Abstract
Purpose
In this meta-analysis, relevant case-control and cohort studies were pooled to evaluate the association between preeclampsia and the risk of autism spectrum disorders (ASDs) in children.
Methods
A search for relevant studies in major databases, including Web of Science, PubMed, and Scopus, was performed up to May 2018. The odds ratios (ORs) or rate ratios (RRs) with 95% confidence intervals (CIs) were extracted from eligible studies to determine the association among studies.
Results
The pooled estimates of ORs and RRs indicated a significant association between preeclampsia and ASD [(OR, 1.36; 95% CI, 1.12–1.60) and (RR, 1.30; 95% CI, 1.20–1.41)].
Conclusions
Despite existing controversy, our findings indicated that preeclampsia was associated with an increased risk of ASD among children.
Keywords: Autism spectrum disorder, Autism, Preeclampsia, Pregnancy
Introduction
The autism spectrum disorders (ASDs) among children are a group of neurodevelopmental disorders that is associated with restricted, repetitive patterns of behavior, verbal and nonverbal communication and social interaction [1]. ASD prevalence has been on the rise in most societies [2]. The pathogenesis of ASD is far from fully known. The evidence show the genetic factors interfere, but non genetic exposures are probably is affected in the pathogenesis of ASD [3]. Some studies reported a number of factors associated with ASD in child such as preterm birth, high maternal age, child sex, small-for-gestational age, low birth weight, hemorrhage antenatal, low Apgar scores, medication use during pregnancy, maternal obesity and in firstborn infants [3,4].
Preeclampsia is a pregnancy complication recognized by hypertension and proteinuria [5]. It is a multisystem disorder that is associated with epigastric pain, liver disease, thrombocytopenia, kidney dysfunction, red blood cell breakdown, and low blood platelet count [6]. Preeclampsia affect 2%–8% of all pregnancies [7].
The association between preeclampsia and risk of ASD has been investigated in previous studies [8]. Although in some studies there was a significant association, but the results among them are inconsistent. A meta-analysis by Wang et al. [9] in 2017 reported that preeclampsia is a risk factor for ASD among children (rate ratio [RR], 1.50; 95% confidence interval [CI], 1.04–2.18). However, this meta-analysis was restricted to 3 cohort studies that may decrease the generalizability of the findings.
This meta-analysis pooled all of case-control and cohort studies to obtain the association between preeclampsia and the risk of ASD children.
Materials and methods
1. Data sources
This meta-analysis was carried out for assessment of the association between preeclampsia and the risk of ASD among children. PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement checklist was applied for reporting of systematic reviews to enhance the quality of reporting [10]. We have performed search for finding relevant studies in major electronic databases including; Web of Science, PubMed, and Scopus up to May 2018.
2. Search strategy
The search strategy was conducted by main terminology including: (Autism or Autism spectrum disorders) and (pre-eclampsia or preeclampsia or pregnancy toxemia or edema-proteinuria-hypertension gestosis).
3. Inclusion and exclusion criteria
Inclusion criteria were all studies of case-control or cohort that identified association between preeclampsia and the risk of ASD among children by systematic search were enrolled. Also, autism diagnostic criteria in present study were International Classification of Diseases (ICD-9), (ICD-10); Diagnostic Statistical Manual of Mental Disorders (DSM-4), (DSM-5); the Autism Diagnostic Observation Schedule (ADOS) and Autism Diagnostic Interview-Revised. Review studies, meta-analysis studies, letter to the review, and case report were exclusion criteria in our study.
4. Data extraction
Two investigators screened title, abstract and full text of studies independently and irrelevant studies were excluded. The disagreements were resolved by discussion between the 2 authors. The data in extraction form were; publications year, first author, country, study design, ASD and preeclampsia diagnostic criteria, sample size, odds ratio (OR), RR, and their associated 95% CIs, adjustment, child's age, and quality of studies.
