Abstract
Sustaining a traumatic brain injury (TBI) during adolescence has a profound effect on brain development and can result in persistent executive functioning deficits in daily life. Cognitive recovery from pediatric-TBI relies on the potential of neuroplasticity, which can be fostered by restorative training-programs. However the structural mechanisms underlying cognitive recovery in the immature brain are poorly understood. This study investigated gray matter plasticity following 2 months of cognitive training in young patients with TBI. Sixteen adolescents in the chronic stage of moderate-severe-TBI (9 male, mean age = 15y8m ± 1y7m) were enrolled in a cognitive computerized training program for 8 weeks (5 times/week, 40 min/session). Pre-and post-intervention, and 6 months after completion of the training, participants underwent a comprehensive neurocognitive test-battery and anatomical Magnetic Resonance Imaging scans. We selected 9 cortical-subcortical Regions-Of-Interest associated with Executive Functioning (EF-ROIs) and 3 control regions from the Desikan-Killiany atlas. Baseline analyses showed significant decreased gray matter density in the superior frontal gyri p = 0.033, superior parietal gyri p = 0.015 and thalamus p = 0.006 in adolescents with TBI compared to age and gender matched controls. Linear mixed model analyses of longitudinal volumetric data of the EF-ROI revealed no strong evidence of training-related changes in the group with TBI. However, compared to the change over time in the control regions between post-intervention and 6 months follow-up, the change in the EF-ROIs showed a significant difference. Exploratory analyses revealed a negative correlation between the change on the Digit Symbol Substitution test and the change in volume of the putamen (r = −0.596, p = 0.015). This preliminary study contributes to the insights of training-related plasticity mechanisms after pediatric-TBI.
Keywords: Pediatric traumatic brain injury, Cognitive rehabilitation, Gray matter, Neural plasticity, Executive function, Linear mixed model analyses
Highlights
-
•
Longitudinal data on cortical – subcortical volume before and after training.
-
•
Post-training significant difference in change between ROI and control regions.
-
•
Post-training significant correlation Digit Symbol Substitution test and putamen.
-
•
Theory of an impaired capacity of plasticity in an immature traumatized brain.
-
•
Exploring plasticity is essential to provide foundation for rehab interventions.
1. Introduction
Pediatric traumatic brain injury (TBI) is one of the most common causes of acquired cognitive and behavioral disabilities in childhood and adolescence, with a long-term detrimental effect on daily executive functioning at home, school and in the social community (Chevignard et al., 2016; Prasad et al., 2017; Sariaslan et al., 2016; Shultz et al., 2016; Treble-Barna et al., 2017). Cognitive recovery from pediatric TBI is strongly related to neurocognitive training and the enhanced ability for reorganization present in the developing brain (Giza and Prins, 2006). The structural neuroplastic mechanisms underlying cognitive recovery are however poorly understood. It is assumed that changes in structural properties of neurons (such as the number of dendritic spines or synapses) may reflect changes in their function (Kolb et al., 2001). Moreover, previous papers in healthy adults suggested that training induced neuroplasticity may resemble developmental plasticity (Thomas and Baker, 2013; Wenger et al. 2017b), characterized by expansion of gray matter volume (based on neurogenesis, glial cell proliferation, dendritic spine growth and synaptogenesis) followed by gradual loss through dendritic and synaptic pruning (Maxwell, 2012; Mills et al., 2016; Sowell et al., 2002; Thomas and Baker, 2013; Treit et al., 2014; Zhou et al., 2015). This expansion–renormalization model for plastic changes post-training may result in a remodeling of activity in efficient neuronal circuits, contributing to improved functional performance (Holtmaat and Svoboda, 2009).
The use of advanced magnetic resonance imaging (MRI) to elucidate brain plasticity after cognitive training has extended in cognitive neuroscience, with an array of potential imaging methods such as task-related functional MRI (Ellis and Turk-Browne, 2018; Kou and Iraji, 2014; Sachs et al., 2017), diffusion MRI (Hutchinson et al., 2018) or voxel-based morphometry (Konstantinou et al., 2016; Lampit et al., 2015). Because of our interest in gray matter volume correlates of cognitive remediation in a longitudinal design, we preferred anatomical T1-weighted MRI scans processed with the longitudinal stream of FreeSurfer (Bernal-Rusiel et al., 2013; Reuter et al., 2012). Prior longitudinal studies with FreeSurfer into gray matter plasticity after cognitive training in healthy adults (Jiang et al., 2016; Lampit et al., 2015; Metzler-Baddeley et al., 2016; Roman et al., 2016) or adults with acquired brain injury (Caeyenberghs et al., 2018; Diez-Cirarda et al., 2017; Han et al., 2014; Han et al., 2017; Lazaridou et al., 2013), have identified opposite findings with either increase or decrease in cortical volume or thickness in task-relevant brain regions. Furthermore, small to moderate correlations between cortical alterations and cognitive performance at post-intervention were obtained, however without suggesting causality between these structural and behavioral changes (Caeyenberghs et al., 2018). To the best of our knowledge, the imaging evidence of gray matter plasticity in adolescents with TBI after cognitive training targeting impaired executive function is not yet provided.
We pre-defined nine cortical and subcortical regions-of-interest (ROI) from the Desikan-Killiany atlas (Desikan et al., 2006) associated with executive function (EF) (including the superior frontal gyrus, caudal part of the middle frontal gyrus, rostral part of the middle frontal gyrus, superior parietal gyrus, inferior parietal gyrus, anterior cingulate gyrus, caudate nucleus, putamen and thalamus) based on prior studies (Alcauter et al., 2014; Anderson and Ylvisaker, 2009; Andre et al., 2016; Breukelaar et al., 2017; Ferguson and Gao, 2014; Fryer et al., 2012; Greven et al., 2015; Hsu et al., 2014; Isbell et al., 2015; Levan et al., 2016; Little et al., 2010; Rosch et al., 2018; Snow, 2016; Velanova et al., 2008; Ware et al., 2016; Young et al., 2015). Additionally, to affirm anatomical specificity, we selected 3 control cortical regions (the primary visual cortex, primary auditory cortex and primary somatosensory cortex) whereby we did not expect to see alterations induced by a cognitive training program.
Our hypotheses were fourfold: (1) Firstly, we hypothesized that adolescents with TBI would show decreased gray matter density at baseline (pre-intervention) in regions associated with executive function compared to typically developing peers. (2) Secondly, we expected to observe an increase of gray matter volume in the ROIs related to EF in the absence of changes in the control regions after 8 weeks of cognitive training. (3) Thirdly, we expected a “re-normalization” of the cortical–subcortical gray matter volume of the EF-ROIs at 6 months follow-up, with a decrease or disappearance of the temporally expansion. (4) Finally, we hypothesized a correlation between changes in gray matter volume and improvements in executive functioning in response to the cognitive training intervention. A better understanding of functional and structural neural plasticity of executive function in the injured adolescent brain, is vitally important to advance appropriate treatment design in adolescents with TBI.
2. Materials and methods
2.1. Participants
We recruited sixteen adolescents (9 males and 7 females, mean age = 15y8m ± 1y7m) with moderate to severe traumatic brain injury (TBI) according to the Mayo Classification System for TBI (Malec et al., 2007) from the Child Rehabilitation Centre Ghent University Hospital, Belgium and the Rehabilitation Centre for children and adolescents Pulderbos, Belgium between March 2015 and January 2017. Causes of brain injury were traffic accidents or sports injuries. All the adolescents with TBI had evidence of a closed head injury with diffuse axonal injuries and cortical encephalomalacia as identified by neuroradiologists on the T1-weighted MRI. Specifically, the adolescents (n = 16) showed DAI in the frontal (n = 14), temporal (n = 13), parietal (n = 11) and occipital lobes (n = 8). Furthermore there were DAI in the corpus callosum (n = 9), brainstem (n = 3) and deep brain nuclei (n = 5). Areas of cortical encephalomalacia were seen in the frontal (n = 8), temporal (n = 8) and parietal (n = 1) cortex.
We included adolescents minimum 1 year and maximum 5 years post-injury, taking into account the rapid period of natural recovery within 1 year post injury (Jaffe et al., 1995; Keenan et al., 2018) and executive functioning ratings remaining relatively stable after this time point (Jaffe et al., 1995; Keenan et al., 2018). The average time between the accident and our study was 2y4m ± 1y2w and the mean age of the participants at the time of injury was 13y4m ± 1y7m. Apart from one participant, all patients with TBI attended a multidisciplinary rehabilitation in the acute phase post-injury (mean duration 11m4w ± 6m1w). At the end of the acute rehabilitation, all the adolescents were referred to a regular school with most of them receiving academic or psychosocial support. During each follow-up appointment after rehabilitation, the adolescents and their parents reported persisting impaired executive functioning in daily living.
The participants with TBI received a drill-based cognitive training with BrainGames, 40 min per session 5 days per week over 8 weeks. This cognitive training program was recently developed by our research group and showed promising results on executive performance in adolescents in the chronic stage of TBI in a small pilot study (Verhelst et al., 2017). Every training session with BrainGames comprised a self-guided module that consisted of 4 games (2 games that tapped into attention and 2 games that loaded on working memory/executive functioning). Task difficulty increased adaptively depending on the level of performance of the trainee. Training data were storaged automatically for each training session on a server, which enabled us to monitor compliance and progress of each participant. Once a week, the adolescents were contacted by phone regarding their training process.
