Abstract
BACKGROUND
Respiratory syncytial virus (RSV) is a leading cause of lower respiratory infections among children.
AIM
To investigate the proportion of RSV and non-RSV respiratory viral infections among hospitalized children ≤ 5 years.
METHODS
Hospitalized children aged < 5 years, with a diagnosis of acute lower respiratory infections (ALRI), admitted between August 2011-August 2013, were included. Cases were defined as laboratory-confirmed RSV and non-RSV respiratory viruses by direct fluorescence assay from the nasopharyngeal wash.
RESULTS
Of 383 1-59 mo old children hospitalized with an acute lower respiratory infection, 33.9% (130/383) had evidence of viral infection, and RSV was detected in 24.5% (94/383). Co-infections with RSV and other respiratory viruses (influenza A or B, adenovirus, para influenza 1, 2 or 3) were seen in children 5.5% (21/383). Over 90% of the RSV-positive children were under 2 years of age. RSV was detected throughout the year with peaks seen after the monsoon season. Children hospitalized with RSV infection were more likely to have been exposed to a shorter duration of breastfeeding of less than 3 mo. RSV positive children had a shorter hospital stay, although there were significant complications requiring intensive care. Use of antibiotics was high among those with RSV and non-RSV viral infections.
CONCLUSION
Our study provides evidence of a high proportion of RSV and other virus-associated ALRI among hospitalized children in India. RSV infection was associated with fewer days of hospital stay compared to other causes of lower respiratory infections. A high level of antibiotic use was seen among all respiratory virus-associated hospitalizations. These results suggest the need for implementing routine diagnostics for respiratory pathogens in order to minimize the use of unnecessary antibiotics and plan prevention strategies among pediatric populations.
Keywords: Respiratory syncytial virus, Acute lower respiratory infections, Children, Epidemiology, India, Respiratory viral infection
Core tip: The study shows that a significant proportion of young children hospitalized for acute lower respiratory tract infection were associated with respiratory syncytial virus (RSV) and other viral infections. Early diagnosis of viral infections using a simple test such as the RSV and viral direct fluorescence assay test, in settings where PCR is not feasible, would be useful in the timely institution of appropriate care, minimization of antibiotic overuse, and appropriate follow-up care for complications and sequelae, potentially leading to a reduction of costs of medical care.
INTRODUCTION
Globally, acute lower respiratory infections (ALRI) are an important cause of morbidity and mortality in children < 5 years of age[1]. Molecular diagnostic methods have identified respiratory syncytial virus (RSV), as the most common viral cause of ALRI-related death; other prominent viruses are human metapneumovirus, parainfluenza viruses, influenza viruses A and B and adenoviruses[2,3]. In 2015, Shi et al[4] reported an estimated annual burden of RSV of 33.1 million globally (22% of all episodes of ALRI) resulting in 3.2 million hospitalizations and 59600 deaths of children < 5 years, with about 99% of RSV-related childhood mortality occurring in low and middle-income countries (LMICs)[4].
Recent literature on pediatric ALRI in LMICs highlights increasing incidence of RSV, a major cause of death of infants < 1 year of age and of ALRI during the first few months of an infant’s life[5]. RSV is also gaining prominence in India; recent hospital-based studies indicate that RSV constitutes up to 16% of children hospitalized with acute lower respiratory tract infections (LRTIs), with incidence highest in infants aged below 6 mo[6]. The common respiratory symptoms are recurrent episodes of wheezing mimicking early childhood asthma and may persist as lung function abnormality till adolescence[7]. Known risk factors include low birth weight, smoking during pregnancy, attendance at a child care facility, crowded household, low parental education, exposure to second-hand smoke, history of atopy and lack of breastfeeding[8]. Furthermore, RSV infections tend to be associated with hospitalization and mortality in high-risk cases[7].
Detection of RSV using rapid, sensitive and specific diagnostic tests aids good clinical management. Viral isolation by tissue culture is regarded as the gold standard but has limited availability. Real-time reverse transcriptase PCR (RT-PCR) is more sensitive but not routinely performed in clinical diagnostic laboratories in LMICs due to high expense, the need for technical expertise and high laboratory standards to prevent contamination[9]. Direct fluorescent antibody assay (DFA) has been used as a simple detection tool for RSV antigen detection[10]. DFA has a sensitivity and specificity of 77.8% and 96.8% respectively and can detect RSV antigens even in conditions where the virus cannot be isolated[10,11].
The objective of the study was to investigate the proportion of RSV and non-RSV respiratory viral infections as a cause of ALRI among 1-59 mo old children admitted to a tertiary care hospital in southern India. The study also sought to assess the seasonality, clinical features, risk factors and outcome of RSV and non-RSV respiratory viral infections among these hospitalized children.