5. Quality assessment
The quality assessment of the selected observational studies was carried out by using an improved Newcastle-Ottawa Scale [11]. Two investigators conducted the quality assessment independently and scores of the papers were divided to low quality (<7 points) and high quality (≥7 points).
6. Statistical analysis
The ORs or RRs with 95% CI were enrolled from eligible studies used as the common measure of association among studies. Heterogeneity across studies was addressed using the I2 statistic. The low, medium, and high heterogeneity was defined by I2 statistic values of 25%, 50%, and 75%, respectively.
Publication bias was assessed using the Begg and Egger’s regression model and by visually funnel plot asymmetry in included studies [12]. All meta-analysis were done using Stata ver. 13 (Stata Corp., College Station, TX, USA).
Results
1. Description of studies
A total of 524 citations yielded in the initial search based on search strategy. After excluding 145 duplicate articles, we have identified articles by title, abstract and full text. At the end, 16 full paper studies were excluded (inappropriate study design, 9; no available sufficient data, 2; and different outcome evaluation method, 5) and 13 articles were retrieved for inclusion in the analysis. The diagram of included studies and selection process are presented in Fig. 1.
Fig. 1.
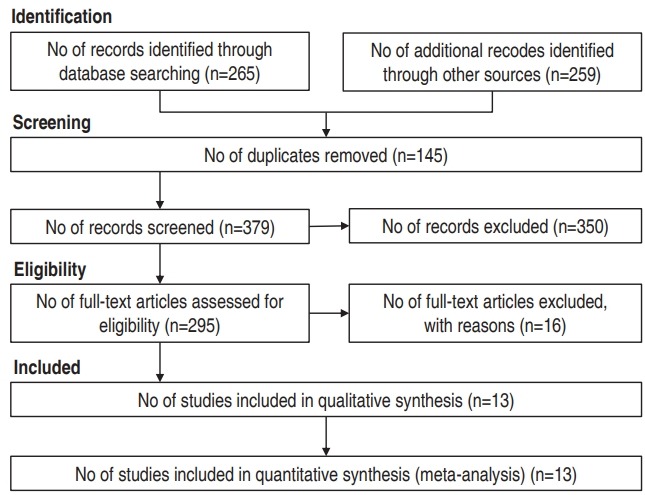
Flowchart of studies extracted through different phases of the systematic review.
Finally, seven studies case-control [3,4,13-17] and sex studies cohort [2,18-22] were included in the meta-analysis with sample size 7,561,696 participants.
The confounder variables of the association between preeclampsia and ASD among children were including maternal age, child’s sex, birth year, year of diagnosis, birth hospital, education, psychosocial disorder during pregnancy, parity, smoking, prenatal care and race (Table 1).
Table 1.
Summary results of the included studies
| Study | Country | Design | Sample size | Estimate | Adjustment | Child age in diagnose (yr) | Autism criteria | Quality |
|---|---|---|---|---|---|---|---|---|
| Buchmayer [3], 2009 | Sweden | Case-control | 7,296 | Odds ratio | Adjusted | <10 | ICD-9, ICD-10 | High |
| Burstyn [18], 2010 | Canada | Cohort | 218,890 | Rate ratio | Adjusted | 4–5 | ICD-9 | High |
| Getahun [2], 2017 | USA | Cohort | 401,660 | Hazard ratio | Adjusted | 3–17 | DSM-4 | High |
| Glasson [4], 2004 | Australia | Case-control | 1,778 | Odds ratio | Crude | <3 | DSM | High |
| Larsson [13], 2005 | Sweden | Case-control | 18,148 | Rate ratio | Crude | <24 | ICD-10 | High |
| Mann [19], 2010 | USA | Cohort | 87,677 | Odds ratio | Adjusted | No data | ICD-9 | High |
| Raz [14], 2015 | USA | Case-control | 1,767 | Odds ratio | Crude | 3 | DSM-5 | High |
| Walker [15], 2015 | USA | Case-control | 867 | Odds ratio | Crude | 2–5 | ADOS; ADI-R | High |
| Moore [20], 2012 | USA | Cohort | 5,979,605 | Odds ratio | Adjusted | 3 | ICD-9 | High |
| Xiang [21], 2015 | USA | Cohort | 332,323 | Hazard ratio | Adjusted | 2.2–8.7 | ICD-9 | High |
| Polo-Kantola [16], 2014 | Finland | Case-control | 5,168 | Odds ratio | Adjusted | 2–17 | ICD-9, ICD-10 | High |
| Langridge [17], 2013 | Australia | Case-control | 377,708 | Odds ratio | Adjusted | 1–17 | DSM-5, DSM-4 | High |
| Dodds [22], 2011 | Canada | Cohort | 128,809 | Relative risk | Adjusted | No data | ICD-9, ICD-10 | High |
ICD-9, International Classification of Diseases, 9th revision; ICD-10, International Classification of Diseases, 10th revision; DSM-5, Diagnostic Statistical Manual of Mental Disorders; ADOS, Autism Diagnostic Observation Schedule; ADI-R, Autism Diagnostic Interview-Revised.
