Abstract
Background
Three-fifths of total deaths in India are attributed to noncommunicable diseases, and coronary heart disease (CHD) is one of the dominant causes. There are only few studies available in India to find confirmed CHD by pragmatic approach. This study aims to find prevalence of confirmed CHD and its risk factors in rural community of central India.
Materials and methods
This was a community-based cross-sectional study during 2013–2014 involving adults ≥60 years from 13 villages in rural central India. We screened CHD on the basis of history and standard 12-lead ECG. Apart from the past documentation of CHD, we diagnosed confirmed CHD in symptomatic patients or with resting ECG changes by means of echocardiography, exercise ECG test or coronary angiography whenever needed.
Results
We screened 1190 of 1415 individuals ≥60 years for CHD. Five hundred eighty were men and 610 were women. Diagnosis of CHD was confirmed in 61 individuals (29 men and 32 women). The prevalence of CHD in individuals older than 60 years was 51.3 per 1000 population. Hypertension was the only independent risk factor associated with CHD, whereas association of diabetes mellitus, obesity, socioeconomic status and smoking with CHD was not significant.
Conclusion
Prevalence of confirmed CHD has increased in agrarian rural community in central India, which requires further studies to find out causative factors.
Keywords: Coronary heart disease, Epidemiology, Rural India
1. Introduction
Noncommunicable diseases (NCDs) were responsible for 70% of total 56.4 million deaths occurred worldwide in 2015, and 81% of them are caused by cardiovascular diseases, cancer and chronic respiratory diseases. Coronary heart disease (CHD), the most prevalent cardiovascular disease and leading killer disease, accounted for 15.5% of total deaths globally.1 CHD has assumed epidemic proportions in India. The Registrar General of India reported diseases of the circulatory system are topmost, constituting one-third of the total medically certified deaths, and CHD accounted 26.9% of these deaths in 2015.2 The increase in the prevalence of cardiovascular disease in India is the largest for any country in the world.3, 4 CHD was the leading cause of death in India, killing more than 12 lakh people in 2012.5
Analysis of population prevalence studies of CHD in India6, 7, 8 highlights the limitations of the ECG-defined (Minnesota code)9 diagnostic criteria in asymptomatic individuals. Progressively increasing ECG-defined CHD has been reported from rural10, 11, 12, 13, 14, 15, 16, 17 and urban India.18, 19, 20, 21, 22, 23 Nonspecific ECG abnormality is a major problem in interpreting resting ECG in these cross-sectional studies. Q wave, considered since long as an infallible sign of myocardial infarction (MI), can be produced by diverse abnormalities. False-positive diagnosis based on nonspecific T wave changes has been well documented particularly in women.24, 25, 26, 27, 28, 29, 30
The rural community–based studies attempting definitive diagnosis of CHD by recruiting exercise ECG test, echocardiography and coronary angiogram are few.12, 16, 31 Hence, this study.
2. Methodology
The study was performed in a periurban community under gram panchayat governance in Wardha district, Maharashtra, in central India during the period between October 2013 and December 2014. It was conducted in 13 villages (Dindoda, Hivra, Karanji Kazi, Khadka, Kutki, Nagapur, Nandura, Pimpalgaon, Takli Kite, Talodi, Tuljapur, Waghala and Yerangaon) which are adopted under a holistic village health assurance programme since 1981 by Kasturba Hospital. This study was a part of a larger community-based, cross-sectional study undertaken to identify the prevalence of CHD, hypertension, diabetes mellitus, metabolic syndrome, stroke and nephropathy in this area.
