Abstract
Background
Many translational MR biomarkers derive from measurements of the water proton longitudinal relaxation rate R1, but evidence for between-site reproducibility of R1 in small-animal MRI is lacking.
Objective
To assess R1 repeatability and multi-site reproducibility in phantoms for preclinical MRI.
Methods
R1 was measured by saturation recovery in 2% agarose phantoms with five nickel chloride concentrations in 12 magnets at 5 field strengths in 11 centres on two different occasions within 1–13 days. R1 was analysed in three different regions of interest, giving 360 measurements in total. Root-mean-square repeatability and reproducibility coefficients of variation (CoV) were calculated. Propagation of reproducibility errors into 21 translational MR measurements and biomarkers was estimated. Relaxivities were calculated. Dynamic signal stability was also measured.
Results
CoV for day-to-day repeatability (N = 180 regions of interest) was 2.34% and for between-centre reproducibility (N = 9 centres) was 1.43%. Mostly, these do not propagate to biologically significant between-centre error, although a few R1-based MR biomarkers were found to be quite sensitive even to such small errors in R1, notably in myocardial fibrosis, in white matter, and in oxygen-enhanced MRI. The relaxivity of aqueous Ni2+ in 2% agarose varied between 0.66 s−1 mM−1 at 3 T and 0.94 s−1 mM−1 at 11.7T.
Interpretation
While several factors affect the reproducibility of R1-based MR biomarkers measured preclinically, between-centre propagation of errors arising from intrinsic equipment irreproducibility should in most cases be small. However, in a few specific cases exceptional efforts might be required to ensure R1-reproducibility.
Abbreviations: Av, Avance; B0, applied magnetic field; CoV, coefficient of variation; DNE, dynamic no enhancement; FISP, fast steady-state free-precession; ICH GCP, International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use Harmonised Tripartite Guideline for Good Clinical Practice; PV, ParaVision; R1, longitudinal relaxation rate; r1, longitudinal relaxativity; rms, root-mean-square; SNR, signal-to-noise ratio; T1, longitudinal relaxation time; T1W, T1-weighted; T2, transverse relaxation time
Keywords: MRI, Hardware stability, Biomarker, Relaxation time, Phantom, Reproducibility, Error propagation
1. Introduction
Many useful MR biomarkers derive from measurements of the water proton longitudinal relaxation time T1, or alternatively the relaxation rate R1 ≡ T1−1. Errors in R1 [1] are common, will propagate, and may damage the reproducibility and accuracy of the resulting MR biomarkers. Although considerable effort has been devoted to measuring and assuring the accuracy of R1 in clinical MR [[2], [3], [4], [5]] systems, there is little evidence for the cross-site reproducibility of R1 measurements in MR systems designed for small-animal research. The lack of standardisation in preclinical imaging has been recognised as an important problem [6,7] which in the worst case could invalidate the findings from animal studies, or confound meta-analyses and translation.
Reproducibility in a valid phantom is an important and ethical prerequisite for reproducible values in vivo. Poor technical validation has been a major impediment to clinical translation of MR biomarkers [8]. An ideal R1 phantom should be traceable [2]; resist biological, chemical and physical deterioration; perform effectively over a range of temperatures convenient and relevant for the users; cover the parameter range expected in subsequent studies; not exhibit physiologically unrepresentative MR characteristics such as radiation damping, convection, unphysiologic T2, excessive self-diffusion, off-resonance chemical shifts, standing waves, or abrupt boundaries; interrogate the entire volume subsequently to be occupied by body parts being imaged; have dimensions suitable for the subject subsequently to be imaged (in this case rats and mice); be convenient for the intended users; and be cost-effective for the intended users. To meet these criteria, nickel agarose phantoms following the design of Christoffersson et al. [9] were used.
Two distinct general approaches to MR standardisation have previously been employed. In the first, which we term “centrally-led”, a central organisation, often independent of the participating sites, is accountable for overall measurement accuracy and reproducibility. They mandate the phantom and acquisition protocol and analyse centrally. They may perform set-up and training at each participating site, instruct sites to repeat aberrant measurements, or even expel sites who cannot achieve the required accuracy. Centrally-led standardisation is common in clinical trials performed to ICH GCP [10,11], or where the MR measurement is regulated as a companion diagnostic [12]. In the second approach, which we term “institution-led”, each investigator is accountable for measurement accuracy in their own centre. They are responsible for their own acquisition and analysis, and for compliance with any guidelines for their chosen phantom. “Institution-led” standardisation is common in academic research and in single-centre studies. Although we expect “centrally-led” standardisation to provide better reproducibility than “institution-led” standardisation, in this work we modelled “institution-led” standardisation as this is more representative of practice in preclinical MR. The study was performed within an international consortium of imaging centres participating in the validation of imaging biomarkers [13], and developing reliable preclinical MR assays which would give comparable results in different laboratories. The aim of this work was to assess the repeatability and reproducibility of R1 in a realistic rodent MR protocol. Simple simulations were performed in order to compare the likely propagation of reproducibility errors into a broad range of R1-derived MR biomarkers.
2. Materials and methods
2.1. Preclinical phantom
Batches of 2% agarose with nickel chloride concentrations respectively of 0.50, 1.04, 2.02, 4.08 and 8.05 mM, with 0.05% sodium azide, were prepared centrally in Berlin and used to create identical phantoms (Supplementary Fig. S3.1) which were distributed to the participating laboratories. The phantoms were prepared and authenticated (supplementary material S3) in July 2017, shipped in August 2017, and the measurements were performed between December 2017 and February 2018.
2.2. MR methods
Thirteen centres involved in an international consortium for the validation of imaging biomarkers for drug safety assessment [13] were invited to participate. Where centres had access to more than one MR system, they were invited to submit data from multiple MR systems. Eleven centres agreed to participate, one of which (G) provided data from two different magnets (G1 and G2): in the analyses, G1 and G2 were treated as if from two different centres. Details of the 12 MR systems are given in Table 1. Eleven of the 12 MR systems (all except B) were in laboratories which regularly and routinely measure MR biomarkers in rodents, intending to translate their findings to create diagnostics or therapeutics to improve human health. Although the use of any particular manufacturer's equipment was not mandated, all participating centres elected to employ Bruker Avance/ParaVision systems. An “institution-led” approach to standardisation was adopted. Pilot studies were performed only in centres B and G. No site training was performed, no quality control was imposed, nor were sites permitted to repeat their measurements to eliminate apparent outliers. Region-of-Interest (RoI) definition and T1 calculation were performed locally.
Table 1.
Equipment used. All equipment was manufactured by Bruker (Rheinstetten, Germany) using Avance (Av) spectrometers and ParaVision (PV) acquisition and analysis software except: (a) Magnet from the companies which formerly traded as Varian, Magnex or Agilent; (b) Transmitter-Receiver from Rapid MR International, Columbus OH USA or Rimpar, Germany.
| Centre | B0/T | Spectrometer | Gradient strength/mT∙m−1 (model) | Radiofrequency transmitter/receiver volume coil (i.d./mm) | Software |
|---|---|---|---|---|---|
| A | 7a | Pharmascan 70/16 US Av III | 375 (B-GA9S) | Quadrature 300 MHz (38)b | PV 6.0 |
| B | 3 | Biospec 3 T Av IIIHD | 900 (B-GA105S HP) | Quadrature 128 MHz (60) | PV 6.0.1 |
| C | 7 | Biospec 70/20 USR Av IIIHD | 660 (B-GA12S HP) | Quadrature 300 MHz (86) | PV 6.0.1 |
| D | 4.7 | Biospec 47/20 USR Av IIIHD | 660 (B-GA12S HP) | Quadrature 200 MHz (72) | PV 6.0.1 |
| E | 7 | Biospec 70/30 USR Av II | 440 (B-GA12S) | Single channel 300 MHz (72) | PV 6.0.1 |
| F | 7a | Biospec 70/20 Av I | 400 (B-GA12) | Single channel 300 MHz (72) | PV 5.1 |
| G1 | 7 | Biospec 70/30 USR Av III | 300 (B-GA12) | Quadrature 300 MHz (90)b | PV 6.0.1 |
| G2 | 4.7 | Biospec 47/40 Av III | 200 (B-GA12S) | Quadrature 200 MHz (90)b | PV 6.0.1 |
| H | 4.7 | Pharmascan 47/16 Av III | 300 (B-G9S) | Single channel 200 MHz (60) | PV 5.1 |
| J | 4.7 | Biospec 47/40 USR Av II | 660 (B-GA12S HP) | Quadrature 200 MHz (72) | PV 6.0.1 |
| K | 9.4a | Biospec 94/30 Av III | 670 (B-GA 12S HP) | Quadrature 400 MHz (87) | PV 6.0.1 |
| L | 11.7 | Biospec 117/16 USR Av III | 750 (B-GA 9S) | Quadrature 500 MHz (72) | PV 6.0.1 |
Centres were asked to measure R1 by saturation recovery using a standard RARE sequence. (Additional measurements using an investigational fast steady-state free-precession (FISP) sequence designed for the consortium's in vivo needs will be reported elsewhere). In an attempt to provide temperature stability and minimise susceptibility artefacts, each phantom was embedded in a cucumber (Supplementary Figs. S3.2 and S3.3). Centres were instructed to “allow the five cucumbered phantoms to come to thermal equilibrium in the magnet bore…[and] measure the temperature of the cucumber flesh in several places and verify thermal equilibrium has been reached.” The temperature of the cucumber flesh around the phantom was measured before and after each acquisition. The entire protocol was run in each centre on two separate days, mean 2.7 days apart (range 1–13).
