Abstract
Background
Elevated levels of proprotein convertase subtilisin/kexin type 9 (PCSK9) have been reported to be related to dyslipidemia, including patients with kidney dysfunction. However, its association with estimated glomerular filtration rate (eGFR) in individuals with normal serum creatinine (SCr) has not been determined.
Methods
A total of 2,089 subjects with normal SCr and without lipid-lowering treatment were consecutively enrolled in this study. Plasma PCSK9 levels were measured by ELISA kit and eGFR was evaluated by the Chronic Kidney Disease Epidemiology Collaboration equation. Subjects were divided into a normal eGFR group (n = 1,205, ≥90 mL/min/1.73 m2) and a decreased eGFR group (n = 884, < 90 mL/min/1.73 m2). Baseline characteristics and laboratory findings were compared between the two groups. Spearman's correlation and linear regression were performed to determine the association between PCSK9 and eGFR.
Results
No significant difference in PCSK9 levels was found between the normal eGFR group and the decreased eGFR group (236.84 ± 67.87 vs. 239.98 ± 68.72 ng/mL, p = 0.303). In Spearman's correlation and multivariable linear regression analysis, no association of PCSK9 levels with eGFR was detected in the total cohort (r = −0.039, p = 0.079; adjusted β = −0.013, p = 0.630). This result remained the same in the subgroups of normal eGFR (r = −0.038, p = 0.190; adjusted β = −0.031, p = 0.367) and decreased eGFR (r = −0.054, p = 0.109; adjusted β = −0.034, p = 0.319).
Conclusion
In this single-center study with moderate sample size, the data showed no relationship of PCSK9 levels with normal or decreased eGFR in untreated patients with normal SCr, suggesting that further studies may be needed to understand the relationship between PCSK9 and lipid disorder in different stage of kidney dysfunction.
Keywords: Cardiovascular disease, PCSK9, Estimated glomerular filtration rate, Dyslipidemia
Introduction
Evidence to date has shown that dyslipidemia plays a crucial role in the development of atherosclerosis and coronary artery disease (CAD) [1]. Previous studies have indicated that patients with kidney disease also exhibit significant alterations in lipid metabolism and that the progression of kidney disease may lead to severe dyslipidemia [2, 3, 4]. Importantly, it is worth noting that disorders in lipid metabolism can also be observed in patients even in the early stage of kidney dysfunction [5, 6]. Recently, proprotein convertase subtilisin/kexin type 9 (PCSK9), a novel protease, has been established as an important regulator in lipid metabolism by regulating low-density lipoprotein receptor expression [7, 8] and its gain of mutation can result in elevated low-density lipoprotein cholesterol (LDL-C) and increased risk of CAD [8, 9]. Hence, considering the effects of PCSK9 and status of kidney function on lipid metabolism, whether PCSK9 is involved in the different stages of the kidney dysfunction needs to be clinically studied.
An increasing number of studies have investigated the potential associations of circulating PCSK9 levels with kidney function. However, the results remained inconsistent. Several studies revealed that plasma PCSK9 levels are influenced by kidney function in severe kidney disease [10, 11, 12, 13], whereas other research found that plasma PCSK9 levels are not associated with kidney function [14, 15]. Indeed, this disparity regarding the relationship of PCSK9 with kidney dysfunction in different studies might be due to confounders, including the different state of kidney injury. Thus, further studies are needed to clarify the correlations between PCSK9 levels and kidney function, especially in different stages of kidney function.
In this study, therefore, we consecutively enrolled 2,089 subjects with normal serum creatinine (SCr) and without lipid-lowering treatment and tried to investigate the relationship between plasma PCSK9 levels and early kidney dysfunction.