2. Main analysis
A forest plot of the association between preeclampsia and ASD is presented in Fig. 2. The pooled estimates of OR and RR indicated a significant association between preeclampsia and ASD among children, respectively (OR, 1.36; 95% CI, 1.12–1.60) and (RR, 1.30; 95% CI, 1.20–1.41). There was medium heterogeneity among the studies reported risk ASD among children based on OR (I2=59.8%, P=0.148). There was not heterogeneity in the studies reported risk ASD among children based on RR (I2=0.0%, P=0.659).
Fig. 2.
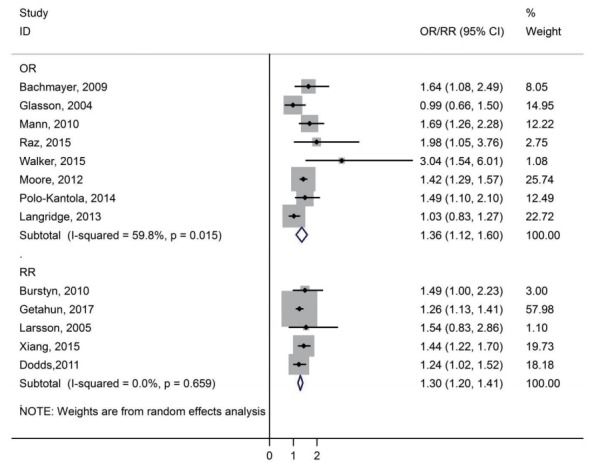
Forest plot of the association between preeclampsia and autism spectrum disorders among children. OR, odds ratio; RR, rate ratio; CI, confidence interval.
The funnel plot graph does not show the clear asymmetry and The P value for Begg and Egger regression were 0.393 and 0.282, respectively. Therefore, we did not observe evidence of the publication bias (Fig. 3).
Fig. 3.
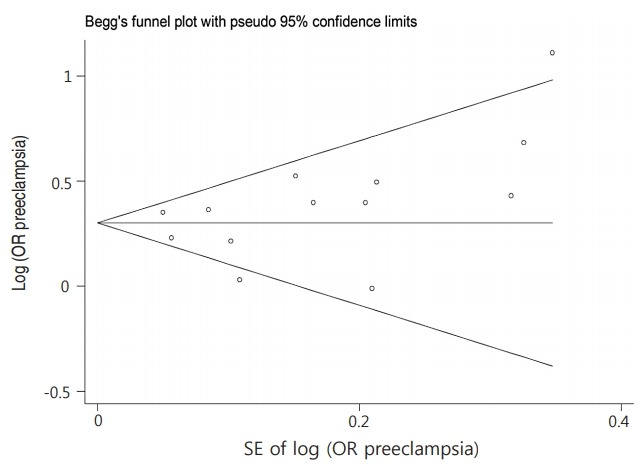
Funnel plot of the association between preeclampsia and autism spectrum disorders among children. OR, odds ratio; SE, standard error.