In ParaVision, the standard RARE T1 saturation-recovery measurement method “T1map_RARE protocol” (Rat/Head/Relaxometry) was invoked. All images were coronal with 58 × 58 mm field of view, 128 × 128 matrix, with a π/2 for 1.16 mm slice selection followed by a π train with RARE factor 8, effective echo time 30 ms, echo spacing 7.5 ms. Signal averaging was not employed and 5 dummy scans were used. Saturation recovery experiments used repetition times TR of 5500, 2000, 1200, 750, 500, 300, 200 and 100 ms giving a scan time of 169 s, not including the dummy scans. Next, a “dynamic-no-enhancement” (DNE) stability series to simulate dynamic contrast-enhanced MRI was run for 5 min (approximately 34 images) with repeated acquisition using the same parameters but with TR fixed at 500 ms.
2.3. Analyses
Each centre conducted measurements independently and was blinded to findings from the other centres until their own results had been submitted. At each centre, T1 values were obtained using a 2-parameter fit in ParaVision from circular 25 mm2 RoIs, i.e. 29 μl volumes, approximately 120 voxels, at three RoI positions. These were: at the isocentre; radially at the edge of the phantom 10 mm from isocentre; and axially at the end of the phantom 12–20 mm from isocentre, denoted respectively by (X,Y,Z) = (0,0,0), (10,0,0) and (0,0,12) mm. The 2-parameter fit assumed zero longitudinal magnetisation at the mid-point of the eighth echo. The resulting T1 values and standard deviation of the fit for each RoI, together with the mean and standard deviation DNE signal for (X,Y,Z) = (0,0,0), were submitted to the core lab in Manchester for further analysis.
At the core lab, root-mean-square (rms) within-centre R1 repeatabilities and between-centre reproducibilities were calculated using Microsoft Excel. Each calculation was performed both using absolute units (i.e. standard deviations with units s−1), and using coefficients of variation (CoV, dimensionless, presented as percentages). This was done because absolute R1 units (s−1) propagate to absolute concentration of relaxive substance and in some instances to absolute biomarker value, while coefficients of variation may be more relevant when biomarker change is considered. Post-hoc tests of significance were made for “effect of day” using Student's t-test, and for “effect of RoI position” by analysis of variance. No correction for multiple comparisons was made but p < 0.01 was considered significant. For each centre, weighted mean R1 values were calculated for each of the five phantoms:
where R1,d,RoI are the R1 values for each of the two days in each of the three RoIs, and wd,RoI are the corresponding weights, derived from the T1 fit in ParaVision:
These weighted mean R1 values were then used to obtain relaxivities by linear regression:
| (1) |
where r1, B0/s−1 ∙ mM−1 is the longitudinal relaxivity of aqueous Ni2+ in 2% agarose at field B0, R1, [Ni]=0, B0/s−1 is the longitudinal relaxation rate of 2% agarose at field B0, and ε is a normally-distributed error term assumed to subsume inter alia any temperature effects.
2.4. Cross-validation
Our “institution-led” study design required each centre to derive its own T1 values. Since centres elected to use the proprietary ParaVision software, a small supplementary study was also performed using an alternative analysis to verify values. Data from one centre were reanalysed. Centre A's data were considered a good test set because they submitted data with both high and low fit errors. For each of the 10 RARE data sets (5 phantoms × 2 days), and for the same three RoIs used in the primary analysis, signal mean and standard deviation were retrieved for each TR value. R1 was calculated using “R” [14] using four expressions of the form:
For three-parameter fits, Minf, M0 and R1 were fitted, while for two-parameter fits M0 was set to zero. For weighted fits, each RoI value y was weighted by w, the inverse of the variance in y, while for unweighted fits w was set to unity. For each of the 30 data sets, each of the four estimates of R1 from “R”, R1R, was compared with the reciprocal T1 from Paravision, R1PV. In each of the four cases:
| (2) |
2.5. Illustrative simulations
Error propagation associated with two standard deviations of R1 reproducibility was estimated for a range of derived measurements and biomarkers, using representative relaxivities and other parameters from the literature. This is conservative as it does not fully eliminate repeatability error. Three general cases were considered: firstly, native R1 (or T1) used as a biomarker; secondly, concentration of endogenous or exogenous paramagnetic substance used as biomarker; and thirdly, biomarkers derived from compartmental models. For Dynamic Contrast-Enhanced (DCE) MRI, the error in precontrast R1 was propagated into the biomarkers for four preclinical case-studies. Representative ‘true’ values of kinetic parameters, pre-contrast R1 values, and appropriate tracer kinetic models were chosen from literature to estimate contrast agent concentration uptake in each tissue type. Simulation parameters are provided in Supplementary Material.
3. Results
Each centre was requested to submit 30 R1 measurements (5 phantoms × 3 locations × 2 days), the results of 10 DNE runs (5 phantoms × 2 days), and the 10 associated temperature measurements (5 phantoms × 2 days). The quality of the exponential fits for the 8 TR values was generally good, although in 15/360 cases the fit error was worse than 5% (9 cases in centre G2, 3 cases in centre A and 3 cases in centre L) (see Fig. 1). All these outliers were included in the analysis and not eliminated. One centre (J) did not provide DNE or temperature measurements in a suitable format, so its results were omitted from any analyses that needed those data. For the other centres, temperatures were recorded to ±0 .1 °C: the mean was 19.3 °C (SD 1.3), the mean deviation in temperature between day 1 and day 2 was 0.65 °C, and the worst deviation 5 °C (centre B, 0.5 mM phantom).
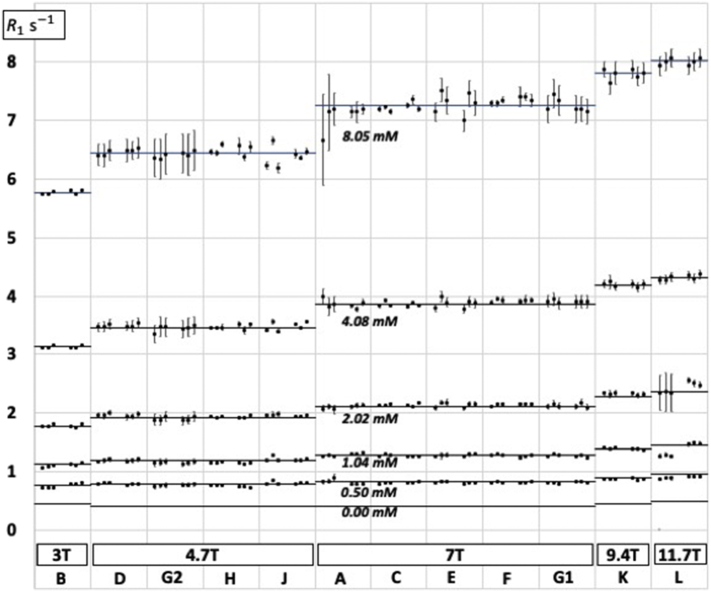
Fig. 1.
R1 measurements (logarithmic axis) for each of centres A–L. Each centre made measurements on five 2% agarose phantoms with different Ni2+ concentrations. The six horizontal lines represent R1 values calculated from the field-dependent relaxivities as explained in Table 2. There are two groups of three data points for each phantom at each centre representing, respectively, days 1 and 2, and RoIs (X,Y,Z) = (0,0,0), (10,0,0) and (0,0,12). Error bars are T1 fit errors from ParaVision.
