Abstract
Purpose: The correlation of advanced cancer with inflammation and/or nutrition factors is well known. Recently, the advanced lung cancer inflammation index (ALI) was developed as a new prognostic tool for patients with advanced lung cancer. In this study, we examined whether ALI results are correlated with prognosis of patients with early stage lung adenocarcinoma who undergo lung resection.
Methods: From January 2009 to December 2014, 544 patients underwent lung resection due to primary lung cancer at Dokkyo Medical University Hospital, of whom 166 with pathological stage IA lung adenocarcinoma were retrospectively investigated in this study. ALI was calculated as follows: Body Mass Index (BMI; kg/m2) × albumin (g/dL)/neutrophil- to-lymphocyte ratio (NLR).
Results: Multivariate analysis revealed that gender, red cell distribution width (RDW), NLR, and ALI were parameters significantly correlated with overall survival (OS). Patients with an ALI value less than 22.2 had an inferior 5-year OS rate as compared to those with a value of 22.2 or higher (p <0.001) as well as an inferior 5-year recurrence-free survival (RFS) rate (p <0.001).
Conclusion: Low ALI was correlated with poor prognosis in patients with stage IA lung adenocarcinoma. Those with an ALI value less than 22.2 should be carefully followed regardless of cancer stage.
Keywords: outcome, thoracic, nutrition
Introduction
Inflammation status is well known to correlate with cancer growth and long-term patient outcome.1–4) Several inflammation markers, including red cell distribution width (RDW),5) neutrophil to lymphocyte ratio (NLR),6) and platelet to lymphocyte ratio (PLR),7) have been reported as useful prognostic factors for patients with non-small-cell lung cancer (NSCLC) who undergo lung resection. In 2003, Forrest and colleagues8) presented a scoring system termed Glasgow Prognostic Score (GPS) that uses the combination of C-reactive protein (CRP) as an inflammation marker and albumin as a nutritional marker, and found that the score was correlated with prognosis of patients with inoperable NSCLC. Thereafter, several reports indicating the correlation of GPS with the prognosis of patients who underwent surgery for NSCLC have been presented.9)
In 2013, Jafri et al.10) developed a new prognostic index termed advanced lung cancer inflammation index (ALI) for patients with advanced lung cancer including small-cell lung cancer. ALI incorporates both inflammation and nutrition factors, the same as GPS, and consists of Body Mass Index (BMI), albumin, and NLR. Its utility has been reported for patients with esophageal cancer, small-cell lung cancer, and malignant lymphoma.11–14) Furthermore, Tomita et al.15) recently reported the utility of ALI for patients with NSCLC who undergo surgical treatment.
The correlation of inflammation and/or nutrition factors with advanced cancer is well known. In this study, we examined whether ALI correlates with prognosis of patients with early stage NSCLC. We analyzed several prognostic factors, including ALI and GPS, as well as others, in patients with pathological stage (p-stage) IA lung adenocarcinoma who underwent a lung resection procedure. Most previous similar reports were based on version 7 of the Union International Contre Cancer (UICC) TNM classification or a former version. Here, we used UICC TNM classification version 8 for analysis, which excludes adenocarcinoma in situ.
Methods
From January 2009 to December 2014, a total of 544 patients underwent lung resection due to primary lung cancer at Dokkyo Medical University Hospital, of whom 166 with p-stage IA lung adenocarcinoma were retrospectively investigated. Patients with acute and/or chronic inflammatory disease during the preoperative period, such as active infection or collagen vascular disease including rheumatoid arthritis, were excluded. The Ethical Committee of Dokkyo Medical University Hospital approved this retrospective study (#28080).
