Abstract
Objective
To evaluate the effect of secukinumab, a fully human anti-interleukin-17A monoclonal antibody, on efficacy, imaging outcomes, and safety through 4 years (208 weeks) in patients with ankylosing spondylitis.
Methods
Patients opting to enrol had completed 2 years’ treatment in the MEASURE 1 core study with subcutaneous secukinumab 150 or 75 mg every 4 weeks (q4Wk), following intravenous loading to Week (Wk) 4, or placebo treatment to Wk16/24. Up-titration from secukinumab 75–150 mg q4Wk was permitted following a protocol amendment. Efficacy is reported for patients originally randomized to secukinumab. Radiographic changes were assessed using the modified Stoke Ankylosing Spondylitis Spinal Score (mSASSS) and changes in MRI measures of inflammation using the Berlin scoring method. Safety and tolerability were evaluated.
Results
Among 274 extension study participants, 89.7% (78/87) and 93.0% (93/100) originally randomized to secukinumab 150 and 75 mg, respectively, completed 208Wk. Through Wk208, Assessment of Spondyloarthritis International Society 20/40 (observed) were 79.7%/60.8% (150 mg), 71.0%/43.5% (75 mg) and 80.0%/76% (up-titrators; n = 25). Mean (s.d.) changes in mSASSS were 1.2 (3.91) (150 mg), 1.8 (4.32) (75 mg) and 1.6 (5.67) (up-titrators). No radiographic progression (mSASSS change from Baseline < 2) was observed in 79% of patients receiving either secukinumab dose. Exposure-adjusted incidence rates per 100 patient-years were: serious infections (1.0), Candida infections (0.4), Crohn’s disease (0.6), ulcerative colitis (0.2), and malignant/unspecified tumours (0.5), with no new safety signals.
Conclusion
Through 4 years, secukinumab provided sustained efficacy on signs and symptoms, and MRI outcomes, a low rate of radiographic progression and a consistent safety profile.
Trial registration
Keywords: ankylosing spondylitis, secukinumab, MEASURE 1, radiographic progression
Rheumatology key messages
First study reporting longer-term effect of secukinumab on radiographic structural progression in ankylosing spondylitis.
Secukinumab 150 mg demonstrated low radiographic progression through 4 years.
Secukinumab 150 mg demonstrated sustained efficacy with a consistent and favourable safety profile through 4 years.
Introduction
AS is a chronic inflammatory disease that primarily affects the axial skeleton, manifesting as inflammatory back pain and stiffness [1]. AS can be associated with progressive irreversible structural damage, which occurs as a result of syndesmophyte formation and ankylosis of the vertebral column [2]. Several risk factors, including smoking, gender, elevated levels of high-sensitivity CRP (hsCRP), and radiographic damage at baseline positively influence the pathologic formation of new bone in the spinal joints of patients with AS [3–5]. Patients with AS experience reduced physical function and quality of life because of these chronic structural changes [6, 7]. The primary goals of AS treatment include maintaining patients’ long-term health-related quality of life and functional status through the control of symptoms and inflammation, as well as the prevention of progressive structural damage [8, 9].
Secukinumab, a fully human anti-interleukin IL-17A monoclonal antibody, demonstrated sustained improvements in the signs and symptoms of AS through 2 years in two Phase 3 studies (MEASURE 1: NCT01358175 and MEASURE 2: NCT01649375), and a low rate of structural radiographic progression in MEASURE 1 [10, 11]. Furthermore, results of the ongoing 3-year extension (NCT01863732) to the 2-year MEASURE 1 core trial (i.e. 5 years total) has provided longer-term data demonstrating sustained efficacy through a total 3 years of treatment [12]. Here, we present an update on the efficacy and safety of secukinumab from the open-label extension of the MEASURE 1 trial through 4 years (208Wk) in patients with AS, including the results of radiographic and MRI analyses.
Methods
Study design and participants
The study design and patient eligibility criteria of this 3-year extension of the MEASURE 1 core trial have been described previously (Supplementary Fig. S1, available at Rheumatology online) [12, 13]. Briefly, subjects ≥18 years with AS fulfilling the modified New York Criteria, and active disease as indicated by a BASDAI score ≥4 [14], and a spinal pain score ≥4 cm (on a 0–10 cm scale) despite prior treatment with NSAIDs were included. Subjects were anti-TNF naïve or anti-TNF IR (i.e. had experienced an inadequate response or stopped treatment for safety/tolerability reasons).
Patients randomized to secukinumab received a 10 mg/kg intravenous (IV) loading dose at baseline, Wk2 and Wk4, and then subcutaneous injections of 150 mg (IV→150 mg) or 75 mg (IV→75 mg) q4Wk from Wk8. The same IV-to-subcutaneous schedule was administered for subjects on placebo up to Wk16 (non-responders) or Wk24 (responders), when subjects were switched to secukinumab, as previously described [13]. Following a protocol amendment, subjects on secukinumab 75 mg not achieving sufficient therapeutic response could be up titrated to 150 mg at investigators’ discretion.
Subjects completing the 2-year core trial were invited to continue their treatment in the open-label 3-year extension, which was conducted in accordance with the principles of the Declaration of Helsinki. All centres received approval from independent ethics committees or institutional review boards. Patients provided written informed consent before starting the study-related procedures. The names of independent ethics committees or institutional review boards that approved the study and the associated approval numbers are listed in Supplementary Table S6, available at Rheumatology online.
Endpoints
Assessments of clinical efficacy and quality of life included Assessment of Spondyloarthritis International Society (ASAS) 20/40 response, ASAS5/6, Ankylosing Spondylitis Disease Activity Score inactive disease, BASDAI, BASMI, BASFI, short form-36 physical component summary and ASAS partial remission.