Alongside the adolescents with TBI, we recruited sixteen age and gender matched control participants without a history of neurological condition (perfect gender match, mean age = 15y7m ± 1y8m) via the social network of the researches, to compare morphological MRI data and cognitive tests results at baseline. The control cohort did not receive the cognitive training program.
Long-term neurocognitive outcome after TBI and cortical volume has been shown to be moderated by familial inheritance and education (Noble et al., 2015; Walhovd et al., 2016). Therefore, we obtained the educational level of the biological parents by calculating the number of years of formal education. The average of the sum of the duration of education of the mother and father was 32y4m ± 1y10m for the typically developing controls which was significantly higher (p < 0.001) than 26y0m ± 5y6m for the adolescents with TBI. Written informed consent was obtained from the adolescents and their parents. The study was approved by the Ethics Committee of the Ghent University Hospital, Belgium.
2.2. Magnetic resonance imaging
2.2.1. MRI acquisition
The participants were scanned at Ghent University Hospital, Belgium, using the 3 T-Siemens Tim TRIO scanner equipped with a 32-channel head coil. A 3D-T1-weighted Magnetization Prepared Rapid Gradient Echo (MPRAGE) was administered with the following scanning parameters: repetition time/echo time = 2250/4.18 ms; acquisition time = 5min14s; flip angle = 9°; field of view = 256 mm; voxel size = 1,0 × 1.0 × 1,0mm3; slab thickness = 176 mm; bandwidth = 150 Hz/pixel). The TBI-adolescents were scanned at three time points: at baseline (pre-intervention, time point 0), within a week of completion of the last training session (post-intervention, time point 1) and 6 months post-intervention (time point 2). The passive control group of typically developing adolescents was scanned only once.
2.2.2. Pre-processing
At baseline (pre-intervention), cross-sectional morphological analyses of the T1-weighted MRI images of all participants were processed using the recon-all function of FreeSurfer version 5.3.0 (http://surfer.nmr.mgh.harvard.edu/), which has been described in detail in previous literature (Fischl, 2012).
To identify training-related morphological changes over the three time points in the TBI-group we used the longitudinal stream of FreeSurfer (Bernal-Rusiel et al., 2013; Reuter et al., 2012). In this longitudinal analysis, spatial normalization was obtained by an inverse and robust consistent registration algorithm, which created an unbiased within-subject template across the 3 time-points (Fischl, 2012; Metzler-Baddeley et al., 2016; Reuter et al., 2012; Tamnes et al., 2017; Thomas and Baker, 2013). To ensure the accuracy of the cortical surface reconstruction by FreeSurfer, all images were visually inspected post-processing and if needed manually corrected.
2.2.3. ROIs selection
We obtained the morphological metrics using the Desikan-Killiany atlas (Desikan et al., 2006). Several MRI and lesion studies offer a large body of evidence that executive functioning (EF) depends on a central executive network (Bettcher et al., 2016; Kim et al., 2017; Nowrangi et al., 2014) including the following important gray matter areas: the prefrontal and parietal cortex, the anterior cingulate cortex (Menon and Uddin, 2010) and basal ganglia (Brooks et al., 2016; Ware et al., 2016). Based on these previous findings, we selected 9 bilateral cortical-subcortical regions of interest (EF-ROIs): the superior frontal gyrus, caudal part of the middle frontal gyrus, rostral part of the middle frontal gyrus, superior parietal gyrus, inferior parietal gyrus, anterior cingulate gyrus, caudate nucleus, putamen and thalamus. Furthermore, we defined the precalcarine cortex (closely corresponding to the primary visual cortex), transverse temporal cortex (closely corresponding to the primary auditory cortex) and the postcentral gyrus (closely corresponding to the primary somatosensory cortex) as control regions. In the present study, we chose not to analyze cortical thickness and surface area separately, but to investigate cortical volumes to reduce the number of comparisons in our small sample size. By using volumes, the same measure for gray matter could be applied in the cortical and subcortical regions of interest. A close examination of the contributions of each hemisphere showed no significant main effect of cerebral hemisphere (right, left) on gray matter volume, and there were no differential symmetry effects across the group (both P > .05). Since diffuse injuries occur over a more widespread area, the bilateral sum of the volumes of these regions was used (Niemann et al., 2014). As such, the remaining analyses were collapsed across hemispheres. Finally, gray matter volumes were corrected for intracranial volume (ICV) (‘estimated total intracranial volume’ from FreeSurfer) (Malone et al., 2015; Nordenskjold et al., 2013; Walhovd et al., 2011) in the cross-sectional statistical analyses at baseline, to ensure that the observed gray matter volume differences between TBI and typically developing adolescents were independent of inter-individual variability in brain size (O'Brien et al., 2011).
2.3. Measures of executive functioning
Training-related improvements in executive functioning in the adolescents with TBI were assessed by means of a comprehensive neurocognitive test battery. In short, the Digit Span forwards and backwards (Donolato et al., 2017) was used to evaluate verbal working memory (working memory span, the number of digits remembered), Flanker task conflict cost (Levin et al., 2004; Sinopoli and Dennis, 2012) to measure response inhibition and selective attention (difference in reaction time between blocks in msec), Continuous Performance Test (Riccio et al., 2002) to assess sustained attention (reaction time in msec), Digit Symbol Substitution Test (Hinton-Bayre and Geffen, 2005) to evaluate associative non-verbal learning and information processing speed (accuracy score, total amount of symbols solved in 120 s); and the Stockings of Cambridge (Jacobs and Anderson, 2002; Kostering et al., 2015; Luciana et al., 2009; Syvaoja et al., 2015) to determine planning and problem solving (total moves needed to copy a pattern of colored balls). Furthermore, we obtained parent-reported symptoms of the Dutch version of the Behavior Rating Inventory of Executive Function (BRIEF) (Donders and DeWit, 2016; Kurowski et al., 2013; Wilson et al., 2011) questionnaire (with lower scores reflecting better daily executive function).
2.4. Statistical analyses
Analyses were performed in Statistical Analysis Software version 9.4.
Cognitive performance at different time points was modeled by linear mixed models. Specifically, longitudinal covariance pattern models were fitted with the cognitive assessments as continuous outcome measures, with group, time and gender as fixed factors, and age and parental education as covariates. Details of the statistical analysis on the neurocognitive assessments can be found in a previous paper (Vander Linden et al., 2018).
Group differences in gray matter volumes for each ROI were investigated by means of linear regression analyses, which included the gray matter volumes as dependent variables; group and gender as fixed factors; and intracranial volume (ICV), age and parental education as covariates (Ardila et al., 2005; Davis-Kean, 2005; Hsu et al., 2014; Kannan et al., 2014; Lenroot et al., 2009; Piccolo et al., 2016; Yeates et al., 2010).
Standardized effect sizes (Glass's delta) were calculated as the ratio of the difference in estimated, adjusted mean volume between controls and TBI-adolescents, and the SD of the control group (Ialongo, 2016).
Linear mixed-effects models (Bernal-Rusiel et al., 2013) were applied to assess training-related changes in gray matter volumes, with random intercepts for subjects, cortical volume as continuous outcome measure, time and gender as fixed factors, and age as covariate. Standardized effect size (Glass's delta) was here calculated as the ratio of the difference in estimated mean volume between two successive time points, and the SD of the earliest time point in the time comparisons (Ialongo, 2016). The standardized effect sizes were interpreted: 0.2–0.49 for a small effect, 0.50–0.79 for a medium effect, and > 0.80 for large effects.
Regional specificity of structural gray matter changes was assessed by analyzing the mean percentage change in gray matter volume over two time periods (T0-T1) and (T1-T2) between test and control regions. Percentage changes in gray matter volumes were calculated by subtracting the baseline volume (T0) from the volume for each region post-intervention (T1), divided by the baseline volume (T0), multiplied by 100% and similarly for the change between post-intervention (T1) and 6 months-follow up (T2). Positive (resp. negative) changes indicate an increase (resp. decrease) in gray matter volume over time. Linear mixed model analyses with random intercept and slope were conducted with the percentage change in gray matter volume as outcome measure and a full factorial model for time points and type of region (EF-ROIs, control).
Correlations between gray matter changes and neurocognitive performance were investigated by calculating Pearson correlation coefficients.
In case of multiple testing, raw p-values were evaluated using the Benjamini-Hochberg procedure (Benjamini and Hochberg, 1995). A False Discovery Rate (FDR) of 10% was considered, allowing 10% of the rejected null hypotheses to be false discoveries (McDonald, 2014). False discovery rate adjusted multiple confidence intervals were reported (Benjamini and Yekutieli, 2005).
3. Results
3.1. Post-intervention changes in executive functioning in adolescents with TBI
Our linear mixed models revealed significant improvements in executive functioning with training on the Digit span forwards (p = 0.002), Flanker conflict cost (p = 0.002), Continuous Performance Test reaction time (p = 0.005), Digit symbol substitution (p = 0.006) and Stockings of Cambridge (p ≤0.001). Furthermore, a significant decrease on the BRIEF-parental scores (p = 0.013) post-intervention could be observed, reflecting an increase in daily executive function. At 6 months follow-up, the improvements remained significant. In addition, a significant improvement in Digit Span Backwards (p ≤0.0001) could be demonstrated in the TBI group at follow-up compared to pre-intervention performance.
