Abstract
Objective
Safety and efficacy of mAbs blocking the IL-6 receptor have been established in RA. This is the first analysis examining safety and tolerability of sarilumab and tocilizumab administered as single or multiple doses in patients with RA within the same study.
Methods
In ASCERTAIN, patients were randomized 1: 1: 2 to 24 weeks’ double-blind sarilumab 150 or 200 mg every 2 weeks s.c. or tocilizumab 4 mg/kg every 4 weeks i.v., increased to 8 mg/kg if clinically indicated. In Study 1309, patients were randomized 1: 1: 1: 1 to single-dose open-label sarilumab 150 or 200 mg s.c. or tocilizumab 4 or 8 mg/kg i.v.
Results
In ASCERTAIN, incidence of treatment-emergent adverse events was similar between sarilumab and tocilizumab. The most common treatment-emergent adverse events were the following: sarilumab: neutropenia [6 patients (12.2%) in the 150 mg group and 8 (15.7%) in the 200 mg group], nasopharyngitis [6 (12.2%) and 3 (5.9%)], and injection-site erythema [4 (8.2%) and 4 (7.8%)]; tocilizumab: accidental overdose [9 (8.8%)], upper respiratory tract infection [7 (6.9%)] and nausea [7 (6.9%)]. Laboratory changes in both studies included decreased neutrophils and platelets and increased transaminases and lipids. In Study 1309, incidence of absolute neutrophil count <1.0 giga/l was similar between sarilumab and tocilizumab, and occurred more frequently in the higher dose groups. No association between decrease in absolute neutrophil count and increased incidence of infection was observed in either study.
Conclusion
No clinically meaningful differences in treatment-emergent adverse events were observed between sarilumab and tocilizumab. Laboratory changes with sarilumab were within the same range as those with tocilizumab.
Trial registration numbers
ASCERTAIN (NCT01768572); Study 1309 (NCT02097524).
Keywords: RA, sarilumab, tocilizumab, subcutaneous, intravenous
Rheumatology key messages
There were no clinically meaningful differences in safety between sarilumab and tocilizumab.
Laboratory changes observed with sarilumab were within the same range as those observed with tocilizumab.
Decreases in absolute neutrophil count were not associated with increased infection risk.
Introduction
The introduction of biologic DMARDs that target inflammatory cytokines, such as TNF or IL-6, has greatly expanded therapeutic options for the treatment of RA and improved treatment outcomes for these patients [1–3]. One of the key cytokines involved in mediating the underlying disease pathophysiology and clinical manifestations of RA is IL-6. The role of IL-6 in the pathophysiology of RA is not completely understood; however, pleiotropic effects of the cytokine have been correlated with disease activity and joint destruction [2]. Furthermore, IL-6 may mediate induction of acute-phase reactants and play a role in activating autoimmune response [1, 2]. Consistent with these observations, blockade of IL-6 signalling mitigates the underlying disease pathophysiology and clinical manifestations of RA [3]. Subsequently, multiple biologics targeting the IL-6 signalling pathway have been developed for the treatment of RA [4–8].
Sarilumab and tocilizumab are monoclonal antibodies directed against the IL-6 receptor (IL-6R). Sarilumab is a human IgG1 monoclonal antibody that binds specifically to both soluble and membrane-bound IL-6R, and it has been shown to inhibit IL-6-mediated signalling through these receptors [8]. Tocilizumab is a recombinant humanized monoclonal antibody also directed against the IL-6R [6]. The efficacy of sarilumab and tocilizumab has previously been demonstrated in patients with moderate-to-severe RA [9, 10]. ASCERTAIN and Study 1309 are the first studies in which sarilumab and tocilizumab were evaluated within the same study.
The Phase 3 ASCERTAIN study was designed to assess the safety, tolerability, pharmacokinetics (PK) and pharmacodynamics (PD) of sarilumab administered s.c. and tocilizumab administered i.v. in a population of patients who had an inadequate response to a prior TNF inhibitor and were receiving conventional synthetic DMARD (csDMARD) background therapy. The Phase 1b Study 1309 was designed to assess the PK and PD, as well as the safety and tolerability, of a single dose of sarilumab s.c. or tocilizumab i.v. in patients with RA who were receiving background MTX. The safety and tolerability assessments of the single-dose study complement the data from the multiple-dose ASCERTAIN study, and together allow for a better understanding of the overall safety profiles of the anti-IL-6R monoclonal antibodies sarilumab and tocilizumab. These studies were designed to report multiple- and single-dose safety and tolerability of sarilumab administered s.c. and of tocilizumab administered i.v. in adults with RA. No inferential statistics were planned.
Methods
Study design
ASCERTAIN (NCT01768572) was an international, multicentre, 24-week, randomized, double-blind, double-dummy, parallel-group, three-arm study assessing the safety and tolerability of sarilumab and tocilizumab in patients with RA and inadequate response to or intolerance of TNF inhibitor (TNF-IR) receiving background csDMARDs. The primary objective of ASCERTAIN was to assess the safety and tolerability of sarilumab and tocilizumab in TNF-IR patients with RA. Patients were randomized 1: 1: 2 to sarilumab 150 or 200 mg every 2 weeks (q2w) s.c., or tocilizumab 4 mg/kg every 4 weeks (q4w) i.v., which could be increased to 8 mg/kg if clinically indicated, consistent with US prescribing information at the time of the study (October 2012) [6]. Patients were also allowed to decrease the dose of tocilizumab from 8 to 4 mg/kg during the course of the study at the discretion of the investigator. Dose adjustments were not allowed for sarilumab. Safety assessments included incidences of treatment-emergent adverse events (TEAEs), adverse events (AEs) of special interest, serious AEs, and potentially clinically significant laboratory abnormalities. During the study, patients who experienced AEs and prespecified laboratory abnormalities [e.g. decreased absolute neutrophil count (ANC), decreased platelet count, elevated alanine aminotransferase (ALT)] could temporarily or permanently discontinue the study drug in accordance with protocol requirements. Laboratory assessments were made at predetermined time points throughout the study (e.g. complete blood count every 2 weeks).
