0 | 79.5 | 82.1 | |
| Clopidogrel | 55.3 | 76.4 | 78.4 | 79.4 | 67.2 | |
| Beta-blocker use, % | ||||||
| Ticagrelor | 50.9 | 71.3 | 73.0 | 85.0 | 75.5 | 77.9 |
| Clopidogrel | 58.9 | 71.0 | 78.0 | 86.9 | 75.4 | 52.4 |
| Statin use, % | Unknown | |||||
| Ticagrelor | 78.6 | 83.8 | 96.8 | 96.1 | 95.2 | |
| Clopidogrel | 69.6 | 79.0 | 97.3 | 96.4 | 96.7 | |
| Glycoprotein IIb/IIIa use, % | Unknown | Unknown | Unknown | |||
| Ticagrelor | 0.4 | 18.3 | 18.2 | |||
| Clopidogrel | 1.8 | 18.7 | 18.4 | |||
| Follow-up, months | 12 | 12 | 12 | 12 | 12 | 6 |
| Adjustment method | PSM | PSM | Bivariate analysis | PSM | PSM | UA |
| Study endpoints | 1), 2), 3), 4), 5), 6) | 1), 3), 4), 5) | 1), 3), 7) | 1), 3), 4), 5), 6), 7) | 1), 3), 4), 5), 6), 7) | 2), 3), 4), 5), 6), |
| Definition of MACCE | 2), 3), 4) | 1), 3), 4) | 1), 3), 7) | 1), 3), 4), 7) | 1), 3), 4), 7) | 2), 3), 4), 7) |
| Definitions of major bleeding | PLATO | Self-defined | None | TIMI | BARC | TIMI |
The table presents only the characteristics for which the published data from most of the studies were available.
RS, retrospective; ACS, acute coronary syndrome; MI, myocardial infarction; PCI, percutaneous coronary intervention; DM, diabetes mellitus; CKD, chronic kidney disease; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; PSM, propensity score matching; UA, unadjusted; MACCEs, major adverse cardiac and cerebrovascular events; PLATO, Platelet Inhibition and Patient Outcomes; TIMI, thrombolysis in myocardial infarction; BARC, Bleeding Academic Research Consortium.
Outcomes: (1) all-cause mortality, (2) cardiovascular mortality, (3) MI, (4) stroke, (5) major bleeding, (6) minor bleeding and (7) repeat PCI.
The quality assessment demonstrated that the methodology of the included studies was not excellent (Table 2). For example, Lee et al did not report data on clinical outcomes in 22.4% of ticagrelor users who switched from their initial P2Y12 antagonist treatment.17 The same limitation was found in another Chinese study.16
Table 2.
Risk of bias assessment of the observational studies.
| Study name, year | Chen et al16 | Lee et al17 | Nur'amin et al18 | Sim et al19 | Wang et al20 | Xin et al21 |
|---|---|---|---|---|---|---|
| Representativeness of the exposed cohort | * | * | – | * | * | * |
| Selection of the non exposed cohort | * | * | * | * | * | * |
| Ascertainment of exposure | * | * | * | * | * | * |
| Demonstration that outcome of interest was not present at start of study | * | * | * | * | * | * |
| Comparability | * | * | * | * | * | – |
| Assessment of outcome | * | * | * | * | * | – |
| Long enough follow-upa | * | * | * | * | * | – |
| Adequacy of follow-up of cohorts | – | – | * | * | – | * |
∗ low risk of bias; – unclear or high risk of bias.
If 1 year or more.