3.2. Cross-sectional analysis of ROI-volumes between TBI and typically developing peers at baseline
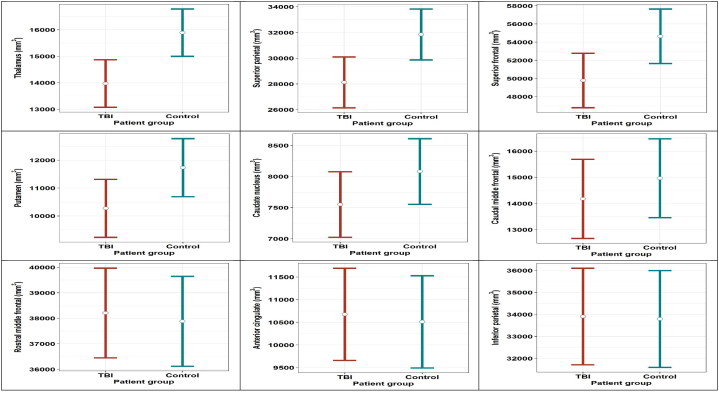
Univariate analyses for each EF-ROI separately revealed significant decreases in the cortical volumes of the thalamus bilateral (−1920.61 mm3, p = .006), the superior parietal gyri (−3719.36 mm3, p = .015) and the superior frontal gyri (−4861.22mm3, p = .033) in TBI-adolescents compared with their matched controls. (Table 1 and Fig. 1).
Table 1.
Pre-intervention statistical comparisons of gray matter volumes (mm3) between groups, corrected for Intra Cranial Volume, age, gender and parental education. Means are estimated for mean values of age (15y7m), ICV (1598276mm3), and parental education (29y2m). Solid p-values are considered statistically significant, applying the Benjamini-Hochberg procedure with FDR 0.10.
| Region of interest | Observed mean volume mm3 (SD) Controls n = 16 |
Observed mean volume mm3 (SD) TBI n = 16 |
Est. diff. in means | FDR-adjusted multiple confidence intervals |
Estimated standardized effect size |
raw p-value |
BH critical value |
||
|---|---|---|---|---|---|---|---|---|---|
| Intra Cranial Volume | 1,602,127.17 | (193,230.55) | 1,594,425.34 | (224,718.35) | 49,184.02 | [−125,070.93; 223,438.98] | 0.25 | 0. 567 | |
| Thalamus | 15,770.84 | (1576.75) | 14,076.41 | (1871.81) | 1920.61 | [469.98; 3371.25] | 1.22 | 0.006 | 0.011 |
| Superior parietal | 32,134.13 | (4413.18) | 27,901.94 | (4944.23) | 3719.36 | [514.92; 6923.80] | 0.84 | 0.015 | 0.022 |
| Superior frontal | 54,904.44 | (6377.49) | 49,597.00 | (4492.14) | 4861.22 | [−3.19; 9725.64] | 0.76 | 0.033 | 0.033 |
| Putamen | 11,522.65 | (1612.18) | 10,474.33 | (1549.18) | 1457.63 | [−229.70; 3144.96] | 0.90 | 0.063 | 0.044 |
| Caudate nucleus | 7858.22 | (1043.18) | 7786.99 | (822.81) | 531.74 | [−323.46; 1386.94] | 0.51 | 0.174 | 0.056 |
| Caudal middle frontal | 15,130.13 | (2442.97) | 13,973.94 | (2256.48) | 788.26 | [−1662.49; 3239.02] | 0.32 | 0.476 | 0.067 |
| Rostral middle frontal | 38,909.56 | (4207.31) | 37,258.44 | (4343.16) | −326.68 | [−3183.00; 2529.64] | −0.08 | 0.799 | 0.078 |
| Anterior cingulate | 10,563.00 | (1272.11) | 10,564.56 | (1780.92) | −163.92 | [−1819.29; 1488.45] | −0.13 | 0.825 | 0.089 |
| Inferior parietal | 35,040.50 | (4920.01) | 32,987.94 | (4175.79) | −110.51 | [−3678.66; 3457.65] | −0.02 | 0.945 | 0.100 |
The results in bold are significant.
Fig. 1.
The estimated mean gray matter volume per region per group (TBI and control), adjusted for age, gender and total intracranial volume, parental education. The error bars represent FDR adjusted confidence intervals.
3.3. Post-intervention change in gray matter volumes with time-point in the TBI-group
In contrast to our second hypothesis, we found no significant volumetric gray matter increases in the 9 ROI's and no meaningful effect sizes between baseline (T0) and immediately post-intervention (T1), or between post-intervention (T1) and 6 months-follow up (T2). Furthermore, there were no significant gray matter alterations in the 3 control regions, which was expected. (Table 2 and Fig. 2).
Table 2.
Mean values and standard deviations of EF-ROIs (mm3) at the 3 time points, effect sizes and p-values post-intervention in TBI group. The Benjamini-Hochberg procedure was applied with FDR 0.10.
| Region of interest | Mean volume mm3 (SD) TBI n = 16 Time 0 |
Mean volume mm3 (SD) TBI n = 16 Time 1 |
Mean volume mm3 (SD) TBI n = 16 Time 2 |
95% Confidence Interval T0-T1 |
95% Confidence Interval T1-T2 |
Stand. Effect size T0-T1 |
Stand Effect size T1-T2 |
p-value T0-T1 |
p-value T1-T2 |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Superior frontal | 50,634.31 | (5365.37) | 50,598.88 | (5379.44) | 50,295.25 | (5348,89) | [−698.82; 769.70] | [−430.63;1037.89] | 0.01 | 0.06 | 0.88 | 0.21 |
| Caud mid front | 14,228.63 | (2754.36) | 14,261.13 | (2789.39) | 14,153.44 | (2790.67) | [−278.32; 213.32] | [−138.13; 353.51] | −0.01 | 0.04 | 0.68 | 0.18 |
| Rostr mid front | 38,345.38 | (5162.16) | 38,146.81 | (4992.51) | 38,017.56 | (5050.60) | [−693.89;1091.01] | [−763.20;1021.70] | 0.04 | 0.03 | 0.50 | 0.66 |
| Sup. parietal | 28,932.00 | (5000.85) | 29,151.19 | (5132.34) | 29,042.06 | (5067.45) | [−579.76; 141.38] | [−251.44; 469.70] | −0.04 | 0.02 | 0.07 | 0.36 |
| Inferior parietal | 33,989.50 | (4068.32) | 34,093.06 | (4125.96) | 33,840.63 | (4074.81) | [−487.40; 280.27] | [−131.39; 636.27] | −0.03 | 0.06 | 0.41 | 0.05 |
| Ant. cingulate | 10,808.38 | (1875.04) | 10,840.13 | (1982.87) | 10,827.88 | (1960.38) | [−235.09; 171.59] | [−191.09; 215.59] | −0.02 | 0.01 | 0.63 | 0.85 |
| Caudate nucleus | 8106.73 | (818.56) | 8131.81 | (829.37) | 8106.12 | (819.43) | [−111.51; 61.33] | [−60.73; 112.11] | −0.03 | 0.03 | 0.38 | 0.36 |
| Putamen | 11,393.54 | (1651.37) | 11,445.10 | (1692.07) | 11,372.50 | (1621.51) | [−197.22; 94.10] | [−73.06; 218.26] | −0.03 | 0.04 | 0.28 | 0.13 |
| Thalamus | 14,315.29 | (1758.37) | 14,337.70 | (1790.40) | 14,270.27 | (1736.71) | [−176.13; 131.32] | [−86.29; 221.16] | −0.01 | 0.04 | 0.65 | 0.18 |
| Pericalcarine | 5232.06 | (882.98) | 5217.38 | (875.33) | 5278.94 | (837.45) | [−163.78; 193.15] | [−240.03; 116.90] | 0.02 | −0.07 | 0.80 | 0.29 |
| Transv.temp.l | 2358.31 | (333.88) | 2346.56 | (334.05) | 2347.88 | (341.00) | [−32.81; 56.31] | [−45.87; 43.25] | 0.04 | 0.00 | 0.42 | 0.93 |
| Postcentral | 22,285,25 | (2776.19) | 22,208.69 | (3025.75) | 22,222.56 | (2867.80) | [−402.37; 555.49] | [−492.81; 465.05] | 0.03 | 0.00 | 0.62 | 0.93 |
Fig. 2.
Longitudinal change in cortical volume of the 9 EF-ROIs (blue) and 3 control regions (brown) in the group with TBI on the 3 time-points. The error bars represent FDR adjusted confidence intervals.
We computed the regional specificity of the gray matter changes by testing interaction between EF-ROI's and control regions. The evolution in mean percentage change (T0-T1) versus (T1-T2) in gray matter volume was significantly different between EF-ROIs and control regions (p = .0016), based on a linear mixed model analysis with random intercept and slope per individual. The differences between control regions and EF-ROIs are visualized in Fig. 3. For the first interval (T0-T1), no statistically significant difference was found when contrasting the EF-ROI to the control regions (p = .124), with an estimated mean increase of 0.2% in the EF-ROI and decrease of 0.3% in the control regions, resulting in an estimated difference in percentage change of 0.5% with 95% CI [−0.1%;1.2%]. For the second interval (T1-T2) a significant difference is found (p = .004), with an estimated mean decrease of 0.5% in the EF-ROI regions and mean increase of 0.5% in the control regions, resulting in an estimated mean difference in percentage change of −1% with 95% CI [−1.7%;-0.3%] contrasting EF-ROI to control regions.
Fig. 3.