Routine laboratory measurements, including CRP, albumin, carcinoembryonic antigen (CEA), RDW, NLR, and PLR, were performed prior to the operation. ALI was calculated as follows: BMI (kg/m2) × albumin (g/dL)/NLR.10) GPS was determined according to the following scoring system. Patients with both increased CRP (>1.0 mg/dL) and hypoalbuminemia (3.5 g/dL) received a score of 2 while those with only one of those parameters received a score of 1 and those with neither of those findings received a score of 0.8)
Cutoff values for ALI, RDW, NLR, and PLR were determined using receiver operating characteristic (ROC) curve analysis to estimate optimal sensitivity, specificity, and area under the curve (AUC) for prediction of death from all causes. Statistical analysis was performed using a chi-square test or Fisher’s exact test for a contingency table. Univariate and multivariate analyses with a COX proportional hazards regression model were used to identify independent risk factors for survival, as well as to estimate the respective hazard ratio (HR) and 95% confidence interval (CI) values for the various factors. When parameters were confounding factors, those analyses were separately performed. The Kaplan–Meier method and a log-rank test were used to compare survival of patients in relation to each parameter. Differences were considered to be significant at p <0.05. All calculations were done using the IBM SPSS statistics software program, version 22.0 (IBM Corporation, Armonk, NY, USA).
Results
The characteristics of the 166 enrolled patients are shown in Table 1. The median observation period was 1653 days (range: 100–3143 days). In all, 25 patients died during the observation period, eight patients due to lung cancer and the others due to other causes such as pneumonia. In total, 18 patients had lung cancer recurrence, 9 loco-regional, 7 distant metastasis (brain 4, bone 2, and multiple organs 1), and 2 unknown. Nine of the 18 patients with recurrence died, 8 from lung cancer and 1 from pneumonia. The cutoff values for ALI, RDW, NLR, and PLR shown by ROC curve analysis were 22.2 (AUC 0.610), 13.3 (AUC 0.695), 3.43 (AUC 0.574), and 120 (AUC 0.566), respectively.
Table 1. Patient characteristics (n = 166).
| Baseline | No. | |
|---|---|---|
| Age (years) | <75 | 117 |
| ≥75 | 49 | |
| Gender | Male | 74 |
| Female | 92 | |
| Brinkman index | 0 | 89 |
| <600 | 37 | |
| ≥600 | 40 | |
| Performance status (ECOG) | 0 | 158 |
| 1 | 4 | |
| 2 | 4 | |
| Body Mass Index | <18.5 | 15 |
| 18.5 to <25 | 111 | |
| ≥25 | 40 | |
| Hugh-Jones classification | 1 | 154 |
| 2 | 9 | |
| 3 | 2 | |
| Surgical procedure | Wedge resection | 22 |
| Segmentectomy | 29 | |
| Lobectomy | 115 | |
| Lymphatic permeation | Absent | 149 |
| Present | 17 | |
| Vascular invasion | Absent | 146 |
| Present | 20 | |
| Pathological stage IA (T factor) | 1 (mi) | 36 |
| (1a) | 38 | |
| 2 (1b) | 56 | |
| 3 (1c) | 36 | |
| Pathological subtype | Minimally invasive adenocarcinoma | 36 |
| Lepidic | 70 | |
| Papillary | 38 | |
| Acinar | 12 | |
| Solid | 6 | |
| Others | 4 | |
| EGFR mutation | Negative | 57 |
| Positive | 47 | |
| Not recorded | 62 | |
| CEA (ng/mL) | ≤5 | 151 |
| >5 | 15 | |
| CRP (mg/dL) | ≤1 | 160 |
| >1 | 6 | |
| Albumin (g/dL) | ≥3.5 | 159 |
| <3.5 | 7 | |
| GPS | 0 | 156 |
| 1 | 7 | |
| 2 | 3 | |
| RDW | <13.3 | 97 |
| ≥13.3 | 69 | |
| PLR | <120 | 69 |
| ≥120 | 97 | |
| NLR | <3.43 | 143 |
| ≥3.43 | 23 | |
| ALI | ≥22.2 | 148 |
| <22.2 | 18 | |
| %VC (%) | ≥80 | 150 |
| <80 | 12 | |
| not recorded | 4 | |
| %FEV1.0 (%) | ≥80 | 136 |
| <80 | 26 | |
| Not recorded | 4 | |
| %DLCO (%) | ≥80 | 150 |
| <80 | 12 | |
| Not recorded | 4 | |
ECOG: eastern cooperative oncology group; EGFR: epidermal growth factor receptor; CEA: carcinoembryonic antigen; CRP: C-reactive protein; GPS: glasgow prognostic score; RDW: red cell distribution width; PLR: platelet-to-lymphocyte ratio; NLR: neutrophil-to-lymphocyte ratio; ALI: advanced lung cancer inflammation index; VC: vital capacity; FEV: forced expiratory volume; DLCO: diffusing capacity of lung carbon monoxide
The relationships between ALI and patient characteristics are shown in Table 2. Patients with an ALI value less than 22.2 were older, had the lower performance status (ECOG-PS) and BMI values, higher Hugh Jones classification, serum CEA, and CRP, lower albumin level, higher GPS, PLR, and NLR, less vital capacity (%), and the higher rate of death.