3.2. Quantitative synthesis
3.2.1. Primary efficacy endpoints
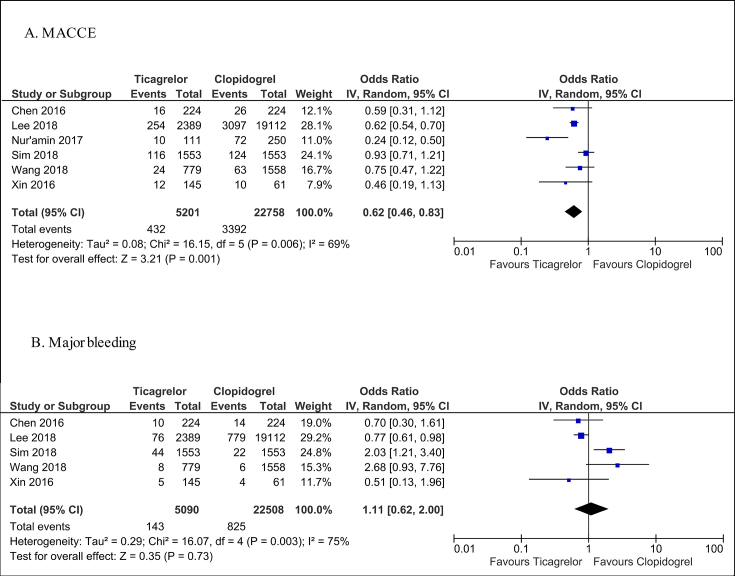
Ticagrelor significantly decreased the risk of MACCEs compared with clopidogrel (OR = 0.62; 95% CI: 0.46–0.83, I2 = 69%, p = 0.001, Fig. 2A). The level of statistical heterogeneity can be rated as substantial.13 Nevertheless, ticagrelor proved its superiority over clopidogrel after performing sensitivity analyses (Table 3, Table 4).
Fig. 2.
Forest plot of ticagrelor versus clopidogrel for primary efficacy (A) and safety (B) endpoints. MACCEs: major adverse cardiac and cerebrovascular events; CI, confidence interval.
Table 3.
Leave-one-out sensitivity analysis for MACCE and major bleeding.
| Study name, year | Ticagrelor versus clopidogrel |
|||||
|---|---|---|---|---|---|---|
| MACCE |
Major bleeding |
|||||
| OR (95% CI) | I2 statistics, % | p-value | OR (95% CI) | I2 statistics, % | p-value | |
| Chen et al16 | 0.62 (0.44, 0.86) | 75 | 0.004 | 1.24 (0.60, 2.56) | 81 | 0.56 |
| Lee et al17 | 0.58 (0.37, 0.92) | 71 | 0.02 | 1.28 (0.62, 2.65) | 63 | 0.50 |
| Nur'amin et al18 | 0.70 (0.55, 0.89) | 53 | 0.003 | – | – | – |
| Sim et al19 | 0.55 (0.41, 0.74) | 47 | 0.0001 | 0.89 (0.53, 1.49) | 46 | 0.65 |
| Wang et al20 | 0.58 (0.41, 0.83) | 75 | 0.003 | 0.95 (0.52, 1.74) | 75 | 0.87 |
| Xin et al21 | 0.63 (0.46, 0.87) | 74 | 0.004 | 1.24 (0.65, 2.37) | 80 | 0.52 |
MACCEs, major adverse cardiac and cerebrovascular events; OR, odds ratio; CI, confidence interval.
Table 4.
Sensitivity analysis including different investigations based on the Korea Acute Myocardial Infarction Registry.
| Study name | Ticagrelor versus clopidogrel |
|||||
|---|---|---|---|---|---|---|
| MACCE |
Major bleeding |
|||||
| OR (95% CI) | I2 statistics, % | p-value | OR (95% CI) | I2 statistics, % | p-value | |
| Choe et al22 | 0.58 (0.48, 0.69) | 35 | <0.0001 | 0.96 (0.66, 1.40) | 57 | 0.83 |
| Kang et al23 | 0.56 (0.46, 0.70) | 36 | <0.0001 | 0.94 (0.63, 1.39) | 44 | 0.75 |
| Park et al24 | 0.60 (0.46, 0.79) | 51 | 0.0003 | 1.12 (0.62, 2.03) | 74 | 0.71 |
MACCEs, major adverse cardiac and cerebrovascular events; OR, odds ratio; CI, confidence interval.
3.2.2. Primary safety endpoints
The meta-analysis demonstrated no differences in the risk of major bleeding between ticagrelor and clopidogrel (OR = 1.11; 95% CI: 0.62–2.00, I2 = 75%, p = 0.73); nevertheless, one should interpret these findings with caution, bearing in mind high heterogeneity of the analysis (Fig. 2B). The sensitivity analyses did not change these findings significantly (Table 3, Table 4).
