Abstract
Continuous glucose monitors (CGM) display real-time glucose values enabling greater glycemic awareness with reduced management burden. Factory-calibrated CGM systems allow for glycemic assessment without the pain and inconvenience of fingerstick glucose testing. Advances in sensor chemistry and CGM algorithms have enabled factory-calibrated systems to have greater accuracy than previous generations of CGM technology. Despite these advances many patients and providers are hesitant about the idea of removing fingerstick testing from their diabetes care. In this commentary, we aim to review the clinical trials on factory-calibrated CGM systems, present the algorithms which facilitate factory-calibrated CGMs to improve accuracy, discuss clinical use of factory-calibrated CGMs, and finally present two cases demonstrating the dangers of utilizing exploits in commercial systems to prolong sensor life.
Keywords: Continuous glucose monitoring, Type 1 diabetes, Factory calibration
Introduction
Subcutaneous Continuous Glucose Monitoring (CGM) utilizes a glucose-oxidase enzyme reaction to measure the glucose concentration in interstitial fluid and estimate glucose concentration in the blood.1,2 The first Food and Drug Administration (FDA)-approved CGM, the Minimed CGM System, was approved in 2000 with a mean absolute relative difference (MARD) between Yellow Springs Instruments Glucose Analyzer (YSI, Yellow Spring, OH) and sensor glucose of 25% (23–27%).3 Over the past 18 years, systems have progressively improved with MARD values in the 12%–16% range with third-generation systems, 13%–14% range with fourth-generation systems, and 9%–11% with fifth-generation systems.4,5 These systems all required user calibrations whereby self-monitoring of blood glucose (SMBG) fingerstick values through a home blood glucose meter (BGM) were used to correlate the sensor signal with a patient's blood glucose value.6 On September 27, 2017 the United States FDA approved the Abbott Freestyle Libre Flash Glucose Monitoring (FGM) System as the first factory-calibrated glucose monitoring system with a published MARD of 11.4%.7 On March 27, 2018 the FDA approved the Dexcom G6 as the first real-time factory-calibrated CGM system with a published MARD of 9.0%.8,9
Factory calibration enables a CGM system to be used by patients without the need to periodically conduct SMBG measurements. Such an advancement enables therapies for type 1 diabetes (T1D) and type 2 diabetes (T2D) to move to a realm of decreased patient burden long thought to be impossible. The ability of a provider to tell a patient “you no longer need to poke your finger” is truly transformative for the field. This advancement has been achieved partly through improvements in sensor chemistry and in device manufacturing processes, however, the major driver has been advancement in the algorithms used within the CGM systems to translate the sensor signal into a glucose value. In this commentary, we aim to demystify this process to better aid clinicians in understanding how factory calibration works, why it is safe, and how various exploits to prolong sensor life may be dangerous.
Review of Clinical Trials of Factory-Calibrated CGM
The Abbott Freestyle Libre FGM calculates a glucose value every 15 min, although it does not report real-time values. Instead, the past 8 h of data are downloaded to the reader when the user scans or “flashes” the Near Field Communication tag. Hoss et al. reported accuracy for a factory-calibrated version of the Libre FGM sensor in 2014.10 They examined 33 subjects with T1D and T2D, each wearing four sensors with a 6.0% coefficient of variation between sensors within a subject across the study. Factory calibration of the sensors produced a MARD of 13.4% with 83.5% of values falling within zone A of the Consensus Error Grid (CEG).11 In 2015 Bailey et al. reported the results of the adult pivotal trial of the Libre FGM system.7 They studied 72 adults with T1D or T2D across four clinical sites wearing the FGM sensor for up to 14 days. Sensor values were compared against SMBG values and reference YSI values.12 The factory-calibrated FGM sensor demonstrated an overall MARD of 11.4% with 85%–89% of values in the CEG zone A, and an average sensor lag time of 4.5 ± 4.8 min.7
The Dexcom G6 CGM calculates a glucose value every 5 min and then reports that value in real-time through Bluetooth communication to a paired receiver, cell phone, or insulin pump. The results of the pivotal trials of the Dexcom G6 have been recently reported by Shah and Wadwa.8,9 In this series of studies, the Dexcom G6 was actually used with once-daily calibration. After the completion of patient use, the raw signal data were reprocessed using the new factory calibration algorithm without additional patient-driven calibrations to demonstrate the accuracy of the system with this build.