Table 2. Relationship between patient characteristics and ALI.
| Baseline characteristic | ALI ≥22.2 | ALI <22.2 | p value | |
|---|---|---|---|---|
| n = 148 | n = 18 | |||
| Age (years) | <75 | 109 | 8 | 0.01 |
| ≥75 | 39 | 10 | ||
| Gender | Male | 82 | 10 | 0.99 |
| Female | 66 | 8 | ||
| Brinkman index | 0 | 80 | 9 | 0.839 |
| <600 | 32 | 5 | ||
| ≥600 | 36 | 4 | ||
| Performance status (ECOG) | 0 | 143 | 15 | <0.001 |
| 1 | 4 | 0 | ||
| 2 | 1 | 3 | ||
| Body Mass Index (kg/m2) | <18.5 | 9 | 6 | 0.001 |
| ≥18.5 to <25 | 101 | 10 | ||
| ≥25 | 38 | 2 | ||
| Hugh-Jones classification | 1 | 140 | 14 | 0.016 |
| 2 | 6 | 3 | ||
| 3 | 1 | 1 | ||
| Surgical procedure | Wedge resection | 18 | 4 | 0.49 |
| Segmentectomy | 26 | 3 | ||
| Lobectomy | 101 | 11 | ||
| Lymphatic permeation | Absent | 134 | 15 | 0.341 |
| Present | 14 | 3 | ||
| Vascular invasion | Absent | 132 | 14 | 0.16 |
| Present | 16 | 4 | ||
| T factor | 1 (mi) | 35 | 1 | 0.197 |
| (1a) | 31 | 7 | ||
| 2 (1b) | 50 | 6 | ||
| 3 (1c) | 32 | 4 | ||
| Pathological subtype | Minimally invasive Adenocarcinoma | 35 | 1 | 0.074 |
| Lepidic | 61 | 9 | ||
| Papillary | 35 | 3 | ||
| Acinar | 10 | 2 | ||
| Solid | 5 | 1 | ||
| Others | 2 | 2 | ||
| CEA (ng/mL) | ≤5 | 138 | 13 | 0.003 |
| >5 | 10 | 5 | ||
| CRP (mg/dL) | ≤1 | 147 | 13 | <0.001 |
| >1 | 1 | 5 | ||
| Albumin (g/dL) | ≥3.5 | 145 | 14 | <0.001 |
| <3.5 | 3 | 4 | ||
| GPS | 0 | 145 | 11 | <0.001 |
| 1 | 2 | 5 | ||
| 2 | 1 | 2 | ||
| RDW | <13.3 | 89 | 8 | 0.202 |
| ≥13.3 | 59 | 10 | ||
| PLR | <120 | 68 | 1 | 0.001 |
| ≥120 | 80 | 17 | ||
| NLR | <3.43 | 140 | 3 | <0.001 |
| ≥3.43 | 8 | 15 | ||
| %VC (%) | ≥80 | 137 | 13 | <0.001 |
| <80 | 7 | 5 | ||
| Not recorded | 4 | 0 | ||
| %FEV1.0 (%) | ≥80 | 119 | 17 | 0.198 |
| <80 | 25 | 1 | ||
| Not recorded | 4 | 0 | ||
| %DLCO (%) | ≥80 | 135 | 15 | 0.112 |
| <80 | 9 | 3 | ||
| Not recorded | 4 | 0 | ||
| Status | Alive | 132 | 9 | <0.001 |
| Dead | 16 | 9 | ||
| Cause of death | Cancer | 7 | 1 | 0.093 |
| Other disease | 9 | 8 | ||
ECOG: eastern cooperative oncology group; CEA: carcinoembryonic antigen; CRP: C-reactive protein; GPS: glasgow prognostic score; RDW: red cell distribution width; PLR: platelet-to-lymphocyte ratio; NLR: neutrophil-to-lymphocyte ratio; ALI: advanced lung cancer inflammation index; VC: vital capacity; FEV: forced expiratory volume; DLCO: diffusing capacity of lung carbon monoxide
As shown in Table 2, 18 patients had an ALI value less than 22.2. During postoperative follow-up, two had the recurrence of lung cancer. Nine of the 18 patients died, one from lung cancer and eight from other cause (pneumonia in two, myocardial infarction in one, chronic heart failure in one, other malignancy in three, and unknown in one).