Subjects and Methods
Study Population
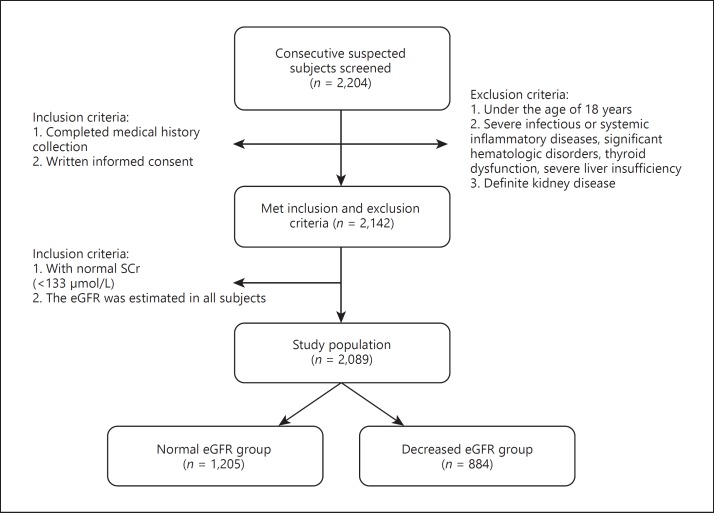
From October 2012 until June 2017, a total of 2,204 consecutive patients were enrolled in the study from our database (registry study on individuals without treatments with lipid-lowering drugs for at least 3 months prior to entrance) described in a previous study [16]. The exclusion criteria were (1) patients with abnormal SCr (≥133 μmol/L) and/or definite kidney disease, (2) patients aged < 18 years, (3) patients with severe infectious or systemic inflammatory disease, and (4) patients with significant hematologic disorders, thyroid dysfunction, or severe liver insufficiency. The study flowchart is shown in Figure 1. Finally, a cohort of 2,089 patients was analyzed.
Fig. 1.
Flowchart of the present study.
Hypercholesterolemia was defined as fasting plasma total cholesterol (TC) ≥5.2 mmol/L (200 mg/dL) and/or triglyceride (TG) ≥1.7 mmol/L (150 mg/dL). Type 2 diabetes mellitus was defined as fasting plasma glucose ≥7.0 mmol/L and/or nonfasting plasma glucose ≥11.1 mmol/L in multiple examinations or subjects with hypoglycemic therapy. Hypertension was defined as repeated measurements of blood pressure ≥140/90 mm Hg three times at least or patients with antihypertensive treatments. CAD was defined as 50% diameter stenosis in at least one of the three major coronary arteries assessed by selective coronary angiography. The body mass index was calculated as body weight (kg) divided by body height (m2). Kidney function was measured as estimated glomerular filtration rate (eGFR) using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation: eGFR = 141 × min(SCr/κ, 1)α × max(SCr/κ, 1)−1.209 × 0.993age × 1.018 [if female] × 1.159 [if black] [17].
Laboratory Analysis
Blood samples were obtained from the cubital vein and collected into EDTA-containing tubes after a 12-h overnight fasting. All samples were subsequently stored at −80°C and analyzed after thawing. Plasma PCSK9 levels were measured by a high-sensitivity, quantitative sandwich enzyme immunoassay (Quantikine ELISA, R&D Systems Europe Ltd.) as described in our previous studies [16, 18, 19]. The lowest limit of detection was 0.096 mg/L. The measurement range of this assay is 0.16–10 ng/mL; intra-assay imprecision (coefficient of variation) is 1.5–2.6% and inter-assay imprecision 2.90–7.09%. The biochemical variables were measured by an automatic biochemistry analyzer (Hitachi7150, Japan). SCr, TG, TC, LDL-C, and high-density lipoprotein cholesterol (HDL-C) were measured using an enzymatic assay. The plasma levels of apolipoprotein A1 (ApoA1) and apolipoprotein B (ApoB) were measured using a turbidimetric immunoassay.
Statistical Analysis
Continuous variables are presented using mean ± SD and categorical variables as percentages. Comparisons of continuous data between groups were performed with Student t test or ANOVA as appropriate. For categorical data, χ2 test or Fisher's exact test were used. The associations of plasma PCSK9 and lipid levels with eGFR were analyzed using Spearman's correlation and multivariate linear regression. A two-sided p value < 0.05 was considered as statistically significant. Statistical analysis was performed with the SPSS 21.0 software (Chicago, IL, USA).
