Abstract
Human papillomaviruses (HPV) cause cancer at a number of vulnerable epithelial sites, including the cervix, the anus and the oropharynx, with cervical cancer being the most significant in terms of numbers. The cervix has a complex epithelial organisation, and comprises the stratified epithelium of the ectocervix, the columnar epithelium of the endocervix, and the cervical transformation zone (TZ). Most cervical cancers arise at the TZ, which is a site where a stratified squamous epithelium can develop via metaplasia from a simple columnar epithelium. It is thought that this process is mediated by the cervical reserve cell, a specialised type of stem cell that is located at the TZ, which has been proposed as the target cell for HPV infection. Reserve cells may be derived from the basal cells of the ectocervix, or may originate from the cuboidal cells found at the squamo columnar junction. It appears that HPV infection of these diverse cell types, including the columnar cells of the endocervix, facilitates deregulated viral gene expression and the development of neoplasia, with different epithelial sites having different cancer risk. It is envisaged that these concepts may explain the vulnerability of the oropharynx, and other TZ regions where HPV-associated cancers arise.
1. High-risk HPV infection and the risk of cancer
High-risk human papillomaviruses infect a diverse range of epithelial sites, but cause cancer at these sites at different frequencies. Thus while cervical cancer accounts for approximately 530,000 cases per year worldwide, of which almost 100% are caused by the virus, HPV-associated cancer of the vulva and vagina amount to around 20,000 cases per year, with HPV-associated penile cancer constituting only 13,000 cases [1]. So what then determines this wide difference in susceptibility to cancer, given that the high-risk papillomaviruses are sexually transmitted, and thus able to access a wide range of genital epithelial sites? Whether a particular epithelial site presents a stratified defensive epithelial barrier to infection is part of the explanation, as HPV-associated cancers, and the precancerous lesions that precede them, cannot develop in the absence of initial infection. However, to explain this fully, we must also appreciate that the epithelial sites where HPV-associated cancers occur most frequently, are not as a rule, the multilayed differentiated epithelia sites that support productive infection. Instead, these are sites with an atypical epithelial organisation, where viral gene expression can become deregulated [2,3], leading to precancerous changes in cell phenotype [4], and over time, to the development of invasive cancer. The transformation zone regions of the cervix and the anus, and the reticulated epithelium of the palatine tonsil, serve specific functions that are required by the host, and as a consequence of this, are particularly vulnerable to HPV-associated transformation. The origin of HPV-associated cancers therefore, is often as much about the epithelial site, as it is about the range of high-risk HPV types that infect it.
2. The cervical transformation zone and the ectocervix
Cervical cancer is classified on the basis of morphologic criteria, as either squamous cell carcinoma, adenocarcinoma, or as one of a number of less common cervical cancers, such as adenosquamous carcinoma [5,6]. Although these divisions are intended to reflect either squamous or glandular disease origins, in reality the approach is imprecise, and offers only limited insight into the natural history of neoplastic disease and the cellular lineages involved. Even so, it appears that high-risk papillomaviruses can infect different epithelial cell types in the cervix [7], and that the consequences of this can be very different.
The cervix consists of at least three distinct epithelial types, of which the cervical transformation zone is the most important with regard to cancer risk [8]. Most cervical cancers arise at this region, which has for many years, been thought to be sustained by a specialised type of stem cell known as the cervical reserve cell [9]. These cells are variably present under the columnar epithelium that lies close to the cervical squamo columnar junction (SCJ), and are involved in driving the process of cervical metaplasia when this is required. Metaplasia is a normal process, by which the columnar epithelium in this region can change into a stratified differentiated epithelium. It occurs at puberty as the endocervix becomes exposed to the acid environment of the vagina, but also occur throughout a woman's life in response to local irritation. For many years, pathologists have suspected that it is the HPV infection of the cervical reserve cell, and the inherent deregulation of viral gene expression that occurs in these cells, that underlies the development of cervical neoplasia. By contrast, HPV infection of the basal epithelial layer that maintain the stratified ectocervix, is thought to result more typically in the regulated HPV expression that facilitates productive infection. Although this model is persuasive, our understanding of the cell types that maintain normal epithelial homeostasis in the vicinity of the SCJ is poorly developed. Interestingly, the reserve cell model has recently received new impetus, with the suggestion that these cells, and the basal cells of the ectocervix, may in fact have a common origin, with their different behaviour being influenced by the stromal microenvironment [10]. As the ectocervix and the transformation zone have distinct origins during embryonic development, this hypothesis deserves attention [11]. However, it has also been suggested that cervical metaplasia is driven not by the reserve cell, but by a group of cuboidal cells located more immediately at the SCJ, with these cells giving rise to a 'reserve cell-like' population beneath them, that is involved in the metaplastic process [12]. Although we are unsure as to how this epithelial site is regulated, it is clear that our conventional model of infection leading primarily to productive infection, is unlikely to hold true here.