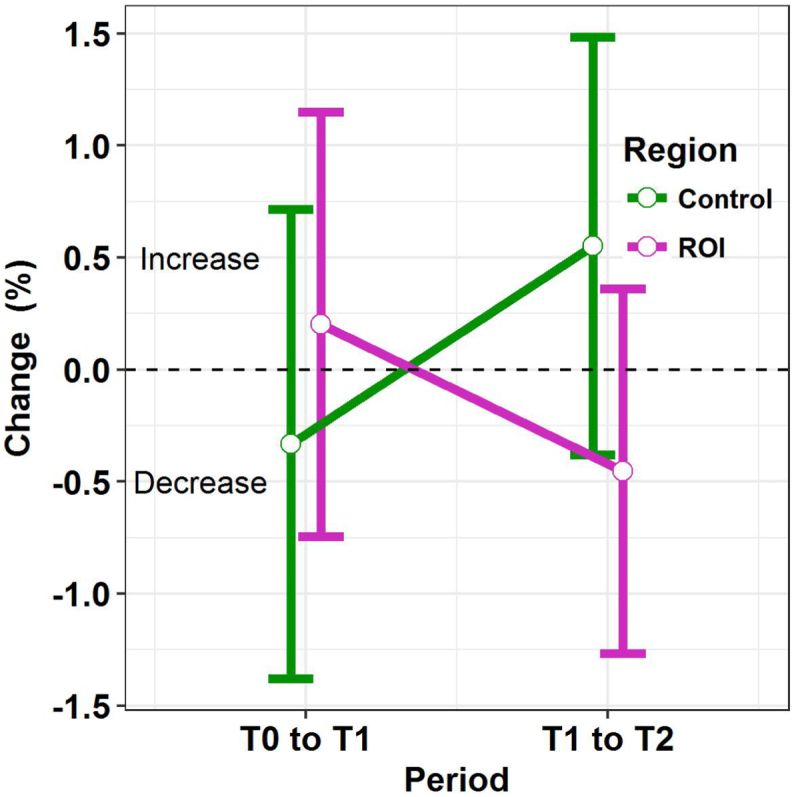
Significant difference in change over time between the 3 control regions and the 9 EF-ROIs.
3.4. Correlation of change in neurocognitive performance with change in cortical and subcortical gray matter volume
Table 3 depicts correlations between changes in performance on neurocognitive assessments and volumetric gray matter alterations in the EF-ROIs after 8 weeks of intensive neurocognitive training (interval T0-T1). Only one explorative significant negative correlation was found between the Digit Symbol Substitution and the volume of the putamen (corr. = − 0.596 p = .015), denoting a more effective interplay of cognitive processing and motor execution is correlated with a smaller volume of the putamen. Important to note, the p-value reported for this correlation was uncorrected for multiple comparisons with a statistical threshold of p < .05.
Table 3.
Pearson correlations and 2-tailed significance between longitudinal change in neurocognitive performance and longitudinal change in cortical and subcortical gray matter. Abbreviations: Corr. = correlation, GEC = Global Executive Composite of BRIEF questionnaire. P-values reported were uncorrected for multiple comparisons.
| Change Time 0 -Time 1 |
Digit span forwards |
Flanker conflict cost |
Cont.Perform.Test reaction time |
Digit symbol substitution |
Stock. of Cambridge |
Brief Gec |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Corr. | P-values | Corr. | P-values | Corr. | P-values | Corr. | P-values | Corr. | P-values | Corr. | P-values | |
| Superior frontal | − 0.179 | 0.508 | − 0.134 | 0.621 | −0.035 | 0.897 | − 0.228 | 0.395 | 0.078 | 0.775 | 0.086 | 0.752 |
| Caudal middle frontal | − 0.184 | 0.495 | − 0.373 | 0.155 | 0.350 | 0.184 | 0.004 | 0.988 | − 0.290 | 0.277 | 0.116 | 0.668 |
| Rostral middle frontal | − 0.276 | 0.300 | 0.017 | 0.950 | − 0.101 | 0.710 | − 0.263 | 0.325 | 0.227 | 0.397 | 0.044 | 0.871 |
| Superior parietal | − 0.122 | 0.652 | 0.008 | 0.976 | − 0.115 | 0.671 | − 0.068 | 0.802 | − 0.237 | 0.377 | 0.140 | 0.605 |
| Inferior parietal | − 0.339 | 0.199 | 0.097 | 0.720 | − 0.455 | 0.077 | − 0.263 | 0.325 | 0.317 | 0.232 | 0.051 | 0.852 |
| Anterior cingulate | − 0.066 | 0.808 | 0.720 | 0.498 | − 0.059 | 0.829 | − 0.347 | 0.188 | − 0.129 | 0.635 | − 0.127 | 0.639 |
| Caudate nucleus | − 0.141 | 0.601 | − 0.103 | 0.703 | 0.040 | 0.882 | − 0.379 | 0.147 | − 0.098 | 0.718 | 0.208 | 0.440 |
| Putamen | 0.036 | 0.895 | − 0.039 | 0.886 | 0.094 | 0.730 | − 0.596 | 0.015 | − 0.088 | 0.745 | 0.078 | 0.773 |
| Thalamus | − 0.067 | 0.804 | 0.242 | 0.368 | − 0.153 | 0.571 | − 0.439 | 0.089 | − 0.068 | 0.802 | −0.268 | 0.315 |
The results in bold are significant.
4. Discussion
A traumatic impact on the adolescent's brain has been implicated in persistent higher-order cognitive deficits. Intensive cognitive training enhances functional and structural neural plasticity in task-related brain regions with the ultimate purpose to ameliorate cognitive functioning. Previous cognitive intervention studies in healthy adults (Jiang et al., 2016; Lampit et al., 2015; Metzler-Baddeley et al., 2016; Roman et al., 2016) or adults with acquired brain injury (Caeyenberghs et al., 2018; Diez-Cirarda et al., 2017; Han et al., 2014; Han et al., 2017; Lazaridou et al., 2013), showed mixed results regarding cortical plasticity, with small correlations between behavioral improvements and neuroplastic changes. To the best of our knowledge, this preliminary study is the first in pediatric TBI literature, investigating training-related gray matter plasticity parallel with cognitive changes in adolescents with TBI.
Our first hypothesis was that adolescents with TBI would show decreased cortical and subcortical volumes in regions associated with executive functioning (EF-ROIs), compared to typically developing peers. Apart from the anterior cingulate gyrus, all EF-ROIs showed smaller volumes in the TBI-group, with a significant difference in the superior part of the frontal gyrus, superior part of the parietal gyrus and the thalamus. These results correspond with prior research of Wilde et al., 2012 in pediatric TBI (n = 20, mean age = 13.6 ± 2.9) (Wilde et al., 2012) showing loss of gray matter volume in the same brain regions using FreeSurfer. We suggest that the main contributor of these gray matter volume reductions is the presence of localized cortical encephalomalacia and diffuse axonal injury (DAI).(Leunissen et al., 2014; Warner et al., 2010).
Our second and third hypothesis was the presence of training-related volumetric alterations in cortical and subcortical regions related to executive function, according to the gray matter expansion–renormalization model for plastic changes post-training (Wenger et al., 2017a). After a period of 8 weeks intense training on several aspects of executive functioning (attention, working memory, inhibition, planning and problem solving) utilizing BrainGames, we observed no significant volumetric changes in the EF-ROIs, despite applying a more liberal threshold (Benjamini-Hochberg procedure with FDR 0.10). As expected (in contrast to the EF-ROIs) there were also no significant training related volumetric changes in the control regions (the precalcarine cortex, transverse temporal and postcentral gyrus). Regional specificity of minor gray matter changes was computed by testing interaction between EF-ROIs and control regions. Change over time in the EF-ROIs was not significantly different from the control regions in the first interval between pre- and immediately post-intervention, but was however significantly different in the second interval between immediately post-intervention and 6 months follow-up.
We suggest there are several reasons for the absence of significant training-induced morphological changes. First, we think we might have missed the time window to capture the gray matter plastic changes. Specifically, the exact timescale of training-dependent gray matter changes is unknown so far. Previous work in healthy adults and acquired brain injury patients (Caeyenberghs et al., 2018) is often limited to 2 assessments (pre- and post-training) due to financial or practical considerations, and the duration between these time points (according to the training duration) is highly variable e.g. ranging from one year (Erickson et al., 2011), two months (Metzler-Baddeley et al., 2016), four weeks (Wenger et al., 2017c), two weeks (Ma et al., 2010), one week (Driemeyer et al., 2008). Wenger et al., 2017a, Wenger et al., 2017c observed a volume expansion of the primary motor cortex after 4 weeks motor learning in healthy adults, which was no longer significant after 7 weeks despite continued training and increased task proficiency (Wenger et al. 2017c). Consistent with these findings, we think that we might have missed a possible early peak of gray matter volume increase and future training studies should include halfway assessments. A second consideration is that, although an adolescent's brain may have an enhanced capacity for plasticity (Ismail et al. 2017; Kolb et al. 2017; Piekarski et al. 2017), the brain damage in young TBI patients hinders experience-dependent developmental plasticity, as suggested by Fineman et al. 2000 (Fineman et al. 2000) and Li et al., 2014 (Li et al. 2014). In other words, we suggest that an injured developing brain has an impaired cortical responsiveness to cognitive training compared to the brain of a healthy peer (Giza and Prins 2006). Therefore, the cortical expansion–renormalization model in healthy adults (Wenger et al. 2017c) may not be readily translated to an injured developing brain. Another explanation for the absence of significant morphological changes might lie in the training regime of the BrainGames. Following our promising results in our feasibility pilot study, we assumed that the cognitive training protocol of BrainGames would also be effective to elicit structural alterations in gray matter volume in adolescents with TBI. Nonetheless, until now the ‘most effective training regime’ (regarding dose, duration, intensity, and timing) to obtain structural alterations in patients with acquired brain injury is still a matter of debate (Caeyenberghs et al. 2018). Based on region specificity (EF-ROIs versus control regions), we did find a significant difference in change over time between post-intervention and 6 months follow-up, which has to be bolstered in future research in larger sample sizes.