3.2.3. Secondary endpoints
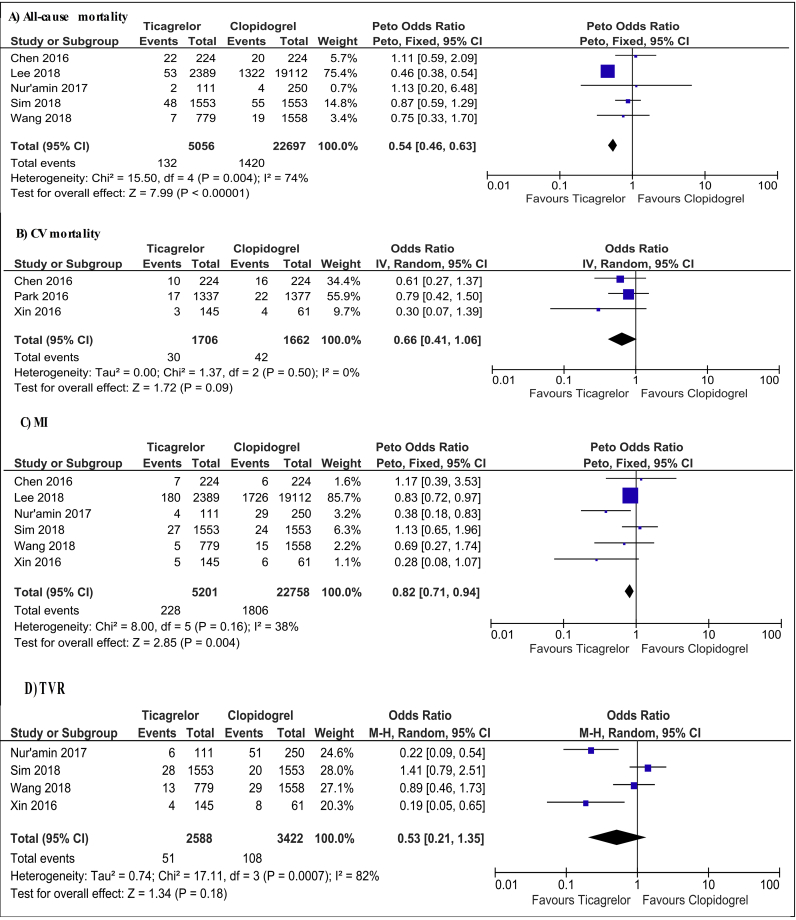
A substantial decline in the risk of all-cause mortality was observed in ticagrelor compared with clopidogrel users with a high level of heterogeneity (OR = 0.54; 95% CI: 0.46–0.63, I2 = 74%, p < 0.0001, Fig. 3A). Of note, after excluding the data provided by Lee et al in the sensitivity analysis, the results of the analysis became insignificant, although with no evidence of heterogeneity (OR = 0.91; 95% CI: 0.67–1.23, I2 = 0%, p = 0.53).
Fig. 3.
Forest plot of ticagrelor versus clopidogrel for secondary endpoints. CV, cardiovascular; MI, myocardial infarction; TVR, target vessel revascularization; CI, confidence interval; IV, inverse variance; M-H, Mantel-Haenszel.
Ticagrelor did not reduce cardiovascular mortality significantly compared with clopidogrel, and the level of heterogeneity was low (OR = 0.66; 95% CI: 0.41–1.06, I2 = 0%, p = 0.09, Fig. 3B).
Ticagrelor was associated with a numerical decrease in the rates of MI as opposed to clopidogrel with evidence of low heterogeneity (OR = 0.82; 95% CI: 0.71–0.94, I2 = 38%, p = 0.004, Fig. 3C). Nevertheless, after removing Lee et al's study in the sensitivity analysis, the pooled OR failed to reach statistical significance (OR = 0.74; 95% CI: 0.51–1.07, I2 = 48%, p = 0.11).
There were no significant differences in the rates of TVR between ticagrelor and clopidogrel users (OR = 0.53; 95% CI: 0.21–1.35, I2 = 82%, p = 0.18, Fig. 3D).