Study 1309 was a multicentre, 6-week, open-label, parallel-group, single-dose safety, PK and PD study of sarilumab and tocilizumab in patients with RA receiving a stable background of MTX. Patients were randomized 1: 1: 1: 1 to sarilumab 150 mg s.c., sarilumab 200 mg s.c., tocilizumab 4 mg/kg i.v. or tocilizumab 8 mg/kg i.v. The primary objective of the study was to describe the PD of ANC, CRP, IL-6 and soluble IL-6R after a single dose of sarilumab s.c. or tocilizumab i.v. in patients with RA receiving a stable dose of MTX. The PK, safety and tolerability of a single dose of active treatment were secondary objectives. Changes in ANC were considered as both a safety parameter and a PD marker as they provide an indication of immediate effects related to circulating drug concentrations. Detailed analyses of all PD markers, PK parameters and the PK/PD relationship will be discussed in a separate article. Laboratory and safety assessments were performed predose and 1, 4 and 8 h after study drug administration on day 1; daily through day 7; every 2 days through day 15; and on days 19, 22, 29 and 43.
Neither ASCERTAIN nor Study 1309 included an s.c. administration arm for tocilizumab as tocilizumab had not been approved for s.c. administration when the studies were designed.
ASCERTAIN and Study 1309 were conducted in accordance with the principles laid down in the Declaration of Helsinki and the International Conference on Harmonisation guidelines for Good Clinical Practice. All protocols and amendments were submitted to independent ethics committees and/or institutional review boards for review and written approval (see Acknowledgements for details). Informed consent was obtained from all patients before the conduct of any study-related procedures.
Patient population
Eligible patients in both studies were ≥18 years of age with an RA diagnosis for ≥3 months as determined by the 2010 revised ACR criteria [11] as well as functional class I–III as categorized by the 1991 revised ACR criteria [12]. All patients had continuous treatment with one or a combination of csDMARDs (ASCERTAIN) or with MTX 10–25 mg/week (Study 1309) for ≥12 consecutive weeks before screening and were on a stable dose for ≥6 (ASCERTAIN) or ≥8 consecutive weeks (Study 1309). Key exclusion criteria included a history of severe systemic RA (e.g. vasculitis, pulmonary fibrosis); juvenile idiopathic arthritis or onset of arthritis before age 16; and past or current autoimmune, inflammatory systemic or localized joint disease other than RA (Supplementary Table S1, available at Rheumatology online).
Statistical analysis
There were no formal hypotheses being tested in these studies and therefore no formal sample-size power calculations. The sample size for these safety studies was based on practical considerations and clinical judgement. In both studies, all randomized patients who received any study drug were included in the analysis and analysed according to the treatment received. Data were analysed using descriptive statistics, and no formal statistical comparisons were performed between groups.
Results
Patient demographic and baseline characteristics
Patients enrolled in ASCERTAIN and Study 1309 were predominantly female (>80% for both studies), with a mean age of 52 and 55 years, respectively (Table 1). ASCERTAIN included patients from the USA, South America, Western Europe, Eastern Europe and Russia (Supplementary Table S2, available at Rheumatology online). All patients in Study 1309 were from the USA. Baseline disease characteristics were generally well balanced among treatment groups in ASCERTAIN; while imbalances in disease characteristics were noted in Study 1309, these were not expected to affect end point analyses in this single-dose study. The most common recorded medical history terms were hypertension (32.2%), cholecystectomy (10.9%), hypothyroidism (10.4%) and tonsillectomy (10.4%) in ASCERTAIN, and postmenopause (49.5%), hypertension (47.5%) and gastrooesophageal reflux disease (24.8%) in Study 1309.
Table 1.
Demographics and baseline characteristics in the multiple-dose, 24-week ASCERTAIN study and single-dose, 6-week Study 1309
| Characteristic | ASCERTAIN | Study 1309 | |||||
|---|---|---|---|---|---|---|---|
| Tocilizumab q4w i.v. + csDMARDs (n = 102)a | Sarilumab 150 mg q2w s.c. + csDMARDs (n = 49) | Sarilumab 200 mg q2w s.c. + csDMARDs (n = 51) | Tocilizumab 4 mg/kg i.v. + MTX (n = 25) | Tocilizumab 8 mg/kg i.v. + MTX (n = 24) | Sarilumab 150 mg s.c. + MTX (n = 26) | Sarilumab 200 mg s.c. + MTX (n = 26) | |
| Age, mean (s.d.), years | 50.4 (13.0) | 54.8 (12.1) | 51.7 (13.1) | 52 (14.0) | 53 (11.4) | 58 (11.1) | 58 (9.2) |
| Female, n (%) | 82 (80.4) | 41 (83.7) | 39 (76.5) | 22 (88.0) | 23 (95.8) | 20 (76.9) | 22 (84.6) |