Results
Characteristics of the Participants
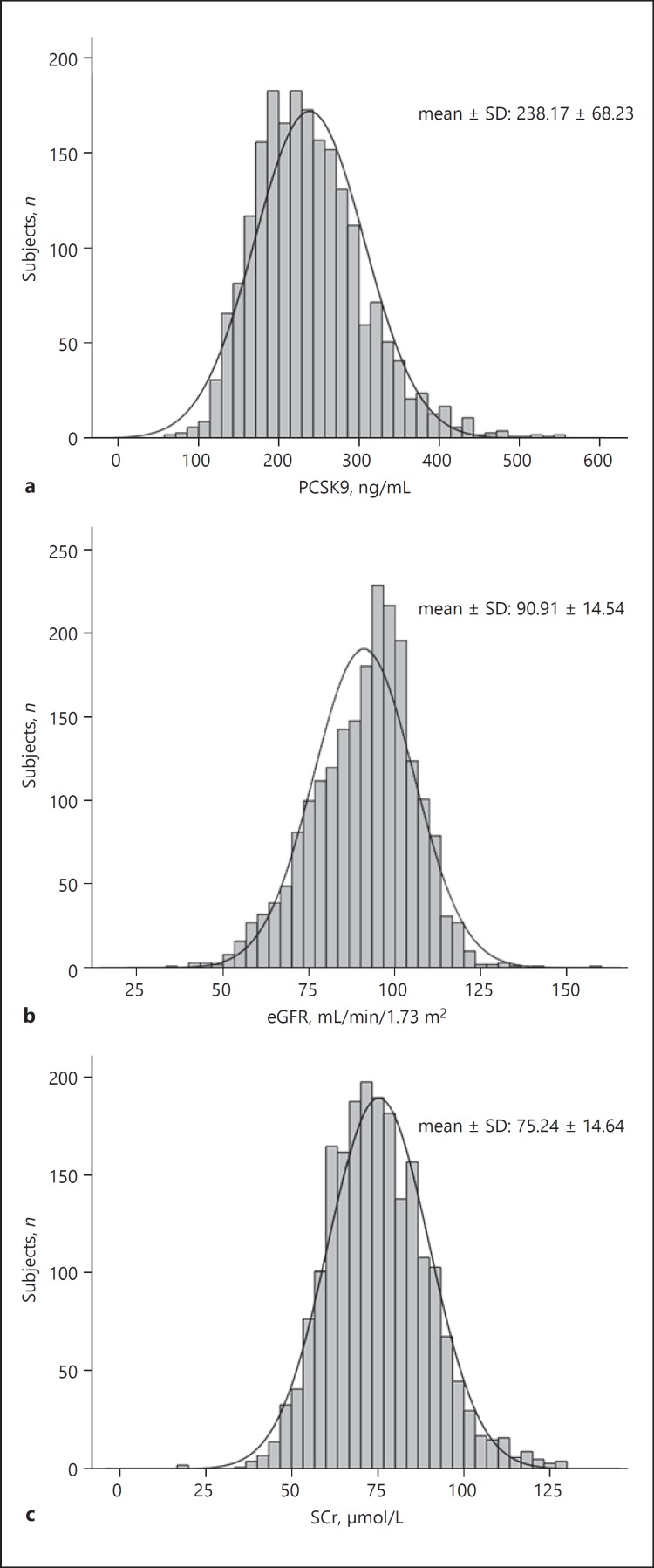
A total of 2,089 subjects who met the study criteria were finally enrolled. The mean age of the study population was 55.8 ± 10.9 years. Patients with hypercholesterolemia accounted for 60.8% of the total cohort, and none of them received any lipid-lowering drug treatment. As shown in Figure 2, the mean levels of PCSK9, eGFR, and SCr in the total cohort were 238.17 ± 68.23 ng/mL, 90.91 ± 14.54 mL/min/1.73 m2, and 75.24 ± 14.64 μmol/L, respective ly. Furthermore, subjects were divided into a normal eGFR group (≥90 mL/min/1.73 m2, n = 1,205) and a decreased eGFR group (< 90 mL/min/1.73 m2, n = 884). As established in Table 1, the subjects were older and the prevalence of hypertension and CAD were higher in the decreased eGFR group compared with the normal eGFR group (p < 0.001, p = 0.014, and p < 0.001, respectively). In addition, the levels of LDL-C, HDL-C, ApoA1, and ApoB were higher in the decreased eGFR group (p = 0.026, p = 0.001, p < 0.001, and p = 0.005, respectively). However, no significant difference in circulating PCSK9 levels was found between the two groups (236.84 ± 67.87 vs. 239.98 ± 68.72 ng/mL, p = 0.303).
Fig. 2.
Distribution of PCSK9 (mean ± SD: 238.17 ± 68.23 ng/mL) (a), eGFR (mean ± SD: 90.91 ± 14.54 mL/min/1.73 m2) (b), and SCr (mean ± SD: 75.24 ± 14.64 μmol/L) (c) in the total cohort.
Table 1.