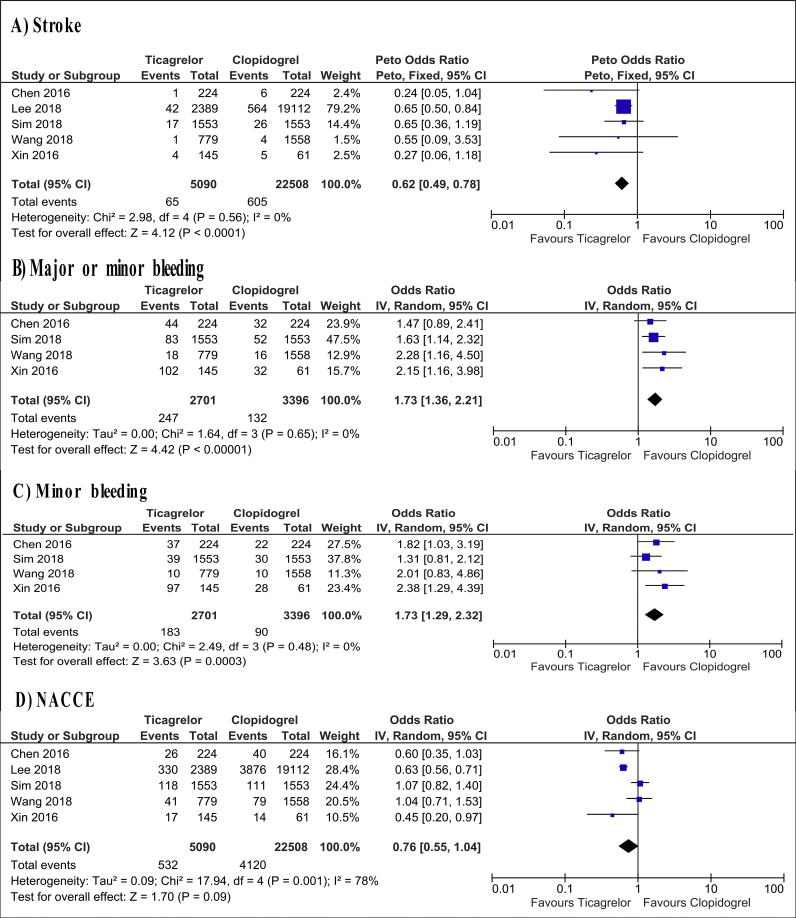
Ticagrelor demonstrated significant superiority over clopidogrel in reducing the risk of stroke with a low level of heterogeneity (OR = 0.62; 95% CI: 0.49–0.78, I2 = 0%, p < 0.0001, Fig. 4A), and this results were consistent in the leave-one-out sensitivity analyses.
Fig. 4.
Forest plot of ticagrelor versus clopidogrel for secondary endpoints. NACCEs, net adverse clinical and cerebral events; CI, confidence interval; IV, inverse variance.
Ticagrelor treatment was found to increase significantly the risk of major/minor bleeding (OR = 1.73; 95% CI: 1.36–2.21, I2 = 0%, p < 0.001) and minor bleeding (OR = 1.73; 95% CI: 1.29–2.32, I2 = 0%, p < 0.001, Fig. 4B and C). Moreover, the summary OR saved its statistical significance in the sensitivity analyses.
The risk of NACCEs was not influenced by ticagrelor compared with clopidogrel, and the level of heterogeneity was substantial (OR = 0.76; 95% CI: 0.55–1.04, I2 = 78%, p = 0.09, Fig. 4D).
Additional analyses with HR chosen as summary measure provided similar findings for all the available outcomes (Table 5).
Table 5.
Analyses of efficacy and safety of ticagrelor compared with clopidogrel with hazard ratio chosen as effect measure.
| Outcomes | Number of studies | HR | (95% CI) | I2 statistics, % | p-value |
|---|---|---|---|---|---|
| Major adverse cardiac and cerebrovascular events | 5 | 0.78 | 0.68–0.89 | 9 | 0.0003 |
| Major bleeding | 2 | 1.20 | 0.44–3.30 | 91 | 0.72 |
| All-cause mortality | 4 | 0.71 | 0.41–1.23 | 78 | 0.22 |
| Cardiovascular mortality | 2 | 0.77 | 0.46–1.30 | 0 | 0.32 |
| Myocardial infarction | 4 | 0.97 | 0.81–1.17 | 0 | 0.77 |
| Stroke | 3 | 0.66 | 0.45–0.96 | 19 | 0.03 |
| Target vessel revascularization | 2 | 0.72 | 0.28–1.87 | 67 | 0.50 |
| Major or minor bleeding | 2 | 1.76 | 1.29–2.40 | 0 | 0.0004 |
| Net adverse cardiac and cerebrovascular events | 2 | 1.09 | 0.87–1.35 | 0 | 0.45 |
HR, hazard ratio; CI, confidence interval.
3.2.4. Subgroup analyses
Subgroup analyses did not reveal any discrepancies in the results for MACCEs, depending on participants' age, sex, presence of diabetes mellitus (DM) or chronic kidney disease (CKD) (Table 6). The similar pattern was observed for major bleeding with exception of female patients who tended to have more major bleeding complications than male patients with ticagrelor treatment (test for subgroup differences I2 = 69.7% when HR was an effect measure).