Three monthly diagnostic village camps were conducted to screen NCDs in these 13 villages. The list of village population was available from the village health assurance programme, which is updated periodically with help of voluntary village health workers appointed by the Kasturba Hospital of Mahatma Gandhi Institute of Medical Sciences, Sevagram. All eligible persons ≥60 years were informed of the diagnostic camp by the village health worker on a day before. A team of trained health professionals conducted the surveys. Structured questionnaire was used to collect information about the individual's demographic details, socioeconomic status, educational status, physical activity and presence of other risk factors for CHD including family history. Physical activity was recorded using the prevalidated scale.32
The socioeconomic status (SES) was recorded using a scale developed to measure the standard of living for National Family Health Survey III.33 It judges SES from material possessions with a possible score ranging from 0 to 67. We made three categories—low socioeconomic status (score 1–24), middle socioeconomic status (score 25–49) and high socioeconomic status (score above 49). Clinical examination was performed by a team of physicians. The anthropometry (weight, waist circumference and hip circumference) and blood pressure were measured. Hypertension was classified as per Joint National Committee (JNC) 2007 guidelines,34 and diabetes mellitus was defined according to the American Diabetes Association criteria for diagnosis. Fasting blood glucose was assessed in all study participants, and for those with impaired fasting glucose, oral glucose tolerance test with 75 g glucose was performed. Obesity was defined as the body mass index (BMI) ≥25kg/m2. Central obesity was defined as waist circumference ≥90 cm in men and ≥80 cm in women or waist-to-hip ratio ≥0.9 for men and ≥0.8 for women.35
Medical health records were scrutinized for MI, unstable angina, coronary artery bypass graft (CABG) surgery, coronary angiography, treatment for CHD and hospital admission for CHD. Survey participants were screened with a high-risk Rose angina questionnaire (RAQ) followed by resting ECG in all study population. Minnesota code and Epstein's criteria were used for diagnosis of CHD.36 All ECG-defined CHD cases and/or those with RAQ angina were further subjected to the exercise ECG test and 2D echocardiography and if required coronary angiography.
3. Categorization of CHD
Confirmed CHD based on the following:
-
i.
Documented evidence of prior acute coronary syndrome (ACS) or treatment for CHD
-
ii.
Documented history of undergoing coronary angioplasty or CABG
-
iii.
More than 50% epicardial coronary stenosis by invasive coronary angiography
-
iv.
ECG showing pathological Q waves (any of Minnesota code 1-1-1 to 1-1-7 or 1-2-1 to 1-2-5 or 1-2-7) supported by exercise ECG test or echocardiographic evidence.
-
v.
Imaging evidence of a region of loss of viable myocardium, that is thinned and has a regional wall motion abnormality (RWMA), in the absence of a nonischaemic cause
-
vi.
RAQ angina plus ECG changes (any of Minnesota codes 4-1-1, 4-1-2, 4-2 or 5-l, 5-2) with positive exercise ECG test or echocardiographic evidence.
-
vii.
RAQ angina plus positive exercise ECG test (exercise-induced horizontal or downsloping ST depression of ≥1 mm at 80 ms from J point).
Suspect CHD: In the absence of criteria for confirmed CHD:
-
i.
RAQ angina without significant ECG changes
-
ii.
ECG changes without RAQ angina
-
iii.
Positive treadmill ECG without RAQ angina.
Non-CHD: absence of all the aforementioned criteria for confirmed or suspect CHD.
4. Statistical analysis
Data entry was performed in MS Excel, and R was used for statistical analysis.37 Data were summarized as proportions for categorical variables and as mean and standard deviation for continuous variables. Chi-square (χ2) test and Fisher's exact test were used for comparison of categorical variables, whereas Student's t-test was used for comparison of continuous variables. Logistic regression was used to find the independent risk factors of CHD, and the final model was created using bidirectional stepwise approach in R. The adjusted odds ratios (ORs) were presented with 95% confidence intervals (CIs). A p-value of less than 0.05 was considered to constitute a statistically significant difference.
5. Results
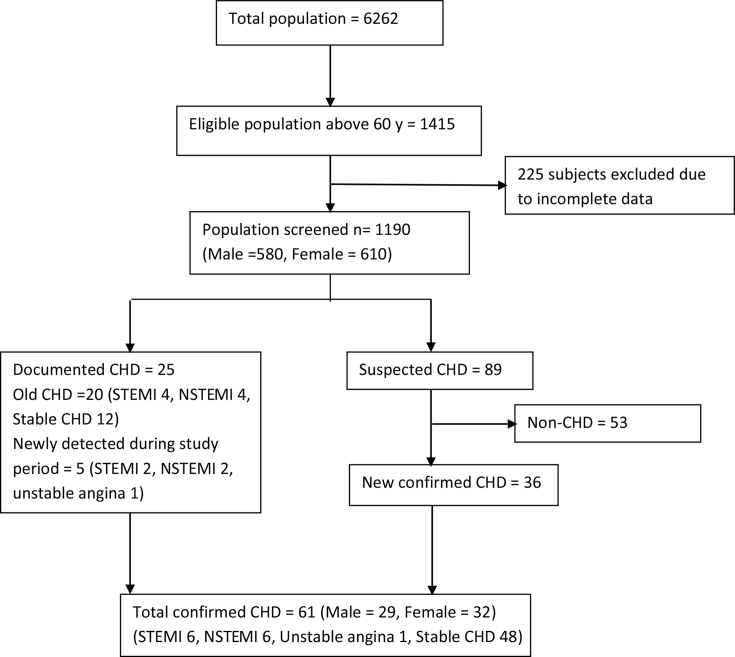
We screened 84% (1190/1415) of the eligible population older than 60 years (Fig. 1). Population screened in each of the villages is Dindoda 90/93, Hivra 62/68, Karanji Kazi 139/187, Khadka 91/107, Kutki 81/102, Nagapur 96/113, Nandura 82/111, Pimpalgaon 86/101, Takli Kite 88/105, Talodi 113/125, Tuljapur 83/106, Waghala 102/111 and Yerangaon 77/86. Only 3.9% belonged to the high socioeconomic status, 49.5% did not undergo any formal school education and none attended college. None of the women smoked, and 64% of the population did not use tobacco in any form, whereas approximately one-third of men reported tobacco use in some form.