| Race, white, n (%) | 94 (92.2) | 47 (95.9) | 46 (90.2) | 21 (84.0) | 20 (83.3) | 22 (84.6) | 21 (80.8) |
| Weight, mean (s.d.), kg | 73.2 (15.4) | 72.6 (17.6) | 77.2 (20.6) | 82.3 (18.8) | 83.4 (18.2) | 82.6 (18.0) | 83.2 (16.9) |
| BMI, mean (s.d.), kg/m2 | 27.3 (5.2) | 27.3 (5.7) | 28.1 (6.6) | 31.6 (6.1) | 30.8 (6.6) | 31.6 (6.8) | 31.1 (6.9) |
| Duration of RA, mean (s.d.), years | 10.8 (8.9) | 13.6 (8.2) | 10.5 (7.6) | 7.2 (8.7) | 12.8 (11.0) | 11.7 (14.0) | 10.7 (11.8) |
| Prior biologic use, n (%) | 102 (100) | 49 (100) | 51 (100) | 2 (8.0) | 7 (29.2) | 4 (15.4) | 3 (11.5) |
| Rheumatoid factor positive, n (%)b | 79 (78.2) | 39 (83.0) | 29 (58.0) | 11 (44.0) | 14 (58.3) | 10 (38.5) | 20 (76.9) |
| ACCPA positive, n (%)b | 82 (84.5) | 41 (87.2) | 36 (70.6) | 10 (40.0) | 11 (45.8) | 14 (53.8) | 18 (69.2) |
| CRP, mean (s.d.) | 24.9 (30.4) | 23.1 (32.1) | 23.8 (29.8) | 7.1 (7.7) | 12.4 (14.1) | 13.1 (18.7) | 8.9 (8.5) |
| Tender joint count (0–68), mean (s.d.) | 23.5 (12.2) | 23.9 (13.0) | 24.7 (12.8) | ||||
| Swollen joint count (0–66), mean (s.d.) | 15.2 (7.6) | 16.0 (8.9) | 16.0 (8.1) | Not assessed | |||
| HAQ-DI, mean (s.d.) | 1.78 (0.63) | 1.63 (0.66) | 1.71 (0.60) | ||||
| DAS28-CRP, mean (s.d.) | 5.91 (1.01) | 5.85 (0.92) | 5.88 (0.97) | ||||
aTocilizumab q4w i.v. starting at 4 mg/kg could be increased to 8 mg/kg based on clinical response as assessed by the investigator. bDenominator for percentage is number with assessment. ACCPA: anti-cyclic citrullinated peptide autoantibody; csDMARD: conventional synthetic DMARD; HAQ-DI: HAQ-Disability Index; q2w: every 2 weeks; q4w: every 4 weeks.
Patient disposition
In ASCERTAIN, 202 patients were randomized (tocilizumab 4/8 mg/kg q4w i.v., n = 102; sarilumab 150 mg q2w s.c., n = 49; sarilumab 200 mg q2w s.c., n = 51). While dose adjustments were not allowed for sarilumab, 62 of 102 patients (60.8%) randomized to tocilizumab 4 mg/kg q4w in ASCERTAIN increased their dose from 4 to 8 mg/kg q4w at least once during the treatment period (Supplementary Fig. S1, available at Rheumatology online); 42 patients (41.2%) increased dose from 4 to 8 mg/kg q4w at week 4. Of the 62 patients who increased their tocilizumab dose to 8 mg/kg q4w, 39 patients who increased dose at week 4 remained at 8 mg/kg q4w for the remainder of the study (identified as the tocilizumab 4/8 mg/kg subset). The other 23 patients were up-titrated to the 8 mg/kg q4w dose at another time (n = 19) and/or did not remain at the 8 mg/kg q4w dose for the duration of the study as they were down-titrated to tocilizumab 4 mg/kg q4w primarily because of adverse safety findings (n = 4). In Study 1309, 101 patients were randomized and treated with single doses of tocilizumab 4 mg/kg i.v. (n = 25), tocilizumab 8 mg/kg i.v. (n = 24), sarilumab 150 mg s.c. (n = 26) or sarilumab 200 mg s.c. (n = 26). In both studies, all patients received concomitant csDMARDs (Supplementary Table S3, available at Rheumatology online).
Safety
Adverse events
Within each study, the incidence of TEAEs was similar between the sarilumab and tocilizumab groups (Table 2). There were few serious AEs in either study, with no discernible patterns. In ASCERTAIN, there were two serious infections in the tocilizumab group and one in the sarilumab 200 mg q2w group; no serious infections were reported in Study 1309. No opportunistic infections were reported in either study. In ASCERTAIN, the most frequently reported TEAEs were neutropenia, injection-site erythema and nasopharyngitis in the sarilumab groups and accidental overdose, upper respiratory tract infection and nausea in the tocilizumab group (Table 3).
Table 2.
Overview of treatment-emergent AEs in the multiple-dose, 24-week ASCERTAIN study and single-dose, 6-week Study 1309
| ASCERTAIN | Study 1309 | ||||||
|---|---|---|---|---|---|---|---|
| AE | Tocilizumab q4w i.v. + csDMARDs (n = 102)a | Sarilumab 150 mg q2w s.c. + csDMARDs (n = 49) | Sarilumab 200 mg q2w s.c. + csDMARDs (n = 51) | Tocilizumab 4 mg/kg i.v. + MTX (n = 25) | Tocilizumab 8 mg/kg i.v. + MTX (n = 24) | Sarilumab 150 mg s.c. + MTX (n = 26) | Sarilumab 200 mg s.c. + MTX (n = 26) |
| AE | 68 (66.7) | 33 (67.3) | 36 (70.6) | 8 (32.0) | 12 (50.0) | 10 (38.5) | 12 (46.2) |
| SAE | 7 (6.9) | 1 (2.0) | 3 (5.9) | 0 | 1 (4.2) | 0 | 0 |
| Serious infection | 2 (2.0) | 0 | 1 (2.0) | 0 | 0 | 0 | 0 |
| AE leading to death | 1 (1.0) | 0 | 0 | 0 | 0 | 0 | 0 |
| AE leading to treatment discontinuation | 4 (3.9) | 6 (12.2) | 8 (15.7) | 0 | 0 | 0 | 0 |
Values are n (%). aTocilizumab q4w i.v. starting at 4 mg/kg could be increased to 8 mg/kg based on clinical response as assessed by the investigator. AE: adverse event; csDMARD: conventional synthetic DMARD; q2w: every 2 weeks; q4w: every 4 weeks; SAE: serious AE.