MATERIALS AND METHODS
Patients and settings
This prospective study was conducted at a tertiary care center in south India between August 2011 and August 2013, on children aged between 1 mo and 5 years, hospitalized for ALRI. The study site, St. John’s Medical College Hospital, Bengaluru, Karnataka, India center to local as well as to referred cases from neighboring states like Andhra Pradesh and Tamil Nadu[12]. ALRI was defined as acute respiratory infection with evidence of respiratory distress and age-specific fast breathing (≤ 2 mo age: ≥ 60 breaths/min; 2-12 mo, ≥ 50 breaths/min; 1-5 years, ≥ 40 breaths/min)[13]. Features of respiratory distress include chest indrawing, stridor, nasal flaring or grunting, inability to breastfeed or drink, with or without danger signs such as central cyanosis, lethargy or unconsciousness. Bronchiolitis was defined as a viral LRTI in children < 2 years of age characterized byclinical features of small airways obstruction causing symptoms of ALRI. Bronchopneumonia was defined as symptoms of ALRI with chest X-ray findings of hyperinflation with bilateral interstitial infiltrates and peribronchial cuffing. Lobar pneumonia was defined as symptoms of ALRI with confluent lobar consolidation. Reactive airway disease was defined as respiratory illness associated with wheeze secondary to allergen exposure. The study excluded children with empyema, hydropneumothorax or tuberculosis and those with non-respiratory causes of respiratory distress. Informed consent was obtained from the caregivers of eligible children. Detailed patient history and clinical observations were recorded. Vital signs and oxygen saturation were monitored and routine laboratory investigations were performed as indicated. Ethical clearance was obtained from the Institutional Ethics Committee (IRB No 134/2008) at St. Johns Medical College Hospital prior to initiating the study.
Sample collection
Nasopharyngeal (NP) wash were collected using a 5 French infant feeding tube cut to 4 cm length, 1.5 mL of sterile saline was quickly introduced into each nostril and immediately aspirated back into a 2 mL disposable sterile syringe. The aspirated sample was transferred into a sterile centrifuge tube, maintained at 4 ºC and transported to the microbiology laboratory immediately on an ice pack (4 °C) within 1 h of collection[14].
Slide preparation and direct immunofluorescence assay
The NP sample was centrifuged and the pallet was smeared on a slide, air dried and placed in cold acetone (-20 ºC) for a minimum of 30 min for fixation. Slides were stained using SimulFluor Respiratory Screen kit (Chemicon International, United States), in accordance with the manufacturer’s instructions.
Statistical analysis
The data was compiled in an excel spreadsheet. Descriptive statistics were reported using mean and standard deviation for variables with a normal distribution, and median for variables without a normal distribution. The association between the presence of RSV infection and RSV-related risk factors were assessed by χ2 test or Fisher’s exact test (for categorical variables) and by independent t-test (for continuous variables). The analysis was done by using the Statistical software STATA/IC version 12.1 and P < 0.05 was considered significant.
RESULTS
Study population
Between August 2011 and August 2013, 9600 children were admitted to the inpatient ward; of these 408 were 1-59 mo old at the time of admission, had ALRI, and were considered eligible for the study. There were 20 caregivers of patients who declined consent citing reasons of non-interest or refusal of permission for the NP wash procedure, and 5 were excluded due to the presence of other diagnoses (2 were diagnosed with pulmonary tuberculosis, and 3 were diagnosed with bacterial pneumonia (one blood culture positive for Staphylococcus aureus and two broncho-alveolar lavage fluid positive for Pseudomonas aeuruginosa and Enterobacter species). The remaining 383 children were included in the study. The median age of subjects was 8 mo (inter quartile range 5-15 mo), and 89.0% were less than 2 years of age. There were 68.7% males, and the subjects resided in Karnataka (64.2%), Andhra Pradesh (19.1%) and Tamil Nadu (15.9%). The majority lived in urban areas (66.8%).
Etiology of respiratory illness
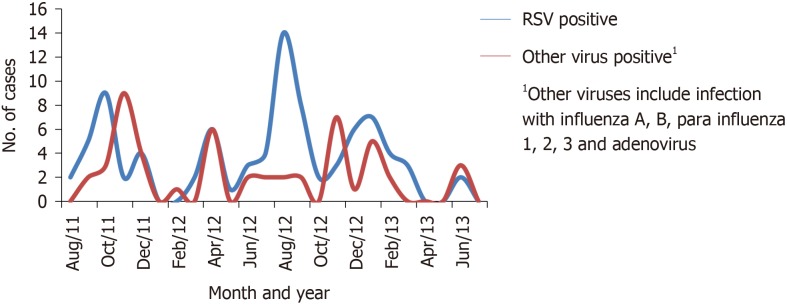
Viral etiology (RSV, influenza A or B, adenovirus, para influenza 1, 2 or 3) was confirmed in 130 (33.9%) of 383 children hospitalized for ALRI. RSV was positive in 94 (24.5%), non-RSV viruses in 57 (14.8%), while co-infection with RSV and non-RSV viruses was seen in 21 (5.5%) children (Table 1). Among the children with RSV infection, 86 (91.5%) children were in the age group 1 mo to 24 mo, and 32 (34%) were children 1-6 mo of age. A peak of RSV positive cases was seen after the rainy season during the months of August through November (Figure 1). A smaller peak was also noted during January and February. The seasonality of other viruses mirrored the pattern of RSV infection.