Fig. 1.
Study flowchart. CHD, coronary heart disease.; STEMI, ST elevated myocardial infaction; NSEMI, non-ST elevated myocardial infaction.
Prevalence of hypertension was 49.5% and diabetes was 5.3%. Around one-tenth of patients were obese (9.3% based on the BMI and 10.2% based on the waist circumference).
We identified 20 cases of CHD through previous documentation, and five symptomatic new cases (acute MI) were detected during the study period. Eighty-nine were identified as suspected CHD based on the initial screening, and 36 of these were confirmed to have CHD, while rest were excluded (non-CHD) owing to absence of objective evidence on evaluation (Fig. 1). In total, prevalence of confirmed CHD in individuals aged 60 years and older in the rural community was 51.3 per 1000 population. Sex distribution was almost equal with a prevalence of 50 per 1000 among men and 52.5 per 1000 among women.
Out of total 61 cases of CHD detected in this study, 6 each had ST-elevation MI and Non- ST-elevation MI, 48 had stable CHD and one had unstable angina (Table 1).
Table 1.
Prevalence of CHD.
| Diagnosis of confirmed CHD | Men |
Women |
Total |
|||
|---|---|---|---|---|---|---|
| Number | Prevalence (per 1000) | Number | Prevalence (per 1000) | Number | Prevalence (per 1000) | |
| Documented CHDa | 15 | 25.9 | 10 | 16.4 | 25 | 21.0 |
| CHD diagnosed by RAQ, ECG and exercise stress test | 3 | 5.2 | 6 | 9.8 | 9 | 7.6 |
| CHD diagnosed by RAQ, ECG, exercise stress test and ECHO findings | 11 | 19.0 | 16 | 26.2 | 27 | 22.7 |
| Total prevalence | 29 | 50.0 | 32 | 52.5 | 61 | 51.3 |
RAQ, Rose angina questionnaire; CHD, coronary heart disease.
Three patients underwent coronary angiography.
Table 2 shows prevalence rate and prevalence rate ratio with various risk factors in consideration. We studied for association of risk factors – sex, educational status, socioeconomic status, physical activity, use of tobacco in any form, presence of hypertension and diabetes as categorical variables and age, BMI, waist circumference and waist hip ratio as continuous variables. Hypertension was found as the lone independent risk factor for CHD both on bivariate analysis as well as logistic regression (adjusted OR 7.07; CI 3.28–15.25, p < 0.001) (Table 2). Risk of CHD is 7 times higher in hypertensive subject as compared to non-hypertensive subject.
Table 2.
Risk factors for CHD.