3.1. Longitudinal relaxation rates and relaxivities
Fig. 1 depicts the individual R1 data, and Table 2 provides mean values. Fig. 2 shows the field dependence of r1 from this work, with additional data points added from the literature [3,9,15,16].
Table 2.
Relaxation rates R1 and relaxivities r1. At each centre R1 (measured) represents the weighted mean of the six measurements (2 days × 3 positions), while R1 (fitted, 0.00 mM) and r1 are respectively the intercept and slope of a linear regression of R1 against [Ni2+]. At 4.7 T and 7 T, where measurements were made at multiple centres, the SD is also given.
| 3.0 T | 4.7 T (SD) N = 4 | 7.0 T (SD) N = 5 | 9.4 T | 11.7 T | |
|---|---|---|---|---|---|
| R1 (measured)/s−1 | |||||
| 0.50 mM | 0.768 | 0.779 (0.023) | 0.808 (0.012) | 0.866 | 0.898 |
| 1.04 mM | 1.123 | 1.171 (0.023) | 1.276 (0.012) | 1.385 | 1.386 |
| 2.02 mM | 1.782 | 1.934 (0.026) | 2.131 (0.013) | 2.330 | 2.518 |
| 4.08 mM | 3.126 | 3.474 (0.019) | 3.881 (0.037) | 4.189 | 4.313 |
| 8.05 mM | 5.762 | 6.443 (0.038) | 7.248 (0.065) | 7.808 | 8.002 |
| R1 (fitted)/s−1 | |||||
| 0.00 mM | 0.438 | 0.404 (0.027) | 0.394 (0.006) | 0.438 | 0.481 |
| Relaxivity r1/s−1 mM−1 | 0.661 | 0.751 (0.006) | 0.852 (0.009) | 0.917 | 0.938 |
Fig. 2.
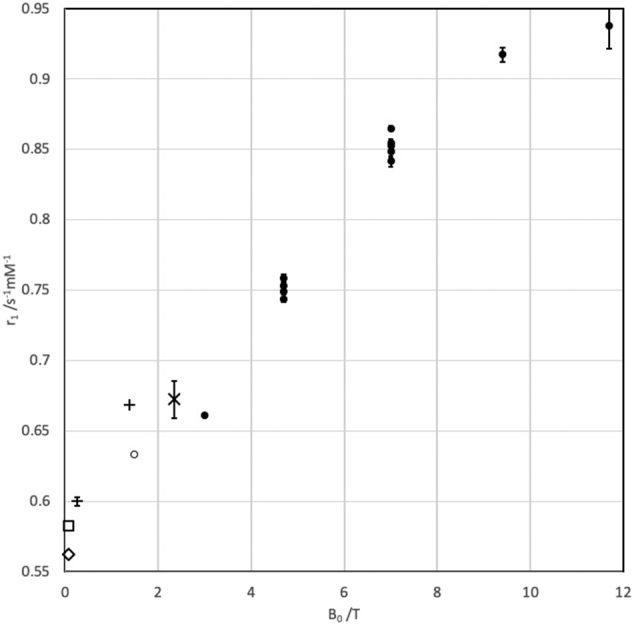
Plot of [Ni2+] relaxivities in 2% agarose against field strength. Closed circles: this work, 19.36 ± 1.20 °C. Open circle: data from initial 1 5 T characterization of the phantom materials (see supplementary material), 21.5 °C. Standard error of fit is shown, although for B0 between 1 5 T and 7 T the standard errors of between 0.19% and 0.48% are not evident as they are smaller than the size of the symbol. Other symbols: estimated from literature. +, parameter c1 in [3], 22 °C. −, estimated, with standard error, from Fig. 1 in [9], 22 °C. ×, estimated, with standard error, from Fig. 4 in [15], 20 °C. ◇, ◻︎, estimated from Fig. 2 in [16], 19 °C and 22 °C respectively.
3.2. Repeatability, reproducibility and linearity
Table 3 shows repeatability and reproducibility. Day-to-day repeatability ranged from 0.025 s−1 (centre D) to 0.097 s−1 (centre A): day-to-day repeatability CoV ranged from 0.76% (centre F) to 5.48% (centre L). In exploratory analysis, the day-to-day repeatability of 2.34% was not markedly improved either if measurements were restricted to the isocentre (2.22%), or if measurements with >1 °C difference in temperature between day 1 and day 2 were excluded (2.05%). No evidence was seen for field dependence of repeatability. For day-to-day repeatability, 2 centres (B, L) showed a statistically significant effect of day, and 4 centres (D, E, G2, K) showed a statistically significant effect of RoI position. Dynamic (DNE) signal stability CoV varied between 0.30% (centre C) and 2.1% (centre L), and in exploratory analyses was not found to be associated with B0, nor with repeatability, nor with the T1 fit error. Between-centre reproducibility of R1 was measured for the 5 phantoms at both 4.7 T and 7 T. Least reproducible, on a CoV basis, was the 0.5 mM phantom at 4.7 T (2.94%, N = 4 centres) or, on an absolute units basis, the 8 mM phantom at 7 T (0.065 s−1, N = 5 centres). In exploratory analysis, reproducibility was not improved if measurements were restricted to the isocentre (all-RoIs rms reproducibility was 0.031 s−1 or 1.4% while isocentre rms reproducibility was 0.064 s−1 or 1.6%).
Table 3.
Repeatability and reproducibility. CoV: coefficient of variation; rms: root mean square. The DNE row shows signal stability for a “dynamic-no-enhancement” (DNE) run of T1-weighted (T1W) acquisitions.
| Number of centres | Number of measurements aggregated | rms error |
||
|---|---|---|---|---|
| Absolute | CoV | |||
| Repeatability | ||||
| R1 fit error | 12 | 360 | 0.105 s−1 | 1.87% |
| R1 day-to-day | 12 | 180 × 2 | 0.056 s−1 | 2.34% |
| R1 isocentre vs. off-centre | 12 | 120 × 3 | 0.059 s−1 | 2.22% |
| DNE T1W signal | 11 | 110 × 34 | – | 0.84% |
| Reproducibility | ||||
| R1 centre-centre | 9 | 45 | 0.031 s−1 | 1.43% |
| R1 centre-centre (isocentre only) | 9 | 45 | 0.064 s−1 | 1.56% |
| Relaxivity centre-centre | 9 | 9 | 0.008 s−1mM−1 | 0.83% |
A measure of the linearity of R1 as a biomarker over the range 0.8–8 s−1 was obtained from the relaxivity Eq. (1): the rms standard error of r1,B0 was 0.6% (range 0.2% in centre B, to 1.7% in centre L, N = 12 centres).
3.3. Comparison of analysis algorithms
R1 values for centre A derived from two-parameter fits performed in “R” and in Paravision were close: mean differences were 0.024% for an unweighted fit and 0.26% for a weighted fit. When three-parameter fits performed in “R” were compared with two-parameter fits performed Paravision, disagreement was greater: 1.67% for an unweighted fit and 1.74% for a weighted fit. Bland-Altman style plots are provided in Supplementary Fig. S5.
3.4. Illustrative propagation to irreproducibility in biomarker values
Illustrative between-centre irreproducibility expected from two standard deviations of the observed R1 reproducibility for a range of derived measurements and biomarkers are given in Table 4.
Table 4.