Table 3.
Frequently reported treatment-emergent AEs in ASCERTAINa
| AE | Tocilizumab q4w i.v. + csDMARDs (n = 102)b | Sarilumab 150 mg q2w s.c. + csDMARDs (n = 49) | Sarilumab 200 mg q2w s.c. + csDMARDs (n = 51) |
|---|---|---|---|
| Infection | 32 (31.4) | 20 (40.8) | 11 (21.6) |
| Nasopharyngitis | 4 (3.9) | 6 (12.2) | 3 (5.9) |
| Urinary tract infection | 6 (5.9) | 4 (8.2) | 2 (3.9) |
| Upper respiratory tract infection | 7 (6.9) | 2 (4.1) | 1 (2.0) |
| Gastroenteritis | 1 (1.0) | 3 (6.1) | 0 |
| Neutropenia | 4 (3.9) | 6 (12.2) | 8 (15.7) |
| Injection-site erythema | 1 (1.0) | 4 (8.2) | 4 (7.8) |
| Accidental overdosec | 9 (8.8) | 1 (2.0) | 3 (5.9) |
| Dizziness | 4 (3.9) | 1 (2.0) | 3 (5.9) |
| Hypercholesterolaemia | 6 (5.9) | 2 (4.1) | 1 (2.0) |
| Nausea | 7 (6.9) | 1 (2.0) | 1 (2.0) |
| RA | 6 (5.9) | 1 (2.0) | 0 |
Values are n (%). aAt least 5% in any treatment group. bTocilizumab q4w i.v. starting at 4 mg/kg could be increased to 8 mg/kg based on clinical response as assessed by the investigator. cAn overdose was defined as the administration of ≥2 sarilumab doses in <11 calendar days or ≥2 tocilizumab doses in <21 calendar days or at least twice of the intended dose within the intended therapeutic interval for sarilumab or tocilizumab. AE: adverse event; csDMARD: conventional synthetic DMARD; q2w: every 2 weeks; q4w: every 4 weeks.
In ASCERTAIN, a numerically higher incidence of TEAEs leading to treatment discontinuation was observed with sarilumab [150 mg q2w, n = 6 (12.2%); 200 mg q2w, n = 8 (15.7%)] than with tocilizumab [n = 4 (3.9%)]. Five patients who received sarilumab discontinued because of laboratory abnormalities (neutropenia, leucopaenia and increased transaminases), which met protocol-defined criteria for discontinuation; none of these laboratory changes were associated with clinical manifestations. Three of these five patients had resumed their s.c. sarilumab injections but were discontinued because their i.v. infusion (placebo for tocilizumab) could not be resumed within the protocol-specified window. No discontinuations due to laboratory abnormalities were reported in the tocilizumab group. Two patients in each of the sarilumab groups and one patient in the tocilizumab group discontinued because of infections. Two patients who received sarilumab 200 mg q2w discontinued because of injection-site reactions and one patient who received sarilumab 200 mg q2w discontinued because of an infusion-related reaction while receiving i.v. placebo. No clinical patterns were observed for the remaining events that led to discontinuation of sarilumab. Two patients discontinued tocilizumab while receiving 8 mg/kg q4w because of worsening RA (n = 1) and acute renal failure (n = 1), and one patient discontinued after one dose of tocilizumab 4 mg/kg because of tremors. There was one death, which was due to septic shock and occurred after one dose of tocilizumab 4 mg/kg. The patient, a 64-year-old woman with history of prior abdominal exploratory laparotomy in 2005 (reason unknown) began to experience bloody diarrhoea and intense abdominal pain 18 days after receiving a single dose of tocilizumab. The patient died the following day despite treatment with antibiotics, vasopressors and mechanical ventilation. ANC 3 days before the event was 4.75 giga/l (baseline 5.33 giga/l). Concomitant medication included prednisone (7.5 mg oral) and naproxen (500 mg oral). The medical report indicted bowel infarction, and the death was adjudicated to be due to septic shock in the context of most probable mesenteric infarction.
In Study 1309, the rates of TEAEs were comparable in the sarilumab and tocilizumab groups and were numerically higher for the higher doses compared with the lower doses of sarilumab (200 mg, 46.2%; 150 mg, 38.5%) and tocilizumab (8 mg/kg, 50.0%; 4 mg/kg, 32.0%). TEAEs occurring in >1 patient in any treatment group were neutropenia, upper respiratory tract infection, headache, thrombocytopenia and RA (Supplementary Table S4, available at Rheumatology online). One patient in the tocilizumab 8 mg/kg group had a serious AE (hip fracture), which was not considered treatment related. No deaths were reported during the study, and no patients in any treatment group discontinued because of an AE.
Laboratory changes
Laboratory changes in both ASCERTAIN and Study 1309 were consistent with IL-6R signalling blockade and included decreases in neutrophil and platelet counts and increases in transaminases and lipids (Table 4).
Table 4.