Table 1.
Respiratory syncytial virus and non-respiratory syncytial viruses associated with lower respiratory tract infection in young children
| RSV | 94 (24.5%) |
| All virus (RSV and/or other virus1) | 130 (34.0%) |
| Co-infection with multiple viruses | 21 (5.5%) |
| Non-RSV viruses alone1 | 57 (14.8%) |
| Virus-negative | 253 (66%) |
| Total enrolled | 383 |
Other viruses include infection with parainfluenza viruses, influenza viruses A and B and adenoviruses. RSV: Respiratory syncytial virus.
Figure 1.
Seasonality patterns of respiratory syncytial virus-positive and non-respiratory syncytial virus viral-associated lower respiratory tract infection in young children. 1Other viruses include infection with influenza A, B, para influenza 1, 2, 3 and adenovirus.
Clinical features and correlates
The predominant physician-assigned clinical diagnosis was bronchiolitis (173/383, 45.1%). Others were bronchopneumonia (135/383, 35.2%), lobar pneumonia (24/383, 6.2%) and reactive airway disease (51/383, 13.3%). Those with RSV infection had fever (23.8%), rhinorrhea (24.6%), cough, (23.5%), chest retractions (25.6%), auscultatory wheeze (22.9%), and auscultatory crepitations (25.7%). Chest radiographs were obtained in 189 of 383 children who had severe ALRI at admission or those who did not respond to first-line treatment. Radiographic abnormalities were detected in 162 children; 135 had evidence of hyperinflation with bilateral interstitial infiltrates suggestive of bronchopneumonia, 24 had focal lung consolidative changes and 3 had evidence of cardiomegaly without lung field abnormalities.
In univariate analysis, RSV infection was significantly associated with being exclusively breastfed for less than 3 mo, compared to those exclusively breastfed for 3-12 mo (unadjusted OR 1.98, 95%CI: 1.69-3.22). There was no significant association between RSV infection and independent variables such as low birth weight, prematurity, complicated neonatal course, family history of asthma, household smoking or indoor wood fuel usage (Tables 2 and 3). There was no significant association between other viruses (influenza A or B, adenovirus, para influenza 1, 2 or 3) and risk factors mentioned in Table 2. Laboratory parameters including total white blood cell count, and absolute lymphocyte count was similar between RSV-positive and RSV-negative patients.
Table 2.
Risk factors for respiratory syncytial virus infection
| Variable | RSV negative (n = 289) | RSV positive (n = 94) | P value | |
| Birth weight (≥ 2500 g) | No | 55 (75.3) | 18 (24.7) | 0.980 |
| Yes | 234 (75.4) | 76 (24.6) | ||
| Breast fed exclusively for ≥ 3 mo | No | 66 (68.0) | 31 (32.0) | 0.049 |
| Yes | 223 (77.9) | 63 (22.1) | ||
| Gestational age (≥ 37 wk) | No | 33 (75.0) | 11 (25.0) | 0.940 |
| Yes | 256 (75.5) | 83 (24.5) | ||
| Neonatal complications | No | 241 (74.8) | 81 (25.2) | 0.522 |
| Yes | 48 (78.6) | 13 (21.4) | ||
| Family history of asthma | No | 256 (75.9) | 81 (24.1) | 0.532 |
| Yes | 33 (71.7) | 13 (28.3) | ||
| History of smoking in the household | No | 220 (73.8) | 78 (26.2) | 0.165 |
| Yes | 69 (81.1) | 16 (18.9) | ||
| Kitchen type | Indoor with partition | 202 (74.2) | 70 (25.8) | 0.484 |
| Indoor without partition | 85 (78.7) | 23 (21.3) | ||
| Open air | 2 (66.6) | 1 (33.4) | ||
| Cooking fuel | Electric | 2 (100) | 0 (0) | 0.695 |
| Kerosene | 6 (66.6) | 3 (33.4) | ||
| Liquefied petroleum gas | 241 (74.8) | 81 (25.2) | ||
| Wood/dung | 40 (80.0) | 10 (20.0) | ||
| PICU admission | No | 245 (75.2) | 81 (24.8) | 0.741 |
| Yes | 44 (77.2) | 13 (22.8) | ||
RSV: Respiratory syncytial virus; PICU: Pediatric intensive care unit.
Table 3.
Risk factors among respiratory viral infection
| Variable | No virus detected (n = 253) | Respiratory viral positive (n = 130) | P value | |
| Birth weight (≥ 2500 g) | No | 48 | 22 | 0.623 |
| Yes | 205 | 108 | ||
| Breast fed exclusively for ≥ 3 mo | No | 198 | 92 | 0.106 |
| Yes | 55 | 38 | ||
| Gestational age (≥ 37 wk) | No | 30 | 14 | 0.752 |
| Yes | 223 | 116 | ||
| Neonatal complications | No | 214 | 111 | 0.836 |
| Yes | 39 | 19 | ||
| Family history of asthma | No | 214 | 114 | 0.751 |
| Yes | 27 | 16 | ||
| History of smoking in the household | No | 195 | 106 | 0.315 |
| Yes | 58 | 24 | ||
| Kitchen type | Indoor with partition | 174 | 99 | 0.313 |
| Indoor without partition | 77 | 30 | ||
| Open air | 2 | 1 | ||
| PICU admission | No | 214 | 112 | 0.683 |
| Yes | 39 | 18 | ||
RSV: Respiratory syncytial virus; PICU: Pediatric intensive care unit.