Wadwa et al. reported results for 262 patients with T1D and T2D ages 6+ years old at 11 sites using the factory calibration algorithm.9 Subjects wore the Dexcom G6 for up to 10 days and underwent frequent sample testing on day 1, 4, 5, 7, or 10. The overall MARD was 10.0% with a similar MARD reported for patients 18+ years old and patients 6–17 years old (9.9% vs. 10.1%). This analysis also looked at the performance of a real-time predictive hypoglycemia alert, which correctly alerted patients 84% of the time within 30 min before impending hypoglycemia <70 mg/dL. Overall, 87% of the sensors lasted for the full 10-day period. The average time lag was 4.5 ± 3.3 min.9
Shah et al. reported results for 62 patients with T1D and T2D ages 6+ years old at four sites using the factory-calibrated algorithm.8 The primary purpose of this study was to evaluate the accuracy of the Dexcom G6 CGM with a new automated sensor applicator, which was hypothesized to decrease pain and inflammation and improve sensor accuracy. Participants wore the Dexcom G6 for up to 10 days and underwent frequent sample testing on day 1, 4, 5, 7, or 10. The overall MARD was 9.0% with better accuracy reported for the adolescents than for adults (7.7% vs. 9.8%). Accuracy was found to be similar for day 1 compared with the other 9 days of sensor use. The average time lag was 3.7 ± 3.1 min.8
Discussion of Factory-Calibrated CGM
How multiple daily-calibrated sensors have been working
Most CGMs, including the Medtronic Guardian 3, Abbott Freestyle Libre, Dexcom G4 Platinum, the Dexcom G5, and Dexcom G6, utilize the previously mentioned glucose oxidase reaction to estimate glucose in the interstitial subcutaneous tissue based on a sensed electrical current.13,14 The measured current is proportional to the concentration of interstitial glucose at the insertion site, however, the relationship between current and glucose concentration changes over time. Thus, to determine concentrations of blood glucose, the current measured at the interstitial site is converted to blood glucose through a calibration function. Due to factors such as manufacturing variability, sensor drift, and biocompatibility (such as changes over time in foreign body response to the sensor), the calibration function needs to be updated based on sensor batch and time since insertion.15–17
In previous generation sensors, such as the G4 Platinum and G5, the parameters of the calibration function are periodically updated, usually every 12 h, by matching output from the calibration function to a reference SMBG measurement to preserve sensor accuracy. In this study, we describe an overview of the calibration method, for which specifics have been previously published.18–20 We note that the methods described here correspond to the most recently published algorithms, which to the best of our knowledge, form the basis of the commercial products. However, it is possible that these algorithms may or may not have been modified between publication and final device manufacturing.
The G4 Platinum algorithm uses a linear function to convert the raw electrical current measured at the interstitial site to a measurement of interstitial glucose. This model assumes that interstitial glucose at some time t, 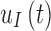 is equal to some multiple, a, of the interstitial current
is equal to some multiple, a, of the interstitial current 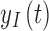 , added white noise
, added white noise 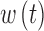 , a linear correction factor, b, and some multiple c, of time-since-insertion,
, a linear correction factor, b, and some multiple c, of time-since-insertion, 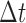 .
.
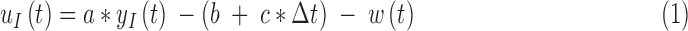 |
The term 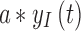 is referred to as the sensor gain, and the
is referred to as the sensor gain, and the 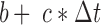 is the offset. Then, to account for the relation between interstitial glucose and blood glucose, the measurement
is the offset. Then, to account for the relation between interstitial glucose and blood glucose, the measurement 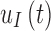 is transformed to a measurement of glucose,
is transformed to a measurement of glucose, 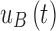 , based on the two-compartment model.15
, based on the two-compartment model.15
This function assumes a linear relationship between the measured interstitial current and the actual glucose value, however, due to the changing environment around the sensor and other factors affecting sensor drift, this linear function is only accurate for about 24 h.15 This results in the parameters of the function needing to be adjusted every 12 h using SMBG measurements to ensure accuracy at the level needed for safe medical decision making.