Finally, we expected a correlation between cognitive changes and neuroplastic alterations in response to the cognitive training intervention. Explorative analyses revealed one negative correlation between performance on the Digit Symbol Substitution and the volume of the putamen. During the completion of the Digit Symbol Substitution Test (the participant has to write down the corresponding symbol under each digit as fast as possible) an effective interplay of cognitive processing and motor execution is required to guarantee good performance (Zihl et al. 2014). The putamen, a subregion in the striatum, primarily assists motor control needed in executive functioning (Arsalidou et al. 2013; Grahn et al. 2008; Haber 2016). This might explain the correlation between the putamen and Digit Symbol Substitution Test. Similarly, Ware et al., found associations between fine motor dexterity and indices of gray matter integrity in the putamen (Ware et al. 2016).
Unexpectedly, the direction of the correlation coefficient between the performance on the Digit Symbol Substitution Test and the Putamen is negative, indicating the smaller the putamen the better the scores of the Digit Symbol Substitution. Previous research in typically developing children has demonstrated that maturation of the putamen during adolescence is characterized by annual volume decline, primarily driven by processes of dendritic-synaptic pruning. (Goddings et al. 2014; Herting et al. 2018; Narvacan et al. 2017; Swagerman et al. 2014). By eliminating unnecessary dendritic connections in the subcortical gray matter, more adequate specific synaptic transmission would be possible, resulting in improved function. Therefore, in adolescents smaller (cortical and) subcortical volumes are correlated with improved functional skills during development. (Dennison et al. 2013; Goddings et al. 2014; Narvacan et al. 2017; Swagerman et al. 2014).
In our training-study, we would have expected a positive correlation coefficient between a temporary expansion of the gray matter volume of the putamen and improvement on the Digit Symbol Substitution Test (Brooks et al. 2016). However, the increase in putamen volume was not significant after 8 weeks cognitive training, despite improved performance on the Digit Symbol Substitution Test. As mentioned above in our discussion, we presume that we might have “missed” a possible gray matter expansion in the putamen during the learning trajectory, that is not present anymore in the maintenance of the trained skills (Reed et al. 2011).
The present study has the following strengths: a well-defined study population of adolescents with TBI, an age and gender matched control cohort, no dropout during the cognitive training, three time points (including a follow-up) for assessments, and mixed model statistical analyses. However, we would like to acknowledge a couple of methodological considerations and limitations. First, our sample size of adolescents with TBI was small and hence there was a lack of power to capture statistically structural training effects. Furthermore, we used a lenient threshold level (FDR at 0.1) to correct for multiple comparisons across regions due to the exploratory nature of the study. Secondly, the absence of a TBI-active control group makes it difficult to control bias and to attribute changes to the training-intervention, however recruitment of an age and gender matched control TBI-group would be a serious practical challenge. Thirdly, we decided to perform the control region selection using the same parcellation scheme of the Desikan-Killiany atlas as the ROI selection, though the Brodmann area labels for primary visual and somatosensory cortices correspond better with the pericalcarine cortex and postcentral gyrus. Finally, although it was not the scope of this study, we acknowledge that clinical-anatomical correlates of executive functioning goes beyond the (sub)cortical localization and EF involves a large-scale distributed brain network (Bettcher et al. 2016; Catani et al. 2012; ffytche and Catani 2005).
In conclusion, while adolescents with TBI showed improvements in executive functioning after cognitive training using multiple neuropsychological assessments, we were not able to establish convincing causality of gray matter structural pre-post changes in this cognitive enhancement. However, region specificity (EF-ROIs versus control regions) provided a significant difference in change over time between post-intervention and 6 months follow up. Furthermore, explorative analyses revealed a negative correlation between changes in the Digit Symbol Substitution Test and the gray matter volume of the putamen, which was interpreted as an effective interplay of cognitive processing and motor execution. Although our major hypotheses were not confirmed, this preliminary study may be considered of value because it contributes to the insights of training-related plasticity mechanisms in pediatric TBI. These results may furnish the scientific investigation of theories emphasizing an impaired capacity of gray matter plasticity in a traumatized brain. Future research involving a larger sample size with early intermittent MRI assessments across the cognitive training program and an equal number of MRI assessments in a matched control cohort, is warranted to corroborate these findings. Exploring brain plasticity in pediatric TBI is essential to provide a foundation for appropriate therapeutic interventions to enhance functional recovery in the injured developing brain.
Author disclosure statement
The authors declared no conflicts of interest with respect to the research, authorship and publication of this article. No competing financial interests exist.
Acknowledgement
This research was supported by a grant (#01 N00214) from the Special Research Fund (BOF) from Ghent University, Belgium.
The authors thank all the children and their parents for their voluntary study participation.
Special thanks to Ghent Institute for Functional and Metabolic Imaging, Child Rehabilitation University Hospital Ghent, Rehabilitation Centre for children and adolescents Pulderbos.
Contributor Information
Vander Linden Catharine, Email: catharine.vanderlinden@uzgent.be.
Verhelst Helena, Email: helena.verhelst@ugent.be.
Deschepper Ellen, Email: ellen.deschepper@ugent.be.
Vingerhoets Guy, Email: guy.vingerhoets@ugent.be.
Deblaere Karel, Email: karel.deblaere@uzgent.be.
Caeyenberghs Karen, Email: karen.caeyenberghs@acu.edu.au.
References
- Alcauter S., Lin W., Smith J.K., Short S.J., Goldman B.D., Reznick J.S., Gilmore J.H., Gao W. Development of thalamocortical connectivity during infancy and its cognitive correlations. J. Neurosci. 2014;34:9067–9075. doi: 10.1523/JNEUROSCI.0796-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson V., Ylvisaker M. Executive function and the frontal lobes: themes for child development, brain insult and rehabilitation. Dev. Neurorehabil. 2009;12:253–254. doi: 10.3109/17518420903086899. [DOI] [PubMed] [Google Scholar]
- Andre J., Picchioni M., Zhang R., Toulopoulou T. Working memory circuit as a function of increasing age in healthy adolescence: a systematic review and meta-analyses. Neuroimage Clin. 2016;12:940–948. doi: 10.1016/j.nicl.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardila A., Rosselli M., Matute E., Guajardo S. The influence of the parents' educational level on the development of executive functions. Dev. Neuropsychol. 2005;28:539–560. doi: 10.1207/s15326942dn2801_5. [DOI] [PubMed] [Google Scholar]
- Arsalidou M., Duerden E.G., Taylor M.J. The Centre of the brain: topographical model of motor, cognitive, affective, and somatosensory functions of the basal ganglia. Hum. Brain Mapp. 2013;34:3031–3054. doi: 10.1002/hbm.22124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 1995:289–300. [Google Scholar]
- Benjamini Y., Yekutieli D. False discovery rate–adjusted multiple confidence intervals for selected parameters. J. Am. Stat. Assoc. 2005;100:71–81. [Google Scholar]
- Bernal-Rusiel J.L., Greve D.N., Reuter M., Fischl B., Sabuncu M.R. Statistical analysis of longitudinal neuroimage data with linear mixed effects models. Neuroimage. 2013;66:249–260. doi: 10.1016/j.neuroimage.2012.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettcher B.M., Mungas D., Patel N., Elofson J., Dutt S., Wynn M., Watson C.L., Stephens M., Walsh C.M., Kramer J.H. Neuroanatomical substrates of executive functions: beyond prefrontal structures. Neuropsychologia. 2016;85:100–109. doi: 10.1016/j.neuropsychologia.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breukelaar I.A., Antees C., Grieve S.M., Foster S.L., Gomes L., Williams L.M., Korgaonkar M.S. Cognitive control network anatomy correlates with neurocognitive behavior: a longitudinal study. Hum. Brain Mapp. 2017;38:631–643. doi: 10.1002/hbm.23401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks S.J., Burch K.H., Maiorana S.A., Cocolas E., Schioth H.B., Nilsson E.K., Kamaloodien K., Stein D.J. Psychological intervention with working memory training increases basal ganglia volume: a VBM study of inpatient treatment for methamphetamine use. Neuroimage Clin. 2016;12:478–491. doi: 10.1016/j.nicl.2016.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caeyenberghs K., Clemente A., Imms P., Egan G., Hocking D.R., Leemans A., Metzler-Baddeley C., Jones D.K., Wilson P.H. Evidence for training-dependent structural neuroplasticity in brain-injured patients: a critical review. Neurorehabil. Neural Repair. 2018;32:99–114. doi: 10.1177/1545968317753076. [DOI] [PubMed] [Google Scholar]