Lateral radiographs of the cervical and lumbar spine were assessed using the modified Stoke Ankylosing Spondylitis Spinal Score (mSASSS, range 0–72, with higher scores indicating greater structural damage) [15], as previously described [10]. MRI of the SI joints and spine was used to investigate the effect of secukinumab on objective signs of inflammation in a subset of anti-TNF-naïve subjects at selected study centres; MRIs were assessed using the Berlin SI joint total oedema score and the Berlin spine score (derived from ASspi-MRI-a results). Radiographs and MRIs obtained at baseline and Wk104 were re-read for patients completing 208Wk of treatment. All imaging data were evaluated by two experienced central readers, blinded to treatment and visit, and mean scores were analysed. The proportion of patients who achieved MRI remission (Berlin spine score of ≤2) up to Wk208 was investigated in a subset of anti-TNF-naïve patients with baseline Berlin spine score of >2 and at least one post-baseline Berlin spine score at Wk156 or 208 [10].
The safety and tolerability of secukinumab were evaluated through Wk208 via assessment of treatment-emergent adverse events (AEs) and serious AEs (SAEs).
Statistical analyses
Clinical efficacy analyses at Wk208 were reported as observed for subjects originally randomized at baseline to secukinumab 150 or 75 mg, with data for the 75 mg group stratified by up-titration status.
Predefined hierarchical variables with secukinumab 150 mg were also analysed using multiple imputation to account for missing data for binary efficacy variables, and mixed-model for repeated measures for continuous variables. Visit and anti-TNF status (naïve or IR) were included in the mixed-model for repeated measures as fixed factors, weight and baseline score as covariates, and visit by baseline score as an interaction term. Multiple imputation or mixed-model for repeated measures were not done for the 75 mg arm because the assumption of missing at random was violated in subjects who up-titrated to secukinumab 150 mg. For patients who discontinued treatment during Wk108–156, the end of treatment visit (i.e. the final assessment 4Wk after last study treatment) was considered as Wk156. For patients who discontinued during Wk160–208, the end of treatment visit was considered as Wk208. Pre-specified analyses in anti-TNF naïve and IR subjects were reported as observed in the secukinumab treatment groups to which subjects were originally randomized.
Imaging data (radiographic/MRI) were reported as observed at each time point assessed. In analyses of the change in mSASSS between timepoints, the data capture window for Wk104 X-rays was extended from 31–743 days to 31–849 days to include 10 and 14 additional patients in the secukinumab 150 and 75 mg groups, respectively. Change from baseline in mSASSS was stratified by baseline demographic and disease characteristics including hsCRP, gender, presence of syndesmophytes and smoking status. Proportion of non-progressors (i.e. subjects achieving change in mSASSS from baseline <2) was also reported.
Safety analyses were reported for all subjects receiving ≥1 dose of secukinumab at any time during the core trial or extension, including those randomized at baseline to secukinumab or placebo. Patients who up-titrated were assessed according to the treatment received at the time of the AE.
Results
Patients
Of the 274 subjects enrolled in this extension study, 89.7% (78/87) and 93.0% (93/100) originally assigned to secukinumab 150 and 75 mg, respectively, completed 208Wk of treatment. Remaining subjects were re-randomized from placebo to either secukinumab dose and were included in safety but not efficacy analyses. 25 subjects originally randomized to secukinumab 75 mg up-titrated to 150 mg at various timepoints from Wk168.
The majority of discontinuations with secukinumab 150 mg (7/9 [77.8%]) and 75 mg (3/7 [42.9%]) were attributed to subject/guardian decision (Supplementary Table S1, available at Rheumatology online). Demographic and baseline characteristics of the extension trial population have been reported previously [12].
Clinical efficacy
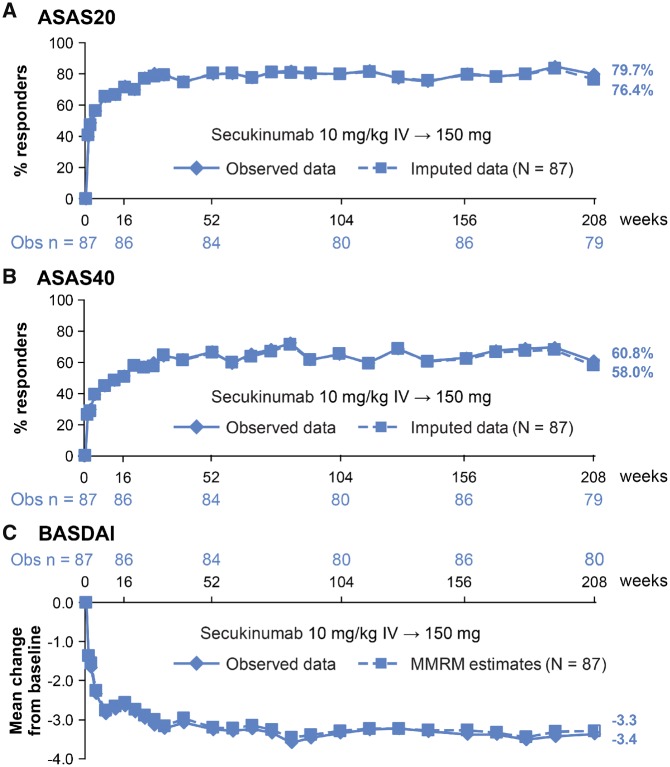
ASAS20/40 response rates at Wk208 were similar to those reported at Wk52 and Wk104 (Fig. 1A and B; Supplementary Table S2, available at Rheumatology online) [10, 12, 13]: 79.7%/60.8% (as observed) and 76.4%/58.0% (multiple imputation) with secukinumab 150 mg. As observed, ASAS20/40 responses with secukinumab 75 mg were 71.0%/43.5% for subjects who did not up-titrate and 80.0%/76.0% for subjects who had a dose increase to 150 mg (Table 1). Secukinumab 150 mg provided sustained efficacy across other clinical endpoints including BASDAI, with consistent findings for observed and imputed analyses (Fig. 1C; Supplementary Table S2, available at Rheumatology online). Numerically improved or comparable as observed responses were seen for most endpoints in subjects who up-titrated from secukinumab 75–150 mg (n = 25) vs those who did not up-titrate (n = 75) (Table 1), except for ASAS partial remission and Ankylosing Spondylitis Disease Activity Score inactive disease.