| Variables | With CHD (n = 61) | Total population (n = 1190) | Prevalence rate (%) | Prevalence rate ratio (95% CI) | Logistic regression |
|
|---|---|---|---|---|---|---|
| Adjusted OR (95% CI) | p-value (LR test) | |||||
| Sex | ||||||
| Male | 29 | 580 | 5 | 1 | 1 | 0.30 |
| Female | 32 | 610 | 5.25 | 1.05 (0.63,1.76) | 0.7 (0.36,1.37) | |
| Education grade | ||||||
| Illiterate | 32 | 589 | 5.4 | 1 | 1 | 0.62 |
| Primary and secondary education | 29 | 601 | 4.83 | 0.88 (0.53,1.48) | 0.87 (0.49,1.52) | |
| Socioeconomic status | ||||||
| Low | 16 | 317 | 5.05 | 1 | 1 | 0.48 |
| Middle | 40 | 827 | 4.84 | 0.96 (0.53–1.73) | 0.94 (0.51,1.73)) | |
| High | 5 | 46 | 10.87 | 2.29 (0.8–6.59) | 1.87 (0.61,5.72) | |
| Physical activity | ||||||
| Sedentary | 39 | 706 | 5.52 | 1 | 1 | 0.92 |
| Moderate | 14 | 311 | 4.5 | 0.81 (0.43–1.51) | 0.9 (0.47,1.73) | |
| Heavy | 8 | 173 | 4.62 | 0.83 (0.38–1.81) | 1.08 (0.48,2.43) | |
| Hypertension | ||||||
| No hypertension | 8 | 592 | 1.33 | 1 | 1 | <0.001 |
| Hypertension | 53 | 590 | 8.98 | 7.3 (3.44,15.5) | 7.07 (3.28,15.25) | |
| Diabetes mellitus | ||||||
| No diabetes mellitus | 54 | 1127 | 4.79 | 1 | 1 | 0.30 |
| Diabetes mellitus | 7 | 63 | 11.11 | 2.48 (1.08,5.71) | 1.61 (0.68,3.83) | |
| Tobacco use | ||||||
| No tobacco | 43 | 764 | 5.6 | 1 | 1 | 0.36 |
| Tobacco (in any form) | 18 | 426 | 4.2 | 0.74 (0.42,1.3) | 0.73 (0.37,1.44) | |
| Age | ||||||
| 60–69 years | 37 | 796 | 4.65 | 1 | 1 | 0.61 |
| 70–79 years | 22 | 326 | 6.75 | 1.48 (0.86,2.56) | 1.27 (0.71,2.26) | |
| ≥80 | 2 | 78 | 2.56 | 0.62 (0.15–2.64) | 0.73 (0.16,3.26) | |
| BMI | ||||||
| BMI < 25 | 52 | 1079 | 4.82 | 1 | 1 | 0.26 |
| BMI ≥ 25 | 9 | 111 | 8.11 | 1.74 (0.83,3.64) | 1.64 (0.71,3.77) | |
| Waist circumference | ||||||
| <90 cm (for men) and <80 cm (for women) | 54 | 1068 | 5.06 | 1 | 1 | 0.49 |
| ≥90 cm (for men) and ≥80 cm (for women) | 7 | 122 | 5.74 | 1.14 (0.51,2.57) | 0.72 (0.28,1.87) | |
| Waist hip ratio | ||||||
| <0.9 (for men) and <0.8 (for women) | 24 | 494 | 4.86 | 1 | 1 | 0.90 |
| ≥0.9 (for men) and ≥0.8 (for women) | 37 | 696 | 5.32 | 1.1 (0.65,1.86) | 0.96 (0.54,1.71) | |
BMI, body mass index; CHD, coronary heart disease; OR, odds ratio; CI, confidence interval; LR, likelihood ratio.
Table 3 shows age-specific prevalence rate of confirmed CHD by sex. In age group 60–69 years prevalence among men was 3.64% and among women was 5.47%. In the age group of 70–79 years prevalence among men was 9.46% and among women was 5.41%. Prevalence in age group ≥80 years among men was 4.44% and among women no patient had confirmed IHD.
Table 3.
Age-specific prevalence by sex.
| Age group | With CHD | Total | Prevalence (%) | |
|---|---|---|---|---|
| 60–69 years | Male | 13 | 357 | 3.64 |
| Female | 24 | 439 | 5.47 | |
| 70–79 years | Male | 14 | 178 | 9.46 |
| Female | 8 | 148 | 5.41 | |
| ≥80 years | Male | 2 | 45 | 4.44 |
| Female | 0 | 23 | 0 | |
CHD, coronary heart disease.
6. Discussion
We found that prevalence of confirmed CHD in individuals above 60 years in rural community of central India was 51.3 per 1000 population. Sex distribution was almost equal with a prevalence of 50 per 1000 among men and 52.5 per 1000 among women.