Outcome
Mean hospital stay was 4.6 d (SD 5.1); and 8.3 d (SD 6.5) in RSV-positive and RSV-negative children, respectively (P = 0.031) (Table 4). The length of PICU stay (P = 0.547), oxygen use (P = 0.176), and qualitative antibiotic use (P = 0.110) were similar in both groups. Antibiotics were used among 56 (59.5%) and 21 (36.8%) of RSV-positive and non-RSV virus-positive children, respectively. Most commonly used first-line antibiotics were amoxicillin/amoxicillin-clavulanic acid/or ceftriaxone. Respiratory complications such as acute respiratory distress syndrome and respiratory failure requiring PICU admission took place among 13.8% (13/94) of RSV-positive, 8.7% (5/57) of non-RSV positive and 16.6% (42/253) of viral negative children. Of the 57 children admitted to PICU, 46 recovered well while 11 had residual respiratory symptoms including persistent tachypnea and persistent oxygen requirement. There was no mortality recorded in this study (Table 5).
Table 4.
Comparison of outcome between respiratory syncytial virus positive and respiratory syncytial virus negative
| Days of |
RSV |
P value | |
| Positive | Negative | ||
| Hospital stay | 4.0 (1.0-17.0) | 4.0 (1.0-65.0) | 0.031 |
| Oxygen use | 1.5 (1.0-9.0) | 2.0 (1.0-65.0) | 0.176 |
| Antibiotic use | 4.0 (1.0-12.0) | 4.0 (1.0-29.0) | 0.303 |
| Nebulization use | 3.0 (1.0-17.0) | 4.0 (1.0-65.0) | 0.012 |
| PICU stay | 2.5 (1.0-10.0) | 2.00 (1.0-20.0) | 0.547 |
RSV: Respiratory syncytial virus; PICU: Pediatric intensive care unit.
Table 5.
Association of signs and symptoms with respiratory syncytial virus infection
| Variable | RSV positive (n = 94) | RSV negative (n = 289) | P value | |
| Fever | Yes | 63 (23.8) | 201 (76.2) | 0.645 |
| No | 31 (26.0) | 88 (74.0) | ||
| Rhinorrhea | Yes | 81 (24.6) | 248 (75.4) | 0.931 |
| No | 13 (24.0) | 41 (76.0) | ||
| Cough | Yes | 85 (23.5) | 276 (76.5) | 0.066 |
| No | 9 (40.9) | 13 (59.1) | ||
| Fast breathing | Yes | 62 (24.5) | 191 (75.5) | 0.981 |
| No | 32 (24.6) | 98 (75.4) | ||
| Wheezing | Yes | 45 (22.9) | 151 (77.1) | 0.461 |
| No | 49 (26.2) | 138 (73.8) | ||
| Auscultatory crepitations | Yes | 73 (25.7) | 210 (76.3) | 0.338 |
| No | 21 (21.0) | 79 (79.0) | ||
| Chest retractions | Yes | 52 (25.6) | 151 (74.4) | 0.604 |
| No | 42 (23.3) | 138 (76.6) | ||
RSV: Respiratory syncytial virus; PICU: Pediatric intensive care unit.
DISCUSSION
Viral-associated respiratory illness among hospitalized children 1-59 mo old at our single center tertiary care hospital in southern India was seen among a third of those with ALRI. RSV was the predominant viral etiological agent, and over a quarter had evidence of mixed viral pathogens in the respiratory tract that may be etiologically linked to the respiratory illness.RSV and other respiratory viruses are a major cause of pediatric ALRI in India. ALRI constitutes one of the principal causes of death among children under-five of the developing countries[8,15] and RSV has been documented as an important cause of ALRI in pediatric age group[8,14]. In India, studies have shown high ALRI incidence rates of 15.0 per 1000 child years in the under-five age group with the incidence being 3.6 times higher among boys as compared to girls[16]. The highest rates of ALRI generally occur in the first year of life. RSV infection in the very young causes substantial complications such as respiratory failure, prolonged hospitalization, and high mortality similar to seasonal influenza. Previous hospital-based studies from India reported hospital RSV prevalence of 11.4-26.0% (22.1%[14]; 20.2%[17]; 21.3%[18]; 12.0%[19]; 26.0%[20]), although the variability in these studies precludes any direct comparison with the present study. RSV-associated ALRI incidence ranging from 2.4% to 21.2% have been reported in different countries[21]. Hospital-based studies have reported a significant association between being male and having RSV-related ALRI[22]. Nair et al[5] concluded in a recent meta-analysis that RSV is the most common cause of childhood LRTI and a major cause of admission to hospital as a result of severe LRTI and that 99.0% of the RSV-related deaths take place among resource-limited countries. The study showed that India, China, Nigeria, Pakistan, and Indonesia together account for a total of 16 million cases of RSV infections, accounting for half of the global cases of under-5 childhood deaths in the world[1].