Overview of laboratory changes in the multiple-dose, 24-week ASCERTAIN study and single-dose, 6-week Study 1309
| ASCERTAIN | Study 1309 | ||||||
|---|---|---|---|---|---|---|---|
| Patients | Tocilizumab q4w i.v. + csDMARDs (n = 102)a | Sarilumab 150 mg q2w s.c. + csDMARDs (n = 49) | Sarilumab 200 mg q2w s.c. + csDMARDs (n = 51) | Tocilizumab 4 mg/kg i.v. + MTX (n = 25) | Tocilizumab 8 mg/kg i.v. + MTX (n = 24) | Sarilumab 150 mg s.c. + MTX (n = 26) | Sarilumab 200 mg s.c. + MTX (n = 26) |
| ANCb | |||||||
| 0.5–<1.0, giga/l | 1 (1.0) | 2 (4.2) | 5 (9.8) | 3 (12.0) | 6 (25.0) | 4 (15.4) | 6 (23.1) |
| <0.5, giga/l | 0 | 1 (2.1) | 0 | 0 | 0 | 0 | 1 (3.8) |
| ALTc | |||||||
| >3–5 × ULN | 3 (3.0) | 2 (4.3) | 2 (3.9) | 0 | 1 (4.2) | 0 | 1 (3.8) |
| >5 × ULN | 0 | 0 | 1 (2.0) | 0 | 1 (4.2) | 0 | 0 |
| Platelet countd | |||||||
| <100, giga/l | 0 | 1 (2.1) | 0 | 0 | 0 | 2 (7.7) | 0 |
Values are n (%). aTocilizumab q4w i.v. starting at 4 mg/kg could be increased to 8 mg/kg based on clinical response as assessed by the investigator. bFor ANC in ASCERTAIN, n = 48 for sarilumab 150 s.c.
cFor ALT in ASCERTAIN, n = 101 for tocilizumab q4w i.v. and n = 47 for sarilumab 150 mg q2w s.c. dFor platelets in ASCERTAIN, n = 48 for sarilumab 150 mg q2w s.c. ALT: alanine aminotransferase; ANC: absolute neutrophil count; csDMARD: conventional synthetic DMARD; ULN: upper limit of normal.
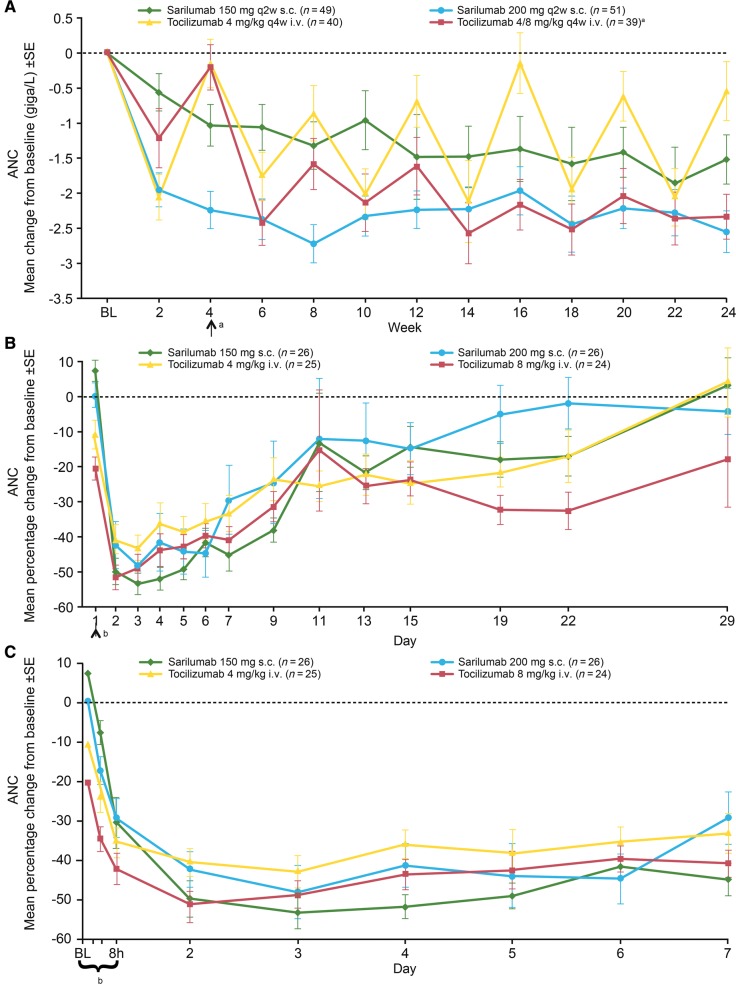
In ASCERTAIN, in which haematology assessments were performed q2w, a numerically higher incidence of ANC <1.0 giga/l was observed with sarilumab administered q2w compared with tocilizumab administered q4w, although the mean change in ANC in the sarilumab groups was within the range observed with tocilizumab (Fig. 1A). At week 24, the mean change in ANC was comparable between patients receiving sarilumab 200 mg q2w and those who increased their tocilizumab dose to 8 mg/kg q4w. There was no evidence of an association between decrease in ANC and increased incidence of infections.
Fig. 1.
Mean change from baseline in ANC ± s.e. by treatment and visit in (A) ASCERTAIN (weeks 1–24), (B) Study 1309 (days 1–29) and (C) Study 1309 (days 1–7)
aPatients in the 4/8 mg/kg q4w i.v. group increased their dose to 8 mg/kg at week 4 and remained on 8 mg/kg for the remainder of the study. bThere were four sampling points on day 1: baseline, 1 h, 4 h and 8 h after dosing. ANC: absolute neutrophil count; BL: baseline; q2w: every 2 weeks; q4w: every 4 weeks.
In Study 1309, a dose-dependent effect on ANC <1.0 giga/l was observed, with a higher incidence reported in the high-dose sarilumab and tocilizumab groups compared with the low-dose groups (Table 4). Over days 1–7, there was no meaningful difference across all treatment groups in the ANC parameters assessed. The time to onset for decreased ANC and magnitude of decrease were similar across the sarilumab and tocilizumab groups for all doses. ANC decreased within a few hours of dosing (Fig. 1B and C). The median time to ANC nadir ranged from 3 to 5 days after administration; mean nadir values ranged from 1.55 to 1.78 giga/l across all treatment groups. The timing of the trend for return to baseline was consistent with the dosing interval for both sarilumab (q2w) and tocilizumab (q4w). By day 15, the approximate time that a second dose of sarilumab would be given in a q2w dosing regimen, ANC values in the sarilumab groups had increased but had not yet returned to baseline. By day 29, the approximate time that a second dose of tocilizumab would be given in a q4w dosing regimen, ANC values had essentially returned to baseline in the tocilizumab 4 mg/kg group but not in the 8 mg/kg group. Decreases in ANC did not appear to be associated with an increased incidence of infections.