There is a wide variation in the observed prevalence of CHD among the Indian population. The prevalence of ECG-defined CHD in rural India varied from as low as 1.69% to as high as 5%.14, 17 It is difficult to compare with other studies owing to heterogeneity in the study settings, design, age group selected and the methods used for detecting CHD. Most of the older studies included ECG-defined CHD. The ECG criteria overestimates the prevalence of CHD because of a large number of false-positive results, lacks diagnostic power in women and may overestimate the prevalence of CHD in women.
Wander et al. conducted a cross-sectional study in the early 90s among individuals older than 30 years who were residing in three villages in rural Punjab. Apart from the Epstein's criteria, they also recruited the clinical judgement and exercise stress test. The prevalence of CHD was found to be 3.1% (37.7/1000 in women and 25.6/1000 in men). Sixty-two percent of individuals were not aware of their disease.12
A 2009 population-based study in three villages of Himachal Pradesh16 used similar methodology and criteria for diagnosing CHD as in our study; however, they included all individuals older than 30 years (mean age 47.4 y) and covered 70% of the target population. Of the total 33 patients, 17 were known to have CHD and 16 were newly diagnosed. Maximum prevalence was found in the age group of 50–59 years in both sexes. However, because of the lack of mention about age-specific prevalence, we could not directly compare our results with those of this study. The overall prevalence of CHD was found to be 40.6 per 1000, 65 per 1000 among men and 16.9 per 1000 among women. In more than half of the patients, awareness about their disease and affordability of angiography in 40% suggests higher socioeconomic status compared with our study where only three of 61 patients could afford angiography.
A cross-sectional community-based study in urban and rural areas of Kerala during the period from January to June 201138 screened a total of 5167 individuals by multistage cluster sampling for age group 20–59 years, and all individuals in the age group 60–79 were selected. Two-fifths of the study population was women. The diagnostic modalities and criteria used for confirmed CHD was similar to the present study. The age-adjusted prevalence of definite CHD was 9 per 1000 in participants younger than 45 years and 68 per 1000 among those older than 45 years. There was no difference between urban and rural areas in the overall prevalence of definite CHD (34 vs. 36 per 1000). The prevalence of definite CHD among men was 13.41% in the age group 60–69 years and 18.8% in the age group 70–79 years and among women, 8.98% in the age group 60–69 years and 12.59% in the age group 70–79 years, while in our study, prevalence of confirmed CHD (similar to definite CHD) among men was 3.64%, 9.46% and 4.44% in the age group 60–69 years, 70–79 years and ≥80 years, respectively. Among women, it was 5.47%, 5.41% and none in age group of 60–69 years, 70–79 years and ≥80 years, respectively. Thus, our study showed markedly less age-specific prevalence rate compared with the Kerala study. This low prevalence may be because of several factors. Our study represents predominantly poor, nonobese and less educated population who survive on hard physical labour. We also noted lower prevalence of traditional risk factors. Moreover, even though three-fifths of population was categorised as sedentary, it is likely to be misclassification as most of them would have engaged in labour-intensive lifestyle throughout their life and adapted sedentary lifestyle after their 60s.
In the same rural area as of the present study, Jajoo et al., in 1983–1984, studied the prevalence of ECG-defined CHD by Epstein's criteria in population ≥30 years, which screened 86.2% of population. Probable CHD prevalence in population ≥30 years was 14.8/1000; however, Q wave–defined CHD (which is close to confirmed CHD in the present study) was present in only five of 2037, i.e., 2/1000 with no symptomatic case of CHD. In population ≥60 years, prevalence of probable CHD (ECG defined) was 31 per 1000. However, there was no Q wave–defined CHD in population ≥60 years.17 There was no recorded case of ACS during or before the study period. These villages are covered under village heath assurance programme from 1981. The free indoor care for any unforeseen illness has facilitated all admissions from these villages to Kasturba Hospital, Sevagram. The first case of acute MI from this area was observed in the year 1992. From our close observations of village health assurance programme, Kasturba Hospital has admitted an increasing number of confirmed CHD cases from this rural community after 1992. The present prevalence rate of confirmed CHD in this study was 51.3 per 1000 population in the age of 60 years and older. These observations point towards increasing prevalence of confirmed CHD in the given rural area. Apart from increase in prevalence of CHD, we have also observed a definite escalation of hypertension, diabetes and chronic kidney disease (unpublished data). With persistent contact with rural community by means of village health assurance scheme, we have not observed significant change in any other major risk factors of CHD.