Propagation of errors using Table 3 reproducibility, with plausible or representative values for a range of important measurements and biomarkers. Actual error propagation varies widely between applications: the values here should therefore be regarded as indicative, but not as a substitute for a thorough analysis of error propagation in any particular setting.
| Measurement or biomarker | Reproducibility error propagated from 2SD of R1 | Notes |
|---|---|---|
| Native R1 (or T1) | 0.062s-1 | |
| Tissue temperature | 1.6–4.6°C | a |
| Contrast agents | ||
| Small non-protein-bound agents e.g. gadoterate, gadopentetate, gadobutrol, relaxivities 3–11s-1mM-1 [[62], [63], [64], [65], [66], [67]]. | 6–21μM | |
| Gadobutrol in plasma at 9.4T [65], relaxivity 4.7s-1mM-1 | 13μM | |
| Gadoxetate, relaxivity [68] 5–17s-1mM-1 | 4–12μM | |
| Ferumoxytol iron oxide nanoparticles, relaxivity [69] of 20s-1(mM Fe)-1 at 1.5 T, monodisperse particle weight of 750kDa [70]. | 3μM (Fe) or 0.2nM (particles) | b |
| Investigational folate dendrimer contrast agent with relaxivity [57] 1646s-1mM-1 | 38nM | c |
| Other substances | ||
| Deoxyhaemoglobin monomer, relaxivity [56] 0.008s-1mM-1 | 7.8mM | d |
| Tempol (investigational radioprotectant), relaxivity [71] 0.2s-1mM-1 | 0.3mM | e |
| Dissolved dioxygen, relaxivity [72] 0.1-0.3s-1mM-1 | 160–470mmHg | f |
| Derived biomarkers | ||
| Transfer constant Ktrans for gadopentetate in rodent glioma, extended Tofts model [[73], [74], [75]] | 0.004min-1 (8%) | g, h |
| Extracellular extravascular fraction ve in rodent glioma, extended Tofts model [[73], [74], [75]] | 0.024 (10%) | g |
| Plasma fraction vp in rodent glioma, extended Tofts model [[73], [74], [75]] | 0.0016 (10%) | g |
| Transfer constant Ki for gadopentetate in transient ischaemia model, Patlak analysis [73,76,77] | 0.0002ml.g-1s-1 (5%) | g |
| Plasma fraction vp, transient ischaemia model, Patlak analysis [73,76,77] | 0.0008 (5%) | g |
| Flow Fp, normal rodent lung, model-free deconvolution [73,78,79] | 0.03min-1 (8%) | g |
| Plasma fraction vp, normal rodent lung, model-free deconvolution [73,78,79] | 0.04 (10%) | g |
| Normal hepatocyte transporter uptake rate constant k1 for gadoxetate, 2-compartment liver model [73,[80], [81], [82]] | 0.0013mM.s-1 (4%) | g, i |
| Normal hepatocyte transporter efflux rate constant k2 for gadoxetate, 2-compartment liver model [73,[80], [81], [82]] | 0.0001s-1 (2%) | g |
| Extracellular extravascular fraction ve, 2-compartment liver model [73,[80], [81], [82]] | 0.016 (7%) | g |
| Albumin concentration | 24μM (~5%) | j |
| Extracellular matrix Fixed Charge Density | 8mM (~4%) | k |
Notes:
Published data [[53], [54], [55]] suggest temperature dependence of tissue R1 in the range 0.013–0.0 39 s−1/°C.
Note that this figure reflects longitudinal relaxivity: transverse relaxivity for this agent is higher so may provide better sensitivity. The particle molarity is only correct if monodispersity is assumed.
This very high relaxivity is per dendrimer molecule, not per Gd.
The physiologic range is up to 17.5 g∙dL−1 (11 mM).
Tempol has been given topically at 400 mM to humans [83] and i.p. at 1.45 mmol/kg to mice [84]. Blood levels reached 3 μM in humans and 3.5 mM in mice.
The physiologic range is 0–100 mmHg in normoxia, 0–600 mmHg in hyperoxia, >1000 mmHg with hyperbaric oxygen.
See supplementary material
Typically drops in Ktrans of >20% are pharmacologically significant [59]
A drop in k1 of 78%–96% was toxicologically significant [80]
For measurements of concentration of substance, the propagated irreproducibility naturally varies with relaxivity, while for “derived” biomarkers the propagated irreproducibilities were generally ≤10%.
4. Discussion
In this work we addressed the repeatability and reproducibility of R1 in MR systems designed and employed for translational in vivo research. We prefer to work with R1 rather than T1, since from a metrology perspective [17], R1 is a ratio variable while T1 is merely an interval variable. No single method for measuring R1 is optimal for all in vivo studies. The most accurate methods (e.g. inversion recovery with long TR and short TE readout) are neither fast nor efficient. In vivo studies involve complex tradeoffs between accuracy, speed, spatial resolution, field of view, need for fat suppression, sensitivity to inflow, sensitivity to motion artefact, biexponential decay, and other confounding behaviours of tissue magnetisation such as T2 and magnetisation transfer. Moreover, even after a specific method is chosen, errors can be very sensitive to pulse sequence parameters such as choice of delays and nutation angles, spoiling and refocussing strategies, mis-set pulses and so on. In this study we elected to use a RARE saturation recovery technique covering the entire field of view, as this is fairly robust and efficient: our findings may not be directly translatable to other commonly used techniques such as Variable Flip Angle [1,18,19] or Look-Locker [1,20,21] which are vulnerable to different confounds, or even to other saturation-recovery techniques with different pulse sequence parameters.
4.1. Repeatability and reproducibility
Previous work in preclinical MR systems has addressed the between-centre reproducibility of apparent diffusion coefficients [22] and volumetrics [23], but there is little evidence on relaxation rates. Clinical MR systems are designed, maintained and operated under Medical Device regulations, but these engineering and regulatory constraints do not apply to preclinical systems, so their reproducibility might differ from clinical reproducibility.
Repeatability [24,25] (ISO 3534:2:3.3.5) refers to the similarity between measurements over a short interval made using the same test object in the same equipment operated by the same investigator. Repeatability is particularly important when the same MR biomarker is measured on successive occasions in the same human or animal, for example before and after treatment. Repeatability depends on signal-to-noise ratio and on factors such as motion artefact, for which phantoms do not model in vivo studies. Reproducibility [24,25] (ISO 3534:2:3.3.10) refers to the similarity between measurements made using test objects in different equipment operated by different investigators. Reproducibility is particularly important when an MR biomarker is measured once in each individual, for example in making a treatment decision in personalised healthcare. The ultimate motivation of this project is to use MR biomarkers to indicate a harmful effect of a drug, in settings where pre-treatment measurements might be unavailable, so reproducibility is the important metric. More generally, it is important to demonstrate reproducibility for multiple animal studies in different laboratories [26] to address the perceived “reproducibility crisis” [27] in translational medicine [28]. In this work, relevant values of R1 reproducibility and repeatability were small, and there was no obvious factor (such as temperature, B0, R1, or centre) that made any one set of measurements worse. Indeed, the error in the exponential fit of signal intensity against TR was numerically the largest error. Several between-centre studies of T1 or R1 reproducibility have been published for clinical equipment [4,5,29,30]: our CoV of 1.43% compares favourably with CoVs recently reported for inversion recovery phantom protocols in clinical systems of 5.5%–8.2% [29].
The relaxivity of Ni(H2O)62+ arises because two of the 3d nickel orbitals are half-filled, creating a high-spin triplet state with two unpaired electrons. At lower fields, below 1 T, populations of the three electron spin states are almost independent of B0, as the Zeeman splittings are dominated by spin-orbit coupling (zero field splitting) and not by the applied field B0. Above 2 T, the Zeeman splittings increase linearly with B0. The relaxivity occurs through proton-electron dipolar mechanisms, with the relevant spectral density being the longitudinal relaxation rate R1,e of the nickel electrons [31]. At low B0, R1,e depends on fluctuations of the zero field splitting which are independent of B0, and previous investigators, working at relatively low fields, reported little field dependence for nickel agarose water proton T1 values [15]. However our data, taken together with previous work (Fig. 2), clearly show a modest increase in relaxivity over the range 0.1–11.7 T.
4.2. Implications for translational research
Repeatability errors (same subject, same device) have previously been extensively studied. Good repeatability in phantoms is a necessary, but not sufficient, condition for good repeatability in vivo, because phantoms seldom model physiologic variability. However reproducibility errors (between centres) are much less studied, but are critically important in translating from single-centre to multi-centre use. Since physiologic variability is largely absorbed in the repeatability error, phantoms can be very informative about reproducibility.
Water proton T1 was arguably the first MR biomarker [[32], [33], [34], [35], [36]]. Native T1 has been reported as a biomarker inter alia in cardiac diseases [37,38], liver diseases [35,39,40], neurology [41], oncology [34,42], in the placenta [43] and in the lung [[44], [45], [46]]. Clinically significant R1 differences (Table 4) usually exceed the expected irreproducibility reported in Table 3. For example: in liver fibrosis 0.1–0.2 s−1 or 10–20% [35,39,40]; in manganese neurotoxicology 0.06 s−1 or 7% [47]; in chronic obstructive pulmonary disease 0.1 s−1 or 10% [44] were clinically significant. In preclinical tumour models, differences of 15–20% were biologically significant [34]. Notably, however, in myocardial fibrosis, differences as small as 0.02–0.03 s−1 or 2–3% may be clinically significant [48,49] and in multiple sclerosis normal-appearing white matter differences of 0.025 s−1 or 2% may be clinically significant [50], so translational animal studies of these conditions may require exceptional efforts to ensure T1 measurements can be validated and qualified for decision-making for these specific indications.