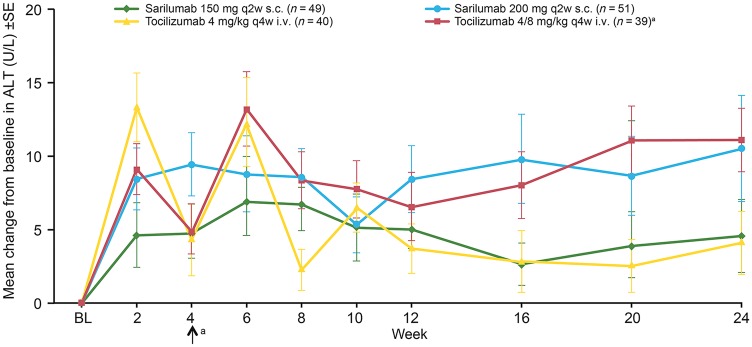
Increased levels of ALT >3× upper limit of normal occurred in few patients in either study. In ASCERTAIN, the mean increase in ALT at week 24 was comparable between the tocilizumab 4/8 mg/kg q4w and sarilumab 200 mg q2w groups, and increases in both groups were slightly greater than in the sarilumab 150 mg q2w group (Fig. 2). Incidence of ALT increases of 3–5× upper limit of normal was comparable among the treatment groups and reversible upon discontinuation of study treatment. Platelet count <100 giga/l was reported in a single patient receiving sarilumab 150 mg q2w in ASCERTAIN and in two patients receiving sarilumab 150 mg in Study 1309; none of the patients experienced bleeding related to decreased platelet count. Incidence of total cholesterol ≥6.2 mmol/l in the sarilumab and tocilizumab groups was generally similar in both studies. In ASCERTAIN, mean increases in low-density lipoprotein cholesterol, high-density lipoprotein cholesterol and triglycerides at week 24 were of similar magnitude in all treatment groups. Lipid-modifying agents (including statins) were initiated on the same day or after the first dose in eight patients (7.8%) in the tocilizumab group, two patients (4.1%) in the sarilumab 150 mg q2w group and two patients (3.9%) in the sarilumab 200 mg q2w group. In Study 1309, small mean increases from baseline in total cholesterol, low-density lipoprotein cholesterol and triglycerides observed at day 15 returned to baseline by day 29 in the sarilumab groups and remained slightly elevated in the tocilizumab groups. There were no notable differences in mean high-density lipoprotein cholesterol values.
Fig. 2.
Mean change from baseline in ALT ± s.e. in ASCERTAIN
aPatients in the 4/8 mg/kg q4w i.v. group increased their dose to 8 mg/kg at week 4 and remained on 8 mg/kg for the remainder of the study. ALT: alanine aminotransferase; BL: baseline; q2w: every 2 weeks; q4w: every 4 weeks.
Discussion
ASCERTAIN and Study 1309 were the first studies in which sarilumab and tocilizumab were evaluated in patients with RA within the same study using multiple and single doses, respectively, providing an opportunity to understand the safety and tolerability of these two agents within the same therapeutic class of IL-6R inhibitors. Overall, no clinically meaningful differences were observed regarding safety between sarilumab and tocilizumab in either study. The incidence and type of TEAEs were similar for the sarilumab and tocilizumab groups in each study.
In ASCERTAIN, the incidence of TEAEs leading to treatment discontinuation was numerically higher with sarilumab than with tocilizumab, but some of these discontinuations were due to protocol-mandated criteria related to laboratory changes; these laboratory changes occurred without clinical manifestations. While most patients in the sarilumab groups who interrupted therapy because of laboratory abnormalities resumed treatment as these abnormalities resolved, three patients were discontinued because the protocol-mandated interval between i.v. infusions had been exceeded. There was no clinical pattern for the non-laboratory TEAEs that led to discontinuation, but the low number of events limits assessment and interpretation of these data.
Upper respiratory tract infections and neutropenia were among the most frequent TEAEs in both studies. Laboratory changes in both studies were consistent with IL-6R inhibition and included increases in lipids and transaminases and decreases in neutrophil counts. Mean changes in ANC in the sarilumab groups were within the ranges observed in the tocilizumab groups.
The association between IL-6 signalling and the PD marker ANC after a single dose of sarilumab or tocilizumab was assessed in Study 1309. This assessment indicated that despite differences in PK profiles due to route of administration and 10-fold lower drug peak concentrations with sarilumab s.c. compared with tocilizumab i.v. (data not shown), similar rapid decreases in ANC (within 4 h), nadir values and median time to nadir values were observed across all four treatment groups. ANC returned towards baseline earlier with sarilumab than with tocilizumab, which is consistent with drug concentration–time profiles. A dose-dependent effect on ANC <1.0 giga/l was observed, with a higher incidence reported in the high-dose sarilumab and tocilizumab groups versus the low-dose groups. Decreased ANC was not associated with an increased incidence of infections.
In ASCERTAIN, tocilizumab was administered i.v. according to US product labelling [6]; patients were treated with 4 mg/kg and the dose was increased at the discretion of the investigator. In the study, 60% of patients received at least one dose of tocilizumab 8 mg/kg. Because of differences in the route of administration (s.c. for sarilumab, i.v. for tocilizumab), PK profiles and dosing intervals (q2w for sarilumab, q4w for tocilizumab) in relation to the sampling schedule (q2w for haematology assessments), numeric differences were observed in the incidence of ANC <1.0 giga/l, a PD marker of IL-6 inhibition, between the sarilumab and tocilizumab groups. Regardless, there was no difference in the rates of infection or serious infection between the tocilizumab and sarilumab groups.