Hypertension emerged to be the only risk factor for CHD with adjusted OR 7.07 (CI 3.28, 15.25) (p < 0.001). We failed to document a statistical association among obesity (as defined by the BMI), waist circumference, diabetes and higher socioeconomic status. In a similar study, Wander et al. also documented hypertension and strong family history as significant risk factors for CHD.12 Krishnan et al. showed significant association between definite CHD and risk factors such as diabetes, hypertension, high serum cholesterol and physical inactivity,38 whereas Rajiv Bharadwaj et al. did not comment on association between CHD and its risk factors.
In present study, the lack of association between CHD and factors such as the socioeconomic status, education status and physical activity could be explained by several reasons. In the age group >60 years in this study, it is likely that these factors will not hold significance. Impact of the socioeconomic status or educational status in modifying dietary practices would have been less in the rural population, which is mostly homogeneous in nature with respect to their dietary practices and physical activity. One-time socioeconomic status captured during the study may not represent what they have experienced over their lifetime. In our study, categorization of three-fifths of population as sedentary would have been misclassification as most of them would have engaged in labour-intensive lifestyle throughout their life and adapted sedentary lifestyle after their 60s.
Our study showed that prevalence of CHD in elderly women (52.5 per 1000) was slightly higher than that in men (50 per 1000), but it was not significantly compared with men. The study conducted previously in the same area during 1983–1984 showed that overall prevalence of CHD by only Epstein's criteria in population ≥30 years was 14.8 per 1000 population, where prevalence in women (20.1 per 1000) was higher compared with men (10.5 per 1000).17
The present study has several strengths. It was a community-based cross-sectional study, which achieved a good coverage of 84% with a sample size of 1190 individuals. Unlike most studies which based diagnosis of CHD on ECG, this study aimed to make a definite diagnosis of CHD by incorporating other diagnostic modalities such as the exercise test, echocardiography and coronary angiography wherever necessary.
Our study has few limitations. Although the sample size in this study was fairly large, the power of the study to detect association was low for several variables. RAQ is heavily dependent on the physical activity status of the individuals. In this study, nearly 60% of all the individuals were leading a sedentary lifestyle. It is possible that CHD was missed in some. However, our approach was pragmatic by including ECG, exercise ECG test, 2D echocardiography and angiography if required. We diagnosed CHD in presence of RWMA in background of appropriate history and ECG changes. However, it is known that RWMA may occur in the absence of CAD, such as in the left bundle branch block, myocarditis, sarcoidosis and stress-induced cardiomyopathy.39 As relation of RWMA and CHD is not straightforward, there might have been misdiagnosis in few.
In conclusion, our study shows that prevalence of confirmed CHD in individuals older than 60 years in the rural community of central India is 51.3 per 1000 population, with nearly equal sex distribution, and hypertension was the only risk factor associated. Comparing with the previous study in the same region and hospital records, there has been increase in prevalence of CHD in the agrarian rural community older than 60 years.
Source of funding
All expenses of investigation and transport for village camps were borne by Mahatma Gandhi Institute of Medical Sciences, Sevagram, India.
Presentation at a meeting
Nil
Conflict of interest
All authors have none to declare.
Acknowledgement
Nil.
Contributor Information
Sheetal Bodkhe, Email: sheetalbodkhe@gmail.com.
Sumedh U. Jajoo, Email: sumedhjajoo@gmail.com.
Ulhas N. Jajoo, Email: ulhasjajoo@gmail.com.
Sheetal Ingle, Email: sheetuingale.8@gmail.com.
Subodh S. Gupta, Email: subodhsgupta@gmail.com.
Bharati A. Taksande, Email: bharati.taksande@gmail.com.