Characteristics of the normal (≥90 mL/min/1.73 m2) and decreased (<90 mL/min/1.73 m2) eGFR groups
| Variables | Total (n = 2,089) |
Normal eGFR (n = 1,205) |
Decreased eGFR (n = 884) |
p value1 | |
|---|---|---|---|---|---|
| Baseline characteristics | |||||
| Age, years | 55.8±10.9 | 51.8±9.7 | 61.4±10.0 | <0.001 | |
| Male | 1,272 (60.9%) | 788 (65.4%) | 484 (54.8%) | <0.001 | |
| Hypertension | 1,152 (55.1%) | 637 (52.9%) | 515 (58.3%) | 0.014 | |
| Body mass index | 25.58±3.27 | 25.67±3.47 | 25.60±3.43 | 0.677 | |
| Hypercholesterolemia | 1,271 (60.8%) | 738 (61.2%) | 533 (60.3%) | 0.660 | |
| Diabetes | 398 (19.1%) | 222 (18.4%) | 176 (19.9%) | 0.393 | |
| Coronary artery disease | 1,234 (59.1%) | 651 (54.0%) | 583 (66.0%) | <0.001 | |
| Laboratory parameters | |||||
| Triglyceride, mmol/L | 1.84±1.26 | 1.96±1.38 | 1.81±1.07 | 0.005 | |
| Total cholesterol, mmol/L | 4.82±0.98 | 4.79±0.98 | 4.86±0.97 | 0.112 | |
| LDL-C, mmol/L | 3.14±0.88 | 3.09±0.91 | 3.19±0.85 | 0.026 | |
| HDL-C, mmol/L | 1.11±0.34 | 1.08±0.33 | 1.13±0.35 | 0.001 | |
| Non-HDL-C, mmol/L | 3.72±0.93 | 3.73±1.04 | 3.87±1.07 | 0.644 | |
| Apolipoprotein A1, g/L | 1.31±0.27 | 1.33±0.29 | 1.38±0.30 | <0.001 | |
| Apolipoprotein B, g/L | 1.02±0.26 | 1.01±0.26 | 1.04±0.25 | 0.005 | |
| Lipoprotein(a), mg/L | 220.44±240.94 | 217.92±244.83 | 224.58±237.65 | 0.534 | |
| Hemoglobin A1c, % | 6.01±0.98 | 5.98±1.02 | 6.06±0.92 | 0.067 | |
| Fasting plasma glucose, mmol/L | 5.59±1.51 | 5.66±1.64 | 5.48±1.28 | 0.417 | |
| Serum creatinine, µmol/L | 75.24±14.64 | 81.29±12.97 | 65.59±11.91 | <0.001 | |
| Blood urea nitrogen, mmol/L | 5.62±1.81 | 5.58±1.50 | 6.05±1.51 | <0.001 | |
| hs-CRP, mg/L | 2.33±2.78 | 2.29±2.77 | 2.38±2.76 | 0.425 | |
| PCSK9, ng/mL | 238.17±68.23 | 236.84±67.87 | 239.98±68.72 | 0.303 | |
Values are expressed as mean ± SD orn (%). Bold indicates statistical significance(p < 0.05). eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; hs-CRP, high-sensitivity C-reactive protein; LDL-C, low-density lipoprotein cholesterol; PCSK9, proprotein convertase subtilisin/kexin type 9; SD, standard deviation.
p values from ANOVA or Kruskal-Wallis test as appropriate for continuous variables and from χ2 test for categorical variables.
Relationship between Plasma Lipid Levels and Renal Function
As shown in Table 2, we found a slightly negative correlation of concentrations of LDL-C, HDL-C, ApoA1, and ApoB with eGFR (r = −0.064, p = 0.014; r = −0.082, p < 0.001; r = −0.080, p < 0.001; and r = −0.080, p < 0.001, respectively) in the total cohort. However, no significant correlations were found between these variables in the subgroup of decreased eGFR (p > 0.05, respectively).
Table 2.
Associations among eGFR, PCSK9, and lipids in the normal (≥90 mL/min/1.73 m2) and decreased (<90 mL/min/1.73 m2) eGFR groups
| Total |
Normal eGFR |
Decreased eGFR |
||||
|---|---|---|---|---|---|---|
| r | p value | r | p value | r | p value | |
| PCSK9 | −0.039 | 0.079 | −0.038 | 0.190 | −0.054 | 0.109 |
| Totalcholesterol | −0.035 | 0.110 | 0.002 | 0.938 | −0.025 | 0.450 |
| Triglyceride | 0.043 | 0.062 | 0.043 | 0.139 | −0.005 | 0.880 |
| LDL-C | −0.064 | 0.014 | −0.027 | 0.553 | −0.031 | 0.421 |
| HDL-C | −0.082 | <0.001 | −0.069 | 0.295 | 0.020 | 0.560 |
| Apolipoprotein A1 | −0.080 | <0.001 | −0.040 | 0.168 | −0.016 | 0.628 |
| Apolipoprotein B | −0.080 | <0.001 | −0.038 | 0.182 | −0.045 | 0.178 |
| Lipoprotein(a) | −0.057 | 0.179 | −0.034 | 0.234 | −0.015 | 0.655 |
Bold indicates statistical significance (p < 0.05). eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; PCSK9, proprotein convertase subtilisin/kexin type 9.