Fig. 1.
(A) ASAS20, (B) ASAS40 responses and (C) mean change from baseline in BASDAI through Wk208
Data are presented for patients originally randomized to secukinumab IV-150 mg. ASAS 20/40 are reported as observed and using multiple imputation. For BASDAI, mean change from baseline is reported as observed data and as least-square mean change (MMRM analysis). ASAS: Assessment of Spondyloarthritis International Society; IV: intravenous; MMRM: mixed model repeated measures; n: number of evaluable patients; N: total number of patients initially randomized to secukinumab 150 mg at baseline; Obs: observed data; Wk: week.
Table 1.
Summary of efficacy data at Wk208 among patients randomized to secukinumab at baseline
| Outcome measure | Missing data considered | Observed data | ||
|---|---|---|---|---|
| Secukinumab IV→150 mg (N = 87) | Secukinumab IV→150 mg (N = 87) | Secukinumab IV→75 mg | ||
| Not up-titrated (N = 75) | Up-titrated to secukinumab 150 mga (N = 25) | |||
| ASAS20 response | 76.4% | 63/79 (79.7%) | 49/69 (71.0%) | 20/25 (80.0%) |
| ASAS40 response | 58.0% | 48/79 (60.8%) | 30/69 (43.5%) | 19/25 (76.0%) |
| ASAS5/6 response | 60.5% | 50/80 (62.5%) | 37/69 (53.6%) | 18/25 (72.0%) |
| ASAS partial remission | 28.8% | 24/79 (30.4%) | 12/69 (17.4%) | 3/25 (12.0%) |
| hsCRP, mean change from baseline (mg/L) | N/A | –8.4 (26.30) | –7.8 (22.59) | –12.4 (18.61) |
| BASDAI, mean change from baseline | –3.3 (0.23) | –3.4 (2.28) (n = 80) | –2.9 (1.79) (n = 69) | –3.4 (1.64) (n = 25) |
| ASDAS inactive disease response | 27.6% | 21/78 (26.9%) | 17/69 (24.6%) | 3/25 (12.0%) |
| BASFI, mean change from baseline | N/A | –2.9 (2.39) (n = 80) | –2.0 (1.81) (n = 69) | –2.6 (1.99) (n = 25) |
| BASMI, mean change from baseline | N/A | –0.5 (1.12) (n = 76) | –0.4 (1.07) (n = 66) | –0.2 (0.80) (n = 25) |
| SF-36 PCS score, mean change from baseline | 7.8 (0.82) | 8.2 (7.93) (n = 80) | 7.0 (6.03) (n = 69) | 8.5 (5.96) (n = 25) |
Binary variables are reported as observed (n/M [%]), where n = number of patients with response and M = number of patients with evaluable data) or using multiple imputation (% responders) to account for missing data in the IV→150 mg treatment arm. For continuous variables, mean change from baseline is reported as observed data (s.d.) or as least-square mean change (s.e.), where mixed-effects model repeated measures analysis was performed on the IV→150 mg treatment arm.
aFollowing the approval of a protocol amendment, patients in the 75 mg group not achieving sufficient therapeutic response could have their dose escalated to 150 mg at the discretion of the investigators; this led to 25 patients originally randomized to secukinumab 75 mg being up-titrated to secukinumab 150 mg at various timepoints starting at Wk168.
ASAS: Assessment of Spondyloarthritis International Society; ASDAS: Ankylosing Spondylitis Disease Activity Score; hsCRP: high-sensitivity CRP; IV: intravenous; N: total number of patients in the extension trial; N/A: not available; SEL: standard error; SF-36 PCS: Short Form-36 physical component summary; Wk: week.
Results for key efficacy endpoints were generally comparable across anti-TNF-naïve and -IR subgroups, except for ASAS40 and ASAS partial remission, which showed a higher proportion of patients in the naïve subgroup (Supplementary Table S3, available at Rheumatology online).
Radiographic outcomes
A total of 71 and 84 subjects in the secukinumab 150 and 75 mg groups, respectively, had X-rays at baseline and Wk208, with 63.4% and 75.0% being male in the secukinumab 150 and 75 mg groups, respectively. Mean (s.d.) mSASSS at baseline was 8.8 (16.23) and 10.7 (17.82) in the secukinumab 150 and 75 mg groups, respectively (Supplementary Table S4, available at Rheumatology online).
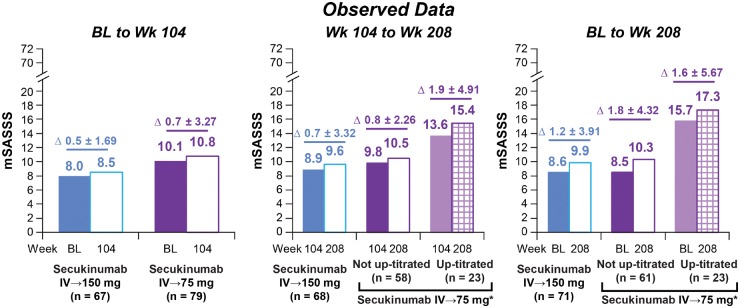
No radiographic progression (mSASSS change from baseline <2) was observed over 208Wk in 78.9% (150 mg) and 78.6% (75 mg) of patients (Fig. 2). Mean (s.d.) change in mSASSS from baseline to Wk208 was numerically lower with secukinumab 150 vs 75 mg: 1.2 (3.91) with secukinumab 150 mg vs 1.8 (4.32) and 1.6 (5.67) with secukinumab 75 mg in subjects who did and did not up-titrate, respectively. Numerically smaller changes were also observed with secukinumab 150 vs 75 mg from baseline to Wk104 and Wk104 to Wk208 (Fig. 3). Changes in mSASSS were consistent for the 67 (150 mg) and 79 (75 mg) patients who had X-ray data available at all three timepoints (Baseline, Wk104 and Wk208; Supplementary Fig. S2, available at Rheumatology online).