Univariate analysis of each factor was performed in regard to overall survival (OS) (Table 3). Gender, CEA, GPS, RDW, NLR, and ALI were each significantly correlated with OS. Since NLR and GPS are confounding factors for ALI, we conducted a separate multivariate analysis, which showed that gender, RDW, NLR, and ALI were significantly correlated with OS (Table 4).
Table 3. Univariate analysis.
| p value | HR | 95% CI | |
|---|---|---|---|
| Age (years) (<75/≥75) | 0.062 | 2.13 | 0.96–4.69 |
| Gender (male/female) | 0.003 | 0.25 | 0.1–0.63 |
| Brinkman index (<600/≥600) | 0.088 | 2.03 | 0.9–4.57 |
| ECOG-PS (0/1-2) | 0.061 | 3.17 | 0.95–10.6 |
| Hugh-Jones classification (1/2–3) | 0.066 | 3.16 | 0.93–10.78 |
| BMI, kg/m2 (≤18.5/>18.5) | 0.217 | 1.97 | 0.67–5.76 |
| Surgical procedure | |||
| (Sublobar resection/lobectomy or greater) | 0.608 | 1.27 | 0.51–3.19 |
| Lymphatic permeation (absent/present) | 0.519 | 1.42 | 0.49–4.15 |
| Vascular invasion (absent/present) | 0.096 | 2.18 | 0.87–5.46 |
| Stage IA (1-2/3) | 0.713 | 0.83 | 0.31–2.22 |
| Histological subtype (lepidic/non-lepidic) | 0.454 | 1.35 | 0.62–2.97 |
| CEA, ng/mL, (≤5/>5) | 0.007 | 3.55 | 1.41–8.92 |
| GPS (0/1-2) | 0.023 | 3.47 | 1.19–10.12 |
| RDW (<13.3/≥13.3) | 0.017 | 2.8 | 1.2–6.5 |
| NLR (<3.43/≥3.43) | 0.002 | 3.87 | 1.66–9.03 |
| PLR (<120/≥120) | 0.063 | 1.01 | 1.0–1.01 |
| ALI (≥22.2/<22.2) | <0.001 | 6.81 | 2.97–15.59 |
| %VC (<80/≥80) | 0.075 | 2.66 | 0.91–7.8 |
| %FEV1 (<80/≥80) | 0.635 | 0.75 | 0.22–2.5 |
| %DLCO (<80/≥80) | 0.213 | 2.16 | 0.64–7.28 |
ECOG: eastern cooperative oncology group; PS: performance status; CEA: carcinoembryonic antigen; GPS: glasgow prognostic score; RDW: red cell distribution width; PLR: platelet-to-lymphocyte ratio; NLR: neutrophilto-lymphocyte ratio; ALI: advanced lung cancer inflammation index; VC: vital capacity; FEV: forced expiratory volume; DLCO: diffusing capacity of lung carbon monoxide; BMI: body mass index; HR: hazard ratio; CI: confidence interval
Table 4. Multivariate analysis.