References
- 1.World Health Organization . vol. 3. 2015. Global Health Observatory (GHO) Data 2015; p. 23. Retrieved. [Google Scholar]
- 2.Report on Medical Certification of Cause of Death. Office of the Registrar General of India, Government of India; 2015. http://www.censusindia.gov.in/2011-Documents/mccd_Report1/MCCD_Report-2015.pdf [Internet] Ministry of Home Affairs, Vital Statistics Division R. K. Puram New Delhi [cited 01/03/2018]. Available from: [Google Scholar]
- 3.Goyal A., Yusuf S. The burden of cardiovascular disease in the Indian subcontinent. Indian J Med Res. 2006;124(3):235–244. [PubMed] [Google Scholar]
- 4.Srinath Reddy K., Shah B., Varghese C., Ramadoss A. Responding to the threat of chronic diseases in India. Lancet. 2005;366(9498):1744–1749. doi: 10.1016/S0140-6736(05)67343-6. [DOI] [PubMed] [Google Scholar]
- 5.Organization W.H. vol. 9. WHO; Geneva: 2014. (Global Health Estimates: Deaths by Cause, Age, Sex and Country, 2000-2012). [Google Scholar]
- 6.Gupta R., Gupta V.P. Meta-analysis of coronary heart disease prevalence in India. Indian Heart J. 1996;48(3):241–245. [PubMed] [Google Scholar]
- 7.Ahmad N., Bhopal R. Is coronary heart disease rising in India? A systematic review based on ECG defined coronary heart disease. Heart. 2005;91(6):719–725. doi: 10.1136/hrt.2003.031047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rao M., Xavier D., Devi P. Prevalence, treatments and outcomes of coronary artery disease in Indians: a systematic review. Indian Heart J. 2015;67(4):302–310. doi: 10.1016/j.ihj.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prineas R.J., Crow R.S., Zhang Z.-M. Springer Science & Business Media; 2009. The Minnesota Code Manual of Electrocardiographic Findings. [Google Scholar]
- 10.Dewan B.D., Malhotra K.C., Gupta S.P. Epidemiological study of coronary heart disease in rural community in Haryana. Indian Heart J. 1974;26(2):68–78. [PubMed] [Google Scholar]
- 11.Chadha S.L.G.N., Radhakrishnan S., Ramachandran K., Kaul U., Tandon R. Prevalence of coronary heart disease and its risk factors in a rural community in Haryana. Indian J Community Med. 1989;14:141–147. [Google Scholar]
- 12.Wander G.S., Khurana S.B., Gulati R. Epidemiology of coronary heart disease in a rural Punjab population--prevalence and correlation with various risk factors. Indian Heart J. 1994;46(6):319–323. [PubMed] [Google Scholar]
- 13.Gupta R., Gupta H.P., Keswani P., Sharma S., Gupta V.P., Gupta K.D. Coronary heart disease and coronary risk factor prevalence in rural Rajasthan. J Assoc Phys India. 1994;42(1):24–26. [PubMed] [Google Scholar]
- 14.Gupta A.K.B.A., Ashotra S., Gupta B.P. Feasibility and training of multipurpose workers in detection, prevention and control of coronary artery disease in apple-belt of Shimla hills. South Asian J Prev Cardiol. 2002;6:17–22. [Google Scholar]
- 15.Chow C., Cardona M., Raju P.K. Cardiovascular disease and risk factors among 345 adults in rural India—the Andhra Pradesh Rural Health Initiative. Int J Cardiol. 2007;116(2):180–185. doi: 10.1016/j.ijcard.2006.03.043. [DOI] [PubMed] [Google Scholar]
- 16.Bhardwaj R., Kandoria A., Marwah R., Vaidya P., Dhiman P., Singh B. Coronary heart disease in rural population of himachal-a population based study. J Assoc Phys India. 2009;57:505–507. [PubMed] [Google Scholar]
- 17.Jajoo U.N., Kalantri S.P., Gupta O.P., Jain A.P., Gupta K. The prevalence of coronary heart disease in rural population from central India. J Assoc Phys India. 1988;36(12):689–693. [PubMed] [Google Scholar]
- 18.Padmavati S. Epidemiology of cardiovascular disease in India. II. Ischemic heart disease. Circulation. 1962;25:711–717. doi: 10.1161/01.cir.25.4.711. [DOI] [PubMed] [Google Scholar]
- 19.Sarvotham S.G., Berry J.N. Prevalence of coronary heart disease in an urban population in northern India. Circulation. 1968;37(6):939–953. doi: 10.1161/01.cir.37.6.939. [DOI] [PubMed] [Google Scholar]
- 20.Singh R.B., Sharma J.P., Rastogi V. Prevalence of coronary artery disease and coronary risk factors in rural and urban populations of north India. Eur Heart J. 1997;18(11):1728–1735. doi: 10.1093/oxfordjournals.eurheartj.a015167. [DOI] [PubMed] [Google Scholar]