Fig. 2.
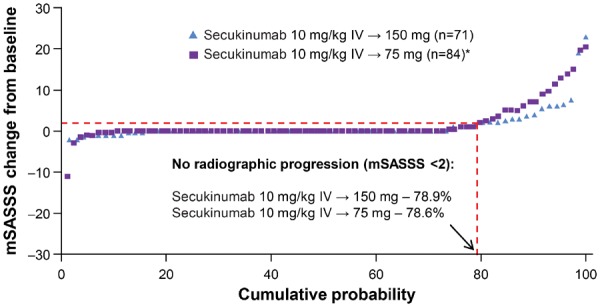
Cumulative probability plot for change from baseline in the mSASSS through Wk208
*Includes 23 patients whose dose was up-titrated from secukinumab 75 mg to 150 mg at various timepoints starting at Wk168, in accordance with a protocol amendment at the discretion of investigators. Data shown as observed. mSASSS: modified Stoke Ankylosing Spondylitis Spine Score; IV: intravenous; n: number of patients with evaluable paired X-rays at Baseline and Wk208; Wk: week.
Fig. 3.
Change in mSASSS between (A) Baseline to Wk104, (B) Wk104 to Wk208, (C) Baseline to Wk208
*Includes 23 patients (22 patients of whom had X-ray data at baseline, Wk104 and Wk208) whose dose was up-titrated from secukinumab 75 mg to 150 mg at various timepoints starting at Wk168. mSASSS score ranges from 0–72; higher scores indicate greater radiographic damage. Δ represents mean (s.d.) difference in mSASSS between timepoints. Baseline and Wk104 X-rays were re-read with Wk208 X-rays to minimize longitudinal variability. BL: baseline; IV: intravenous; mSASSS: modified Stoke Ankylosing Spondylitis Spine Score; n: number of patients with assessments at both timepoints; Wk: week.
Mean mSASSS changes from baseline at Wk208 were numerically greater in subjects with vs without baseline syndesmophytes, in subjects with elevated (>5 mg/L) vs normal (≤5 mg/L) baseline hsCRP, and in males vs females across both secukinumab doses, regardless of up-titration. Compared with patients who had never smoked, ever-smokers (ie previous/current smokers) had numerically higher mean mSASSS changes in subjects with secukinumab 75 mg, regardless of up-titration (Table 2).
Table 2.
Mean mSASSS change from baseline to Wk208 in different subgroups of patients (observed data)
| mSASSS, mean (s.d.) | Secukinumab IV→150mg | Secukinumab IV→75 mg | ||||
|---|---|---|---|---|---|---|
| Not up-titrated (N = 75) | Up-titrated to secukinumab 150 mga (N = 25) | |||||
| Syndesmophytes at baseline | ||||||
| Yes (n = 32) | No (n = 39) | Yes (n = 32) | No (n = 29) | Yes (n = 11) | No (n = 12) | |
| Baseline | 18.8 (19.63) | 0.2 (0.71) | 16.2 (17.12) | 0.1 (0.30) | 32.6 (24.18) | 0.3 (0.62) |
| Change at Wk208 from baseline | 2.1 (4.61) | 0.5 (3.10) | 3.1 (5.56) | 0.2 (1.13) | 3.3 (8.04) | 0.0 (0.00) |
| Elevated hsCRPb at baseline | ||||||
| Yes (n = 48) | No (n = 23) | Yes (n = 37) | No (n = 24) | Yes (n = 17) | No (n = 6) | |
| Baseline | 10.6 (17.75) | 4.4 (10.91) | 11.8 (16.72) | 3.4 (9.15) | 18.8 (25.80) | 7.0 (10.84) |
| Change at Wk208 from baseline | 1.8 (4.64) | 0.2 (0.90) | 2.3 (4.35) | 0.9 (4.23) | 2.8 (5.66) | − 1.8 (4.49) |
| Male | ||||||
| Yes (n = 45) | No (n = 26) | Yes (n = 43) | No (n = 18) | Yes (n = 20) | No (n = 3) | |
| Baseline | 12.1 (18.75) | 2.5 (6.48) | 10.8 (16.82) | 3.1 (4.76) | 17.8 (24.24) | 1.7 (1.53) |
| Change at Wk208 from baseline | 1.5 (3.98) | 0.8 (3.80) | 2.3 (4.89) | 0.6 (2.17) | 1.8 (6.06) | 0.0 (0.00) |
| Ever-smoker at baseline | ||||||
| Yes (n = 21) | No (n = 50) | Yes (n = 24) | No (n = 37) | Yes (n = 8) | No (n = 15) | |
| Baseline | 8.7 (13.31) | 8.6 (17.20) | 14.1 (16.11) | 4.9 (12.71) | 7.3 (9.67) | 20.2 (27.15) |
| Change at Wk208 from baseline | 0.9 (1.94) | 1.4 (4.49) | 2.0 (4.32) | 1.6 (4.38) | 3.8 (6.86) | 0.4 (4.78) |
Data shown as mean (s.d.). mSASSS ranges from 0 to 72, with higher scores indicating greater radiographic damage.
aFollowing the approval of a protocol amendment, patients in the 75 mg group not achieving sufficient therapeutic response could have their dose escalated to 150 mg at the discretion of the investigators; this led to 25 patients originally randomized to secukinumab 75 mg being up-titrated to secukinumab 150 mg at various timepoints starting at Wk168. bElevated hsCRP: >5 mg/L; normal hsCRP: ≤5 mg/L.
hsCRP: high-sensitivity CRP; IV: intravenous; mSASSS: modified Stoke Ankylosing Spondylitis Spine Score; n: number of patients with evaluable paired X-rays at baseline and Wk208; Wk: week.