In India, routine laboratory diagnoses of viral ALRI remains unavailable and unexplored, even in tertiary care centers. Viral etiology of ALRI largely remains unknown and most cases are empirically treated with antibiotics. Gold standard test like viral isolation and RT-PCR are more sensitive but not routinely performed in clinical diagnostic laboratories in LMICs due to high expense LMICs due to high expense. However, in resource-limited settings, DFA still has value for the diagnosis of ALRI[23]. Compared to culture, DFA has sensitivities of 72% to 94% and specificities of 95% to 100%[24]. The DFA kit used in this study, SimulFluor Respiratory Screen Kit (Chemicon) was easy to perform and results were available within 4-5 h. It tested for a panel of seven respiratory viruses; RSV, Influenza A, and B, Parainfluenza 1 to 3 and Adenovirus[10,11]. Limitations of DFA include the degree of subjectivity in the evaluation of the result, as well as the need for a high level of technical skill and sample quality for the assay[25].
Early diagnosis facilitates early management and helps combat ALRIs. In India, antibiotics use in pediatric ALRI is very common even if a viral cause is suspected. A Cochrane review on the effectiveness of antibiotics in children fewer than two years old diagnosed with bronchiolitis did not find enough evidence to support the use of antibiotics[26]. Antibiotics can be used if there is clear documented evidence of secondary bacterial infections[8]. Early diagnosis of a viral respiratory infection has the potential to reduce the rampant use of antibiotics.
As established by the results, this study demonstrates that exclusive breastfeeding for over 3 mo of age, seems to have some protective effect against RSV and other respiratory viral infections. Significance between exclusive breastfeeding and decreased incidence of RSV positive illnesses have been reported[27]. However, there is no association between exclusive breastfeeding and wheezing illnesses associated with RSV as wheezing can be secondary to a maternal history of allergy and asthma or pet exposure[28]. This possibly can be explained by the fact that RSV is caused by infection and breastfeeding is often thought to confer some protection against it[29], whereas wheezing may be immune to any protective effects of breastfeeding since high IgE levels have been found to share an association with the presence of wheezing.
Our finding showed a high rate of respiratory complications in 13.8% (13/94), 8.7% (5/57) and 16.6% (42/253) of children with RSV positive, non-RSV positive and viral negative results. Out of these, 6 children were mechanically ventilated with a median of 4 d (range 3-5 d). Our findings were consistent with previous studies done by Kholy et al[30] in which 10% (24/240) of patients were admitted to PICU with a median duration of 6.5 d and 14 patients required mechanical ventilation.
Limitations of the study
In this study, we focused only on the viral causes of ALRI as they are the most common etiological agents and there are limited studies from south India. However, we did not address key questions about bacterial etiology and the possible role of viral and bacterial co-infections. We had a small sample size which was insufficient to make finer observations regarding differences in age, clinical profile, or factors determining severity between specific viral species.
We find that a significant proportion of young children hospitalized for acute LRTI is associated with RSV and other viral infections. Early diagnosis of viral infections using a simple test such as the RSV and viral DFA test, in settings where PCR is not feasible, would be useful in the timely institution of appropriate care, minimization of antibiotic overuse, and appropriate follow-up care for complications and sequelae, potentially leading to a reduction of costs of medical care.
ARTICLE HIGHLIGHTS
Research background
Respiratory syncytial virus (RSV) is the most frequent agent of viral-associated acute lower respiratory diseases (ALRI) and is known to be associated with hospitalization and mortality among high-risk cases.
Research motivation
Early diagnosis of viral infections using a simple test such as the RSV and viral fluorescent antibody assay (DFA) test, in settings where PCR is not feasible, would be useful in the timely institution of appropriate supportive care, minimization of antibiotic overuse, and appropriate follow-up care for complications and sequelae, potentially leading to a reduction of costs of medical care.
Research objectives
The principal objective of the study was to investigate the proportion of RSV and non-RSV respiratory viral infections as a cause of ALRI among 1-59 mo old children admitted to a tertiary care hospital in India. The study also assesses the seasonality, clinical features, risk factors and outcome of RSV and non-RSV respiratory viral infections among these hospitalized children.
Research methods
The prospective study was conducted on hospitalized children aged < 5 years, with a diagnosis of acute lower respiratory infections (ALRI), admitted between August 2011-August 2013, were included. Nasopharyngeal (NP) swabs were obtained from eligible children, and transported to the laboratory in suitable media. Slides were prepared from the media, and DFA staining was performed using SimulFluor Respiratory Screen kit on NP wash samples.