3. Refining our model of the cellular origins of cervical cancer
The conventional model of cervical cancer progression that is often shown in text books, pays little attention to the vulnerability of the different epithelial sites where HPV-associated cancers occur. Our refined model clearly indicates that cervical cancer should not be considered as one disease, but is instead a heterogeneous group of cancers, with at least three progression routes that depend on the nature of the initially infected cell (Fig. 1). Persistent HPV infection, and the deregulation of normal viral gene expression, are unifying risk factors that affect all three infection sites. This line of thinking suggests that high risk HPV infection of the ectocervix has a progression risk similar to that of vaginal and/or penile infections, with slow progression over time from LSIL to HSIL, and then to cancer. Infection of the columnar epithelium close to the SCJ, where metaplasia can occur, is associated with a higher risk of cancer progression (Fig. 1). It is unclear at present, whether infections at this site need always to progress from LSIL, or even if such low grade lesions actually equate to productive infections. Our recent observations suggest in fact, that non-productive LSIL are amongst the variety of low-grade disease that can develop at both the cervix and the anus [13,14], and that some of these disease pathologies arise from reserve cell infection. Despite their importance however, not all infections are confined to the ectocervix and transformation zone, and it is becoming clear that high risk HPV types can also infect the columnar cells of the endocervix proper [15], a site which has no capacity to support productive LSIL (see Fig. 2).
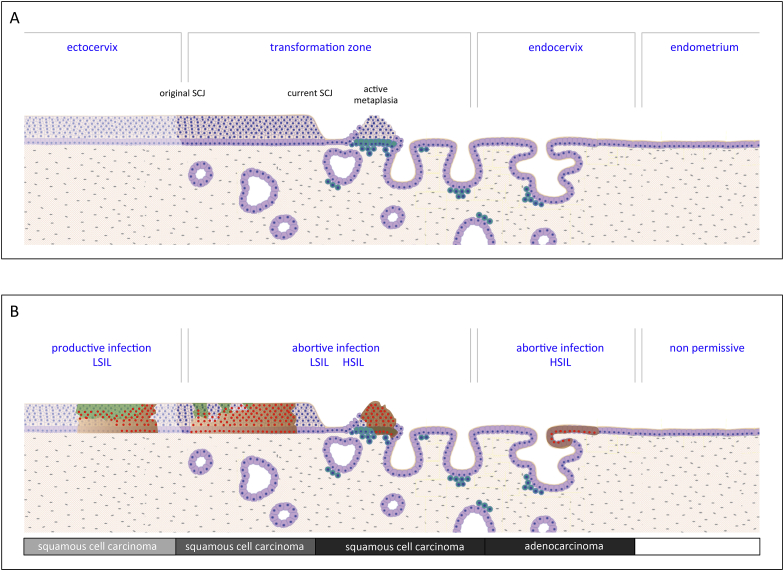
Fig. 1.