A second class of imaging biomarkers attaches a specific interpretation of the observed longitudinal relaxation, for example in arterial spin labelling [51,52] or in MR thermometry [[53], [54], [55]]. Thirdly, R1 is commonly used to determine the spatially resolved in vivo concentration of an exogenous or endogenous paramagnetic substance of known relaxivity. Relaxivity can be field-, tissue- and temperature- dependent, and varies over many orders of magnitude between relaxive substances: from <10−2 s−1 mM−1 for deoxyhaemoglobin monomer [56] to >103 s−1 mM−1 reported for certain investigational polymetallated contrast agents [57]. R1 errors propagate to low micromolar errors in typical gadolinium- or manganese-based contrast agents. However, propagation of errors may be more significant for techniques based on lower-relaxivity substances. For example in oxygen-enhanced MRI, which measures hyperoxia-induced changes in deoxyhaemoglobin and dissolved oxygen concentration via change in R1 [45,58], meticulous standardisation is warranted. From Table 4, error propagation might also be important for studies of therapeutic nitroxyls and perhaps for thermometry.
Finally, there are many biomarkers derived indirectly from contrast agent concentration, using a physiologic model. These include measures of perfusion and permeability in tumours, infarcts, synovitis or lung disease; myocardial extracellular volume, cartilage fixed charge density in osteoarthritis, and liver transporter function in toxicology. All biomarkers are also measured in animal models, often aiming to assist the design and interpretation of clinical studies, so it is important to understand the validity of these measurements in preclinical systems. Table 4 includes a representative selection of such MR biomarkers, with simple assessments of how instrumentation-derived irreproducibility in R1 might propagate. For example, the measured between-centre uncertainty in precontrast R1 translates to at most 10% between-centre uncertainty in the biomarkers derived from DCE-MRI (Table 4). This error is smaller than the typical day-to-day repeatability error, and in itself would have little effect on the interpretation of change in parameters such as Ktrans, because treatment effects are typically much >10% [59].
A realistic assessment of propagation of errors is complex and beyond the scope of this work. In particular, in compartmental models, reproducibility errors and repeatability errors are not completely independent. We omitted from consideration terms which primarily affect repeatability, such as error cancellation with post-contrast R1, additional R1 errors that arise in the presence of contrast agent (e.g. signal saturation, limited water exchange), and in vivo effects (e.g. inflow, breathing motion, bolus dispersion, partial volume). Nevertheless, Table 4 provides comparative order-of magnitude assessments to highlight cases in which the variance seen in our study might be important. With this caveat, in myocardial fibrosis, in normal-appearing multiple sclerosis white mater, and in oxygen-enhanced MRI, R1-based MR biomarkers would be quite sensitive even to such small errors in R1 unless additional acquisition and analysis methods are designed to reduce the impact of error propagation. An example of this is the use of dynamic time series in OE-MRI that determine ∆R1(t) by referencing the time-varying signal to a baseline R1 measurement, thereby reducing the degrees of freedom in the measurement and subsequent error propagation [60]. Similar approaches have been common in DCE-MRI for many years.
4.3. Study limitations
-
(1)
This study was performed using only one vendor's equipment, Bruker Avance I, II or III systems running Paravision 5 or 6, representing a typical range of equipment for preclinical MR biomarker research at the time when the study was performed (2017–18). The findings may not be translatable to other vendors' equipment.
-
(2)
Only one pulse sequence (saturation recovery with RARE readout) was employed. This was chosen [61] in a compromise between accuracy and speed. However the assumption of zero longitudinal magnetisation at the mid-point of the eighth echo may be invalid if B1 is imperfect, and the findings may not be translatable to other sequences with different B1 sensitivity.
-
(3)
The accuracy of our data was not verified using an external standard, such as spectroscopic inversion-recovery.
-
(4)
A common problem for MR phantoms is temperature dependence. In addition to ambient room temperature, heat is imparted to the phantom from the shims during the working day, from the pulsed gradients, and directly from radiofrequency power deposited by the pulse. Data at 1.5 T [4] and 2.35 T [15] show R1 temperature dependencies in the range −1.3%/°C to +0.7%/°C; data at 0. 08 T [16] show an r1 temperature dependence of 0.006 s−1 mM−1/°C. Although temperatures were measured in this study, no direct measurement was made of the agarose temperature itself during MR data acquisition, and exploratory analyses did not reveal temperature as a confound.
-
(5)
In order to address the question of reproducibility in normal academic practice, our study modelled “institution-led” standardisation. No site training was performed, no quality control was imposed, nor were sites permitted to repeat their measurements to eliminate apparent outliers. We did not verify that all scanners were performing optimally, and indeed SNR estimated from the DNE runs did not show the anticipated variation with B0 or coil design. RoIs and T1 calculations were performed locally. Possibly, “centrally-led” standardisation rigorously imposed by a core lab might further improve reproducibility.
-
(6)
No phantom study can fully model the in vivo measurement. Nevertheless, a well-designed phantom study sets a lower limit on the error to be expected from measurements in living animals.
4.4. Conclusions
Using nickel agarose phantoms in typical preclinical MR systems, R1 exhibited adequate reproducibility for most purposes. Reproducibility (and repeatability) of <0.06 s−1 and < 2.4% was readily achieved. These small technical (instrumentation-derived) errors in R1 measurement mostly do not contribute biologically significant errors into R1-based MR biomarkers. However, in a small number of very demanding applications, such as myocardial fibrosis, white mater, or oxygen-enhanced MRI, the accuracy of R1-based MR biomarkers would be quite sensitive even to such small errors in R1, therefore in these cases further work may be needed to adequately standardise R1 data acquisition and analysis.
Conflicts of interest
CG, GS and SZ are employees of Bayer AG, a for-profit company providing MR contrast agents. PDH is an employee at Antaros Medical, a for-profit company providing MR biomarker services. SK and KS are employees of Bruker BioSpin MRI GmbH, a for-profit company which is the manufacturer of the MR systems used in the study. JCW receives compensation from Bioxydyn Ltd., a for-profit company providing MR biomarker services.
Acknowledgements
The research leading to these results received funding from the Innovative Medicines Initiatives 2 Joint Undertaking under grant agreement No 116106 (IB4SD-TRISTAN). This Joint Undertaking receives support from the European Union's Horizon 2020 research and innovation programme and EFPIA. This work was also supported by the CRUK and EPSRC Cancer Imaging Centre in Cambridge and Manchester funding to The University of Manchester (grant C8742/A18097). The MRI scanner at the University of Manchester is supported by the UK BBSRC (BB/F011350/1).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mri.2019.03.008.
Contributor Information
John C. Waterton, Email: john.waterton@bioxydyn.com.
Catherine D.G. Hines, Email: catherine.hines@merck.com.
Paul D. Hockings, Email: paul.hockings@antarosmedical.com.
Iina Laitinen, Email: iina.laitinen@sanofi.com.
Sabina Ziemian, Email: sabina.ziemian@bayer.com.
Simon Campbell, Email: simon.x.campbell@gsk.com.
Michael Gottschalk, Email: michael.gottschalk@med.lu.se.
Claudia Green, Email: claudia.green@bayer.com.
Michael Haase, Email: michael.v.haase@gsk.com.
Katja Hassemer, Email: katja.hassemer@sanofi.com.
Sascha Koehler, Email: Sascha.Koehler@bruker.com.
William Lloyd, Email: william.lloyd@manchester.ac.uk.
Yanping Luo, Email: yanping.luo@abbvie.com.
Irma Mahmutovic Persson, Email: irma.mahmutovic_persson@med.lu.se.
James P.B. O'Connor, Email: james.o'connor@manchester.ac.uk.
Lars E. Olsson, Email: lars_e.olsson@med.lu.se.
Kashmira Pindoria, Email: kashmira.2.pindoria@gsk.com.
Jurgen E. Schneider, Email: J.E.Schneider@leeds.ac.uk.
Steven Sourbron, Email: S.Sourbron@leeds.ac.uk.
Denise Steinmann, Email: denise.steinmann@sanofi.com.