Research results
The median age of subjects was 8 mo (inter quartile range 5-15 mo), and 89.0% were less than 2 years of age. Viral etiology (RSV, influenza A or B, adenovirus, para influenza 1, 2 or 3) was confirmed in 33.9% (130/383) of children hospitalized for ALRI. RSV was positive in 24.5% (94/383) non-RSV viruses in 14.8% (57/383) while co-infection with RSV and non-RSV viruses was seen in 5.5% (21/383) children. A peak of RSV positive cases was seen after the rainy season during the months of August through November. The RSV infection was significantly associated with being exclusively breastfed for less than 3 mo. There was no significant association between RSV infection and independent variables such as low birth weight, prematurity, complicated neonatal course, family history of asthma, household smoking or indoor wood fuel usage. Mean hospital stay was 4.6 ± 5.1 d and 8.3 ± 6.5 d in RSV-positive and RSV-negative children, respectively. The respiratory complications such as acute respiratory distress syndrome and respiratory failure requiring PICU admission, were 13.8% (13/94), 8.7% (5/57) and 16.6% (42/253) of children with RSV positive, non-RSV positive and viral negative results.
Research conclusions
A high proportion of RSV and other virus-associated ALRI were seen among hospitalized children in India. The study demonstrates that exclusive breastfeeding for over 3 mo of age, may have a protective effect against RSV and other respiratory viral infections. The viral DFA test was easy to perform and results were available within 4-5 h.
Research perspectives
Early diagnosis of viral infections using a simple test such as the RSV and would be useful in the timely institution of appropriate supportive care, minimization of antibiotic overuse, and appropriate follow-up care for complications and sequelae, potentially leading to a reduction of costs of medical care.
ACKNOWLEDGEMENTS
We thank all medical staff and faculty members from the Department of Paediatrics and Microbiology at St. John’s Medical College Hospital for extending their support for this study.
Footnotes
Institutional review board statement: Ethical clearance was obtained from the Institutional Ethics Committee (IRB No 134/2008) at St. Johns Medical College Hospital prior to initiating the study.
Informed consent statement: Informed consent was obtained from the caregivers of eligible children.
Conflict-of-interest statement: The authors declare no conflict of interest.
Manuscript source: Unsolicited manuscript
Peer-review started: November 30, 2018
First decision: January 9, 2019
Article in press: February 27, 2019
Specialty type: Pediatrics
Country of origin: India
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Kenar T, Pandey A, Pavone P, Yang L S-Editor: Ji FF L-Editor: A E-Editor: Song H
Contributor Information
Sandesh Kini, Department of Paediatrics, Kasturba Medical College, Manipal Academy of Higher Education, Manipal 576104, Karnataka, India.
Bhuvanesh Sukhlal Kalal, Department of Biochemistry, Yenepoya Medical College, Yenepoya (Deemed to be University), Mangaluru 575018, Karnataka, India.
Sara Chandy, Pushpagiri Research Centre, Pushpagiri Institute of Medical Science and Research Centre, Thiruvalla 689101, Kerala, India.
Ranjani Shamsundar, Department of Microbiology, St. John’s Medical College, Bengaluru 560034, Karnataka, India.
Anita Shet, International Vaccine Access Center, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD 21205, United States. ashet1@jhu.edu.
References
- 1.Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu J, Lawn JE, Cousens S, Mathers C, Black RE. Global, regional, and national causes of under-5 mortality in 2000-15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet. 2016;388:3027–3035. doi: 10.1016/S0140-6736(16)31593-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oumei H, Xuefeng W, Jianping L, Kunling S, Rong M, Zhenze C, Li D, Huimin Y, Lining W, Zhaolan L, Xinmin L, Hua X, Zhiyan J, Yanning L, Yan H, Baoqing Z, Xiaochun F, Chunhui H, Yonghong J, Xue Z, Wei W, Zi W. Etiology of community-acquired pneumonia in 1500 hospitalized children. J Med Virol. 