Relationship between Plasma Lipid Levels and PCSK9 Levels
Spearman's correlation of PCSK9 and lipids in different level of eGFR is shown in Table 3. In the total cohort, PCSK9 levels were positively correlated with TC (r = 0.238, p < 0.001), LDL-C (r = 0.217, p < 0.001), ApoA1 (r = 0.138, p < 0.001), and ApoB (r = 0.209, p < 0.001). In the subgroup with different eGFR levels, the positive relationship between plasma lipid levels and PCSK9 levels remained significant (p < 0.05, respectively).
Table 3.
Correlations between PCSK9 and lipids in the normal (≥90 mL/min/1.73 m2) and decreased (<90 mL/min/1.73 m2) eGFR groups
| Total | Normal eGFR | Decrease | s eGFR | |||
|---|---|---|---|---|---|---|
| r | p value | r | p value | r | p value | |
| Total cholesterol | 0.238 | <0.001 | 0.275 | <0.001 | 0.187 | <0.001 |
| Triglyceride | 0.085 | <0.001 | 0.069 | 0.017 | 0.109 | 0.001 |
| LDL-C | 0.217 | <0.001 | 0.252 | <0.001 | 0.169 | <0.001 |
| HDL-C | 0.131 | <0.001 | 0.173 | <0.001 | 0.072 | 0.034 |
| Apolipoprotein A1 | 0.138 | <0.001 | 0.155 | <0.001 | 0.110 | 0.001 |
| Apolipoprotein B | 0.209 | <0.001 | 0.229 | <0.001 | 0.181 | <0.001 |
| Lipoprotein(a) | 0.059 | 0.009 | 0.084 | 0.004 | 0.016 | 0.647 |
Bold indicates statistical significance (p < 0.05). eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; PCSK9, proprotein convertase subtilisin/kexin type 9.
Relationship between Plasma PCSK9 Levels and Renal Function
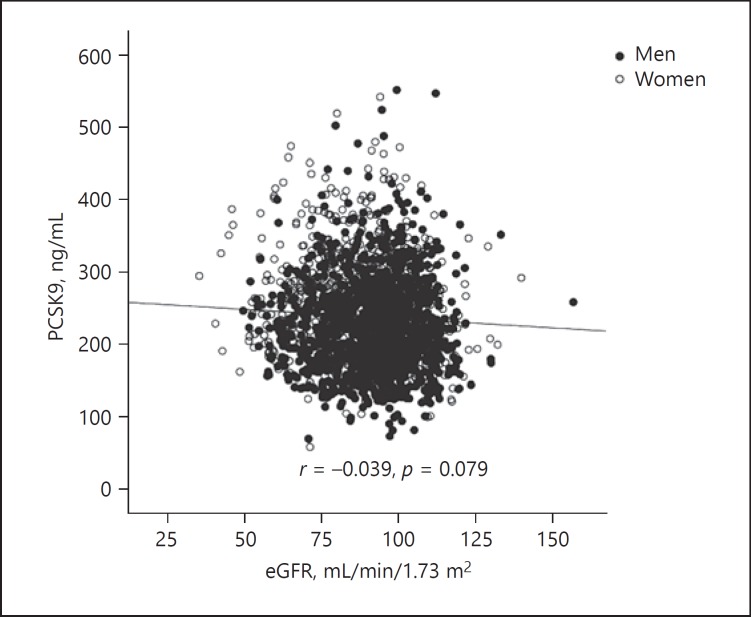
As shown in Figure 3, there were no associations between PCSK9 levels and eGFR in the total cohort (r = −0.039, p = 0.079). Moreover, in the subgroups of normal or decreased eGFR, no significant relationship was found between PCSK9 levels and eGFR (r = −0.038, p = 0.190; r = −0.054, p = 0.109, respectively). As shown in Table 4, we further investigate their relationship in the decreased eGFR group using multivariate linear regression adjusted for age, sex, hypertension, diabetes, history of CAD, and hypercholesterolemia, and again no associations between PCSK9 levels and eGFR were found (unadjusted β = −0.069, p = 0.071; adjusted β = −0.034, p = 0.319). In addition, we found a weakly negative correlation between PCSK9 and SCr levels in the total cohort (r = −0.127, p < 0.001; unadjusted β = −0.147, p < 0.001). However, this relationship disappeared after adjusting for the established confounders (adjusted β = −0.024, p = 0.252).