To adjust the parameters a, b, c of the calibration function, a calibration algorithm is used. In essence, this algorithm begins with an “average value” for each parameter a, b, c, which are specific to the day since insertion. These averages were identified by averaging best-fit parameters for 72 patients of previously collected data consisting of measured interstitial current and glucose measurements obtained through YSI.12
These averages provide a good starting point, however, due to sensor variability between batches and individual's wearing the sensor, the parameters are adjusted from the averages to minimize the difference between the glucose identified using the new calibration function, and the last two SMBG measurements. This process enables the sensor to “check” how well the calibration function is working by comparing the sensor glucose value identified from passing the interstitial current through the calibration function to a “ground truth” glucose value from the SMBG. It has been documented that SMBG values are imperfect reference standards, as is discussed in a later section on fingerstick testing.
How factory-calibrated G6 works
To remove the need for SMBG calibrations, the G6 uses a calibration function, which corrects for sensor drift over the 10-day wear period by keeping track of the day since insertion and adjusting the calibration function, which converts interstitial current to glucose, based on the day. The adjustments are hardcoded and based on how much an “average” sensor would drift. Additionally, rather than the near function used in previous models, which accounts for drift over 24h, the G6 uses a calibration function that has time-varying functions for sensor offset and gain, which can account for drift over 10 days.15,18 The new function used to transform interstitial current, 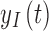 , to interstitial glucose,
, to interstitial glucose, 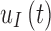 , utilizes device-dependent functions,
, utilizes device-dependent functions, 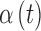 and
and 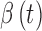 , which describe the sensor drift over time. Specifically, the DG6 uses the following calibration function, rather than (1) which is used by the G4 Platinum:
, which describe the sensor drift over time. Specifically, the DG6 uses the following calibration function, rather than (1) which is used by the G4 Platinum:
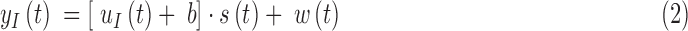 |
Here 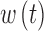 still represents additive noise, but the sensor offset and gain are calculated using
still represents additive noise, but the sensor offset and gain are calculated using 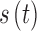 , which represents sensor sensitivity, and is device specific, combined with a term, b, which is the baseline of the glucose profile. The sensitivity function, which now utilizes the device-dependent nonlinear functions
, which represents sensor sensitivity, and is device specific, combined with a term, b, which is the baseline of the glucose profile. The sensitivity function, which now utilizes the device-dependent nonlinear functions 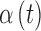 and
and 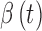 , and model parameters
, and model parameters 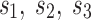 , is shown below:
, is shown below:
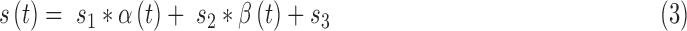 |
Incorporation of the potentially nonlinear time-varying functions, 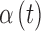 and
and 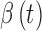 , which account for sensor sensitivity and drift is what enables the DG6 to maintain accuracy over the 10-day use window without requiring user calibration.15
, which account for sensor sensitivity and drift is what enables the DG6 to maintain accuracy over the 10-day use window without requiring user calibration.15
Rather than adjusting parameters of the calibration function with every incoming SMBG, the parameters are identified based on an initial factory calibration and “average values” of best-fit parameters. The distribution of average values comes from best-fit parameters, which were identified for a data set consisting of measured values of interstitial current from the CGM and reference YSI measurements from a group of clinical trial subjects.15 These average values provide the starting point for the parameters, which are then adjusted slightly during the warm-up period using the factory calibration to account for manufacturing variability between batches. For the G6 in particular, the factory calibration information is stored in the sensor code.
Removing the necessity of SMBG calibrations provides a large benefit to the patient in terms of ease of use and cost, however, it heightens the dependence on the initial factory calibration and on-label use of the sensor. In particular, to maintain high accuracy, the calibration function inside the G6 relies on accurate information of time since insertion, since the sensor never “checks” how well the calibration function is performing for a specific individual during wear. For a given sensor, the same level of electrical current could translate to a significantly different BG value on day 1 versus day 10 due to sensor drift and biocompatibility.16–18 Hence, off-label resetting of the calibration-free device during the initial 10-day window could result in the dangerous condition of the CGM translating a measured current to a glucose value of 160 mg/dL when the actual value is 60 mg/dL, or vice versa, by using the wrong gain. Case series illustrating this exact phenomenon are presented in the final section of this commentary.
