Abstract
High income, high socioeconomic status, and affluence increase breast cancer incidence. Socioeconomic status in USA breast cancer studies has been assessed by block-group socioeconomic measures. A block group is a portion of a census tract with boundaries that segregate, as far as possible, socioeconomic groups. In this study, we used US Census income data instead of block groups to gauge socioeconomic status of breast cancer patients in relationship with incidence, prognostic markers, and survival. US state breast cancer incidence and mortality data are from the U.S. Cancer Statistics Working Group, United States Cancer Statistics: 1999–2011. Three-Year-Average Median Household Income by State, 2010 to 2012, is from the U.S. Census Bureau, Current Population Survey, 2011 to 2013 Annual Social and Economic Supplements. County incomes are from the 2005–2009 American Community Survey of the U.S. Census Bureau. The American Community Survey is an ongoing statistical survey that samples a small percentage of the population yearly. Its purpose is to provide communities the information they need to plan investments and services. Breast cancer county incidence and survival data are from the National Cancer Institute’s Surveillance, Epidemiology and End Results Program (SEER) data base. We analyzed SEER data from 198 counties in California, Connecticut, Georgia, Hawaii, Iowa, New Mexico, Utah, and Washington. SEER uses the Collaborative Stage (CS) Data Collection System. We have retained the SEER CS variables. There was a significant relationship of income with breast cancer incidence in 50 USA states and the District of Columbia in White women (r = 0.623, p < 0.001). There was a significant relationship between node involvement and income in Whites in 198 USA counties. Income was significantly correlated with 5-year relative survival in Whites with localized breast cancer. Income was not correlated with 5-year survival of Black race (p = 0.364) or other races (p = 0.624). The multivariate general linear model with income as covariate, 5-year survival by race as a dependent variable, showed a significant effect of income and White race on 5-year survival (p < 0.001), unrelated to Black race (p = 0.780) or other races (p = 0.618). In men, we found a nonsignificant positive correlation between county breast cancer incidence and income (r = 0.098, p = 0.168). Breast cancer risk factors, such as delayed childbirth, less breast-feeding, and use of hormone supplements, are more common in affluent women. Affluent women are more likely to have mammograms, which detect many cancers that might not otherwise be diagnosed. In addition, women in certain affluent ethnic groups—Ashkenazi Jews, Icelanders and the Dutch—are more likely to carry genetic mutations known to predispose to breast cancer. We hypothesize that women with more income can afford better cancer care and survive longer than poorer women. But our hypothesis does not explain why this effect should be limited to White women; or why node involvement increased with income in White women but not in Blacks or Hispanics. Further studies may be worthwhile.
Keywords: affluence, breast cancer, incidence, income
High income, high socioeconomic status, and affluence increase breast cancer incidence. Madigan et al. reported that women in the upper two-thirds of the USA population by income had an age-adjusted breast cancer risk of 1.7, while in the lower third their risk was not elevated (1). In Marin County, north of San Francisco, the median per capita income is more than twice as high as that of the USA as a whole, and between 1990 and 1999 Marin County breast cancer incidence increased six times more rapidly than in comparison areas (2). However, a study of women in San Francisco suggested that affluence increases breast cancer incidence only in Hispanic women (3). In addition, breast cancer incidence is increasing in lower income countries (4,5).
Socioeconomic status in USA breast cancer studies has been assessed by block-group socioeconomic measures. A block group is a portion of a census tract with boundaries that segregate, as far as possible, socioeconomic groups (3). Income data, an excellent mark of socioeconomic status, is not recorded in the National Cancer Institute’s Surveillance, Epidemiology and End Results Program (SEER) data base or other cancer registries (6).
In this study, we used U.S. Census income data instead of block groups to gauge socioeconomic status of breast cancer patients in relationship with incidence, prognostic markers, and survival.
METHODS
The USA state breast cancer incidence and mortality data are from the U.S. Cancer Statistics Working Group, United States Cancer Statistics: 1999–2011 (7). Three-Year-Average Median Household Income by State, 2010–2012, is from the U.S. Census Bureau, Current Population Survey, 2011 to 2013 Annual Social and Economic Supplements.
County incomes are from the 2005–2009 American Community Survey of the U.S. Census Bureau (8). The American Community Survey is an ongoing statistical survey that samples a small percentage of the population yearly. Its purpose is to provide communities the information they need to plan investments and services.