Fig. 3.
Relationship between PCSK9 levels and eGFR in the total cohort (r = −0.039, p = 0.079).
Table 4.
Linear regression analysis of the association between eGFR and PCSK9 levels in the decreased eGFR group (<90 mL/min/1.73 m2)
| Unadjusted |
Adjusted1 |
|||
|---|---|---|---|---|
| β | p value | β | p value | |
| Total cholesterol | −0.017 | 0.613 | −0.008 | 0.812 |
| LDL-C | −0.016 | 0.628 | −0.011 | 0.737 |
| Apolipoprotein B | −0.051 | 0.131 | −0.068 | 0.054 |
| PCSK9 | −0.069 | 0.071 | −0.034 | 0.319 |
eGFR, estimated glomerular filtration rate; LDL-C, low-density lipoprotein cholesterol; PCSK9, proprotein convertase subtilisin/kexin type 9.
Adjusted for age, sex, hypertension, diabetes, history of coronary artery disease and hypercholesterolemia.
Discussion
It has been reported that there are significant lipoprotein disorders in patients with kidney dysfunction, but the mechanism is not fully understood. PCSK9 is a key regulating protein of lipid metabolism, and we hypothesized that PCSK9 might be involved in the lipid changes of kidney dysfunction even in the early stage. We decided to study the relationship between circulating PCSK9 levels and kidney function in a Chinese cohort without lipid-lowering therapy. We used the CKD-EPI equation to evaluate kidney function, which takes into account sex, age, ethnicity, and SCr concentration [17]. Importantly, it has lower bias, especially at a eGFR > 60 mL/min/1.73 m2 [17]. Interestingly, the data showed that although elevated LDL-C levels were found in subjects with decreased eGFR, there was no significant increment of circulating PCSK9 levels. Apparently, our study may provide meaningful information with regard to the association among PCSK9, kidney function, and dyslipidemia in subjects with clinically normal SCr.
Indeed, PCSK9 is a serine protease mainly produced and released in the circulation by the liver and also expressed by the intestine and kidney partly. Besides, PCSK9 is also cleared from extrahepatic tissues, including the kidneys [20]. Therefore, the progression of kidney dysfunction may influence the expression or clearance of circulating PCSK9 levels, or in other words contribute to the change in plasma concentrations of PCSK9. Previous studies have reported that elevated PCSK9 concentrations are strongly associated with increased LDL-C levels and the development of atherosclerosis [8, 16, 18, 19]. Consequently, PCSK9 inhibitors have become a novel therapeutic target for the therapy of hypercholesterolemia, which has been demonstrated to reduce the risk of cardiovascular disease [21, 22]. Given the possible interactions among plasma levels of PCSK9, lipids, and kidney function, studies might be of clinical interest.
Notably, in recent years, several clinical and experimental studies have examined the relationship between circulating PCSK9 levels and kidney function. A study conducted by Konarzewski et al. [10] revealed that plasma PCSK9 and lipids levels in CKD patients were higher compared with those in the control group, and a strong negative correlation between PCSK9 levels and eGFR was found. Moreover, Abujrad et al. [11] showed that hemodialysis treatment could significantly reduce LDL-C and PCSK9 levels in CKD patients. The data from Jin et al. [13] also indicated that there were higher plasma PCSK9 concentrations in patients with nephrotic syndrome. Another research demonstrated that PCSK9 was correlated with hypercholesterolemia in nephrotic syndrome [12]. In addition, considering that circulating PCSK9 concentrations may be influenced by lipid-lowering treatments [23], Tecson et al. [24] did not find any association between PCSK9 and LDL-C in a mixed population of statin-treated and nontreated patients. Morena et al. [14] conducted a study in CKD patients without statin treatment. Unfortunately, they found that plasma PCSK9 levels did not vary significantly between different CKD stages and that PCSK9 levels were not associated with eGFR. Another study conducted by Rogacev et al. [15] in 729 patients who received statins showed that no correlation between eGFR and PCSK9 levels existed. Based on the disparity of studies, it is necessary to further examine the relationship between PCSK9 and kidney function at different stages in different populations.
It has also been demonstrated that patients with impaired kidney function show significant alterations in lipoprotein metabolism. These changes could be observed in the early stages of kidney disease [25, 26], and serious kidney dysfunction led to the development of severe dyslipidemia [2, 3, 27]. In our study, we found a higher LDL-C level in the decreased eGFR group compared to the normal eGFR group. However, no difference in PCSK9 levels was found, which suggests that dyslipidemia in patients with mild kidney dysfunction may not be involved in the PCSK9 pathway in our present study on 2,089 Chinese untreated individuals. However, we could not reach a definite conclusion, but the data suggested that more studies might be needed to further examine the relationship between PCSK9 and the early lipid abnormality in kidney dysfunction.