2018;90:421–428. doi: 10.1002/jmv.24963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brini I, Guerrero A, Hannachi N, Bouguila J, Orth-Höller D, Bouhlel A, Boughamoura L, Hetzer B, Borena W, Schiela B, Von Laer D, Boukadida J, Stoiber H. Epidemiology and clinical profile of pathogens responsible for the hospitalization of children in Sousse area, Tunisia. PLoS One. 2017;12:e0188325. doi: 10.1371/journal.pone.0188325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi T, McAllister DA, O'Brien KL, Simoes EAF, Madhi SA, Gessner BD, Polack FP, Balsells E, Acacio S, Aguayo C, Alassani I, Ali A, Antonio M, Awasthi S, Awori JO, Azziz-Baumgartner E, Baggett HC, Baillie VL, Balmaseda A, Barahona A, Basnet S, Bassat Q, Basualdo W, Bigogo G, Bont L, Breiman RF, Brooks WA, Broor S, Bruce N, Bruden D, Buchy P, Campbell S, Carosone-Link P, Chadha M, Chipeta J, Chou M, Clara W, Cohen C, de Cuellar E, Dang DA, Dash-Yandag B, Deloria-Knoll M, Dherani M, Eap T, Ebruke BE, Echavarria M, de Freitas Lázaro Emediato CC, Fasce RA, Feikin DR, Feng L, Gentile A, Gordon A, Goswami D, Goyet S, Groome M, Halasa N, Hirve S, Homaira N, Howie SRC, Jara J, Jroundi I, Kartasasmita CB, Khuri-Bulos N, Kotloff KL, Krishnan A, Libster R, Lopez O, Lucero MG, Lucion F, Lupisan SP, Marcone DN, McCracken JP, Mejia M, Moisi JC, Montgomery JM, Moore DP, Moraleda C, Moyes J, Munywoki P, Mutyara K, Nicol MP, Nokes DJ, Nymadawa P, da Costa Oliveira MT, Oshitani H, Pandey N, Paranhos-Baccalà G, Phillips LN, Picot VS, Rahman M, Rakoto-Andrianarivelo M, Rasmussen ZA, Rath BA, Robinson A, Romero C, Russomando G, Salimi V, Sawatwong P, Scheltema N, Schweiger B, Scott JAG, Seidenberg P, Shen K, Singleton R, Sotomayor V, Strand TA, Sutanto A, Sylla M, Tapia MD, Thamthitiwat S, Thomas ED, Tokarz R, Turner C, Venter M, Waicharoen S, Wang J, Watthanaworawit W, Yoshida LM, Yu H, Zar HJ, Campbell H, Nair H RSV Global Epidemiology Network. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet. 2017;390:946–958. doi: 10.1016/S0140-6736(17)30938-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O'Brien KL, Roca A, Wright PF, Bruce N, Chandran A, Theodoratou E, Sutanto A, Sedyaningsih ER, Ngama M, Munywoki PK, Kartasasmita C, Simões EA, Rudan I, Weber MW, Campbell H. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375:1545–1555. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saha S, Pandey BG, Choudekar A, Krishnan A, Gerber SI, Rai SK, Singh P, Chadha M, Lal RB, Broor S. Evaluation of case definitions for estimation of respiratory syncytial virus associated hospitalizations among children in a rural community of northern India. J Glob Health. 2015;5:010419. doi: 10.7189/jogh.05.020419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Resch B. Burden of respiratory syncytial virus infection in young children. World J Clin Pediatr. 2012;1:8–12. doi: 10.5409/wjcp.v1.i3.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi T, Balsells E, Wastnedge E, Singleton R, Rasmussen ZA, Zar HJ, Rath BA, Madhi SA, Campbell S, Vaccari LC, Bulkow LR, Thomas ED, Barnett W, Hoppe C, Campbell H, Nair H. Risk factors for respiratory syncytial virus associated with acute lower respiratory infection in children under five years: Systematic review and meta-analysis. J Glob Health. 2015;5:020416. doi: 10.7189/jogh.05.020416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Midgley CM, Haynes AK, Baumgardner JL, Chommanard C, Demas SW, Prill MM, Abedi GR, Curns AT, Watson JT, Gerber SI. Determining the Seasonality of Respiratory Syncytial Virus in the United States: The Impact of Increased Molecular Testing. J Infect Dis. 2017;216:345–355. doi: 10.1093/infdis/jix275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shafik CF, Mohareb EW, Youssef FG. Comparison of direct fluorescence assay and real-time rt-PCR as diagnostics for respiratory syncytial virus in young children. J Trop Med. 2011;2011:781919. doi: 10.1155/2011/781919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang YW, Crowe Jr JC. Washington, DC: American Society for Microbiology Press; 2007. Respiratory syncytial virus and human metapneumovirus. In: Murray PR, Baron EJ, Jorgensen JH, Landry ML, Pfaller MA (ed.). Manual of clinical microbiology, vol. 2. 9th ed; pp. 1361–1377. [Google Scholar]
- 12.Kalal BS, Puranik P, Nagaraj S, Rego S, Shet A. Scrub typhus and spotted fever among hospitalised children in South India: Clinical profile and serological epidemiology. Indian J Med Microbiol. 2016;34:293–298. doi: 10.4103/0255-0857.188315. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization (WHO) 2016. Department of child and adolescent health and development. (CAH). Integrated management of childhood illness (IMCI) Technical seminar acute respiratory infections. Available from: URL: http://www.who.int/maternal_child_adolescent/documents/pdfs/cah_01_10_ts_ari.pdf. [Google Scholar]
- 14.Gupta S, Shamsundar R, Shet A, Chawan R, Srinivasa H. Prevalence of respiratory syncytial virus infection among hospitalized children presenting with acute lower respiratory tract infections. Indian J Pediatr. 2011;78:1495–1497. doi: 10.1007/s12098-011-0491-0. [DOI] [PubMed] [Google Scholar]