3. Subgroup analysis
We carried out subgroup analyses based on type of results presentation (crude/adjusted). The pooled results based on OR in crude and adjusted studies were 1.47 (0.79–2.16) and 1.38 (1.13–1.63), respectively. There was significant association in adjusted studies in Table 2.
Table 2.
Results of the subgroup analysis of the preeclampsia and autism spectrum disorders (studies based on odds ratio [OR])
| Subgroups | Studies |
||
|---|---|---|---|
| No. of studies | OR (95% CI) | I2 | |
| Crude analysis | 4 | 1.47 (0.79–2.16) | 43.0% |
| Adjusted analysis | 5 | 1.38 (1.13–1.63) | 65.9% |
CI, confidence interval.
4. Quality of the studies
The present epidemiologic surveys had high-quality according to the Newcastle-Ottawa Scale (Table 1).
Discussion
The present meta-analysis is based on epidemiologic studies published regarding risk of ASD among children born from mothers with history of preeclampsia in pregnancy. We found a significant association between preeclampsia and the risk of ASD among children. Pooled estimate of included studies showed that OR and RR of ASD among children of preeclampsia mothers were 1.36 and 1.30, respectively. There was no heterogeneity in the studies reported risk of ASD among children based on RR, while heterogeneity in the studies reported risk of ASD based on OR was medium.
A meta-analysis by Wang et al. [9] in 2017 showed that preeclampsia is a risk factor for ASD among children (RR, 1.50; 95% CI, 1.04–2.18). In this meta-analysis, data from 3 cohort studies were extracted. However, this meta-analysis was restricted to cohort studies. Also, low number of studies may decrease the generalizability of the findings.
Other study in this field by Dachew et al. [23] in 2018 was conducted. They reported that preeclampsia increased the risk of ASD in offspring (RR, 1.32; 95% CI, 1.20–1.45). This study searched databases of PubMed, Embase, and PsycINFO. However, this meta-analysis limited to 10 studies and they was not searched Web of Science database.
Preeclampsia is a multisystem disorder in second half of pregnancy that can lead to mother and fetal morbidity and mortality. The main cause of preeclampsia is unknown. Some studies confirmed the hypothesis that longer exposure to preeclampsia during early fetal life may associate with neurodevelopment at a critical stage of fetal brain development that may lead to behavioral disorders. However, more studies are needed to assess the effects of preeclampsia on brain development.
The inadequate oxygenation in placenta with poor placental development frequently occurs in the preeclampsia, and probably leads to intrauterine growth restriction and infants born small for gestational age. Also, suboptimal uteroplacental perfusion is due to abnormal trophoblast differentiation during embryogenesis and the effects of vascular compromise progress. Bilayer foldings of abnormal trophoblast are associate with ASD [24]. Therefore, preeclampsia can increase risk for developing autistic disorders [3,19].
According to 2 meta-analyses, obesity is a risk factor for preeclampsia and ASD [25,26]. Therefore, increasing obesity prevalence has paralleled the increase in preeclampsia and ASD.
Our meta-analysis has three limitations. First, all of included studies were conducted at settings with good resources so that from 13 studies included, 6 surveys performed in United States, 2 in Sweden, 4 in Australia and Canada, and 1 study in Finland, therefore generalizability of finding to all setting is questionable. Second, all of studies were not reported adjusted results, the prenatal or perinatal risk factors including Apgar score and gestational age might be considered as confounding factors in this issue. Therefore, our overall estimated OR or RR is prone to effect of confounders. Finally, data that were presented by enrolled studies were not sufficient to perform some subgroup analysis based on confounding variables. However, our findings indicated based on epidemiological studies with sample size 7,561,696 participants that preeclampsia can increase the risk of ART among children.