There were some limitations to the present study. Firstly, it was a single-center study, and selection bias might have been present. Besides, we did not enroll patients with severely decreased eGFR levels. Whether the results are consistent in such a population needs to be further studied. Finally, the relatively small sample size and the single measurement of circulating PCSK9 levels may also be limitations.
Conclusions
In this cohort study on 2,089 Chinese subjects without lipid-lowering treatment and with normal SCr, we did not found an association between circulating PCSK9 concentrations and mildly decreased eGFR. Our data suggest that PCSK9 might not be involved in the development of dyslipidemia in patients with mild kidney dysfunction but normal SCr. Further studies are needed to confirm our findings.
Statement of Ethics
The protocol of this study complied with the Declaration of Helsinki. The study was approved by the hospital ethics review board (Fu Wai Hospital and National Center for Cardiovascular Diseases, Beijing, China). Informed consent was obtained from all enrolled subjects.
Disclosure Statement
The authors state that no conflict of interest exists in this paper.
Acknowledgments
This study was partly supported by the Capital Health Development Fund (201614035) and the CAMS Major Collaborative Innovation Project (2016-I2M-1-011) awarded to Dr. Jian-Jun Li.
References
- 1.European Association for Cardiovascular Prevention & Rehabilitation, Reiner Z, Catapano AL, De Backer G, Graham I, Taskinen MR, et al. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) Eur Heart J. 2011;32:1769–1818. doi: 10.1093/eurheartj/ehr158. [DOI] [PubMed] [Google Scholar]
- 2.Marino A, Tannock LR. Role of dyslipidemia in patients with chronic kidney disease. Postgrad Med. 2013;125:28–37. doi: 10.3810/pgm.2013.07.2676. [DOI] [PubMed] [Google Scholar]
- 3.Mikolasevic I, Zutelija M, Mavrinac V, Orlic L. Dyslipidemia in patients with chronic kidney disease: etiology and management. Int J Nephrol Renovasc Dis. 2017;10:35–45. doi: 10.2147/IJNRD.S101808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wahl P, Ducasa GM, Fornoni A. Systemic and renal lipids in kidney disease development and progression. Am J Physiol Renal Physiol. 2016;310:F433–F445. doi: 10.1152/ajprenal.00375.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sechi LA, Catena C, Zingaro L, Melis A, De Marchi S. Abnormalities of glucose metabolism in patients with early renal failure. Diabetes. 2002;51:1226–1232. doi: 10.2337/diabetes.51.4.1226. [DOI] [PubMed] [Google Scholar]
- 6.Fliser D, Pacini G, Engelleiter R, Kautzky-Willer A, Prager R, Franek E, et al. Insulin resistance and hyperinsulinemia are already present in patients with incipient renal disease. Kidney Int. 1998;53:1343–1347. doi: 10.1046/j.1523-1755.1998.00898.x. [DOI] [PubMed] [Google Scholar]
- 7.Tavori H, Fan D, Blakemore JL, Yancey PG, Ding L, Linton MF, et al. Serum proprotein convertase subtilisin/kexin type 9 and cell surface low-density lipoprotein receptor: evidence for a reciprocal regulation. Circulation. 2013;127:2403–2413. doi: 10.1161/CIRCULATIONAHA.113.001592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giunzioni I, Tavori H, Covarrubias R, Major AS, Ding L, Zhang Y, et al. Local effects of human PCSK9 on the atherosclerotic lesion. J Pathol. 2016;238:52–62. doi: 10.1002/path.4630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abifadel M, Varret M, Rabes JP, Allard D, Ouguerram K, Devillers M, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003;34:154–156. doi: 10.1038/ng1161. [DOI] [PubMed] [Google Scholar]
- 10.Konarzewski M, Szolkiewicz M, Sucajtys-Szulc E, Blaszak J, Lizakowski S, Swierczynski J, et al. Elevated circulating PCSK-9 concentration in renal failure patients is corrected by renal replacement therapy. Am J Nephrol. 2014;40:157–163. doi: 10.1159/000365935. [DOI] [PubMed] [Google Scholar]