- Catani M., Dell'acqua F., Bizzi A., Forkel S.J., Williams S.C., Simmons A., Murphy D.G., Thiebaut de Schotten M. Beyond cortical localization in clinico-anatomical correlation. Cortex. 2012;48:1262–1287. doi: 10.1016/j.cortex.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Chevignard M., Francillette L., Toure H., Brugel D., Meyer P., Vannier A.L., Opatowski M., Watier L. Academic outcome, participation and health-related quality of life following childhood severe traumatic brain injury: results of a prospective longitudinal study: the seven-year follow-up of the TGE cohort. Ann. Phys. Rehabil. Med. 2016;59S:e133. [Google Scholar]
- Davis-Kean P.E. The influence of parent education and family income on child achievement: the indirect role of parental expectations and the home environment. J. Fam. Psychol. 2005;19:294–304. doi: 10.1037/0893-3200.19.2.294. [DOI] [PubMed] [Google Scholar]
- Dennison M., Whittle S., Yucel M., Vijayakumar N., Kline A., Simmons J., Allen N.B. Mapping subcortical brain maturation during adolescence: evidence of hemisphere- and sex-specific longitudinal changes. Dev. Sci. 2013;16:772–791. doi: 10.1111/desc.12057. [DOI] [PubMed] [Google Scholar]
- Desikan R.S., Segonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D., Buckner R.L., Dale A.M., Maguire R.P., Hyman B.T., Albert M.S., Killiany R.J. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Diez-Cirarda M., Ojeda N., Pena J., Cabrera-Zubizarreta A., Lucas-Jimenez O., Gomez-Esteban J.C., Gomez-Beldarrain M.A., Ibarretxe-Bilbao N. Increased brain connectivity and activation after cognitive rehabilitation in Parkinson's disease: a randomized controlled trial. Brain Imaging Behav. 2017;11:1640–1651. doi: 10.1007/s11682-016-9639-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donders J., DeWit C. Parental ratings of daily behavior and child cognitive test performance after pediatric mild traumatic brain injury. Child Neuropsychol. 2016:1–17. doi: 10.1080/09297049.2016.1161015. [DOI] [PubMed] [Google Scholar]
- Donolato E., Giofre D., Mammarella I.C. Differences in verbal and Visuospatial forward and backward order recall: a review of the literature. Front. Psychol. 2017;8:663. doi: 10.3389/fpsyg.2017.00663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driemeyer J., Boyke J., Gaser C., Buchel C., May A. Changes in gray matter induced by learning--revisited. PLoS ONE. 2008;3 doi: 10.1371/journal.pone.0002669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis C.T., Turk-Browne N.B. Infant fMRI: a model system for cognitive neuroscience. Trends Cogn. Sci. 2018;22:375–387. doi: 10.1016/j.tics.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson K.I., Voss M.W., Prakash R.S., Basak C., Szabo A., Chaddock L., Kim J.S., Heo S., Alves H., White S.M., Wojcicki T.R., Mailey E., Vieira V.J., Martin S.A., Pence B.D., Woods J.A., McAuley E., Kramer A.F. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. U. S. A. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson B.R., Gao W.J. Development of thalamocortical connections between the mediodorsal thalamus and the prefrontal cortex and its implication in cognition. Front. Hum. Neurosci. 2014;8:1027. doi: 10.3389/fnhum.2014.01027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ffytche D.H., Catani M. Beyond localization: from hodology to function. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2005;360:767–779. doi: 10.1098/rstb.2005.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fineman I., Giza C.C., Nahed B.V., Lee S.M., Hovda D.A. Inhibition of neocortical plasticity during development by a moderate concussive brain injury. J. Neurotrauma. 2000;17:739–749. doi: 10.1089/neu.2000.17.739. [DOI] [PubMed] [Google Scholar]
- Fischl B. FreeSurfer. Neuroimage. 2012;62:774–781. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer S.L., Mattson S.N., Jernigan T.L., Archibald S.L., Jones K.L., Riley E.P. Caudate volume predicts neurocognitive performance in youth with heavy prenatal alcohol exposure. Alcohol. Clin. Exp. Res. 2012;36:1932–1941. doi: 10.1111/j.1530-0277.2012.01811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giza C.C., Prins M.L. Is being plastic fantastic? Mechanisms of altered plasticity after developmental traumatic brain injury. Dev. Neurosci. 2006;28:364–379. doi: 10.1159/000094163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddings A.L., Mills K.L., Clasen L.S., Giedd J.N., Viner R.M., Blakemore S.J. The influence of puberty on subcortical brain development. Neuroimage. 2014;88:242–251. doi: 10.1016/j.neuroimage.2013.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahn J.A., Parkinson J.A., Owen A.M. The cognitive functions of the caudate nucleus. Prog. Neurobiol. 2008;86:141–155. doi: 10.1016/j.pneurobio.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Greven C.U., Bralten J., Mennes M., O'Dwyer L., van Hulzen K.J., Rommelse N., Schweren L.J., Hoekstra P.J., Hartman C.A., Heslenfeld D., Oosterlaan J., Faraone S.V., Franke B., Zwiers M.P., Arias-Vasquez A., Buitelaar J.K. Developmentally stable whole-brain volume reductions and developmentally sensitive caudate and putamen volume alterations in those with attention-deficit/hyperactivity disorder and their unaffected siblings. JAMA Psychiatry. 2015;72:490–499. doi: 10.1001/jamapsychiatry.2014.3162. [DOI] [PubMed] [Google Scholar]
- Haber S.N. Corticostriatal circuitry. Dialogues Clin. Neurosci. 2016;18:7–21. doi: 10.31887/DCNS.2016.18.1/shaber. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han K., Davis R., Chapman S., Krawczyk D. Cortical thickness changes of individuals with chronic traumatic brain injury following strategy-based reasoning training. Arch. Phys. Med. Rehabil. 2014;95:e7. [Google Scholar]
- Han K., Davis R.A., Chapman S.B., Krawczyk D.C. Strategy-based reasoning training modulates cortical thickness and resting-state functional connectivity in adults with chronic traumatic brain injury. Brain Behav. 2017;7 doi: 10.1002/brb3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting M.M., Johnson C., Mills K.L., Vijayakumar N., Dennison M., Liu C., Goddings A.L., Dahl R.E., Sowell E.R., Whittle S., Allen N.B., Tamnes C.K. Development of subcortical volumes across adolescence in males and females: a multisample study of longitudinal changes. Neuroimage. 2018;172:194–205. doi: 10.1016/j.neuroimage.2018.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton-Bayre A., Geffen G. Comparability, reliability, and practice effects on alternate forms of the digit symbol substitution and symbol digit modalities tests. Psychol. Assess. 2005;17:237–241. doi: 10.1037/1040-3590.17.2.237. [DOI] [PubMed] [Google Scholar]
- Holtmaat A., Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat. Rev. Neurosci. 2009;10:647–658. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- Hsu N.S., Novick J.M., Jaeggi S.M. The development and malleability of executive control abilities. Front. Behav. Neurosci. 2014;8:221. doi: 10.3389/fnbeh.2014.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson E.B., Schwerin S.C., Avram A.V., Juliano S.L., Pierpaoli C. Diffusion MRI and the detection of alterations following traumatic brain injury. J. Neurosci. Res. 2018;96:612–625. doi: 10.1002/jnr.24065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ialongo C. Understanding the effect size and its measures. Biochem. Med. 2016;26:150–163. doi: 10.11613/BM.2016.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isbell E., Fukuda K., Neville H.J., Vogel E.K. Visual working memory continues to develop through adolescence. Front. Psychol. 2015;6:696. doi: 10.3389/fpsyg.2015.00696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail F.Y., Fatemi A., Johnston M.V. Cerebral plasticity: windows of opportunity in the developing brain. Eur. J. Paediatr. Neurol. 2017;21:23–48. doi: 10.1016/j.ejpn.2016.07.007. [DOI] [PubMed] [Google Scholar]
- Jacobs R., Anderson V. Planning and problem solving skills following focal frontal brain lesions in childhood: analysis using the tower of London. Child Neuropsychol. 2002;8:93–106. doi: 10.1076/chin.8.2.93.8726. [DOI] [PubMed] [Google Scholar]
- Jaffe K.M., Polissar N.L., Fay G.C., Liao S. Recovery trends over three years following pediatric traumatic brain injury. Arch. Phys. Med. Rehabil. 1995;76:17–26. doi: 10.1016/s0003-9993(95)80037-9. [DOI] [PubMed] [Google Scholar]