Diversity of HPV Infection Sites at the Cervix, and the Consequence of Infection. A) The cervix is comprised of the conventional stratified epithelium of the ectocervix, the transformation zone, and the columnar epithelium of the endocervix, which lies adjacent to the endometrium. The reserve cells, which are shown in turquoise, lie under the columnar epithelium of the transformation zone, and play a role in normal metaplasia, a process that leads to the formation of a new stratified epithelium when required. This is shown between the original and current squamocolumnar junction (SCJ). B) The consequence of HPV infection differs depending on the site of infection. Current thinking suggests that the ectocervix is a site where productive HPV infection and LSIL is supported, and that the other sites are associated with different level of deregulated HPV gene expression. The typical molecular phenotypes observed at these sites can be revealed using biomarkers E4 (green), p16 (brown) and MCM/Ki67 (red). The higher grade phenotypes observed in the transformation zone and the endocervix are potential precursors of cervical cancer (squamous cell carcinoma and adenocarcinoma), and develops more often at theses sites when compared to the ectocervix. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
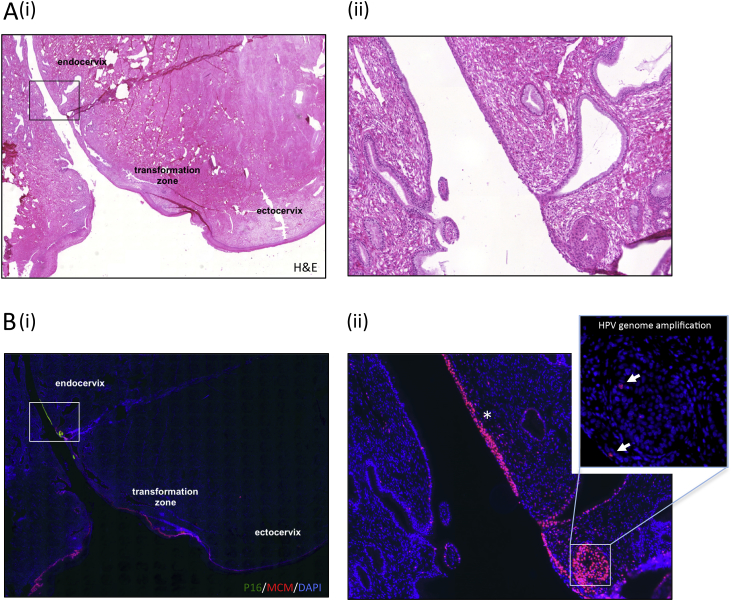
Fig. 2.
Endocervical HPV Infection and its Consequences. A)(i) Histology shows the three cervical regions, with a region of neoplasia highlighted in the boxed area. (ii) The high power image of this region reveals that the neoplasia has arisen in an area of columnar epithelium, and that the epithelium on the adjacent side of the os is normal. B)(i) Immunofluorescence stain to show the cell cycle marker MCM in red, and p16, which is used as a surrogate marker of deregulated HPV gene expression (transforming infections) in green. (ii) The extent of cell cycle activity is revealed by the MCM staining (red; marked with asterisk). The epithelium is undifferentiated, with only very limited evidence of HPV genome amplification in the centre of a HPV infected crypt, which is shown in the inset. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. Infection of the endocervix and the development of adenocarcinoma
Unlike the ectocervix and the metaplastic epithelium of the transformation zone, the endocervix comprises a single layer of mucin-secreting columnar cells organised into a network of crypts. Cancers arising at such epithelial sites may still be classified as squamous cell carcinoma, despite their cellular origin, as diagnosis depends largely on the epithelial thickness within the neoplasia. The concept of HPV deregulation, and its dependence on the nature of the infected cell and its local microenvironment, is perhaps easiest to appreciate at the endocervix. This is an epithelial site that lacks an ability to stratify and to support the full productive papillomavirus life cycle, and from our recent studies, appears to be a site where papillomavirus gene expression is deregulated. A third route to cancer is thus relevant here, which does not invoke the need for a LSIL precursor. Curiously, the range of HPV types that cause adenocarcinoma, is a subset of a much wider group that is responsible for cervical squamous cell carcinoma. Two members of the Alpha 7 species (HPV18 & 45) cause around 45% of cases, with HPV16, (an Alpha 9 species member), causing a similar number [16]. This restricted range of HPV types is apparent at other sites of non-productive infection, including the oropharynx [17,18]. A final point to make, is that adenocarcinomas themselves are a heterogeneous group, and that HPVs are not the exclusive cause of cancer here [19]. In fact, the data suggests that there may be both permissive and non-permissive cell types within the columnar cells of the endocervix and endometrium.