Breast cancer county incidence and survival data are from the National Cancer Institute’s SEER data base. We analyzed SEER data from 198 counties in California, Connecticut, Georgia, Hawaii, Iowa, New Mexico, Utah, and Washington. SEER uses the Collaborative Stage (CS) Data Collection System (9–13). We have retained the SEER CS variables.
Alcohol consumption in women by USA state is from the Centers for Disease Control, State-Specific Weighted Prevalence Estimates of Alcohol Use Among Women 18–44 Years of Age, Behavioral Risk Factor Surveillance System, 2012 (14).
RESULTS
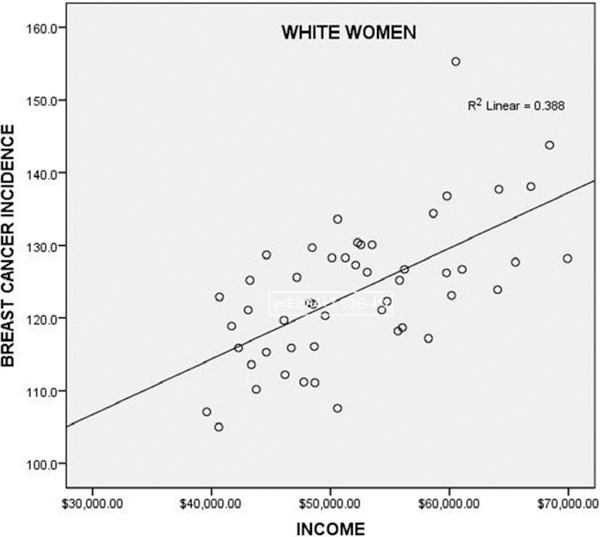
There was a significant relationship of income with breast cancer incidence in 50 USA states and the District of Columbia in White women (r = 0.623, p < 0.001, Fig. 1). The relationship of income to incidence was not significant in Black women (r = −0.176, p = 0.221) and is of borderline significance in Hispanics (r = 0.277, p = 0.051). There was no significant correlation of income with mortality (deaths per 100,000) in Whites (r = 0.172, p = 0.227), Blacks (r = −0.154, p = 0.393), or Hispanics (r = −0.042, p = 0.867). Multivariate linear regression indicated that the relationship with income of breast cancer incidence in White women was significant (β = 0.371, p = 0.001) and independent of the relationship with alcohol consumption (β = 0.518, p < 0.001).
Figure 1.
Breast cancer incidence in White women versus three-year-average median household income by USA state, 2010 to 2012, in 50 USA states and the District of Columbia. The relationship is significant (p < 0.001). The outlier (155.3 cases per 100,000) is the District of Columbia, which has the highest breast cancer incidence in the USA.
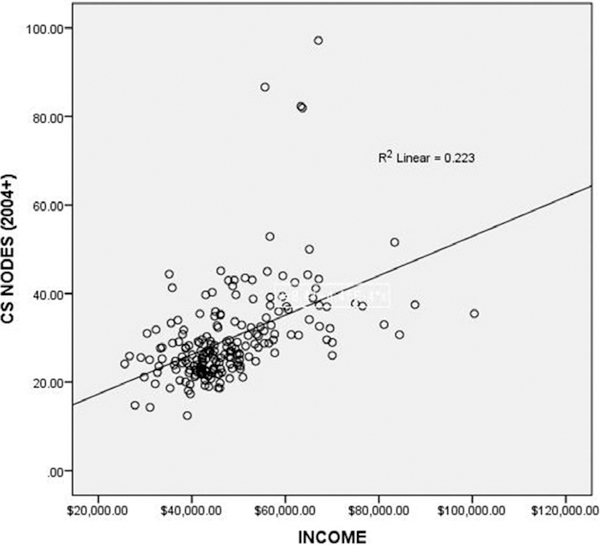
There was a significant relationship between node involvement and income in Whites in 198 USA counties (Fig. 2). The multivariate general linear model with income as covariate, node involvement by race as dependent variable, showed a significant effect of income and White race on node involvement (p = 0.004), unrelated to Black race (p = 0.183) or other races (American Indian, Alaskan Native, Asian, Pacific Islander, p = 0.165).
Figure 2.
Node involvement in White women with breast cancer (SEER 2004 Collaborative Staging) versus income in 198 USA counties. The relationship is significant (p < 0.001). The four outliers are from Hawaii: Hawaii County, Honolulu County, Kauai County, and Maui County.