In conclusion, despite existing controversy, our findings indicated that preeclampsia is associated with increased risk of ASD among children.
Acknowledgments
We thank from Hamadan University of Medical Sciences for financial support in this study.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Bailey A, Phillips W, Rutter M. Autism: towards an integration of clinical, genetic, neuropsychological, and neurobiological perspectives. J Child Psychol Psychiatry. 1996;37:89–126. doi: 10.1111/j.1469-7610.1996.tb01381.x. [DOI] [PubMed] [Google Scholar]
- 2.Getahun D, Fassett MJ, Peltier MR, Wing DA, Xiang AH, Chiu V, et al. Association of perinatal risk factors with autism spectrum disorder. Am J Perinatol. 2017;34:295–304. doi: 10.1055/s-0036-1597624. [DOI] [PubMed] [Google Scholar]
- 3.Buchmayer S, Johansson S, Johansson A, Hultman CM, Sparén P, Cnattingius S. Can association between preterm birth and autism be explained by maternal or neonatal morbidity? Pediatrics. 2009;124:e817–25. doi: 10.1542/peds.2008-3582. [DOI] [PubMed] [Google Scholar]
- 4.Glasson EJ, Bower C, Petterson B, de Klerk N, Chaney G, Hallmayer JF. Perinatal factors and the development of autism: a population study. Arch Gen Psychiatry. 2004;61:618–27. doi: 10.1001/archpsyc.61.6.618. [DOI] [PubMed] [Google Scholar]
- 5.Global Burden of Metabolic Risk Factors for Chronic Diseases Collaboration (BMI Mediated Effects), Lu Y, Hajifathalian K, Ezzati M, Woodward M, Rimm EB, et al. Metabolic mediators of the effects of body-mass index, overweight, and obesity on coronary heart disease and stroke: a pooled analysis of 97 prospective cohorts with 1∙8 million participants. Lancet. 2014;383:970–83. doi: 10.1016/S0140-6736(13)61836-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woodham PC, Brittain JE, Baker AM, Long DL, Haeri S, Camargo CA, Jr, et al. Midgestation maternal serum 25-hydroxyvitamin D level and soluble fms-like tyrosine kinase 1/placental growth factor ratio as predictors of severe preeclampsia. Hypertension. 2011;58:1120–5. doi: 10.1161/HYPERTENSIONAHA.111.179069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kramer MS, Berg C, Abenhaim H, Dahhou M, Rouleau J, Mehrabadi A, et al. Incidence, risk factors, and temporal trends in severe postpartum hemorrhage. Am Obstet Gynecol. 2013;209:449. doi: 10.1016/j.ajog.2013.07.007. e1-7. [DOI] [PubMed] [Google Scholar]
- 8.Adam I, Haggaz AD, Mirghani OA, Elhassan EM. Placenta previa and pre-eclampsia: analyses of 1645 cases at medani maternity hospital, Sudan. Front Physiol. 2013;4:32. doi: 10.3389/fphys.2013.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang C, Geng H, Liu W, Zhang G. Prenatal, perinatal, and postnatal factors associated with autism: a meta-analysis. Medicine (Baltimore) 2017;96:e6696. doi: 10.1097/MD.0000000000006696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peters JP, Hooft L, Grolman W, Stegeman I. Reporting quality of systematic reviews and meta-analyses of otorhinolaryngologic articles based on the PRISMA Statement. PLoS One. 2015;10:e0136540. doi: 10.1371/journal.pone.0136540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. Ottawa Hospital Research Institute; 2019. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [Internet] [cited 2018 May 14]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Google Scholar]