Klaus Strobel, Email: Klaus.Strobel@bruker.com.
Sirisha Tadimalla, Email: S.Tadimalla@leeds.ac.uk.
Irvin Teh, Email: I.Teh@leeds.ac.uk.
Andor Veltien, Email: Andor.Veltien@radboudumc.nl.
Xiaomeng Zhang, Email: xiaomeng.zhang@abbvie.com.
Gunnar Schütz, Email: gunnar.schuetz@bayer.com.
Appendix A. Supplementary data
Supplementary material
References
- 1.Stikov N., Boudreau M., Levesque I.R., Tardif C.L., Barral J.K., Pike G.B. On the accuracy of T1 mapping: searching for common ground. Magn Reson Med. 2015;73:514–522. doi: 10.1002/mrm.25135. [DOI] [PubMed] [Google Scholar]
- 2.Keenan K.E., Ainslie M., Barker A.J., Boss M.A., Cecil K.M., Charles C. Quantitative magnetic resonance imaging phantoms: a review and the need for a system phantom. Magn Reson Med. 2018;79:48–61. doi: 10.1002/mrm.26982. [DOI] [PubMed] [Google Scholar]
- 3.Captur G., Gatehouse P., Keenan K.E., Heslinga F.G., Bruehl R., Prothmann M. A medical device-grade T1 and ECV phantom for global T1 mapping quality assurance - the T1 mapping and ECV standardization in cardiovascular magnetic resonance (T1MES) program. J Cardiovasc Magn Reson. 2016;18:1–20. doi: 10.1186/s12968-016-0280-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vassiliou V.S., Heng E.L., Gatehouse P.D., Donovan J., Raphael C.E., Giri S. Magnetic resonance imaging phantoms for quality-control of myocardial T1 and ECV mapping: specific formulation, long-term stability and variation with heart rate and temperature. J Cardiovasc Magn Reson. 2016;18:1–12. doi: 10.1186/s12968-016-0275-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lerski R.A., McRobbie D.W., Straughan K., Walker P.M., de Certaines J.D., Bernard A.M. V. Multi-center trial with protocols and prototype test objects for the assessment of MRI equipment. Magn Reson Imaging. 1988;6:201–214. doi: 10.1016/0730-725x(88)90451-1. [DOI] [PubMed] [Google Scholar]
- 6.Mannheim J.G., Kara F., Doorduin J., Fuchs K., Reischl G., Liang S. Standardization of small animal imaging—current status and future prospects. Mol Imaging Biol. 2018;20:716–731. doi: 10.1007/s11307-017-1126-2. [DOI] [PubMed] [Google Scholar]
- 7.Osborne D.R., Kuntner C., Berr S., Stout D. Guidance for efficient small animal imaging quality control. Mol Imaging Biol. 2017;19:485–498. doi: 10.1007/s11307-016-1012-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Connor J.P.B., Aboagye E.O., Adams J.E., Aerts H.J.W.L., Barrington S.F., Beer A.J. Imaging biomarker roadmap for cancer studies. Nat Rev Clin Oncol. 2017;14:169–186. doi: 10.1038/nrclinonc.2016.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christoffersson J.O., Olsson L.E., Sjöberg S. Nickel-doped agarose gel phantoms in MR imaging. Acta Radiol. 1991;32:426–431. [PubMed] [Google Scholar]
- 10.International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use ICH harmonised tripartite guideline: guideline for good clinical practice E6(R1) 1996. https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf
- 11.Waterton J.C., Ho M., Nordenmark L.H., Jenkins M., DiCarlo J., Guillard G. Repeatability and response to therapy of dynamic contrast-enhanced magnetic resonance imaging biomarkers in rheumatoid arthritis in a large multicentre trial setting. Eur Radiol. 2017;27:3662–3668. doi: 10.1007/s00330-017-4736-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.ResonanceHealth FerriScan®-R2-MRI measurement of liver Iron concentration. 2015. http://www.resonancehealth.com/images/files/FerriScan/FerriScan Fact Sheet Mar 2015.pdf (accessed November 1, 2018)
- 13.TRISTAN Translational imaging in drug safety assessment 2017. www.imi-tristan.eu
- 14.R_Core_Team Vienna, Austria: R Foundation for Statistical Computing; R: a language and environment for statistical computing. https://www.R-project.org/
- 15.Kraft K.A., Fatouros P.P., Clarke G.D., Kishore P.R.S. An MRI phantom material for quantitative relaxometry. Magn Reson Med. 1987;5:555–562. doi: 10.1002/mrm.1910050606. [DOI] [PubMed] [Google Scholar]
- 16.Howe F.A. Relaxation times in paramagnetically doped agarose gels as a function of temperature and ion concentration. Magn Reson Imaging. 1988;6:263–270. doi: 10.1016/0730-725x(88)90400-6. [DOI] [PubMed] [Google Scholar]
- 17.Sullivan D.C., Obuchowski N.A., Kessler L.G., Raunig D.L., Gatsonis C., Huang E.P. Metrology standards for quantitative imaging biomarkers. Radiology. 2015;277:813–825. doi: 10.1148/radiol.2015142202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christensen K.A., Grant D.M., Schulman E.M., Walling C. Optimal determination of relaxation times of fourier transform nuclear magnetic resonance. Determination of spin-lattice relaxation times in chemically polarized species. J Phys Chem. 1974;78:1971–1977. [Google Scholar]
- 19.Kingsley P.B. Methods of measuring spin-lattice (T1) relaxation times: an annotated bibliography. Concepts Magn Reson. 1999;11:243–276. [Google Scholar]
- 20.Look D.C., Locker D.R. Nuclear spin-lattice relaxation measurements by tone-burst modulation. Phys Rev Lett. 1968;20:987–989. [Google Scholar]
- 21.Messroghli D.R., Radjenovic A., Kozerke S., Higgins D.M., Sivananthan M.U., Ridgway J.P. Modified Look-Locker inversion recovery (MOLLI) for high-resolutionT1 mapping of the heart. Magn Reson Med. 2004;52:141–146. doi: 10.1002/mrm.20110. [DOI] [PubMed] [Google Scholar]
- 22.Doblas S., Almeida G.S., Blé F.X., Garteiser P., Hoff B.A., McIntyre D.J.O. Apparent diffusion coefficient is highly reproducible on preclinical imaging systems: evidence from a seven-center multivendor study. J Magn Reson Imaging. 2015;42:1759–1764. doi: 10.1002/jmri.24955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee Y.C., Fullerton G.D., Baiu C., Lescrenier M.G., Goins B.A. Preclinical multimodality phantom design for quality assurance of tumor size measurement. BMC Med Phys. 2011;11(1) doi: 10.1186/1756-6649-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.QIBA Technical Perfornance Working Group Quantitative imaging biomarkers: a review of statistical methods for technical performance assessment. Stat Methods Med Res. 2015;24:27–67. doi: 10.1177/0962280214537344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.ISO . vol. 3. 2006. Statistics - vocabulary and symbols. Part 2: Applied statistics. ISO 3534–2; p. 125. [Google Scholar]
- 26.Voelkl B., Vogt L., Sena E.S., Würbel H. Reproducibility of preclinical animal research improves with heterogeneity of study samples. PLoS Biol. 2018;16 doi: 10.1371/journal.pbio.2003693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baker M., Penny D. Is there a reproducibility crisis? Nature. 2016;533:452–454. doi: 10.1038/533452a. [DOI] [PubMed] [Google Scholar]
- 28.Goodman S.N., Fanelli D., Ioannidis J.P.A. What does research reproducibility mean? Sci Transl Med. 2016;8:341ps12. doi: 10.1126/scitranslmed.aaf5027. [DOI] [PubMed] [Google Scholar]
- 29.Bane O., Hectors S.J., Wagner M., Arlinghaus L.L., Aryal M.P., Cao Y. Accuracy, repeatability, and interplatform reproducibility of T<ce:inf>1</ce:inf> quantification methods used for DCE-MRI: results from a multicenter phantom study. Magn Reson Med. 2018;79:2564–2575. doi: 10.1002/mrm.26903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jerome N.P., Orton M.R., Parkes H.G., Winfield J.M., Boss M.A., Leach M.O. Development of a temperature-controlled phantom for magnetic resonance quality assurance of diffusion, dynamic, and relaxometry measurements. Med Phys. 2016;43:2998–3007. doi: 10.1118/1.4948997. [DOI] [PubMed] [Google Scholar]