Table 6.
Subgroup analysis.
| Study name | Ticagrelor versus clopidogrel |
|||||
|---|---|---|---|---|---|---|
| MACCE | ||||||
| OR (95% CI) | I2 statistics, % | p-value | HR (95% CI) | I2 statistics, % | p-value | |
| Age | N/Aa | |||||
| Elderly | 0.81 (0.63; 1.05) | 0 | 0.11 | |||
| Nonelderly | 0.56 (0.20; 1.59) | 76 | 0.27 | |||
| Sex | N/A | |||||
| Male | 0.72 (0.38; 1.36) | 51 | 0.31 | |||
| Female | 0.94 (0.29; 3.02) | 56 | 0.92 | |||
| DM status | N/A | |||||
| DM | 0.73 (0.60; 0.89) | 0 | 0 | |||
| Non-DM | 0.82 (0.68; 0.99) | 0 | 0.04 | |||
| CKD status | N/A | |||||
| CKD | 0.86 (0.62; 1.17) | 0 | 0.33 | |||
| Non-CKD | 0.65 (0.29; 1.43) | 46 | 0.28 | |||
| Major bleeding | ||||||
| Age | ||||||
| Elderly | 1.23 (0.74; 2.03) | 52 | 0.43 | 1.65 (0.72; 3.79) | 65 | 0.24 |
| Nonelderly | 1.35 (0.34; 5.38) | 87 | 0.67 | 1.10 (0.63; 1.90) | 60 | 0.75 |
| Sex | ||||||
| Male | 0.91 (0.41; 2.02) | 83 | 0.82 | 0.99 (0.59; 1.64) | 60 | 0.96 |
| Female | 1.72 (0.78; 3.81) | 62 | 0.18 | 1.84 (1.19; 2.86) | 0 | 0.007 |
| DM status | ||||||
| DM | 1.03 (0.49; 2.15) | 59 | 0.95 | 1.00 (0.68; 1.47) | 9 | 0.99 |
| Non-DM | 1.30 (0.38; 4.42) | 91 | 0.68 | 1.45 (0.50; 4.18) | 87 | 0.49 |
| CKD status | ||||||
| CKD | 1.30 (0.26; 6.51) | 75 | 0.75 | 1.44 (0.40; 5.17) | 61 | 0.57 |
| Non-CKD | 1.24 (0.53; 2.87) | 86 | 0.62 | 1.33 (0.75; 2.36) | 70 | 0.33 |
MACCEs, major adverse cardiac and cerebrovascular events; OR, odds ratio; CI, confidence interval; HR, hazard ratio; N/A, not available; DM, diabetes mellitus; CKD, chronic kidney disease.
The original report did not provide crude event data.
3.2.5. Additional sensitivity analysis
As several groups of scientists conducted their studies investigating the data from the KAMIR-NIH registry, we performed the sensitivity analysis by including these studies one by one instead of data from Sim et al. However, no major changes was detected in pooled OR for MACCEs and major bleeding (Table 4).
In addition, the estimated effect size did not altered significantly after eliminating the studies without propensity score matching (MACCEs: OR = 0.72; 95% CI: 0.56–0.93, I2 = 62%, p = 0.01; major bleeding: OR = 1.24; 95% CI: 0.65–2.37, I2 = 80%, p = 0.52).
4. Discussion
Although several meta-analyses of RCTs devoted to this subject have been published recently, this review seems to be the first meta-analysis of observational studies on ticagrelor use in Asian patients with ACS in real-world practice. As ticagrelor did not reduce significantly the risk of all-cause mortality, cardiovascular death, MI and TVR, we conclude that the decrease in the MACCE rates was driven mainly by the decline in the stroke incidence in ticagrelor compared with clopidogrel users, which was consistent even after excluding Lee et al's study. In addition, while ticagrelor was not associated with the major bleeding risk, it was, nevertheless, accompanied with a considerable rise in major/minor and minor bleeding as opposed to clopidogrel.
The recent meta-analyses of RCTs demonstrated a strong association of ticagrelor with an increase in major bleeding among Asians without any ischaemic benefits.7, 8 Notably, only two properly designed RCTs with a sample size of 1907 patients are available at present.9, 25 Strong inclusion and exclusion criteria, small sample sizes, low rates of events, a nonsuperiority design of Goto et al's trial and a retrospective design of the Asian subanalysis of the PLATO trial make it difficult to extrapolate their findings on general Asian population. From this point of view, the fact that our meta-analysis included two large national-wide observational studies obviously increased the external validity of our results.