MRI
Reductions from baseline in the Berlin SI joint total oedema and Berlin spine scores were seen across both doses at Wk104 and 208, with numerically greater decreases in Berlin SI joint total oedema and Berlin spine scores at Wk104 with secukinumab 150 vs 75 mg (Supplementary Table S5, available at Rheumatology online). Reduction in these scores was sustained through Wk208, with the reduction from baseline in Berlin SI joint total oedema score being numerically greater with secukinumab 150 than 75 mg at Wk208. MRI remission was achieved in 33.3% (1/3) and 62.5% (5/8) of patients treated with secukinumab 150 mg and 75 mg, respectively, at Wk208. However, given the small number of anti-TNF-naïve patients meeting the baseline analysis criteria (i.e. Berlin spine score >2), generalizable conclusions regarding MRI remission with secukinumab cannot be drawn.
Safety
Safety analyses of patients originally randomized to secukinumab and placebo patients re-randomized to secukinumab at Wk16/24 were combined into ‘Any secukinumab 150 mg’ and ‘Any secukinumab 75 mg’ groups. Across the entire treatment period (secukinumab exposure [mean (s.d.)]: 1242.6 (524.05) days in ‘Any secukinumab group’), the most common AEs with secukinumab were viral upper respiratory infections (Table 3). SAE incidence (95% CI) was 11.9% (8, 17.2) and 20.1% (14.7, 26.9) in the ‘Any secukinumab 150 and 75 mg groups’, respectively. Discontinuations due to AEs were infrequent and comparable between the two doses. There were two deaths during the study, which have been reported previously [10, 12]. No suicides were reported. There was one mild AE of latent tuberculosis infection and one SAE of primary tuberculoma through Wk208 in patients treated with secukinumab 75 mg (Table 3).
Table 3.
Safety and tolerability of secukinumab during the entire treatment period through Wk208
| Variable | Any secukinumab 150 mg (N = 218)a | Any secukinumab 75 mg (N = 179)a | Any secukinumab (N = 360) |
|---|---|---|---|
| Total exposure to study treatment (days)b | 1002.7 (617.93) | 1278.0 (504.58) | 1242.6 (524.05) |
| Number of patients with event, n (%) | |||
| Any AE | 175 (80.3) | 151 (84.4) | 313 (86.9) |
| SAEc | 36 (20.1) | 26 (11.9) | 62 (17.2) |
| Any AE leading to discontinuationd | 19 (8.9) | 13 (7.3)e | 32 (8.9) |
| Death, n (%)f | 0 (0.0) | 2 (1.1) | 2 (0.6) |
| Common AEs (seen in >5% of subjects on secukinumab), n (%) | |||
| Viral upper respiratory tract infection | 46 (21.1) | 36 (20.1) | 80 (22.2) |
| Diarrhoea | 29 (13.3) | 25 (14.0) | 52 (14.4) |
| Headache | 25 (11.5) | 26 (14.5) | 51 (14.2) |
| Upper respiratory tract infection | 19 (8.7) | 29 (16.2) | 48 (13.3) |
| Influenza | 23 (10.6) | 19 (10.6) | 42 (11.7) |
| Pharyngitis | 23 (10.6) | 13 (7.3) | 36 (10.0) |
| Dyslipidaemia | 14 (6.4) | 18 (10.1) | 32 (8.9) |
| Arthralgia | 17 (7.8) | 16 (8.9) | 32 (8.9 |
| Back pain | 19 (8.7) | 12 (6.7) | 31 (8.6) |
| Oropharyngeal pain | 17 (7.8) | 14 (7.8) | 31 (8.6) |
| Cough | 15 (6.9) | 12 (6.7) | 27 (7.5) |
| Ankylosing spondylitis | 14 (6.4) | 11 (6.1) | 25 (6.9) |
| Nausea | 11 (5.0) | 12 (6.7) | 23 (6.4) |
| Bronchitis | 14 (6.4) | 9 (5.0) | 23 (6.4) |
| Nasopharyngitis | 14 (6.4) | 9 (5.0) | 23 (6.4) |
| Leukopenia | 8 (3.7) | 14 (7.8) | 22 (6.1) |
| Uveitis | 13 (6.0) | 9 (5.0) | 21 (5.8) |
| Urinary tract infection | 11 (5.0) | 10 (5.6) | 21 (5.8) |
| Hypertension | 8 (3.7) | 13 (7.3) | 21 (5.8) |
| Gastroenteritis | 9 (4.1) | 10 (5.6) | 19 (5.3) |
| Selected AEs of interest, n (exposure-adjusted incidence rate per 100 patient-years) | |||
| Serious infections | 3 (0.5) | 9 (1.5) | 12 (1.0) |
| Candida infections | 3 (0.5) | 2 (0.3) | 5 (0.4) |
| Crohn’s disease | 2 (0.3) | 5 (0.8) | 7 (0.6) |
| Ulcerative colitis | 1 (0.2) | 1 (0 2) | 2 (0.2) |
| MACE (adjudicated) | 2 (0.3) | 5 (0.8) | 7 (0.6) |
| Malignant/unspecified tumours | 4 (0.7) | 2 (0.3) | 6 (0.5) |
| Neutropenia | 3 (0.5) | 5 (0.8) | 8 (0.7) |
| Uveitis | 2.3 | 1.5 | 1.8 |
aIncludes patients originally randomized to secukinumab and those re-randomized from placebo to secukinumab at Wk16/24; AE data in patients who up-titrated from secukinumab 75–150 mg are attributed to their dose at the time of AE onset.
bMean (s.d.).
cInclude deaths.
dUp to Wk104; 2 additional patients discontinued secukinumab after Wk104.
eIncludes one case of new-onset latent tuberculosis infection and one case of primary pulmonary tuberculoma.
AEs: treatment-emergent adverse events; IV: intravenous; MACE: major adverse cardiac events; n, number of subjects who received ≥1 dose of secukinumab at any time during the core trial or extension, including those randomized at baseline to secukinumab or placebo; SAE: serious adverse event; TB: tuberculosis; Wk, week.