| GPS-NLR model | ALI model | |||||
|---|---|---|---|---|---|---|
| p value | HR | 95% CI | p value | HR | 95% CI | |
| Gender (male/female) | 0.007 | 0.27 | 0.1–0.7 | 0.002 | 0.22 | 0.09–0.58 |
| CEA, ng/mL (≤5/>5) | 0.109 | 2.23 | 0.84–5.93 | 0.235 | 1.8 | 0.68–4.72 |
| GPS (0/1-2) | 0.754 | 1.24 | 0.32–4.77 | |||
| RDW (<13.3/≥13.3) | 0.012 | 3.11 | 1.28–7.55 | 0.023 | 2.78 | 1.15–6.69 |
| NLR (<3.43/≥3.43) | 0.011 | 3.91 | 1.36–11.26 | |||
| ALI (≥22.2/<22.2) | <0.001 | 7.55 | 3.03–18.8 | |||
CEA: carcinoembryonic antigen; GPS: Glasgow prognostic score; RDW: red cell distribution width; NLR: neutrophil-to-lymphocyte ratio; ALI: advanced lung cancer inflammation index; HR: hazard ratio; CI: confidence interval
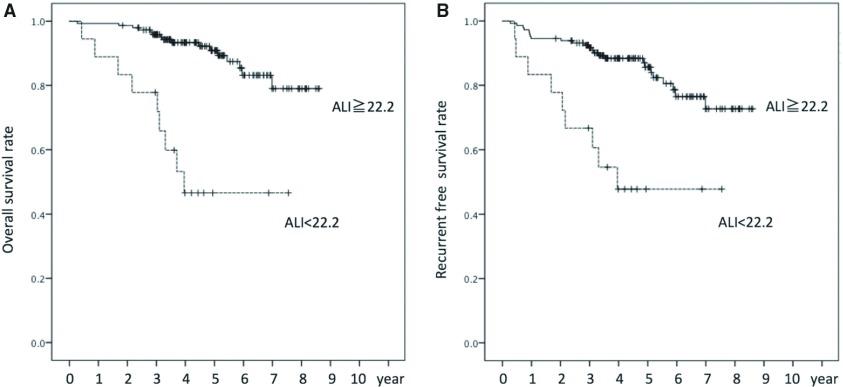
Patients with an ALI value less than 22.2 had an inferior 5-year OS rate as compared to those with a value of 22.2 or higher (46.5% vs. 90.9%, p <0.001) (Fig. 1A) while those with an RDW value less than 13.3 and those with an NLR value less than 3.43 had superior 5-year OS rates as compared to those with values of 13.3 or higher and 3.43 or higher, respectively (RDW: 93.1% vs. 76.8%, p = 0.005; NLR: 89.6% vs. 62.6%, p = 0.001).
Fig. 1. Overall (A) and recurrence-free (B) survival following surgery. ALI: advanced lung cancer inflammation index.
We also conducted analyses of recurrence-free survival (RFS) using the same cutoff values. Patients with an ALI value less than 22.2 had an inferior 5-year RFS rate as compared to those with a value of 22.2 or higher (47.7% vs. 85.6%, p <0.001) (Fig. 1B) while those with an RDW value less than 13.3 and those with an NLR value less than 3.43 had superior 5-year RFS rates as compared to those with values of 13.3 or higher and 3.43 or higher, respectively (RDW: 88.6% vs. 71.5%, p = 0.005; NLR: 84.1% vs. 64.7%, p = 0.001).
Discussion
When cancer advances, muscle and fat burden are decreased, resulting in body weight loss, which increases the risk of mortality.16) BMI17,18) and serum albumin level19) have been shown to be useful parameters for evaluation of nutritional condition while malnutrition evaluated with those parameters is a risk factor for NSCLC.19) Although the detailed mechanism remains unclear, malnutrition likely causes deterioration of the immune system.15) An increase in NLR is due to increased neutrophils or a decrease in lymphocytes. Neutrophils produce cytokines, which inhibit lymphocyte-mediated immune activity comprised of natural killer T cells or activated T cells.12,13) Previous meta-analysis findings revealed that an increase in NLR is correlated with inferior prognosis in NSCLC patients,20,21) with a similar result reported in cases of surgically resected NSCLC.22) The ALI consists of BMI, serum albumin, and NLR; thus, a correlation of decrease in ALI with poor prognosis in patients with advanced cancer is considered reasonable. We investigated early stage cancer cases (stage IA) in the present study and found that ALI was correlated with prognosis shown by OS as well as RFS. Our findings indicate the importance of malnutrition and inflammatory status in regard to prognosis, even in early stage cases because of a possible latent malignant potential.