The inconsistency in the results between the observational investigations can be partially explained by the different characteristics of the study population among the reported studies. For example, among studies with low risk of bias, statins tended to be administered more frequently in investigations, which failed to demonstrate the efficacy of ticagrelor. As a result, ticagrelor might not reach statistical significance in MACCE prevention in these studies because of low rates of events.
In addition, a positive effect of ticagrelor on MACCEs tended to be detected in the studies where study population had a higher prevalence of DM, CKD, dyslipidaemia and chronic heart failure as opposed to the studies with a neutral effect of ticagrelor on MACCEs (Table 1). Consequently, it can be assumed that the baseline risk profile of patients with ACS should be bearing in mind while administering ticagrelor. This conception might be further supported by recently published data. Based on the nationwide KAMIR-NIH registry, Sim et al found that ticagrelor proved its superiority over clopidogrel in prevention of all-cause death only in patients with high (>140 points) Global Registry of Acute Coronary Events risk score. Moreover, the benefits of ticagrelor in patients with high ischaemic risk were demonstrated if bleeding risk according to the ACUITY-HORIZONS score was low (below 20 points).19 In addition, Wang et al indicated that ticagrelor reduced the MACCE incidence in individuals with low baseline bleeding risk according to the CRUSADE score. On the other hand, the increase of major bleeding was shown only in patients with moderate to high bleeding potential.20 Further large-scale studies are required to establish the best strategies of ticagrelor use depending on the baseline risk estimation.
The discrepancies in the findings among the retrieved studies also can be explained by different study endpoint definitions. Lee et al considered as major bleeding the cases with intracranial haemorrhage or major gastrointestinal bleeding.17 Sim et al, meanwhile, used thrombolysis in myocardial infarction (TIMI) definition for major bleeding, which included a decrease in a haemoglobin level of more than 50 g/l.19 Therefore, Lee et al could underestimate the real impact of ticagrelor on major bleeding according to the TIMI scale. Given the fact that the true magnitude of bias could not be evaluated, our data on the safety of ticagrelor should be considered with caution.
Our meta-analysis suggested that ticagrelor had a substantial protective effect on stroke in Asian patients with ACS. Given the consistency between the included observational studies, these results are unlikely to be due to a play of chance.16, 17, 18, 19, 20, 21 Of note, ticagrelor treatment increased, although insignificantly, the rates of stroke in the PLATO trial (HR = 1.17; 95% CI: 0.91–1.52), Asian subanalysis of the PLATO trial (HR = 1.01; 95% CI: 0.44–2.32) and Goto et al's trial (HR = 1.50; 95% CI: 0.54–4.23).3, 9, 25 This discrepancy is likely to be related to the aforementioned limitations of RCTs. In addition, our findings are consistent with recently published data, claiming the benefits of ticagrelor for stroke prevention.26, 27
Concerning the subgroup analyses, ticagrelor seemed to increase the major bleeding complications among female as opposed to male patients. Although the findings from subgroup analyses could be misleading,13 conflicting and limited data from other trials call for high-quality studies to investigate the gender differences in bleeding risk with ticagrelor treatment, especially in Asian population.9, 28, 29
Another direction for future research is the application of reduced doses of ticagrelor in Asians. Although recent studies provided promising findings, the current evidence is somewhat insufficient. The future large-scale investigations are warranted to reach definite conclusions.30, 31, 32
4.1. Limitations
There are some limitations of this review that should be mentioned. First, selection biases are prone for observational studies and could not be avoided in their pooled analysis. Second, our meta-analysis was limited by a low number of the included studies. Third, all the studies in the meta-analysis had a retrospective design. Consequently, further high-quality researches studies are needed to ascertain our findings.
5. Conclusions
This meta-analysis suggested that ticagrelor might reduce the risk of MACCEs mainly by decreasing the stroke incidence in Asian patients with ACS. Compared with clopidogrel, ticagrelor did not demonstrate a significant effect on the rates of all-cause cardiovascular mortality, MI, TVR, NACCEs and major bleeding. Ticagrelor was associated with a considerable rise in the major and minor bleeding complications.
However, further high-quality studies are of utmost importance, especially on the use of ticagrelor, depending on the baseline risk profile of patients.
Conflicts of interest
All authors have none to declare.