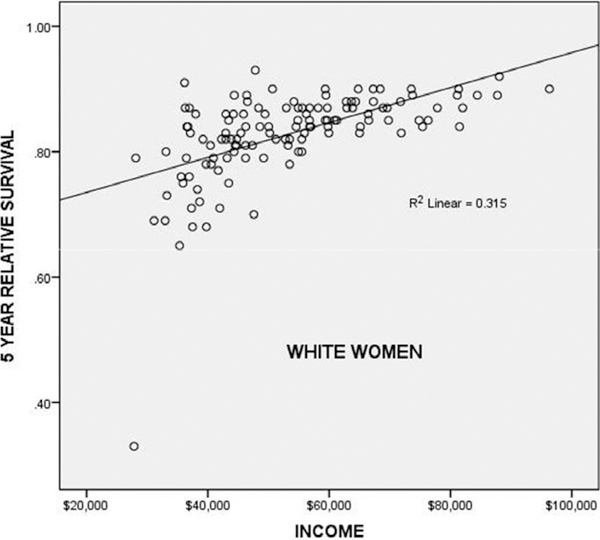
Although income was not significantly correlated with mortality, as noted above, income was significantly correlated with a 5-year relative survival in Whites with localized breast cancer (p < 0.001, Fig. 3). Localized means breast cancer localized to the breast, not involving the nodes. Income was not correlated with a 5-year survival of Black race (p = 0.364) or other races (p = 0.624). The multivariate general linear model with income as covariate, 5-year survival by race as a dependent variable, showed a significant effect of income and White race on a 5-year survival (p < 0.001), unrelated to Black race (p = 0.780) or other races (p = 0.618).
Figure 3.
Income versus 5-year relative survival of White women in 137 USA counties. The correlation was significant (p < 0.001). The outlier (32% 5-year relative survival) is DeBaca County, New Mexico.
In men, we found a nonsignificant positive correlation between SEER county breast cancer incidence and income (r = 0.098, p = 0.168).
DISCUSSION
Breast cancer has generally been considered a disease of affluence, possibly because risk factors. such as delayed childbirth, less breast-feeding, and use of hormone supplements. are more common in affluent women. Affluent women are more likely to have mammograms, which detect many cancers that might not otherwise be diagnosed. In addition, women in certain affluent ethnic groups—Ashkenazi Jews, Ice-landers and the Dutch—are more likely to carry genetic mutations that predispose to breast cancer. Nevertheless, breast cancer mortality is higher in Black women than White women (6,15).
Alcohol is a well-documented breast cancer risk factor, probably due to increased estrogens and androgens, as well as a direct carcinogenic effect on breast tissue (16). Drinking at least two glasses of wine a day (or the equivalent alcohol) is correlated with both affluence and higher breast cancer risk. But we found that the relationship with income of breast cancer incidence in White women was independent of the relationship with alcohol consumption.
In Figure 2, the four outliers are from Hawaii County, Honolulu County, Kauai County, and Maui County. Meng et al. report that native Hawaiian and Filipino women have a higher risk of dying from breast cancer within 5 years than women of other ethnic groups (17). The elevated incidence of nodes in affluent women would correspond to the elevated mortality, especially if some of the native Hawaiian and Filipino women were classified as White in SEER, even though SEER includes a designation for Asian-Pacific Islanders.
As was mentioned, cancer registries do not contain breast cancer incidence data and corresponding socioeconomic status data as defined by income. Our analysis based on income confirms the relationship of breast cancer incidence to affluence but only in White women, not Blacks, and a borderline relationship in Hispanics.
Ansell found that income was associated with a relative risk of death in breast cancer of 1.6 and that this effect was marginally significant (p = 0.06) in a multivariate analysis including Black race, estrogen receptor, distant disease, and age (18). In contrast, we found that income increases 5-year survival, but only in White women. We were not able to include therapy (radiation versus not, systemic therapy versus not), stage, and/or tumor size/grade in a multivariate analysis because of SEERstat limitations.
We hypothesize that women with more income can afford better cancer care and survive longer than poorer women. But our hypothesis does not explain why this effect should be limited to White women; or why node involvement increased with income in White women but not in Blacks or Hispanics.