- Jiang L., Cao X., Li T., Tang Y., Li W., Wang J., Chan R.C., Li C. Cortical thickness changes correlate with cognition changes after cognitive training: evidence from a Chinese community study. Front. Aging Neurosci. 2016;8:118. doi: 10.3389/fnagi.2016.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan N., Ramaiah R., Vavilala M.S. Pediatric neurotrauma. Int. J. Crit. Illn. Inj. Sci. 2014;4:131–137. doi: 10.4103/2229-5151.134152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan H.T., Clark A.E., Holubkov R., Cox C.S., Ewing-Cobbs L. Psychosocial and executive function recovery trajectories one year after Pediatric traumatic brain injury: the influence of age and injury severity. J. Neurotrauma. 2018;35:286–296. doi: 10.1089/neu.2017.5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N.Y., Wittenberg E., Nam C.S. Behavioral and neural correlates of executive function: interplay between inhibition and updating processes. Front. Neurosci. 2017;11:378. doi: 10.3389/fnins.2017.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B., Brown R., Witt-Lajeunesse A., Gibb R. Neural compensations after lesion of the cerebral cortex. Neural Plast. 2001;8:1–16. doi: 10.1155/NP.2001.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B., Harker A., Gibb R. Principles of plasticity in the developing brain. Dev. Med. Child Neurol. 2017;59:1218–1223. doi: 10.1111/dmcn.13546. [DOI] [PubMed] [Google Scholar]
- Konstantinou N., Pettemeridou E., Seimenis I., Eracleous E., Papacostas S.S., Papanicolaou A.C., Constantinidou F. Assessing the relationship between neurocognitive performance and brain volume in chronic moderate-severe traumatic brain injury. Front. Neurol. 2016;7:29. doi: 10.3389/fneur.2016.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostering L., Schmidt C.S., Egger K., Amtage F., Peter J., Kloppel S., Beume L.A., Hoeren M., Weiller C., Kaller C.P. Assessment of planning performance in clinical samples: reliability and validity of the tower of London task (TOL-F) Neuropsychologia. 2015;75:646–655. doi: 10.1016/j.neuropsychologia.2015.07.017. [DOI] [PubMed] [Google Scholar]
- Kou Z., Iraji A. Imaging brain plasticity after trauma. Neural Regen. Res. 2014;9:693–700. doi: 10.4103/1673-5374.131568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurowski B.G., Wade S.L., Kirkwood M.W., Brown T.M., Stancin T., Cassedy A., Taylor H.G. Association of parent ratings of executive function with global- and setting-specific behavioral impairment after adolescent traumatic brain injury. Arch. Phys. Med. Rehabil. 2013;94:543–550. doi: 10.1016/j.apmr.2012.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampit A., Hallock H., Suo C., Naismith S.L., Valenzuela M. Cognitive training-induced short-term functional and long-term structural plastic change is related to gains in global cognition in healthy older adults: a pilot study. Front. Aging Neurosci. 2015;7:14. doi: 10.3389/fnagi.2015.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazaridou A., Astrakas L., Mintzopoulos D., Khanicheh A., Singhal A.B., Moskowitz M.A., Rosen B., Tzika A.A. Diffusion tensor and volumetric magnetic resonance imaging using an MR-compatible hand-induced robotic device suggests training-induced neuroplasticity in patients with chronic stroke. Int. J. Mol. Med. 2013;32:995–1000. doi: 10.3892/ijmm.2013.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot R.K., Schmitt J.E., Ordaz S.J., Wallace G.L., Neale M.C., Lerch J.P., Kendler K.S., Evans A.C., Giedd J.N. Differences in genetic and environmental influences on the human cerebral cortex associated with development during childhood and adolescence. Hum. Brain Mapp. 2009;30:163–174. doi: 10.1002/hbm.20494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leunissen I., Coxon J.P., Caeyenberghs K., Michiels K., Sunaert S., Swinnen S.P. Subcortical volume analysis in traumatic brain injury: the importance of the fronto-striato-thalamic circuit in task switching. Cortex. 2014;51:67–81. doi: 10.1016/j.cortex.2013.10.009. [DOI] [PubMed] [Google Scholar]
- Levan A., Black G., Mietchen J., Baxter L., Brock Kirwan C., Gale S.D. Right frontal pole cortical thickness and executive functioning in children with traumatic brain injury: the impact on social problems. Brain Imaging Behav. 2016;10:1090–1095. doi: 10.1007/s11682-015-9472-7. [DOI] [PubMed] [Google Scholar]
- Levin H.S., Hanten G., Zhang L., Swank P.R., Hunter J. Selective impairment of inhibition after TBI in children. J. Clin. Exp. Neuropsychol. 2004;26:589–597. doi: 10.1080/13803390409609783. [DOI] [PubMed] [Google Scholar]
- Li N., Yang Y., Glover D.P., Zhang J., Saraswati M., Robertson C., Pelled G. Evidence for impaired plasticity after traumatic brain injury in the developing brain. J. Neurotrauma. 2014;31:395–403. doi: 10.1089/neu.2013.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little D.M., Kraus M.F., Joseph J., Geary E.K., Susmaras T., Zhou X.J., Pliskin N., Gorelick P.B. Thalamic integrity underlies executive dysfunction in traumatic brain injury. Neurology. 2010;74:558–564. doi: 10.1212/WNL.0b013e3181cff5d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciana M., Collins P.F., Olson E.A., Schissel A.M. Tower of London performance in healthy adolescents: the development of planning skills and associations with self-reported inattention and impulsivity. Dev. Neuropsychol. 2009;34:461–475. doi: 10.1080/87565640902964540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L., Wang B., Narayana S., Hazeltine E., Chen X., Robin D.A., Fox P.T., Xiong J. Changes in regional activity are accompanied with changes in inter-regional connectivity during 4 weeks motor learning. Brain Res. 2010;1318:64–76. doi: 10.1016/j.brainres.2009.12.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malec J.F., Brown A.W., Leibson C.L., Flaada J.T., Mandrekar J.N., Diehl N.N., Perkins P.K. The mayo classification system for traumatic brain injury severity. J. Neurotrauma. 2007;24:1417–1424. doi: 10.1089/neu.2006.0245. [DOI] [PubMed] [Google Scholar]
- Malone I.B., Leung K.K., Clegg S., Barnes J., Whitwell J.L., Ashburner J., Fox N.C., Ridgway G.R. Accurate automatic estimation of total intracranial volume: a nuisance variable with less nuisance. Neuroimage. 2015;104:366–372. doi: 10.1016/j.neuroimage.2014.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell W.L. Traumatic brain injury in the neonate, child and adolescent human: an overview of pathology. Int. J. Dev. Neurosci. 2012;30:167–183. doi: 10.1016/j.ijdevneu.2011.12.008. [DOI] [PubMed] [Google Scholar]
- McDonald J. 3rd edn. Sparky House; Baltimore: 2014. Handbook of Biological Statistics. [Google Scholar]
- Menon V., Uddin L.Q. Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzler-Baddeley C., Caeyenberghs K., Foley S., Jones D.K. Task complexity and location specific changes of cortical thickness in executive and salience networks after working memory training. Neuroimage. 2016;130:48–62. doi: 10.1016/j.neuroimage.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills K.L., Goddings A.L., Herting M.M., Meuwese R., Blakemore S.J., Crone E.A., Dahl R.E., Guroglu B., Raznahan A., Sowell E.R., Tamnes C.K. Structural brain development between childhood and adulthood: convergence across four longitudinal samples. Neuroimage. 2016;141:273–281. doi: 10.1016/j.neuroimage.2016.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narvacan K., Treit S., Camicioli R., Martin W., Beaulieu C. Evolution of deep gray matter volume across the human lifespan. Hum. Brain Mapp. 2017;38:3771–3790. doi: 10.1002/hbm.23604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemann C., Godde B., Staudinger U.M., Voelcker-Rehage C. Exercise-induced changes in basal ganglia volume and cognition in older adults. Neuroscience. 2014;281:147–163. doi: 10.1016/j.neuroscience.2014.09.033. [DOI] [PubMed] [Google Scholar]
- Noble K.G., Houston S.M., Brito N.H., Bartsch H., Kan E., Kuperman J.M., Akshoomoff N., Amaral D.G., Bloss C.S., Libiger O., Schork N.J., Murray S.S., Casey B.J., Chang L., Ernst T.M., Frazier J.A., Gruen J.R., Kennedy D.N., Van Zijl P., Mostofsky S., Kaufmann W.E., Kenet T., Dale A.M., Jernigan T.L., Sowell E.R. Family income, parental education and brain structure in children and adolescents. Nat. Neurosci. 2015;18:773–778. doi: 10.1038/nn.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordenskjold R., Malmberg F., Larsson E.M., Simmons A., Brooks S.J., Lind L., Ahlstrom H., Johansson L., Kullberg J. Intracranial volume estimated with commonly used methods could introduce bias in studies including brain volume measurements. Neuroimage. 2013;83:355–360. doi: 10.1016/j.neuroimage.2013.06.068. [DOI] [PubMed] [Google Scholar]
- Nowrangi M.A., Lyketsos C., Rao V., Munro C.A. Systematic review of neuroimaging correlates of executive functioning: converging evidence from different clinical populations. J. Neuropsychiatr. Clin. Neurosci. 2014;26:114–125. doi: 10.1176/appi.neuropsych.12070176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien L.M., Ziegler D.A., Deutsch C.K., Frazier J.A., Herbert M.R., Locascio J.J. Statistical adjustments for brain size in volumetric neuroimaging studies: some practical implications in methods. Psychiatry Res. 2011;193:113–122. doi: 10.1016/j.pscychresns.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolo L.R., Merz E.C., He X., Sowell E.R., Noble K.G. Age-related differences in cortical thickness vary by socioeconomic status. PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0162511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piekarski D.J., Johnson C.M., Boivin J.R., Thomas A.W., Lin W.C., Delevich K., E M.G., Wilbrecht L. Does puberty mark a transition in sensitive periods for plasticity in the associative neocortex? Brain Res. 2017;1654:123–144. doi: 10.1016/j.brainres.2016.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad M.R., Swank P.R., Ewing-Cobbs L. Long-term school outcomes of children and adolescents with traumatic brain injury. J. Head Trauma Rehabil. 2017;32:E24–E32. doi: 10.1097/HTR.0000000000000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed A., Riley J., Carraway R., Carrasco A., Perez C., Jakkamsetti V., Kilgard M.P. Cortical map plasticity improves learning but is not necessary for improved performance. Neuron. 2011;70:121–131. doi: 10.1016/j.neuron.2011.02.038. [DOI] [PubMed] [Google Scholar]