Why Removing Fingersticks is Beneficial
Although SMBG calibrations give CGM users a sense of control over the accuracy of their CGM readings, it is important to consider the literature on SMBG accuracy itself. A 2017 study by Ekhlaspour investigated the comparative accuracy of 17 commercially available BGMs using venous blood samples, thereby eliminating error from skin contamination.21 These BGMs were compared against YSI values for reference. This investigation demonstrated a range in MARD from 5.6% to 20.8% across these commercial devices. Of the 17 devices tested, 47% had a MARD <10%, whereas 53% had a MARD 13%–20.8%. A separate analysis by Klonoff and the Diabetes Technology Society looked at the accuracy of 18 commercially approved BGMs compared against YSI using capillary blood glucose testing.22 This analysis looked at three different accuracy studies. They found that 6 of the 18 systems met the predetermined accuracy standard in all 3 studies, 5 met it in 2 of 3 studies, 3 met it in 1 of 3 studies, and 4 of the commercially available devices did not meet accuracy standards in any of the 3 studies. Taken together, these two studies highlight the wide variability in accuracy among commercially approved BGM devices and that many devices fall outside the recommended range for accuracy.
Beyond research, real-world device use is important to consider. Among pediatric patients in particular, unclean fingertips can produce significant pseudohyperglycemia by falsely elevating the measured SMBG value.23–25 Several studies conducted on fingerstick-assessed SMBG values after handling fruit have demonstrated that contamination can falsely elevate assessed values by >250 mg/dL.25 Equally important is that cleaning the fingertip with one or even five alcohol swabs before testing did not eliminate the contamination effect for most fruits.25 This form of error is highly important to consider when discussing SMBG testing for CGM calibration as entering a falsely high reference value will bias the CGM reading falsely high, thus limiting the reporting of true hypoglycemia.
There are two main take-away points from this discussion of SMBG accuracy. First, even conventional SMBG testing is subject to error and possibly seriously erroneous glycemic assessment in the setting of fingertip contamination. Second, SMBG values may be a highly error-based form of calibration of CGM systems. By supplying erroneous forms of “truth” to the CGM systems we limit their potential accuracy thereby reducing rather than improving patient utility and safety.
Factory-Calibrated CGM in Clinical Practice
The implications of factory-calibrated CGM intersects significantly with the ability of CGM systems to replace SMBG for diabetes decision making. Taken together, the near removal of SMBG from clinical care with CGM dramatically changes patient and provider practices for diabetes management and support. In the real world, some individuals still chose to manually calibrate factory-calibrated devices. Furthermore, as part of routine diabetes care, many individuals have been in the practice of dosing insulin off of CGM systems not approved for replacement SMBG, however, there are no published data quantifying the extent of this practice.
To implement factory-calibrated CGM into standard clinical practice, it is incumbent on health care providers and diabetes educators to make two critical changes. The first is to alter clinical practices to enhance uptake and use of CGM data for clinical diabetes care. Technologies like CGM continue to be a barrier for providers and diabetes educators who feel ill equipped to handle the magnitude of data available for diabetes care.26 To better manage it, practices will need to be restructured to facilitate use of CGM as primary glucose monitoring for patient care visits. This will involve training staff on new downloading and data preparation practices, and new methods for interpreting sensor glucose levels. Ongoing integration of CGM with pump downloads and other diabetes data may ease the transition as well.27
Second, providers and educators must retune their education priorities for CGM utility and ensuing diabetes management. The most critical education point is explaining the theoretical harm in restarting these factory-calibrated CGMs, in particular as it relates to previous systems. Although the vast majority of individuals need not be exposed to the calculations presented above, patients do need a clear understanding of why factory-calibrated devices, which rely on accurate knowledge of the time since insertion, will degrade in accuracy when used beyond their commercial labeling, leading to the possibility of real harm. The highest concerns for sensors being restarted with inappropriate calibration schema include administering inappropriate doses of insulin in response to erroneous sensor glucose levels, inappropriately treating hypoglycemia or pseudohypoglycemia with carbohydrates or glucagon, missed alerts, and missed detection of hypoglycemia resulting in severe hypoglycemia. More research is warranted to characterize the frequency and severity of these risks.