5. Extending the cervical cancer model to other epithelial sites
The general principles, outlined here for the cervix, can be applied to other vulnerable epithelial sites where the proportion of cancers attributable to high risk HPV infection is high. The crypts of the palatine tonsil are one such example, where HSIL and cancer may develop without the need for a productive LSIL precursor [20]. As seen with the cervix, the particular characteristics of the tonsil, including its reticulated, or open, epithelial structure, most likely contribute to its vulnerability to infection. Subsequent to this, the 'porous' basal membrane of the tonsillar crypt, which is necessary for flow of lymphocytes, may inadvertently facilitate the movement of HPV infected epithelial cells into the stroma and beyond.
6. Conclusions
In contrast to our understanding of HPV protein function, our knowledge of the epithelial sites where high risk HPV types cause cancer is very limited. It appears that the specialised epithelial cells that constitute these sites, must control viral gene expression in different ways, with the cellular microenvironment influencing this further. To properly understand HPV-cancer associations, there is a need to understand the molecular pathways that control epithelial homeostasis at these sites, and the consequences of viral gene expression on this control. Such studies have enormous potential, and are expected to provide new opportunities for disease treatment and cure.
Acknowledgements
The authors thank Olaf Reich and Sigrid Regauer (Graz University Medical School, Austria), Jacob Bornstein (Bar-Ilan University Faculty of Medicine, Nahariya, Israel) and Margaret Stanley (University of Cambridge, UK) for their insight and helpful discussions. The writing of this article was facilitated by funding from the UK Medical Research Council grant MR/S024409/1 (Understanding the Epithelial Site-Specific Origin of HPV Neoplasia and its Control) awarded to JD and HG.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pvr.2019.04.005.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Kreimer A.R., Pierce Campbell C.M., Lin H.Y., Fulp W., Papenfuss M.R. Incidence and clearance of oral human papillomavirus infection in men: the HIM cohort study. Lancet. 2013;382:877–887. doi: 10.1016/S0140-6736(13)60809-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Egawa N., Egawa K., Griffin H., Doorbar J. Human papillomaviruses; epithelial tropisms, and the development of neoplasia. Viruses. 2015;7:3863–3890. doi: 10.3390/v7072802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doorbar J., Egawa N., Griffin H., Kranjec C., Murakami I. Human papillomavirus molecular biology and disease association. Rev. Med. Virol. 2015;25(Suppl 1):2–23. doi: 10.1002/rmv.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kranjec C., Doorbar J. Human papillomavirus infection and induction of neoplasia: a matter of fitness. Curr. Opin. Virol. 2016;20:129–136. doi: 10.1016/j.coviro.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Benedet J.L., Bender H., Jones H., 3rd, Ngan H.Y., Pecorelli S. FIGO staging classifications and clinical practice guidelines in the management of gynecologic cancers. FIGO Committee on Gynecologic Oncology. Int. J. Gynaecol. Obstet. 2000;70:209–262. [PubMed] [Google Scholar]
- 6.Quddus M.R., Manna P., Sung C.J., Kerley S., Steinhoff M.M. Prevalence, distribution, and viral burden of all 15 high-risk human papillomavirus types in adenosquamous carcinoma of the uterine cervix: a multiplex real-time polymerase chain reaction-based study. Hum. Pathol. 2014;45:303–309. doi: 10.1016/j.humpath.2013.07.048. [DOI] [PubMed] [Google Scholar]