- 21.Mohan V., Deepa R., Rani S.S., Premalatha G., Chennai Urban Population S. Prevalence of coronary artery disease and its relationship to lipids in a selected population in South India: the Chennai Urban Population Study (CUPS No. 5) J Am Coll Cardiol. 2001;38(3):682–687. doi: 10.1016/s0735-1097(01)01415-2. [DOI] [PubMed] [Google Scholar]
- 22.Latheef S.A., Subramanyam G. Prevalence of coronary artery disease and coronary risk factors in an urban population of Tirupati. Indian Heart J. 2007;59(2):157–164. [PubMed] [Google Scholar]
- 23.Gupta S.P., Malhotra K.C. Urban--rural trends in the epidemiology of coronary heart disease. J Assoc Phys India. 1975;23(12):885–892. [PubMed] [Google Scholar]
- 24.Blackburn H., Keys A., Simonson E., Rautaharju P., Punsar S. The electrocardiogram in population studies: a classification system. Circulation. 1960;21(6):1160–1175. doi: 10.1161/01.cir.21.6.1160. [DOI] [PubMed] [Google Scholar]
- 25.Higgins I., Kannel W., Dawber T. The electrocardiogram in epidemiological studies: reproducibility, validity, and international comparison. Br J Prev Soc Med. 1965;19(2):53. doi: 10.1136/jech.19.2.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rossouw J., Weich H., Steyn K., Kotze J., Kotze T. The prevalence of ischaemic heart disease in three rural South African communities. J Chron Dis. 1984;37(2):97–106. doi: 10.1016/0021-9681(84)90051-1. [DOI] [PubMed] [Google Scholar]
- 27.Patel D., Winterbotham M., Sutherland S., Britt R., Keil J., Sutton G. Comparison of methods to assess coronary heart disease prevalence in South Asians. Natl Med J India. 1997;10(5):210–213. [PubMed] [Google Scholar]
- 28.Rose G., Baxter P., Reid D., McCartney P. Prevalence and prognosis of electrocardiographic findings in middle-aged men. Heart. 1978;40(6):636–643. doi: 10.1136/hrt.40.6.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sketch M.H., Mohiuddin S.M., Lynch J.D., Zencka A.E., Runco V. Significant sex differences in the correlation of electrocardiographic exercise testing and coronary arteriograms. Am J Cardiol. 1975;36(2):169–173. doi: 10.1016/0002-9149(75)90521-4. [DOI] [PubMed] [Google Scholar]
- 30.Fisch C. Abnormal ECG in clinically normal individuals. Jama. 1983;250(10):1321–1323. [PubMed] [Google Scholar]
- 31.Jajoo U.N., Rao K.P., Kalantri S.P., Gupta O.P., Jain A.P. False positive ECG and coronary heart disease. J Assoc Phys India. 1993;41(4):205–207. [PubMed] [Google Scholar]
- 32.Agarwal S., Agarwal A., Agarwal K., Agarwal D., Bansal A. Physical activity and pregnancy outcome in rural undernourished women. Indian Pediatr. 2001;38(9):1017–1021. [PubMed] [Google Scholar]
- 33.International Institute for Population Sciences . International Institute for Population Sciences; 2007. India National Family Health Survey (NFHS-3), 2005-06. [Google Scholar]
- 34.Chobanian A.V., Bakris G.L., Black H.R. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. Jama. 2003;289(19):2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 35.Misra A., Chowbey P., Makkar B. 2009. Consensus Statement for Diagnosis of Obesity, Abdominal Obesity and the Metabolic Syndrome for Asian Indians and Recommendations for Physical Activity, Medical and Surgical Management. [PubMed] [Google Scholar]
- 36.Epstein F.H., Ostrander L.D., Jr., Johnson B.C. Epidemiological studies of cardiovascular disease in a total community--tecumseh, Michigan. Ann Intern Med. 1965;62:1170–1187. doi: 10.7326/0003-4819-62-6-1170. [DOI] [PubMed] [Google Scholar]
- 37.R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2015. R: A Language and Environment for Statistical Computing. [Google Scholar]
- 38.Krishnan M., Zachariah G., Venugopal K. Prevalence of coronary artery disease and its risk factors in Kerala, South India: a community-based cross-sectional study. BMC Cardiovasc Disord. 2016;16(1):12. doi: 10.1186/s12872-016-0189-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lang R.M., Badano L.P., Mor-Avi V. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the european association of cardiovascular imaging. J Am Soc Echocardiogr. 2015;28(1):1–39. doi: 10.1016/j.echo.2014.10.003. e14. [DOI] [PubMed] [Google Scholar]