Based on our findings of GPS and NLR as confounding factors with ALI, we conducted separate multivariate analyses. Those revealed that male gender, high RDW, and high NLR, the same as low ALI, were significantly associated with poor prognosis, which are findings consistent with previous investigations of patients with resected NSCLC.5,7,15) RDW changes are due to iron metabolism deterioration, inhibition of response to erythropoietin, and shortened turnover of red blood cells, which are induced by inflammatory conditions.23) Furthermore, high RDW has been reported to be a parameter of poor prognosis in NSCLC cases.23)
Using ROC curve analysis, we determined a cutoff value of 22.2 for ALI. Jafri and colleagues10) reported a cutoff value of 18 in analysis of 173 cases with advanced NSCLC and systemic metastasis while Tomita et al.15) demonstrated a cutoff value of 37.66 in their report of resected NSCLC, and Kim et al. 13) reported that of 31.1 and He et al. 12) that of 19.5 in patients with small-cell lung cancer. Thus, our present analysis resulted in a value within the range of those found in other studies.
Age, performance status, Hugh-Jones score, serum CEA level, GPS, PLR, percentage of vital capacity (%VC), and alive status were different between patients with high and low ALI. Elevated CEA may represent the potential of lung adenocarcinoma malignancy even in a pathological early stage. Since GPS and PLR are also parameters representing nutritional and inflammatory status, their elevated levels may highlight patients with the poor prognosis from others, such as with ALI. In this study, only 2 of the 18 patients with an ALI value less than 22.2 had the recurrence of lung cancer. Nine of the 18 patients died, 5 from benign disease and 1 from lung cancer and 3 in other malignancy. During the postoperative follow-up, we should pay attention to other disease control for the patients with an ALI value less than 22.2. Unfortunately, we do not have postoperative data of ALI, but our data suggested that preoperative ALI might predict postoperative death in all causes. In fact, Proctor et al.24) reported that albumin and CRP are prognostic markers for cardiovascular and neurovascular diseases. ALI, consisting of BMI, albumin, and NLR, will be a prognostic factor of not only lung cancer but all causes of death as well as other inflammation or nutrition markers, such as GPS. Furthermore, there is no known report describing the relationship between %VC and ALI. We speculated that some types of inflammation may decrease vital capacity because of 5 of our patients with ALI lower than 22.2 and %VC lower than 80%, a history of pneumonia was noted in one patient and another was complicated with interstitial lung disease.
Limitations of this study include its nature as a single institute retrospective examination and the small patient population. Accumulation of additional cases is necessary to clarify the utility of ALI as a prognostic factor in patients with stage IA lung adenocarcinoma.
Conclusion
Low ALI was correlated with poor prognosis of stage IA lung adenocarcinoma patients. Those with an ALI less than 22.2 should be carefully followed, regardless of stage. During the postoperative follow-up for the patients with an ALI value less than 22.2, we should pay attention to other disease control.
Disclosure Statement
None was declared.
References
- 1).Coussens LM, Werb Z. Inflammation and cancer. Nature 2002; 420: 860-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Grange JM, Krone B, Mastrangelo G. Infection, inflammation and cancer. Int J Cancer 2011; 128: 2240-1. [DOI] [PubMed] [Google Scholar]
- 3).Proctor MJ, Morrison DS, Talwar D, et al. A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow Inflammation Outcome Study. Eur J Cancer 2011; 47: 2633-41. [DOI] [PubMed] [Google Scholar]
- 4).McMillan DC. The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev 2013; 39: 534-40. [DOI] [PubMed] [Google Scholar]
- 5).Warwick R, Mediratta N, Shackcloth M, et al. Preoperative red cell distribution width in patients undergoing pulmonary resections for non-small-cell lung cancer. Eur J Cardiothorac Surg 2014; 45: 108-13. [DOI] [PubMed] [Google Scholar]
- 6).Tomita M, Shimizu T, Ayabe T, et al. Elevated preoperative inflammatory markers based on neutrophil-to-lymphocyte ratio and C-reactive protein predict poor survival in resected non-small cell lung cancer. Anticancer Res 2012; 32: 3535-8. [PubMed] [Google Scholar]