- 7.van der Marel J., van Baars R., Alonso I., Del Pino M., van de Sandt M. Oncogenic human papillomavirus-infected immature metaplastic cells and cervical neoplasia. Am. J. Surg. Pathol. 2014;38:470–479. doi: 10.1097/PAS.0000000000000174. [DOI] [PubMed] [Google Scholar]
- 8.Reich O., Regauer S., McCluggage W.G., Bergeron C., Redman C. Defining the cervical transformation zone and squamocolumnar junction: can we reach a common colposcopic and histologic definition? Int. J. Gynecol. Pathol. 2017;36:517–522. doi: 10.1097/PGP.0000000000000381. [DOI] [PubMed] [Google Scholar]
- 9.Martens J.E., Smedts F.M., Ploeger D., Helmerhorst T.J., Ramaekers F.C. Distribution pattern and marker profile show two subpopulations of reserve cells in the endocervical canal. Int. J. Gynecol. Pathol. 2009;28:381–388. doi: 10.1097/PGP.0b013e31819932f8. [DOI] [PubMed] [Google Scholar]
- 10.Cindrilla Chumduri T.F., Gurumurthy R.K., Berger H., Koster S., Brinkmann V., Klemm U., Mollenkopf H.-J., Herbst H., Mangler M., Meyer T.F. bioRxiv; 2018. Transition of Wnt Signaling Microenvironment Delineates the Squamo-Columnar Junction and Emergence of Squamous Metaplasia of the Cervix. [Google Scholar]
- 11.Martens J.E., Smedts F., van Muyden R.C., Schoots C., Helmerhorst T.J. Reserve cells in human uterine cervical epithelium are derived from mullerian epithelium at midgestational age. Int. J. Gynecol. Pathol. 2007;26:463–468. doi: 10.1097/pgp.0b013e31803c7c18. [DOI] [PubMed] [Google Scholar]
- 12.Herfs M., Yamamoto Y., Laury A., Wang X., Nucci M.R. A discrete population of squamocolumnar junction cells implicated in the pathogenesis of cervical cancer. Proc. Natl. Acad. Sci. U.S.A. 2012;109:10516–10521. doi: 10.1073/pnas.1202684109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leeman A., Pirog E.C., Doorbar J., van de Sandt M.M., van Kemenade F.J. Presence or absence of significant HPVE4 expression in high-grade Anal intraepithelial neoplasia with p16/ki-67 positivity indicates distinct patterns of neoplasia: a study combining immunohistochemistry and laser capture microdissection PCR. Am. J. Surg. Pathol. 2018;42:463–471. doi: 10.1097/PAS.0000000000000984. [DOI] [PubMed] [Google Scholar]
- 14.Griffin H., Soneji Y., Van Baars R., Arora R., Jenkins D. Stratification of HPV-induced cervical pathology using the virally encoded molecular marker E4 in combination with p16 or MCM. Mod. Pathol. 2015;28:977–993. doi: 10.1038/modpathol.2015.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reich O., Regauer S. Thin HSIL of the cervix: detecting a variant of high-grade squamous intraepithelial lesions with a p16INK4a antibody. Int. J. Gynecol. Pathol. 2017;36:71–75. doi: 10.1097/PGP.0000000000000311. [DOI] [PubMed] [Google Scholar]
- 16.Doorbar J., Quint W., Banks L., Bravo I.G., Stoler M. The biology and life-cycle of human papillomaviruses. Vaccine. 2012;30(Suppl 5):F55–F70. doi: 10.1016/j.vaccine.2012.06.083. [DOI] [PubMed] [Google Scholar]
- 17.Lechner M., Fenton T.R. The genomics, epigenomics, and transcriptomics of HPV-associated oropharyngeal cancer-understanding the basis of a rapidly evolving disease. Adv. Genet. 2016;93:1–56. doi: 10.1016/bs.adgen.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Michaud D.S., Langevin S.M., Eliot M., Nelson H.H., Pawlita M. High-risk HPV types and head and neck cancer. Int. J. Cancer. 2014;135:1653–1661. doi: 10.1002/ijc.28811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pirog E.C. Cervical adenocarcinoma: diagnosis of human papillomavirus-positive and human papillomavirus-negative tumors. Arch. Pathol. Lab Med. 2017;141:1653–1667. doi: 10.5858/arpa.2016-0356-RA. [DOI] [PubMed] [Google Scholar]
- 20.Westra W.H. The morphologic profile of HPV-related head and neck squamous carcinoma: implications for diagnosis, prognosis, and clinical management. Head Neck Pathol. 2012;6(Suppl 1):S48–S54. doi: 10.1007/s12105-012-0371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.