- Reuter M., Schmansky N.J., Rosas H.D., Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012;61:1402–1418. doi: 10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio C.A., Reynolds C.R., Lowe P., Moore J.J. The continuous performance test: a window on the neural substrates for attention? Arch. Clin. Neuropsychol. 2002;17:235–272. [PubMed] [Google Scholar]
- Roman F.J., Lewis L.B., Chen C.H., Karama S., Burgaleta M., Martinez K., Lepage C., Jaeggi S.M., Evans A.C., Kremen W.S., Colom R. Gray matter responsiveness to adaptive working memory training: a surface-based morphometry study. Brain Struct. Funct. 2016;221:4369–4382. doi: 10.1007/s00429-015-1168-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosch K.S., Crocetti D., Hirabayashi K., Denckla M.B., Mostofsky S.H., Mahone E.M. Reduced subcortical volumes among preschool-age girls and boys with ADHD. Psychiatry Res. 2018;271:67–74. doi: 10.1016/j.pscychresns.2017.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs M., Kaplan J., Der Sarkissian A., Habibi A. Increased engagement of the cognitive control network associated with music training in children during an fMRI Stroop task. PLoS ONE. 2017;12 doi: 10.1371/journal.pone.0187254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sariaslan A., Sharp D.J., D'Onofrio B.M., Larsson H., Fazel S. Long-term outcomes associated with traumatic brain injury in childhood and adolescence: a Nationwide Swedish cohort study of a wide range of medical and social outcomes. PLoS Med. 2016;13 doi: 10.1371/journal.pmed.1002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz E.L., Hoskinson K.R., Keim M.C., Dennis M., Taylor H.G., Bigler E.D., Rubin K.H., Vannatta K., Gerhardt C.A., Stancin T., Yeates K.O. Adaptive functioning following pediatric traumatic brain injury: relationship to executive function and processing speed. Neuropsychology. 2016;30:830–840. doi: 10.1037/neu0000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinopoli K.J., Dennis M. Inhibitory control after traumatic brain injury in children. Int. J. Dev. Neurosci. 2012;30:207–215. doi: 10.1016/j.ijdevneu.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow P.J. The structural and functional Organization of Cognition. Front. Hum. Neurosci. 2016;10:501. doi: 10.3389/fnhum.2016.00501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell E.R., Trauner D.A., Gamst A., Jernigan T.L. Development of cortical and subcortical brain structures in childhood and adolescence: a structural MRI study. Dev. Med. Child Neurol. 2002;44:4–16. doi: 10.1017/s0012162201001591. [DOI] [PubMed] [Google Scholar]
- Swagerman S.C., Brouwer R.M., de Geus E.J., Hulshoff Pol H.E., Boomsma D.I. Development and heritability of subcortical brain volumes at ages 9 and 12. Genes Brain Behav. 2014;13:733–742. doi: 10.1111/gbb.12182. [DOI] [PubMed] [Google Scholar]
- Syvaoja H.J., Tammelin T.H., Ahonen T., Rasanen P., Tolvanen A., Kankaanpaa A., Kantomaa M.T. Internal consistency and stability of the CANTAB neuropsychological test battery in children. Psychol. Assess. 2015;27:698–709. doi: 10.1037/a0038485. [DOI] [PubMed] [Google Scholar]
- Tamnes C.K., Herting M.M., Goddings A.L., Meuwese R., Blakemore S.J., Dahl R.E., Güroğlu B., Raznahan A., Sowell E.R., Crone E.A., Mills K.L. Development of the cerebral cortex across adolescence: a multisample study of inter-related longitudinal changes in cortical volume, surface area, and thickness. J. Neurosci. 2017;37:3402–3412. doi: 10.1523/JNEUROSCI.3302-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C., Baker C.I. Teaching an adult brain new tricks: a critical review of evidence for training-dependent structural plasticity in humans. Neuroimage. 2013;73:225–236. doi: 10.1016/j.neuroimage.2012.03.069. [DOI] [PubMed] [Google Scholar]
- Treble-Barna A., Zang H., Zhang N., Taylor H.G., Yeates K.O., Wade S. Long-term neuropsychological profiles and their role as mediators of adaptive functioning after traumatic brain injury in early childhood. J. Neurotrauma. 2017;34:353–362. doi: 10.1089/neu.2016.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treit S., Zhou D., Lebel C., Rasmussen C., Andrew G., Beaulieu C. Longitudinal MRI reveals impaired cortical thinning in children and adolescents prenatally exposed to alcohol. Hum. Brain Mapp. 2014;35:4892–4903. doi: 10.1002/hbm.22520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Linden C., Verhelst H., Deschepper E., Vingerhoets G., Deblaere K., Caeyenberghs K. 2018. Cognitive Training Benefit Depends on Brain Injury Location in Adolescents with Traumatic Brain Injury: A Pilot Study. (European journal of physical and rehabilitation medicine) [DOI] [PubMed] [Google Scholar]
- Velanova K., Wheeler M.E., Luna B. Maturational changes in anterior cingulate and frontoparietal recruitment support the development of error processing and inhibitory control. Cereb. Cortex. 2008;18:2505–2522. doi: 10.1093/cercor/bhn012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhelst H., Vander Linden C., Vingerhoets G., Caeyenberghs K. How to train an injured brain? a pilot feasibility study of home-based computerized cognitive training. Games Health J. 2017;6:28–38. doi: 10.1089/g4h.2016.0043. [DOI] [PubMed] [Google Scholar]
- Walhovd K.B., Westlye L.T., Amlien I., Espeseth T., Reinvang I., Raz N., Agartz I., Salat D.H., Greve D.N., Fischl B., Dale A.M., Fjell A.M. Consistent neuroanatomical age-related volume differences across multiple samples. Neurobiol. Aging. 2011;32:916–932. doi: 10.1016/j.neurobiolaging.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhovd K.B., Krogsrud S.K., Amlien I.K., Bartsch H., Bjornerud A., Due-Tonnessen P., Grydeland H., Hagler D.J., Jr., Haberg A.K., Kremen W.S., Ferschmann L., Nyberg L., Panizzon M.S., Rohani D.A., Skranes J., Storsve A.B., Solsnes A.E., Tamnes C.K., Thompson W.K., Reuter C., Dale A.M., Fjell A.M. Neurodevelopmental origins of lifespan changes in brain and cognition. Proc. Natl. Acad. Sci. U. S. A. 2016;113:9357–9362. doi: 10.1073/pnas.1524259113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware A.L., Kulesz P.A., Williams V.J., Juranek J., Cirino P.T., Fletcher J.M. Gray matter integrity within regions of the dorsolateral prefrontal cortical-subcortical network predicts executive function and fine motor dexterity in spina bifida. Neuropsychology. 2016;30:492–501. doi: 10.1037/neu0000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner M.A., Youn T.S., Davis T., Chandra A., Marquez de la Plata C., Moore C., Harper C., Madden C.J., Spence J., McColl R., Devous M., King R.D., Diaz-Arrastia R. Regionally selective atrophy after traumatic axonal injury. Arch. Neurol. 2010;67:1336–1344. doi: 10.1001/archneurol.2010.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenger E., Brozzoli C., Lindenberger U., Lovden M. Expansion and renormalization of human brain structure during skill acquisition. Trends Cogn. Sci. 2017;21:930–939. doi: 10.1016/j.tics.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenger E., Kuhn S., Verrel J., Martensson J., Bodammer N.C., Lindenberger U., Lovden M. Repeated structural imaging reveals nonlinear progression of experience-dependent volume changes in human motor cortex. Cereb. Cortex. 2017;27:2911–2925. doi: 10.1093/cercor/bhw141. [DOI] [PubMed] [Google Scholar]
- Wilde E.A., Merkley T.L., Bigler E.D., Max J.E., Schmidt A.T., Ayoub K.W., McCauley S.R., Hunter J.V., Hanten G., Li X., Chu Z.D., Levin H.S. Longitudinal changes in cortical thickness in children after traumatic brain injury and their relation to behavioral regulation and emotional control. Int. J. Dev. Neurosci. 2012;30:267–276. doi: 10.1016/j.ijdevneu.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson K.R., Donders J., Nguyen L. Self and parent ratings of executive functioning after adolescent traumatic brain injury. Rehabil. Psychol. 2011;56:100–106. doi: 10.1037/a0023446. [DOI] [PubMed] [Google Scholar]
- Yeates K.O., Taylor H.G., Walz N.C., Stancin T., Wade S.L. The family environment as a moderator of psychosocial outcomes following traumatic brain injury in young children. Neuropsychology. 2010;24:345–356. doi: 10.1037/a0018387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J.M., Powell T.L., Morgan B.R., Card D., Lee W., Smith M.L., Sled J.G., Taylor M.J. Deep grey matter growth predicts neurodevelopmental outcomes in very preterm children. Neuroimage. 2015;111:360–368. doi: 10.1016/j.neuroimage.2015.02.030. [DOI] [PubMed] [Google Scholar]
- Zhou D., Lebel C., Treit S., Evans A., Beaulieu C. Accelerated longitudinal cortical thinning in adolescence. Neuroimage. 2015;104:138–145. doi: 10.1016/j.neuroimage.2014.10.005. [DOI] [PubMed] [Google Scholar]
- Zihl J., Fink T., Pargent F., Ziegler M., Buhner M. Cognitive reserve in young and old healthy subjects: differences and similarities in a testing-the-limits paradigm with DSST. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0084590. [DOI] [PMC free article] [PubMed] [Google Scholar]