References
- 1.Gurbel P.A., Bliden K.P., Butler K. Randomized double-blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease. Circulation. 2009;120:2577–2585. doi: 10.1161/CIRCULATIONAHA.109.912550. [DOI] [PubMed] [Google Scholar]
- 2.Storey R.F., Husted S., Harrington R.A. Inhibition of platelet aggregation by AZD6140, a reversible oral P2Y12 receptor antagonist, compared with clopidogrel in patients with acute coronary syndromes. J Am Coll Cardiol. 2007;50(19):1852–1856. doi: 10.1016/j.jacc.2007.07.058. [DOI] [PubMed] [Google Scholar]
- 3.Wallentin L., Becker R.C., Budaj A. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 4.Levine G.N., Bates E.R., Bittl J.A. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2016;68(10):1082–1115. doi: 10.1016/j.jacc.2016.03.513. [DOI] [PubMed] [Google Scholar]
- 5.Valgimigli M., Bueno H., Byrne R.A. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS. The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2017;0:1–48. [Google Scholar]
- 6.Mak K.H., Bhatt D.L., Shao M. Ethnic variation in adverse cardiovascular outcomes and bleeding complications in the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) study. Am Heart J. 2009;157:658–665. doi: 10.1016/j.ahj.2008.08.031. [DOI] [PubMed] [Google Scholar]
- 7.Misumida N., Aoi S., Moon S., Ziada K.M., Abdel-latif A. Ticagrelor versus clopidogrel in East Asian patients with acute coronary syndrome: systematic review and meta-analysis. Cardiovasc Revascularization Med. 2018;19(6):689–694. doi: 10.1016/j.carrev.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 8.Wu B., Lin H., Tobe R.G., Zhang L., He B. Ticagrelor versus clopidogrel in East-Asian patients with acute coronary syndromes: a meta-analysis of randomized trials. J Comp Eff Res. 2017;7(3):281–291. doi: 10.2217/cer-2017-0074. Epub 2017 Nov 2. [DOI] [PubMed] [Google Scholar]
- 9.Goto S., Huang C., Park S., Emanuelsson H., Kimura T. Ticagrelor vs. clopidogrel in Japanese, Korean and Taiwanese patients with acute coronary syndrome — randomized, double-blind, phase III PHILO study. Circ J. 2015;79:2452–2460. doi: 10.1253/circj.CJ-15-0112. [DOI] [PubMed] [Google Scholar]
- 10.Wang H., Wang X. Efficacy and safety outcomes of ticagrelor compared with clopidogrel in elderly Chinese patients with acute coronary syndrome. Ther Clin Risk Manag. 2016;12:1101–1105. doi: 10.2147/TCRM.S108965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yao G., Su G., Li K., Li B., Dong H. Comparative study of ticagrelor and clopidogrel in therapeutic effect of acute myocardial infarction patients undergoing percutaneous coronary intervention. Saudi J Biol Sci. 2017;24(8):1818–1820. doi: 10.1016/j.sjbs.2017.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moher D., Liberati A., Tetzlaff J., Altman D.G., The PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins J., Green S., editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (Updated March 2011) The Cochrane Collaboration; 2011. http://handbook.cochrane.org Available from: [Google Scholar]
- 14.Wells GA, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale for assessing the quality of nonrandomised studies in meta-analyses. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 1 October 2018.