Exposure-adjusted incidence rates (EAIRs, per 100 subject-years) through Wk208 are presented in Table 3. The 12 serious infections through Wk208 were reported previously [10, 12]. Candida infections were reported in five secukinumab-treated patients through Wk208, with one new case in Wk156–208 [10]. Through 208Wk, there were seven Crohn’s disease cases; neither of the two patients reporting Crohn’s disease in Wk156–208 had a history of inflammatory bowel disease. One case was a non-SAE that resolved with treatment; the other, SAE, was unresolved, resulting in treatment discontinuation. Two patients reported ulcerative colitis through Wk208, one of which was reported through Wk156 [12]. The second non-SAE was moderate in severity and occurred in Wk156–208 in a patient without pre-existing history; the unresolved case resulted in treatment discontinuation. Six cases of malignant/unspecified tumours were reported through Wk208, with one occurring in Wk156–208 [10, 12]. Prior history of uveitis was reported in 17% (62/371) of the MEASURE 1 study population at baseline [10]. Through Wk208, uveitis was reported by 21 secukinumab-treated patients, of whom 12 had pre-existing history and nine were de novo cases; no SAEs of uveitis were reported during Wk156–208. The seven adjudicated major adverse cardiac events were reported previously [10, 12].
Discussion
These results demonstrate the sustained efficacy of secukinumab in both clinical and radiographic outcomes through 4 years in the MEASURE 1 study, the first study reporting the longer-term effect of secukinumab on radiographic structural progression in AS; previous publications reported efficacy and radiographic outcomes from the 2-year core study [10].
Clinical improvements were sustained across all endpoints through Wk208, with numerically greater improvements with secukinumab 150 vs 75 mg. This is consistent with the 3-year results [12], where decreased efficacy was observed with 75 vs 150 mg (the licensed dose in AS), particularly with higher-hurdle endpoints (e.g. ASAS partial remission). These results represent the longest-term efficacy results reported for an IL-17 inhibitor in AS and are notable for demonstrating no evidence of decreasing efficacy with long-term use in both observed and imputed analyses, in contrast to the secondary treatment failure reported with long-term use of anti-TNF agents [16, 17]. This finding with secukinumab, a fully human monoclonal antibody, is consistent with the reported low rates of immunogenicity (rates of treatment-emergent anti-drug antibodies typically <1%) [18].
Among subjects originally randomized to secukinumab 75 mg, numerically improved responses were observed on most endpoints in those who up-titrated to secukinumab 150 mg vs those who did not up-titrate. These results should be interpreted with caution due to the relatively small number of subjects who up-titrated and the limited observation window. As patients were allowed to up-titrate at investigators’ discretion at any point after Wk156 following a protocol amendment, there was a wide distribution of exposure time on secukinumab 150 mg (4–40 Wk), which may explain why a consistent pattern was not seen across all endpoints (eg ASAS partial remission) in up-titrated subjects. End-of-study results to be reported at the conclusion of the 3-year extension trial should confirm whether this trend continues in the setting of longer-term up-titration of 1–2 years in all up-titrated subjects. Whether treatment with higher doses of secukinumab (300 mg q4Wk) has an even stronger effect on radiographic progression is unknown; a recent study showed a trend towards better clinical effects in some patients [19].
Sustained improvements were seen in anti-TNF-naïve and -IR subgroups, with similar improvements observed across both subgroups in all endpoints, except for ASAS40 and ASAS partial remission, where improvements were greater in anti-TNF-naïve subjects. The results demonstrate that secukinumab is a suitable longer-term AS treatment option for both biologic-naïve subjects as first-line biologic therapy and those who experience an inadequate response or intolerance to anti-TNF agents.
This study represents the first long-term (4-year) data from a controlled study of a biologic in AS to suggest a dose-response effect for structural progression, with a numerically lower mSASSS change from baseline with secukinumab 150 vs 75 mg, regardless of up-titration, through Wk208. In the GO-RAISE study, which evaluated two doses of the anti-TNF golimumab through 4 years, no benefit was observed with the higher dose: mean (s.d.) change from baseline in mSASSS with 50 vs 100 mg were 0.9 (2.7) vs 0.9 (3.9) over 2 years, and 1.3 (4.1) vs 2.0 (5.6) over 4 years, respectively [20].
Results of the current study also demonstrate no radiographic progression (change from baseline in mSASSS <2) in almost 80% of secukinumab-treated patients through Wk208; this finding is consistent with the MEASURE 1 core study, in which ∼80% of secukinumab-treated patients experienced no radiographic structural progression through 2 years (change in mSASSS < smallest detectable change = 1.83) [10]. Results of the core and extension studies can’t be directly compared as only 74% of core study participants enrolled in the extension study. Additionally, baseline and Wk104 X-rays were re-read for patients completing 208Wk. As expected, and consistent with the core study findings, mean mSASSS changes through Wk208 were numerically higher in patients with known risk factors for progression, including baseline syndesmophytes, male gender and elevated hsCRP [3–5, 10].
Prevention of progressive structural damage is a key objective of AS treatment [8, 9]. Previous studies have failed to show a reduction in radiographic structural progression over 2 years in subjects treated with anti-TNF agents vs historical control-matched subjects from an anti-TNF naïve population [21–23], despite recent certolizumab data demonstrating low mSASSS progression rates over 4 years and non-progression in 80.6% of patients [24]. Furthermore, longitudinal data from cohort studies suggest a longer duration of anti-TNF therapy is required before a positive effect on radiographic structural damage is observed [20, 25, 26]. In these analyses, secukinumab 150 mg, the approved dose in AS, demonstrated a sustained low mean change from baseline in mSASSS through 4 years (i.e. 0.5 over 2 years and 1.2 over 4 years). Direct comparisons between the current structural progression results and those of previous studies cannot be made because of differences in the study designs and populations; however, comparative data will become available from SURPASS, an ongoing 2-year randomized and active-controlled head-to-head study comparing radiographic structural progression with secukinumab vs an anti-TNF agent in AS [27].