- 15.Pinzón-Rondón ÁM, Aguilera-Otalvaro P, Zárate-Ardila C, Hoyos-Martínez A. Acute respiratory infection in children from developing nations: a multi-level study. Paediatr Int Child Health. 2016;36:84–90. doi: 10.1179/2046905515Y.0000000021. [DOI] [PubMed] [Google Scholar]
- 16.Krishnan A, Amarchand R, Gupta V, Lafond KE, Suliankatchi RA, Saha S, Rai S, Misra P, Purakayastha DR, Wahi A, Sreenivas V, Kapil A, Dawood F, Pandav CS, Broor S, Kapoor SK, Lal R, Widdowson MA. Epidemiology of acute respiratory infections in children - preliminary results of a cohort in a rural north Indian community. BMC Infect Dis. 2015;15:462. doi: 10.1186/s12879-015-1188-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bharaj P, Sullender WM, Kabra SK, Mani K, Cherian J, Tyagi V, Chahar HS, Kaushik S, Dar L, Broor S. Respiratory viral infections detected by multiplex PCR among pediatric patients with lower respiratory tract infections seen at an urban hospital in Delhi from 2005 to 2007. Virol J. 2009;6:89. doi: 10.1186/1743-422X-6-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh AK, Jain A, Jain B, Singh KP, Dangi T, Mohan M, Dwivedi M, Kumar R, Kushwaha RA, Singh JV, Mishra AC, Chhaddha MS. Viral aetiology of acute lower respiratory tract illness in hospitalised paediatric patients of a tertiary hospital: one year prospective study. Indian J Med Microbiol. 2014;32:13–18. doi: 10.4103/0255-0857.124288. [DOI] [PubMed] [Google Scholar]
- 19.Saxena S, Singh D, Zia A, Umrao J, Srivastava N, Pandey A, Singh S, Bhattacharya P, Kumari R, Kushwaha R, Dhole TN. Clinical characterization of influenza A and human respiratory syncytial virus among patients with influenza like illness. J Med Virol. 2017;89:49–54. doi: 10.1002/jmv.24607. [DOI] [PubMed] [Google Scholar]
- 20.Yeolekar LR, Damle RG, Kamat AN, Khude MR, Simha V, Pandit AN. Respiratory viruses in acute respiratory tract infections in Western India. Indian J Pediatr. 2008;75:341–345. doi: 10.1007/s12098-008-0035-4. [DOI] [PubMed] [Google Scholar]
- 21.Fall A, Dia N, Cisse el HA, Kiori DE, Sarr FD, Sy S, Goudiaby D, Richard V, Niang MN. Epidemiology and Molecular Characterization of Human Respiratory Syncytial Virus in Senegal after Four Consecutive Years of Surveillance, 2012-2015. PLoS One. 2016;11:e0157163. doi: 10.1371/journal.pone.0157163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mishra P, Nayak L, Das RR, Dwibedi B, Singh A. Viral Agents Causing Acute Respiratory Infections in Children under Five: A Study from Eastern India. Int J Pediatr. 2016;2016:7235482. doi: 10.1155/2016/7235482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simões EA, DeVincenzo JP, Boeckh M, Bont L, Crowe JE, Jr, Griffiths P, Hayden FG, Hodinka RL, Smyth RL, Spencer K, Thirstrup S, Walsh EE, Whitley RJ. Challenges and opportunities in developing respiratory syncytial virus therapeutics. J Infect Dis. 2015;211 Suppl 1:S1–S20. doi: 10.1093/infdis/jiu828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Popow-Kraupp T, Aberle JH. Diagnosis of respiratory syncytial virus infection. Open Microbiol J. 2011;5:128–134. doi: 10.2174/1874285801105010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anestad G. Surveillance of respiratory viral infections by rapid immunofluorescence diagnosis, with emphasis on virus interference. Epidemiol Infect. 1987;99:523–531. doi: 10.1017/s0950268800068023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farley R, Spurling GK, Eriksson L, Del Mar CB. Antibiotics for bronchiolitis in children under two years of age. Cochrane Database Syst Rev. 2014;(10):CD005189. doi: 10.1002/14651858.CD005189.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vereen S, Gebretsadik T, Hartert TV, Minton P, Woodward K, Liu Z, Carroll KN. Association between breast-feeding and severity of acute viral respiratory tract infection. Pediatr Infect Dis J. 2014;33:986–988. doi: 10.1097/INF.0000000000000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Celedón JC, Litonjua AA, Ryan L, Platts-Mills T, Weiss ST, Gold DR. Exposure to cat allergen, maternal history of asthma, and wheezing in first 5 years of life. Lancet. 2002;360:781–782. doi: 10.1016/S0140-6736(02)09906-3. [DOI] [PubMed] [Google Scholar]
- 29.Stein RT, Sherrill D, Morgan WJ, Holberg CJ, Halonen M, Taussig LM, Wright AL, Martinez FD. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet. 1999;354:541–545. doi: 10.1016/S0140-6736(98)10321-5. [DOI] [PubMed] [Google Scholar]
- 30.El Kholy AA, Mostafa NA, El-Sherbini SA, Ali AA, Ismail RI, Magdy RI, Hamdy MS, Soliman MS. Morbidity and outcome of severe respiratory syncytial virus infection. Pediatr Int. 2013;55:283–288. doi: 10.1111/ped.12051. [DOI] [PubMed] [Google Scholar]