- 15.Tierney J.F., Stewart L.A., Ghersi D. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8(suppl 1):16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen I., Lee C., Fang C. Efficacy and safety of ticagrelor versus clopidogrel in acute coronary syndrome in Taiwan: a multicenter retrospective pilot study. J Chin Med Assoc. 2016;79(10):521–530. doi: 10.1016/j.jcma.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 17.Lee C., Cheng C., Yang Y.K., Chao T., Chen J., Li Y. Cardiovascular and bleeding risks in acute myocardial infarction newly treated with ticagrelor vs. clopidogrel in Taiwan. Circ J. 2018;82:747–756. doi: 10.1253/circj.CJ-17-0632. [DOI] [PubMed] [Google Scholar]
- 18.Nur'amin H.W., Dwiprahasto I., Kristin E. Effectiveness of ticagrelor compared to clopidogrel in reducing the risk of major adverse cardiovascular events in patients with coronary heart disease after percutaneous coronary intervention. Int J Pharm Pharm Sci. 2017;9(9):178–183. [Google Scholar]
- 19.Sim D.S., Jeong M.H., Kim H.S. Utility of GRACE and ACUITY-HORIZONS risk scores to guide dual antiplatelet therapy in Korean patients with acute myocardial infarction undergoing drug-eluting stenting. J Cardiol. 2018;72(411):419. doi: 10.1016/j.jjcc.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 20.Wang H.Y., Li Y., Xu X.M., Li J., Han Y.L. Impact of baseline bleeding risk on efficacy and safety of ticagrelor versus clopidogrel in Chinese patients with acute coronary syndrome undergoing percutaneous coronary intervention. Chin Med J Engl. 2018;131(17):2017–2024. doi: 10.4103/0366-6999.239306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xin Y., Zhang H., Li Y. Efficacy and safety of ticagrelor versus clopidogrel with different dosage in high-risk patients with acute coronary syndrome. Int J Cardiol. 2017;228:275–279. doi: 10.1016/j.ijcard.2016.11.160. [DOI] [PubMed] [Google Scholar]
- 22.Choe J.C., Cha K.S., Ahn J. Comparison of prescription rates and clinical outcomes in acute coronary syndrome patients who underwent percutaneous coronary intervention using different P2Y12 inhibitors in a large observational study. Int J Cardiol. 2019;274:21–26. doi: 10.1016/j.ijcard.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 23.Kang J., Han J.-K., Ahn Y. Third-generation P2Y12 inhibitors in East Asian acute myocardial infarction patients: a Nationwide Prospective Multicentre study. Thromb Haemost. 2018;118(3):591–600. doi: 10.1055/s-0038-1626697. [DOI] [PubMed] [Google Scholar]
- 24.Park K.-H., Jeong M.H., Ahn Y. Comparison of short-term clinical outcomes between ticagrelor versus clopidogrel in patients with acute myocardial infarction undergoing successful revascularization; from Korea Acute Myocardial Infarction Registry – National Institute of Health. Int J Cardiol. 2016;215:193–200. doi: 10.1016/j.ijcard.2016.04.044. [DOI] [PubMed] [Google Scholar]
- 25.Kang H., Clare R.M., Gao R. Ticagrelor versus clopidogrel in Asian patients with acute coronary syndrome: a retrospective analysis from the Platelet Inhibition and Patient Outcomes (PLATO) Trial. Am Heart J. 2015;169(6):899–905.e1. doi: 10.1016/j.ahj.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 26.Malhotra K., Goyal N., Kasunich A.S. Ticagrelor for stroke prevention in patients with vascular risk factors: a systematic review and meta-analysis. J Neurol Sci. 2018 Jul 15;390:212–218. doi: 10.1016/j.jns.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Bonaca M.P., Goto S., Bhatt D.L. Prevention of stroke with ticagrelor in patients with prior myocardial infarction. Circulation. 2016;134:861–871. doi: 10.1161/CIRCULATIONAHA.116.024637. [DOI] [PubMed] [Google Scholar]
- 28.Xanthopoulou I., Davlouros P., Deftereos S. Gender-related differences in antiplatelet treatment patterns and outcome: insights from the GReekAntiPlatElet Registry. 2017;35(4):e12270. doi: 10.1111/1755-5922.12270. [DOI] [PubMed] [Google Scholar]
- 29.Lau E.S., Braunwald E., Murphy S.A. Potent P2Y12 inhibitors in men versus women. A colloborative meta-analysis of randomized trials. J Am Coll Cardiol. 2017;69(12):1549–1559. doi: 10.1016/j.jacc.2017.01.028. [DOI] [PubMed] [Google Scholar]
- 30.Lee Y.J., Kim H., Choi J. Evaluation of pharmacokinetic, pharmacodynamic, efficacy, and safety data of low-dose ticagrelor versus standard dose in East Asians: a systematic review. Ther Clin Risk Manag. 2018;14:83–93. doi: 10.2147/TCRM.S152276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi K.-N., Jin H.-Y., Shin H.-C. Comparison of the antiplatelet effects of once and twice daily low-dose ticagrelor and clopidogrel after percutaneous coronary intervention. Am J Cardiol. 2017;120(2):201–206. doi: 10.1016/j.amjcard.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 32.He M.J., Liu B., Sun D.H. One-quarter standard-dose ticagrelor better than standard-dose clopidogrel in Chinese patients with stable coronary artery disease: a randomized, single-blind, crossover clinical study. Int J Cardiol. 2016;215:209–213. doi: 10.1016/j.ijcard.2016.04.087. [DOI] [PubMed] [Google Scholar]