MRI analyses demonstrated that secukinumab reduced objective signs of spinal inflammation in active AS, and that these improvements were sustained through 208Wk of therapy. These results were consistent with the reduction in hsCRP levels, another objective measure of inflammation, seen over 4 years of secukinumab treatment.
Secukinumab showed a favourable safety profile through 4 years, with similar EAIRs for selected AEs (e.g. serious infections, Candida, Crohn’s disease, uveitis), as previously reported [10, 12]. Uveitis is a common extra-articular manifestation of spondyloarthritis with an estimated prevalence of 33.2% in patients with AS. The EAIRs of uveitis reported in AS patients treated with TNF inhibitors are 2.6–3.5 per 100 patient-years [28, 29]. The EAIR of uveitis in this study was 1.8 per 100 patient-years, suggesting a low to comparable incidence of uveitis in secukinumab-treated AS patients.
Furthermore, no new safety risks were identified during this extended secukinumab treatment analysis.
Limitations of this 4-year extension study include the lack of a control group beyond Wk16/24 and the potential for selection bias among subjects choosing to enter the extension study. However, the lower 75 mg dose has utility as a randomized comparator arm of a less effective dose that demonstrates numerical dose-separation on multiple endpoints, including radiographic progression. Although, the results of the present analysis show a trend for numerically improved clinical efficacy and radiographic outcomes with secukinumab 150 vs 75 mg, the study was not powered for such a comparison.
The results of this ongoing 5-year study build on the extension study findings previously reported from the MEASURE 1 study at 3 years and confirm the sustained efficacy and known safety profile of secukinumab through Wk208 (4 years) [12].
Supplementary Material
Acknowledgements
Novartis Pharma AG supported this analysis. The authors thank Aisling O’Keeffe, PhD, and John Gallagher of Novartis Ireland Ltd, Dublin, Ireland and Santoshkumar Tota of Novartis, India for providing medical writing support, which was funded by Novartis in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3). J.B., X.B., A.D. and P.E. were involved in the design of the study. J.B., A.D. and D.P. enrolled subjects into the study. All authors contributed to the analysis and interpretation of the data. Each draft of the manuscript was reviewed by all authors and all authors approved the final version.
Funding: This clinical trial was sponsored by Novartis Pharma AG.
Disclosure statement: J.B. has received grant/research support from: Abbvie (Abbott), Amgen, BMS, Boehringer, Celgene, Celltrion, Centocor, Chugai, EBEWE Pharma, Medac, MSD (Schering-Plough), Mundipharma, Novartis, Pfizer (Wyeth), Roche, Sanofi-Aventis and UCB, served as a consultant for: Abbvie (Abbott), Amgen, BMS, Boehringer, Celgene, Celltrion, Centocor, Chugai, EBEWE Pharma, Medac, MSD (Schering-Plough), Mundipharma, Novartis, Pfizer (Wyeth), Roche, Sanofi-Aventis and UCB, and received speakers bureau fees from: Abbvie (Abbott), Amgen, BMS, Boehringer, Celgene, Celltrion, Centocor, Chugai, EBEWE Pharma, Medac, MSD (Schering-Plough), Mundipharma, Novartis, Pfizer (Wyeth), Roche, Sanofi-Aventis and UCB. X.B. has received grant/research support from: AbbVie, Merck, Pfizer, UCB, Novartis and Chugai, served as a consultant for: AbbVie, Merck, Pfizer, UCB, Novartis and Chugai, and received speakers bureau fees from: AbbVie, Merck, Pfizer, UCB, Novartis and Chugai. A.D. has received grant/research support from: AbbVie, Eli Lilly, GSK, Janssen, Novartis, Pfizer and UCB, and served as a consultant for: AbbVie, Eli Lilly, Janssen, Novartis, Pfizer and UCB. D.P. has received grant/research support from: AbbVie, MSD and Novartis, served as a consultant for: AbbVie, BMS, MSD, Novartis, Pfizer and UCB, and received speaker’s bureau fees from: AbbVie, BMS, Janssen, MSD, Novartis, Pfizer and UCB. P.E. has served as a consultant for: AbbVie, BMS, Merck, Novartis, Pfizer, Roche and UCB. E.M.Dd is a contractor for Novartis. Z.T. is an employee and shareholder of Novartis. B.P. is an employee and shareholder of Novartis.