- 31.Helm L. Relaxivity in paramagnetic systems: theory and mechanisms. Prog Nucl Magn Reson Spectrosc. 2006;49:45–64. [Google Scholar]
- 32.Damadian R. Tumor detection by nuclear magnetic resonance. Science. 1971;171:1151–1153. doi: 10.1126/science.171.3976.1151. [DOI] [PubMed] [Google Scholar]
- 33.Runge V.M., Clanton J.A., Smith F.W., Hutchison J., Mallard J., Partain C.L. Nuclear magnetic resonance of iron and copper disease states. AJR Am J Roentgenol. 1983;141:943–948. doi: 10.2214/ajr.141.5.943. [DOI] [PubMed] [Google Scholar]
- 34.McSheehy P.M.J., Weidensteiner C., Cannet C., Ferretti S., Laurent D., Ruetz S. Quantified tumor T1 is a generic early-response imaging biomarker for chemotherapy reflecting cell viability. Clin Cancer Res. 2010;16:212–225. doi: 10.1158/1078-0432.CCR-09-0686. [DOI] [PubMed] [Google Scholar]
- 35.Banerjee R., Pavlides M., Tunnicliffe E.M., Piechnik S.K., Sarania N., Philips R. Multiparametric magnetic resonance for the non-invasive diagnosis of liver disease. J Hepatol. 2014;60:69–77. doi: 10.1016/j.jhep.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Doyle F.H., Gore J.C., Pennock J.M. Relaxation rate enhancement observed in vivo by NMR imaging. J Comput Assist Tomogr. 1981;5:295–296. [Google Scholar]
- 37.Bulluck H., Maestrini V., Rosmini S., Abdel-Gadir A., Treibel T.A., Castelletti S. Myocardial T1 mapping. Circ J. 2015;79:487–494. doi: 10.1253/circj.CJ-15-0054. [DOI] [PubMed] [Google Scholar]
- 38.Moon J.C., Messroghli D.R., Kellman P., Piechnik S.K., Robson M.D., Ugander M. Myocardial T1 mapping and extracellular volume quantification: a Society for Cardiovascular Magnetic Resonance (SCMR) and CMR working Group of the European Society of cardiology consensus statement. J Cardiovasc Magn Reson. 2013;15 doi: 10.1186/1532-429X-15-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heye T., Yang S.-R., Bock M., Brost S., Weigand K., Longerich T. MR relaxometry of the liver: significant elevation of T1 relaxation time in patients with liver cirrhosis. Eur Radiol. 2012;22:1224–1232. doi: 10.1007/s00330-012-2378-5. [DOI] [PubMed] [Google Scholar]
- 40.Kim K.A., Park M.S., Kim I.S., Kiefer B., Chung W.S., Kim M.J. Quantitative evaluation of liver cirrhosis using T1 relaxation time with 3 tesla MRI before and after oxygen inhalation. J Magn Reson Imaging. 2012;36:405–410. doi: 10.1002/jmri.23620. [DOI] [PubMed] [Google Scholar]
- 41.Deoni S.C.L. Magnetic resonance relaxation and quantitative measurement in the brain. Methods Mol Biol. 2011;711:65–108. doi: 10.1007/978-1-61737-992-5_4. [DOI] [PubMed] [Google Scholar]
- 42.O'Connor J.P.B., Carano R.A.D., Clamp A.R., Ross J., Ho C.C.K., Jackson A. Quantifying antivascular effects of monoclonal antibodies to vascular endothelial growth factor: insights from imaging. Clin Cancer Res. 2009;15:6674–6682. doi: 10.1158/1078-0432.CCR-09-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wright C., Morris D.M., Baker P.N., Crocker I.P., Gowland P.A., Parker G.J. Magnetic resonance imaging relaxation time measurements of the placenta at 1.5T. Placenta. 2011;32:1010–1015. doi: 10.1016/j.placenta.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alamidi D.F., Morgan A.R., Hubbard Cristinacce P.L., Nordenmark L.H., Hockings P.D., Lagerstrand K.M. COPD patients have short lung magnetic resonance T1 relaxation time. COPD J Chronic Obstr Pulm Dis. 2016;13:153–159. doi: 10.3109/15412555.2015.1048851. [DOI] [PubMed] [Google Scholar]
- 45.Zurek M., Johansson E., Risse F., Alamidi D., Olsson L.E., Hockings P.D. Accurate T1 mapping for oxygen-enhanced MRI in the mouse lung using a segmented inversion-recovery ultrashort echo-time sequence. Magn Reson Med. 2014;71:2180–2185. doi: 10.1002/mrm.24876. [DOI] [PubMed] [Google Scholar]
- 46.Alamidi D.F., Kindvall S.S.I., Hubbard Cristinacce P.L., McGrath D.M., Young S.S., Naish J.H. T1 relaxation time in lungs of asymptomatic smokers. PLoS One. 2016:11. doi: 10.1371/journal.pone.0149760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bowler R.M., Yeh C.L., Adams S.W., Ward E.J., Ma R.E., Dharmadhikari S. Association of MRI T1 relaxation time with neuropsychological test performance in manganese- exposed welders. Neurotoxicology. 2018;64:19–29. doi: 10.1016/j.neuro.2017.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bull S., White S.K., Piechnik S.K., Flett A.S., Ferreira V.M., Loudon M. Human non-contrast T1 values and correlation with histology in diffuse fibrosis. Heart. 2013;99:932–937. doi: 10.1136/heartjnl-2012-303052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakamori S., Bui A.H., Jang J., El-Rewaidy H.A., Kato S., Ngo L.H. Increased myocardial native T 1 relaxation time in patients with nonischemic dilated cardiomyopathy with complex ventricular arrhythmia. J Magn Reson Imaging. 2017;47:779–786. doi: 10.1002/jmri.25811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Steenwijk M.D., Vrenken H., Jonkman L.E., Daams M., Geurts J.J., Barkhof F. High-resolution T1-relaxation time mapping displays subtle, clinically relevant, gray matter damage in long-standing multiple sclerosis. Mult Scler J. 2016;22:1279–1288. doi: 10.1177/1352458515615953. [DOI] [PubMed] [Google Scholar]
- 51.Alsop D.C., Detre J.A., Golay X., Günther M., Hendrikse J., Hernandez-Garcia L. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med. 2015;73:102–116. doi: 10.1002/mrm.25197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Petersen E.T., Mouridsen K., Golay X. The QUASAR reproducibility study, part II: results from a multi-center arterial spin labeling test-retest study. Neuroimage. 2010;49:104–113. doi: 10.1016/j.neuroimage.2009.07.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matsumoto R., Oshio K., Jolesz F.A. Monitoring of laser- and freezing-induced ablation in the liver with T1-weighted MR imaging. J Magn Reson Imaging. 1992;2:555–562. doi: 10.1002/jmri.1880020513. [DOI] [PubMed] [Google Scholar]
- 54.Rieke V., Pauly K.B. MR thermometry. J Magn Reson Imaging. 2008;27:376–390. doi: 10.1002/jmri.21265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cline H.E., Hynynen K., Hardy C.J., Watkins R.D., Schenck J.F., Jolesz F.A. MR temperature mapping of focused ultrasound surgery. Magn Reson Med. 1994;31:628–636. doi: 10.1002/mrm.1910310608. [DOI] [PubMed] [Google Scholar]
- 56.Hales P.W., Kirkham F.J., Clark C.A. A general model to calculate the spin-lattice (T1) relaxation time of blood, accounting for haematocrit, oxygen saturation and magnetic field strength. J Cereb Blood Flow Metab. 2016;36:370–374. doi: 10.1177/0271678X15605856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Konda S.D., Aref M., Wang S., Brechbiel M., Wiener E.C. Specific targeting of folate-dendrimer MRI contrast agents to the high affinity folate receptor expressed in ovarian tumor xenografts. MAGMA. 2001;12:104–113. doi: 10.1007/BF02668091. [DOI] [PubMed] [Google Scholar]
- 58.Linnik I.V., Scott M.L.J., Holliday K.F., Woodhouse N., Waterton J.C., O'Connor J.P.B. Noninvasive tumor hypoxia measurement using magnetic resonance imaging in murine U87 glioma xenografts and in patients with glioblastoma. Magn Reson Med. 2014;71:1854–1862. doi: 10.1002/mrm.24826. [DOI] [PubMed] [Google Scholar]