- 12.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larsson HJ, Eaton WW, Madsen KM, Vestergaard M, Olesen AV, Agerbo E, et al. Risk factors for autism: perinatal factors, parental psychiatric history, and socioeconomic status. Am J Epidemiol. 2005;161:916–25. doi: 10.1093/aje/kwi123. [DOI] [PubMed] [Google Scholar]
- 14.Raz R, Roberts AL, Lyall K, Hart JE, Just AC, Laden F, et al. Autism spectrum disorder and particulate matter air pollution before, during, and after pregnancy: a nested case-control analysis within the Nurses' Health Study II Cohort. Environ Health Perspect. 2015;123:264–70. doi: 10.1289/ehp.1408133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walker CK, Krakowiak P, Baker A, Hansen RL, Ozonoff S, Hertz-Picciotto I. Preeclampsia, placental insufficiency, and autism spectrum disorder or developmental delay. JAMA Pediatr. 2015;169:154–62. doi: 10.1001/jamapediatrics.2014.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polo-Kantola P, Lampi KM, Hinkka-Yli-Salomäki S, Gissler M, Brown AS, Sourander A. Obstetric risk factors and autism spectrum disorders in Finland. J Pediatr. 2014;164:358–65. doi: 10.1016/j.jpeds.2013.09.044. [DOI] [PubMed] [Google Scholar]
- 17.Langridge AT, Glasson EJ, Nassar N, Jacoby P, Pennell C, Hagan R, et al. Maternal conditions and perinatal characteristics associated with autism spectrum disorder and intellectual disability. PLoS One. 2013;8:e50963. doi: 10.1371/journal.pone.0050963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burstyn I, Sithole F, Zwaigenbaum L. Autism spectrum disorders, maternal characteristics and obstetric complications among singletons born in Alberta, Canada. Chronic Dis Can. 2010;30:125–34. [PubMed] [Google Scholar]
- 19.Mann JR, McDermott S, Bao H, Hardin J, Gregg A. Pre-eclampsia, birth weight, and autism spectrum disorders. J Autism Dev Disord. 2010;40:548–54. doi: 10.1007/s10803-009-0903-4. [DOI] [PubMed] [Google Scholar]
- 20.Moore GS, Kneitel AW, Walker CK, Gilbert WM, Xing G. Autism risk in small- and large-for-gestational-age infants. Am J Obstet Gynecol. 2012;206:314. doi: 10.1016/j.ajog.2012.01.044. e1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiang AH, Wang X, Martinez MP, Walthall JC, Curry ES, Page K, et al. Association of maternal diabetes with autism in offspring. JAMA. 2015;313:1425–34. doi: 10.1001/jama.2015.2707. [DOI] [PubMed] [Google Scholar]
- 22.Dodds L, Fell DB, Shea S, Armson BA, Allen AC, Bryson S. The role of prenatal, obstetric and neonatal factors in the development of autism. J Autism Dev Disord. 2011;41:891–902. doi: 10.1007/s10803-010-1114-8. [DOI] [PubMed] [Google Scholar]
- 23.Dachew BA, Mamun A, Maravilla JC, Alati R. Pre-eclampsia and the risk of autism-spectrum disorder in offspring: meta-analysis. Br J Psychiatry. 2018;212:142–7. doi: 10.1192/bjp.2017.27. [DOI] [PubMed] [Google Scholar]
- 24.Kolevzon A, Gross R, Reichenberg A. Prenatal and perinatal risk factors for autism: a review and integration of findings. Arch Pediatr Adolesc Med. 2007;161:326–33. doi: 10.1001/archpedi.161.4.326. [DOI] [PubMed] [Google Scholar]
- 25.Li YM, Ou JJ, Liu L, Zhang D, Zhao JP, Tang SY. Association between maternal obesity and autism spectrum disorder in offspring: a metaanalysis. J Autism Dev Disord. 2016;46:95–102. doi: 10.1007/s10803-015-2549-8. [DOI] [PubMed] [Google Scholar]
- 26.Poorolajal J, Jenabi E. The association between body mass index and preeclampsia: a meta-analysis. J Matern Fetal Neonatal Med. 2016;29:3670–6. doi: 10.3109/14767058.2016.1140738. [DOI] [PubMed] [Google Scholar]