The rapid and similar decrease in ANC with these IL-6R inhibitors is potentially explained by redistribution from the vascular compartment and results in the uptake and removal of circulating neutrophils without affecting their function [13]. In vitro evidence suggests that IL-6 increases circulating neutrophils by releasing them from marginated pools in bone marrow [14]. Thus, an anti-IL-6R agent may potentially reverse this effect. The rapid onset and recovery of the decrease in neutrophil count observed in Study 1309 provides support for this mechanism, and a PD model that implemented neutrophil margination with ANC-specific tolerance describes the mechanism of transient decreases in ANC observed with IL-6R inhibitors [15].
One limitation of ASCERTAIN is that robust characterization of the neutrophil kinetics (including identification of the nadir) after multiple doses of sarilumab and tocilizumab, as was done in Study 1309 after single-dose administration, could not be performed because of less frequent PK and PD sampling. Therefore, Study 1309 provides valuable insight into the similarities in changes in neutrophil parameters that occur with the two IL-6R inhibitors.
In summary, IL-6R inhibition leads to transient decreases in ANC, likely due to margination of neutrophils from the circulation where the neutrophils remain present and maintain their functionality if triggered. This phenomenon of margination is further supported by the fact that no clinically meaningful consequence of the decrease in ANC by IL-6R inhibitors has been identified.
Conclusions
Overall, no clinically meaningful differences were observed with regard to safety between sarilumab and tocilizumab in either study, including the incidence and types of TEAEs. Laboratory changes (e.g. decrease in ANC and increase in ALT) noted in the sarilumab groups were within the same range as those noted in the tocilizumab groups. Although numeric differences in incidence of ANC <1.0 giga/l between the sarilumab and tocilizumab groups were observed in ASCERTAIN, considering the results of Study 1309, this most likely reflects differences in PK (related to route of administration; s.c. for sarilumab and i.v. for tocilizumab) and dosing interval (q2w for sarilumab versus q4w for tocilizumab) in relation to the sampling schedule (q2w). Decreased ANC was not associated with an increased incidence of infection.
Supplementary Material
Acknowledgements
The authors thank the patients who participated in this study, the co-investigators for their contribution to the study, and the staff at the participating centres. The authors thank Christine Xu, PhD, Sanofi Genzyme, and Xiaoping Zhu, Regeneron, for critical review of the data and manuscript. Editorial assistance was provided under the direction of the authors by William S. Turner, PhD, Todd Parker, PhD, and Jennifer Rossi, MA, ELS, MedThink SciCom, and by Adelphi Communications Ltd, and funded by Sanofi Genzyme and Regeneron Pharmaceuticals, Inc.
The specific ethical bodies that approved this study were: CIE Para Ensayos en Farmacologia Clínica Dr Luis M. Zieher Jose E. Uriburu, Buenos Aires, Argentina; Dim Clínica Privada Belgrano 136, Buenos Aires, Argentina; Comité de Ética CAICICIAP Rodriguez 1198, Santa Fe, Argentina; Comité de Ética San Isidro Av. Libertador 16958, Buenos Aires, Argentina; Commissie voor Medische Ethiek/Klinisch Onderzoek, Leuven, Belgium; Ethics Committee of Hospital das Clinicas da Univ. Federal do Parana Rua General Carneiro, Parana, Brazil; Comissão Nacional de Ética em Pesquisa Unidade II – Ministério da Saúde, Brasilia, Distrito Federal, Brazil; Ethics Committee of Faculdade de Medicina de São Jose do Rio Preto Av. Brigadeiro Faria Lima, São Paulo, Brazil; Eticka Komise Krajska Nemocnice Liberec, Liberec, Czech Republic; Eticka Komise Revmatologickeho Ustavu, Czech Republic; Tallinn Medical Research Ethics Committee, Tallinn, Estonia; HUS Medisiininen Eettinen Toimikunta Biomedicum, Helsinki, Finland; Klin. Pharm. Etikai Bizottság, Budapest, Hungary; Ethics Committee of Rambam Medical Center, Haifa, Israel; Ethics Committee of Tel Aviv Sourasky Medical Center, Tel Aviv, Israel; Ethics Committee of Assaf Harofe Medical Center, Tzrifin, Israel; Azienda Ospedaliera Universitaria Careggi Comitato Etico Area, Firenze, Italy; Comitato Etico Regionale SEZ.N.2 Sperimentazione Dei Medicinali, Genova, Italy; Comitato Etico Ospedale San Martino, Genova, Italy; Sperimentazione Az. Osp.L. Sacco di Milano, Milano, Italy; Comité Bioética Para la Investigación Clínica S.C., México, D.F., Mexico; Comision de Investigación Ética y Bioseguridad, México D.F., Mexico; Comité de Ética en Investigación de Grupo Calyde S.C.P., Mérida, Yucatán, Mexico; Comité de Ética e Investigación del Hospital Aranda de la Parra SA de CV Hidalgo, León, Guanajuato, Mexico; Comité de Ética en Investigación del Hospital Civil de Guadalajara Fray, Guadalajara, Jalisco, Mexico; METC voor het Slotervaartziekenhuis en Reade, Amsterdam, The Netherlands; Regional Komité for Medisinsk og Helsefaglig forskningsetikk REK, Oslo, Norway; Komisja Bioetyczna przy Akademii Medycznej, Lubelskie, Poland; National Ethics Committee for Clinical Trial of the Medicine, Bucharest, Romania; Ethics Board at Ministry of Health of the Russian Federation, Moscow, Russian Federation; LEC of the Kemerovo State Medical Academy, Kemerovo, Russian Federation; LEC of Applied Medicine, Moscow, Russian Federation; LEC City Clinical Moscow Botkin Ho, Moscow, Russian Federation; LEC of the City Clinical Hospital 71, Moscow, Russian Federation; LEC of Ava-Peter LLC, St Petersburg, Russian Federation; CEIC Hospital Vall d’Hebron, Barcelona, Spain; Regionala Etikprövningsnämnden I, Stockholm, Sweden; South East Coast – Brighton and Sussex REC NRES Committee London – West London National Research Ethics Service, London, UK; Compass IRB, Mesa, AZ, USA; NYU Langone Medical Center, NY, NY, USA; and Partners Human Research Committee, Boston, MA, USA.