- 59.O'Connor J.P.B., Jackson A., Parker G.J.M., Roberts C., Jayson G.C. Dynamic contrast-enhanced MRI in clinical trials of antivascular therapies. Nat Rev Clin Oncol. 2012;9:167–177. doi: 10.1038/nrclinonc.2012.2. [DOI] [PubMed] [Google Scholar]
- 60.Morgan A.R., Parker G.J.M., Roberts C., Buonaccorsi G.A., Maguire N.C., Hubbard Cristinacce P.L. Feasibility assessment of using oxygen-enhanced magnetic resonance imaging for evaluating the effect of pharmacological treatment in COPD. Eur J Radiol. 2014;83:2093–2101. doi: 10.1016/j.ejrad.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 61.Kingsley P.B. Signal intensities and T1 calculations in multiple-Echo sequences with imperfect pulses. Concepts Magn Reson. 1999;11:29–49. [Google Scholar]
- 62.Lohrke J., Frenzel T., Endrikat J., Alves F.C., Grist T.M., Law M. 25 years of contrast-enhanced MRI: developments, current challenges and future perspectives. Adv Ther. 2016;33:1–28. doi: 10.1007/s12325-015-0275-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.De Leõn-Rodríguez L.M., Martins A.F., Pinho M.C., Rofsky N.M., Sherry A.D. Basic MR relaxation mechanisms and contrast agent design. J Magn Reson Imaging. 2015;42:545–565. doi: 10.1002/jmri.24787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rohrer M., Bauer H., Mintorovitch J., Requardt M., Weinmann H.J. Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Invest Radiol. 2005;40:715–724. doi: 10.1097/01.rli.0000184756.66360.d3. [DOI] [PubMed] [Google Scholar]
- 65.Fries P., Müller A., Seidel R., Robert P., Denda G., Menger M.D. P03277-a new approach to achieve high-contrast enhancement: initial results of an experimental extracellular gadolinium-based magnetic resonance contrast agent. Invest Radiol. 2015;50:835–842. doi: 10.1097/RLI.0000000000000192. [DOI] [PubMed] [Google Scholar]
- 66.Pintaske J., Martirosian P., Graf H., Erb G., Lodemann K.P., Claussen C.D. Relaxivity of gadopentetate dimeglumine (Magnevist), gadobutrol (Gadovist), and gadobenate dimeglumine (MultiHance) in human blood plasma at 0.2, 1.5, and 3 Tesla. Invest Radiol. 2006;41:213–221. doi: 10.1097/01.rli.0000197668.44926.f7. [DOI] [PubMed] [Google Scholar]
- 67.Shen Y., Goerner F.L., Snyder C., Morelli J.N., Hao D., Hu D. T1 relaxivities of gadolinium-based magnetic resonance contrast agents in human whole blood at 1.5, 3, and 7 T. Invest Radiol. 2015;50:330–338. doi: 10.1097/RLI.0000000000000132. [DOI] [PubMed] [Google Scholar]
- 68.Schuhmann-Giampieri G., Schmitt-Willich H., Press W.R., Negishi C., Weinmann H.J., Speck U. Preclinical evaluation of Gd-EOB-DTPA as a contrast agent in MR imaging of the hepatobiliary system. Radiology. 1992;183:59–64. doi: 10.1148/radiology.183.1.1549695. [DOI] [PubMed] [Google Scholar]
- 69.Knobloch G., Colgan T., Wiens C.N., Wang X., Schubert T., Hernando D. Relaxivity of ferumoxytol at 1.5 T and 3.0 T. Invest Radiol. 2017;53:257–263. doi: 10.1097/RLI.0000000000000434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Food and Drug Administration of the United States of America FERAHEME® (ferumoxytol injection), for intravenous use. HIGHLIGHTS OF PRESCRIBING INFORMATION. 2009. http://www.feraheme.com/pdfs/Feraheme-Prescribing-Information.pdf
- 71.Hyodo F., Matsumoto S., Devasahayam N., Dharmaraj C., Subramanian S., Mitchell J.B. Pulsed EPR imaging of nitroxides in mice. J Magn Reson. 2009;197:181–185. doi: 10.1016/j.jmr.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hausser R., Noack F. Kernmagnetische Relaxation und Korrelation im System Wasser -Sauerstoff. Z Naturforschg. 1965;20:1668–1675. [Google Scholar]
- 73.McGrath D.M., Bradley D.P., Tessier J.L., Lacey T., Taylor C.J., Parker G.J.M. Comparison of model-based arterial input functions for dynamic contrast-enhanced MRI in tumor bearing rats. Magn Reson Med. 2009;61:1173–1184. doi: 10.1002/mrm.21959. [DOI] [PubMed] [Google Scholar]
- 74.Larsson C., Kleppestø M., Grothe I., Vardal J., Bjørnerud A. T1 in high-grade glioma and the influence of different measurement strategies on parameter estimations in DCE-MRI. J Magn Reson Imaging. 2015;42:97–104. doi: 10.1002/jmri.24772. [DOI] [PubMed] [Google Scholar]
- 75.Chwang W.B., Jain R., Bagher-Ebadian H., Nejad-Davarani S.P., Iskander A.S.M., VanSlooten A. Measurement of rat brain tumor kinetics using an intravascular MR contrast agent and DCE-MRI nested model selection. J Magn Reson Imaging. 2014;40:1223–1229. doi: 10.1002/jmri.24469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abo-Ramadan U., Durukan A., Pitkonen M., Marinkovic I., Tatlisumak E., Pedrono E. Post-ischemic leakiness of the blood-brain barrier: a quantitative and systematic assessment by Patlak plots. Exp Neurol. 2009;219:328–333. doi: 10.1016/j.expneurol.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 77.Merali Z., Huang K., Mikulis D., Silver F., Kassner A. Evolution of blood-brain-barrier permeability after acute ischemic stroke. PLoS One. 2017;12 doi: 10.1371/journal.pone.0171558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jakob P.M., Hillenbrand C.M., Wang T., Schultz G., Hahn D., Haase A. Rapid quantitative lung 1H T1 mapping. J Magn Reson Imaging. 2001;14:795–799. doi: 10.1002/jmri.10024. [DOI] [PubMed] [Google Scholar]
- 79.Mistry N.N., Qi Y., Hedlund L.W., Johnson G.A. Ventilation/perfusion imaging in a rat model of airway obstruction. Magn Reson Med. 2010;63:728–735. doi: 10.1002/mrm.22221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ulloa J.L., Stahl S., Yates J., Woodhouse N., Kenna J.G., Jones H.B. Assessment of gadoxetate DCE-MRI as a biomarker of hepatobiliary transporter inhibition. NMR Biomed. 2013;26:1258–1270. doi: 10.1002/nbm.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Karageorgis A., Lenhard S., Yerby B., Forsgren M., Liachenko S. A multi-center preclinical study of gadoxetate DCE-MRI in rats as a biomarker of drug induced inhibition of liver transporter function. PLoS One. 2018:e0197213. doi: 10.1371/journal.pone.0197213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chow A.M., Gao D.S., Fan S.J., Qiao Z., Lee F.Y., Yang J. Measurement of liver T1 and T2 relaxation times in an experimental mouse model of liver fibrosis. J Magn Reson Imaging. 2012;36:152–158. doi: 10.1002/jmri.23606. [DOI] [PubMed] [Google Scholar]
- 83.Metz J.M., Smith D., Mick R., Lustig R., Mitchell J., Cherakuri M. A phase I study of topical tempol for the prevention of alopecia induced by whole brain radiotherapy. Clin Cancer Res. 2004;10:6411–6417. doi: 10.1158/1078-0432.CCR-04-0658. [DOI] [PubMed] [Google Scholar]
- 84.Hahn S.M., Tochner Z., Krishna C.M., Glass J., Wilson L., Samuni A. Tempol, a stable free radical, is a novel murine radiation protector. Cancer Res. 1992;52:1750–1753. [PubMed] [Google Scholar]
- 85.Richardson O.C., Bane O., Scott M.L.J., Tanner S.F., Waterton J.C., Sourbron S.P. Gadofosveset-based biomarker of tissue albumin concentration: technical validation in vitro and feasibility in vivo. Magn Reson Med. 2015;73:244–253. doi: 10.1002/mrm.25128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bashir A., Gray M.L., Burstein D. Gd-DTPA2- as a measure of cartilage degradation. Magn Reson Med. 1996;36:665–673. doi: 10.1002/mrm.1910360504. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material