- 11.Abujrad H, Mayne J, Ruzicka M, Cousins M, Raymond A, Cheesman J, et al. Chronic kidney disease on hemodialysis is associated with decreased serum PCSK9 levels. Atherosclerosis. 2014;233:123–129. doi: 10.1016/j.atherosclerosis.2013.12.030. [DOI] [PubMed] [Google Scholar]
- 12.Haas ME, Levenson AE, Sun X, Liao WH, Rutkowski JM, de Ferranti SD, et al. The role of proprotein convertase subtilisin/kexin type 9 in nephrotic syndrome-associated hypercholesterolemia. Circulation. 2016;134:61–72. doi: 10.1161/CIRCULATIONAHA.115.020912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin K, Park BS, Kim YW, Vaziri ND. Plasma PCSK9 in nephrotic syndrome and in peritoneal dialysis: a cross-sectional study. Am J Kidney Dis. 2014;63:584–589. doi: 10.1053/j.ajkd.2013.10.042. [DOI] [PubMed] [Google Scholar]
- 14.Morena M, Le May C, Chenine L, Arnaud L, Dupuy AM, Pichelin M, et al. Plasma PCSK9 concentrations during the course of nondiabetic chronic kidney disease: relationship with glomerular filtration rate and lipid metabolism. J Clin Lipidol. 2017;11:87–93. doi: 10.1016/j.jacl.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Rogacev KS, Heine GH, Silbernagel G, Kleber ME, Seiler S, Emrich I, et al. PCSK9 plasma concentrations are independent of GFR and do not predict cardiovascular events in patients with decreased GFR. PLoS One. 2016;11:e0146920. doi: 10.1371/journal.pone.0146920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li S, Guo YL, Xu RX, Zhang Y, Zhu CG, Sun J, et al. Association of plasma PCSK9 levels with white blood cell count and its subsets in patients with stable coronary artery disease. Atherosclerosis. 2014;234:441–445. doi: 10.1016/j.atherosclerosis.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li S, Zhang Y, Xu RX, Guo YL, Zhu CG, Wu NQ, et al. Proprotein convertase subtilisin-kexin type 9 as a biomarker for the severity of coronary artery disease. Ann Med. 2015;47:386–393. doi: 10.3109/07853890.2015.1042908. [DOI] [PubMed] [Google Scholar]
- 19.Zhao X, Zhang HW, Li S, Zhang Y, Xu RX, Zhu CG, et al. Association between plasma proprotein convertase subtilisin/kexin type 9 concentration and coronary artery calcification. Ann Clin Biochem. 2018;55:158–164. doi: 10.1177/0004563217695351. [DOI] [PubMed] [Google Scholar]
- 20.Norata GD, Tibolla G, Catapano AL. Targeting PCSK9 for hypercholesterolemia. Annu Rev Pharmacol Toxicol. 2014;54:273–293. doi: 10.1146/annurev-pharmtox-011613-140025. [DOI] [PubMed] [Google Scholar]
- 21.Cohen JC, Boerwinkle E, Mosley TH Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354:1264–1272. doi: 10.1056/NEJMoa054013. [DOI] [PubMed] [Google Scholar]
- 22.Kathiresan S, Myocardial Infarction Genetics Consortium A PCSK9 missense variant associated with a reduced risk of early-onset myocardial infarction. N Engl J Med. 2008;358:2299–2300. doi: 10.1056/NEJMc0707445. [DOI] [PubMed] [Google Scholar]
- 23.Careskey HE, Davis RA, Alborn WE, Troutt JS, Cao G, Konrad RJ. Atorvastatin increases human serum levels of proprotein convertase subtilisin/kexin type 9. J Lipid Res. 2008;49:394–398. doi: 10.1194/jlr.M700437-JLR200. [DOI] [PubMed] [Google Scholar]
- 24.Tecson KM, Panettiere-Kennedy KS, Won JI, Garg P, Olugbode O, McCullough PA. Relation between proprotein convertase subtilisin/kexin type 9 and directly measured low-density lipoprotein cholesterol. Proc (Bayl Univ Med Cent) 2017;30:16–20. doi: 10.1080/08998280.2017.11929514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med. 2004;164:659–663. doi: 10.1001/archinte.164.6.659. [DOI] [PubMed] [Google Scholar]
- 26.Attman PO, Samuelsson O, Alaupovic P. Lipoprotein metabolism and renal failure. Am J Kidney Dis. 1993;21:573–592. doi: 10.1016/s0272-6386(12)80030-8. [DOI] [PubMed] [Google Scholar]
- 27.Lin TH, Chuang SY, Chu CY, Lee WH, Hsu PC, Su HM, et al. The impact of chronic kidney disease on lipid management and goal attainment in patients with atherosclerosis diseases in Taiwan. Int J Med Sci. 2014;11:381–388. doi: 10.7150/ijms.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]