- 7).Kim SH, Lee HW, Go SI, et al. Clinical significance of the preoperative platelet count and platelet-to-lymphocyte ratio (PLT-PLR) in patients with surgically resected non-small cell lung cancer. Oncotarget 2016; 7: 36098-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Forrest LM, McMillan DC, McArdle CS, et al. Evaluation of cumulative prognostic scores based on the systemic inflammatory response in patients with inoperable non-small-cell lung cancer. Br J Cancer 2003; 89: 1028-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Yotsukura M, Ohtsuka T, Kaseda K, et al. Value of the glasgow prognostic score as a prognostic factor in resectable non-small cell lung cancer. J Thorac Oncol 2016; 11: 1311-8. [DOI] [PubMed] [Google Scholar]
- 10).Jafri SH, Shi R, Mills G. Advance lung cancer inflammation index (ALI) at diagnosis is a prognostic marker in patients with metastatic non-small cell lung cancer (NSCLC): a retrospective review. BMC Cancer 2013; 13: 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Feng JF, Huang Y, Chen QX. A new inflammation index is useful for patients with esophageal squamous cell carcinoma. Onco Targets Ther 2014; 7: 1811-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).He X, Zhou T, Yang Y, et al. Advanced lung cancer inflammation index, a new prognostic score, predicts outcome in patients with small-cell lung cancer. Clin Lung Cancer 2015; 16: e165-71. [DOI] [PubMed] [Google Scholar]
- 13).Kim EY, Kim N, Kim YS, et al. Prognostic significance of modified advanced lung cancer inflammation index (ALI) in patients with small cell lung cancer_ comparison with original ALI. PLoS One 2016; 11: e0164056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Park YH, Yi HG, Lee MH, et al. Prognostic value of the pretreatment advanced lung cancer inflammation index (ALI) in diffuse large B cell lymphoma patients treated with R-CHOP chemotherapy. Acta Haematol 2017; 137: 76-85. [DOI] [PubMed] [Google Scholar]
- 15).Tomita M, Ayabe T, Nakamura K. The advanced lung cancer inflammation index is an independent prognostic factor after surgical resection in patients with non-small-cell lung cancer. Interact Cardiovasc Thorac Surg 2018; 26: 288-92. [DOI] [PubMed] [Google Scholar]
- 16).McMillan DC. An inflammation-based prognostic score and its role in the nutrition-based management of patients with cancer. Proc Nutr Soc 2008; 67: 257-62. [DOI] [PubMed] [Google Scholar]
- 17).Tewari N, Martin-Ucar AE, Black E, et al. Nutritional status affects long term survival after lobectomy for lung cancer. Lung Cancer 2007; 57: 389-94. [DOI] [PubMed] [Google Scholar]
- 18).Nakagawa T, Toyazaki T, Chiba N, et al. Prognostic value of body mass index and change in body weight in postoperative outcomes of lung cancer surgery. Interact Cardiovasc Surg 2016; 23: 560-6. [DOI] [PubMed] [Google Scholar]
- 19).Jin Y, Zhao L, Peng F. Prognostic impact of serum albumin levels on the recurrence of stage I non-small cell lung cancer. Clinics (Sao Paulo) 2013; 68: 686-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Yin Y, Wang J, Wang X, et al. Prognostic value of the neutrophil to lymphocyte ratio in lung cancer: a meta-analysis. Clinics 2015; 70: 524-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Peng B, Wang YH, Liu YM, et al. Prognostic significance of the neutrophil to lymphocyte ratio in patients with non-small cell lung cancer: a systemic review and meta-analysis. Int J Clin Exp Med 2015; 8: 3098-106. [PMC free article] [PubMed] [Google Scholar]
- 22).Sarraf KM, Belcher E, Raevsky E, et al. Neutrophil/lymphocyte ratio and its association with survival after complete resection in non-small cell lung cancer. J Thorac Cardiovasc Surg 2009; 137: 425-8. [DOI] [PubMed] [Google Scholar]
- 23).Koma Y, Onishi A, Matsuoka H, et al. Increased red blood cell distribution width associates with cancer stage and prognosis in patients with lung cancer. PLoS One 2013; 8: e80240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Proctor MJ, McMillan DC, Horgan PG, et al. Systemic inflamemation predicts all-cause mortality: a Glasgow inflammation outcome study. PLoS One 2015; 10: 1-12. doi:10.1371/journal.pone.0116206. [DOI] [PMC free article] [PubMed] [Google Scholar]