P.E., Y.L. and H.vH. contributed to the conception of the study. P.E., J.P., Y.L. and H.vH. contributed to the design of the study. P.E., J.R. and A.S. contributed to the acquisition of data. N.L. and R.W. contributed to the analysis of data. All authors contributed to the interpretation of data. P.E., J.P., Y.L. and C.P.-R. contributed to the drafting of the manuscript. All authors critically revised the manuscript for important intellectual content and approved the manuscript for publication.
Funding: This study was sponsored by Sanofi Genzyme and Regeneron Pharmaceuticals, Inc.
Disclosure statement: P.E. has received research grants and consulting fees from Abbott, AbbVie, Bristol-Myers Squibb, Pfizer, UCB, Merck Sharp & Dohme, Roche, Novartis, Samsung, Takeda, Eli Lilly, Sanofi and Regeneron Pharmaceuticals, Inc. J.P., N.M.H.G., A.P. and R.W. are employees of Regeneron Pharmaceuticals, Inc., and may hold stock and/or stock options in the company. Y.L., C.P.-R., H.vH. and N.L. are employees of Sanofi Genzyme and may hold stock and/or stock options in the company. The other authors have declared no conflicts of interest.
References
- 1. Choy E. Understanding the dynamics: pathways involved in the pathogenesis of rheumatoid arthritis. Rheumatology (Oxford) 2012;51:v3–11. [DOI] [PubMed] [Google Scholar]
- 2. Srirangan S, Choy EH. The role of interleukin 6 in the pathophysiology of rheumatoid arthritis. Ther Adv Musculoskelet Dis 2010;2:247–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yoshida Y, Tanaka T. Interleukin 6 and rheumatoid arthritis. Biomed Res Int 2014;2014:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Smolen JS, Weinblatt ME, Sheng S, Zhuang Y, Hsu B. Sirukumab, a human anti-interleukin-6 monoclonal antibody: a randomised, 2-part (proof-of-concept and dose-finding), phase II study in patients with active rheumatoid arthritis despite methotrexate therapy. Ann Rheum Dis 2014;73:1616–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weinblatt ME, Mease P, Mysler E et al. . The efficacy and safety of subcutaneous clazakizumab in patients with moderate-to-severe rheumatoid arthritis and an inadequate response to methotrexate: results from a multinational, phase IIb, randomized, double-blind, placebo/active-controlled, dose-ranging study. Arthritis Rheumatol 2015;67:2591–600. [DOI] [PubMed] [Google Scholar]
- 6. Actemra (tocilizumab) injection for intravenous use injection, for subcutaneous use [package insert]. South San Francisco, CA, USA: Genentech, Inc., 2012. [Google Scholar]
- 7. Dörner T, Weinblatt M, Beneden KV et al. . FRI0239 Results of a phase 2b study of vobarilizumab, an anti-interleukin-6 receptor nanobody, as monotherapy in patients with moderate to severe rheumatoid arthritis. Ann Rheum Dis 2017;76:575. [Google Scholar]
- 8. KEVZARA [package insert]. Bridgewater, NJ, USA: Sanofi-aventis US LLC, 2017. [Google Scholar]
- 9. Genovese MC, Fleischmann R, Kivitz AJ et al. . Sarilumab plus methotrexate in patients with active rheumatoid arthritis and inadequate response to methotrexate: results of a Phase III study. Arthritis Rheum 2015;67:1424–37. [DOI] [PubMed] [Google Scholar]
- 10. Genovese MC, McKay JD, Nasonov EL et al. . Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: the tocilizumab in combination with traditional disease-modifying antirheumatic drug therapy study. Arthritis Rheum 2008;58:2968–80. [DOI] [PubMed] [Google Scholar]
- 11. Aletaha D, Neogi T, Silman AJ et al. . 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 2010;69:1580–8. [DOI] [PubMed] [Google Scholar]
- 12. Hochberg MC, Chang RW, Dwosh I et al. . The American College of Rheumatology 1991 revised criteria for the classification of global functional status in rheumatoid arthritis. Arthritis Rheum 1992;35:498–502. [DOI] [PubMed] [Google Scholar]
- 13. Summers C, Rankin SM, Condliffe AM et al. . Neutrophil kinetics in health and disease. Trends Immunol 2010;31:318–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gibiansky L, Frey N. Linking interleukin-6 receptor blockade with tocilizumab and its hematological effects using a modeling approach. J Pharmacokinet Pharmacodyn 2012;39:5–16. [DOI] [PubMed] [Google Scholar]
- 15. Kovalenko P, Paccaly A, Boyapati A, Xu C, Davis JD, DiCioccio AT. Pharmacodynamic (PD) model of neutrophil margination to describe transient effect of sarilumab on absolute neutrophil count (ANC) in patients with RA after single-dose administration. Abstracts of the Annual Meeting of the Population Approach Group in Europe, PAGE 26, 2017, Abstr 7284. https://www.page-meeting.org/default.asp?abstract=7284 (26 November 2018, date last accessed). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.