One expects that affluence will correlate positively with improved access to screening and healthcare. Yet we arrived at the contradictory conclusions that affluent women, although more likely to be screened, are more likely to have node -positive disease, yet more likely to have improved 5-year survival compared to other groups. Obviously, a highly screened population will have a higher incidence of node positivity, since more attention to the patient and more elaborate screening techniques are more likely to detect nodes than the simple axillary palpation afforded to low income patients. Moreover, better access to care and better care given to affluent women can no doubt overcome the 5-year survival disadvantage of node-positive disease. Nevertheless, further studies may be worthwhile.
Acknowledgments
FUNDING
None.
Footnotes
CONFLICTS OF INTEREST
No conflicts of interest.
REFERENCES
- 1.Madigan MP, Ziegler RG, Benichou J, Byrne C, Hoover RN. Proportion of breast cancer cases in the United States explained by well-established risk factors. J Natl Cancer Inst 1995;87:1681–5. [DOI] [PubMed] [Google Scholar]
- 2.Clarke CA, Glaser SL, West DW, et al. Breast cancer incidence and mortality trends in an affluent population: Marin County, California, USA, 1990–1999. Breast Cancer Res 2002;4:R13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krieger N, Quesenberry C Jr, Peng T, et al. Social class, race/ethnicity, and incidence of breast, cervix, colon, lung, and prostate cancer among Asian, Black, Hispanic, and White residents of the San Francisco Bay Area, 1988–92 (United States). Cancer Causes Control 1999;10:525–37. [DOI] [PubMed] [Google Scholar]
- 4.Porter P “Westernizing” women’s risks? Breast cancer in lower-income countries. N Engl J Med 2008;358:213–6. [DOI] [PubMed] [Google Scholar]
- 5.Hall SA, Rockhill B. Race, poverty, affluence, and breast cancer. Am J Public Health 2002;92:1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krieger N Is breast cancer a disease of affluence, poverty, or both? The case of African American women. Am J Public Health 2002;92:611–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.US Cancer Statistics Working Group. United States cancer statistics: 1999–2011 incidence and mortality web-based report. Atlanta, GA, 2014. [Google Scholar]
- 8.US Census Bureau. American Community Survey 2005–2009, 2014. Available at: http://wwwcensusgov/acs/www/ (accessed on October 24, 2014).
- 9.Collaborative Staging Task Force. Collaborative Stage Data Collection System. Chicago, IL: AJCC, Collaborative Staging, 2012. [Google Scholar]
- 10.Johnson CH. The SEER Program Coding and Staging Manual 2004 Cancer Statistics Branch, Surveillance Research Program, Division of Cancer Control and Population Sciences, National Cancer Institute, National Institutes of Health, Public Health Service, US Department of Health and Human Services, 2004.
- 11.Manual CS. Coding Instructions. Collaborative Staging Task Force of the American Joint Committee on Cancer NIH Publication, 2007 (04–5496).
- 12.Wu XC, Yu Q, Andrews PA, et al. Comparisons of directly coded SEER Summary Stage 2000 and Collaborative Staging Derived SEER Summary Stage 2000. J Registry Manag 2009;37:137–40. [PubMed] [Google Scholar]
- 13.Howlader N, Chen VW, Ries LAG, et al. Overview of breast cancer collaborative stage data items–their definitions, quality, usage, and clinical implications: a review of SEER data for 2004–2010. Cancer 2014;120:3771–80. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. State-Specific Alcohol Consumption Rates for 2012. Fetal Alcohol Spectrum Disorders. Available at: http://www.cdc.gov/ncbddd/fasd/monitor_table.html (accessed October 31, 2014).
- 15.American Cancer Society. Breast Cancer Facts & Figures 2011–2012. Atlanta, GA: American Cancer Society, 2012. [Google Scholar]
- 16.Singletary KW, Gapstur SM. Alcohol and breast cancer: review of epidemiologic and experimental evidence and potential mechanisms. JAMA 2001;286:2143–51. [DOI] [PubMed] [Google Scholar]
- 17.Meng L, Maskarinec G, Wilkens L. Ethnic differences and factors related to breast cancer survival in Hawaii. Int J Epidemiol 1997;26:1151–8. [DOI] [PubMed] [Google Scholar]
- 18.Ansell D, Whitman S, Lipton R, Cooper R. Race, income, and survival from breast cancer at two public hospitals. Cancer 1993;72:2974–8. [DOI] [PubMed] [Google Scholar]