References
- 1. Braun J, Sieper J. Ankylosing spondylitis. Lancet 2007;369:1379–90. [DOI] [PubMed] [Google Scholar]
- 2. Baraliakos X, Listing J, Rudwaleit M et al. . Progression of radiographic damage in patients with ankylosing spondylitis: defining the central role of syndesmophytes. Ann Rheum Dis 2007;66:910–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ramiro S, van der Heijde D, van Tubergen A et al. . Higher disease activity leads to more structural damage in the spine in ankylosing spondylitis: 12-year longitudinal data from the OASIS cohort. Ann Rheum Dis 2014;73:1455–61. [DOI] [PubMed] [Google Scholar]
- 4. Baraliakos X, Listing J, von der Recke A, Braun J. The natural course of radiographic progression in ankylosing spondylitis–evidence for major individual variations in a large proportion of patients. J Rheumatol 2009;36:997–1002. [DOI] [PubMed] [Google Scholar]
- 5. Maas F, Spoorenberg A, Brouwer E et al. . Spinal radiographic progression in patients with ankylosing spondylitis treated with TNF-a blocking therapy: a prospective longitudinal observational cohort study. PLoS One 2015;10:e0122693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Landewe R, Dougados M, Mielants H, van der Tempel H, van der Heijde D. Physical function in ankylosing spondylitis is independently determined by both disease activity and radiographic damage of the spine. Ann Rheum Dis 2009;68:863–7. [DOI] [PubMed] [Google Scholar]
- 7. Yang X, Fan D, Xia Q et al. . The health-related quality of life of ankylosing spondylitis patients assessed by SF-36: a systematic review and meta-analysis. Qual Life Res 2016;25:2711–23. [DOI] [PubMed] [Google Scholar]
- 8. van der Heijde D, Ramiro S, Landewe R et al. . 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis 2017;76:978–91. [DOI] [PubMed] [Google Scholar]
- 9. Ward MM, Deodhar A, Akl EA et al. . American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network 2015 recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondyloarthritis. Arthritis Care Res 2016;68:151–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Braun J, Baraliakos X, Deodhar A et al. . Effect of secukinumab on clinical and radiographic outcomes in ankylosing spondylitis: 2-year results from the randomised phase III MEASURE 1 study. Ann Rheum Dis 2017;76:1070–7. [DOI] [PubMed] [Google Scholar]
- 11. Marzo-Ortega H, Sieper J, Kivitz A et al. . Secukinumab and sustained improvement in signs and symptoms of patients with active ankylosing spondylitis through two years: results from a Phase III study. Arthritis Care Res 2017;69:1020–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baraliakos X, Kivitz AJ, Deodhar AA et al. . Long-term effects of interleukin-17A inhibition with secukinumab in active ankylosing spondylitis: 3-year efficacy and safety results from an extension of the Phase 3 MEASURE 1 trial. Clin Exp Rheumatol 2018;36:50–5. [PubMed] [Google Scholar]
- 13. Baeten D, Sieper J, Braun J et al. . Secukinumab, an interleukin-17A inhibitor, in ankylosing spondylitis. N Engl J Med 2015;373:2534–48. [DOI] [PubMed] [Google Scholar]
- 14. Garrett S, Jenkinson T, Kennedy LG et al. . A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol 1994;21:2286–91. [PubMed] [Google Scholar]
- 15. Creemers MC, Franssen MJ, van’t Hof MA et al. . Assessment of outcome in ankylosing spondylitis: an extended radiographic scoring system. Ann Rheum Dis 2005;64:127–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bartelds GM, Krieckaert CL, Nurmohamed MT et al. . Development of antidrug antibodies against adalimumab and association with disease activity and treatment failure during long-term follow-up. JAMA 2011;305:1460–8. [DOI] [PubMed] [Google Scholar]
- 17. Vincent FB, Morand EF, Murphy K et al. . Antidrug antibodies (ADAb) to tumour necrosis factor (TNF)-specific neutralising agents in chronic inflammatory diseases: a real issue, a clinical perspective. Ann Rheum Dis 2013;72:165–78. [DOI] [PubMed] [Google Scholar]
- 18. Reich K, Blauvelt A, Armstrong A et al. . Secukinumab, a fully human anti-interleukin-17A monoclonal antibody, exhibits minimal immunogenicity in patients with moderate-to-severe plaque psoriasis. Br J Dermatol 2017;176:752–8. [DOI] [PubMed] [Google Scholar]
- 19. Pavelka K, Kivitz A, Dokoupilova E et al. . Efficacy, safety, and tolerability of secukinumab in patients with active ankylosing spondylitis: a randomized, double-blind phase 3 study, MEASURE 3. Arthritis Res Ther 2017;19:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Braun J, Baraliakos X, Hermann KG et al. . The effect of two golimumab doses on radiographic progression in ankylosing spondylitis: results through 4 years of the GO-RAISE trial. Ann Rheum Dis 2014;73:1107–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van der Heijde D, Landewe R, Einstein S et al. . Radiographic progression of ankylosing spondylitis after up to two years of treatment with etanercept. Arthritis Rheum 2008;58:1324–31. [DOI] [PubMed] [Google Scholar]
- 22. van der Heijde D, Landewe R, Baraliakos X et al. . Radiographic findings following two years of infliximab therapy in patients with ankylosing spondylitis. Arthritis Rheum 2008;58:3063–70. [DOI] [PubMed] [Google Scholar]
- 23. van der Heijde D, Salonen D, Weissman BN et al. . Assessment of radiographic progression in the spines of patients with ankylosing spondylitis treated with adalimumab for up to 2 years. Arthritis Res Ther 2009;11:R127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van der Heijde D, Baraliakos X, Hermann KA et al. . Limited radiographic progression and sustained reductions in MRI inflammation in patients with axial spondyloarthritis: 4-year imaging outcomes from the RAPID-axSpA phase III randomised trial. Ann Rheum Dis 2018;77:699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Maas F, Arends S, Brouwer E et al. . Reduction in spinal radiographic progression in ankylosing spondylitis patients receiving prolonged treatment with tumor necrosis factor inhibitors. Arthritis Care Res (Hoboken) 2017;69:1011–9. [DOI] [PubMed] [Google Scholar]
- 26. Haroon N, Inman RD, Learch TJ et al. . The impact of tumor necrosis factor alpha inhibitors on radiographic progression in ankylosing spondylitis. Arthritis Rheum 2013;65:2645–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marzo-Ortega H, McGonagle D, O’Connor P, Emery P. Efficacy of etanercept in the treatment of the entheseal pathology in resistant spondylarthropathy: a clinical and magnetic resonance imaging study. Arthritis Rheum 2001;44:2112–7. [DOI] [PubMed] [Google Scholar]
- 28. Wendling D, Joshi A, Reilly P et al. . Comparing the risk of developing uveitis in patients initiating anti-tumor necrosis factor therapy for ankylosing spondylitis: an analysis of a large US claims database. Curr Med Res Opin 2014;30:2515–21. [DOI] [PubMed] [Google Scholar]
- 29. Deodhar A, Miceli-Richard C, Baraliakos X et al. . Low incidence of both new-onset and flares of uveitis in secukinumab-treated patients with ankylosing spondylitis: clinical trial and post-marketing safety analysis. Ann Rheum Dis 2018;77:999. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.