Another significant educational priority is cautiously normalizing the use of factory-calibrated CGMs for medical decision making. This runs counter to decades of entrenched education on using CGM as supplementary diabetes information, and thus is a difficult concept to change for both clinicians and individuals with diabetes. Unambiguous education and endorsement from health care providers on safe and efficient use of factory-calibrated CGMs for medical decision making will be beneficial to individual patients and the diabetes community at large. Major paradigm shifts in glucose monitoring have occurred before, with the last one being four decades ago from urine testing to blood glucose testing. Similarly, uptake to contemporary CGM technologies may be slow and difficult in transition; but will likely decrease burden of diabetes management for patients. Health care providers and diabetes educators must be prepared to provide relevant information and support to patients to transition safety and appropriately to factory-calibrated CGM for medical decision making.
Patient Cases of Restarting the Dexcom G6
In an attempt to better understand the impact of restarting a factory-calibrated CGM, we reviewed data from two adults with T1D who regularly restart their Dexcom G6 systems without providing SMBG calibration values. As this practice is contrary to FDA labeling and believed by our group to be unsafe, we did not attempt to prospectively gather such data. Neither CGM user performed SMBG testing on a routine basis to provide adequate data to compare SMBG against CGM values. Data were voluntarily provided by these patients and had been deidentified to comply with HIPAA standards. Case 1 is a 25-year-old female with T1D for over 10 years and Case 2 is a 36-year-old male with T1D for over 10 years.
For factory-calibrated CGMs worn beyond the recommended 10-day period, we analyzed the average and distribution of the sensor glucose values across all days, with focus on comparing the true days 1–2 of wear (48 h) with days 11–12 of wear (the first 48 h after the restart) with each comparison consisting of ∼576 values per time period. We focused on the first 2 days of wear and first 2 days after restart due to the known wound reaction effects seen with initial sensor placement.28 The factory calibration function must account for this reaction on true days 1 and 2. With a sensor restart, the calibration function is correcting for a wound reaction, which is in fact not occurring. Due to this effect, we hypothesized that restarting the sensors would produce higher average glucose values with greater glycemic variability as the calibration function was being falsely informed that the sensor was a day 1–2 sensor when in fact it was being used beyond day 10.
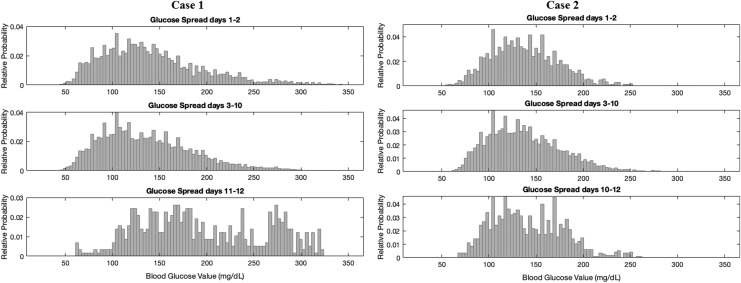
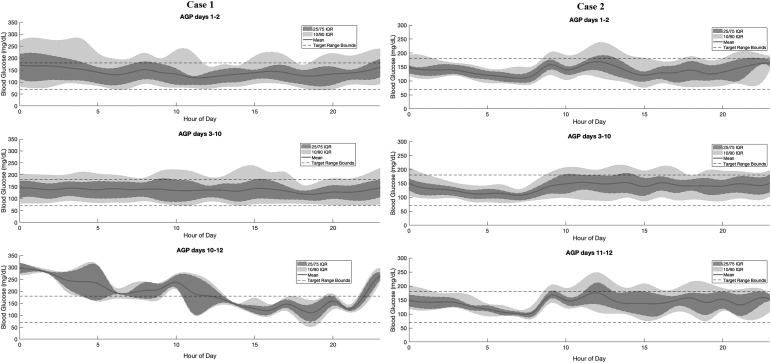
The results of these case reports are shown in Figures 1, 2, and Table 1. Both Case 1 and Case 2 had significantly higher average sensor glucose (SG) values, with higher SG variability after the sensor restart than on true days 1–2, as well as days 3–10 of wear. Calculating Kullback–Leibler divergences of the distributions showed that the restart distributions were significantly different from the true day 1–2 distributions with P-values <0.01 for all individuals, while distributions of days 1–2 with days 3–10 are more similar to one another. The Kullback–Leibler divergence is a distribution-wise asymmetric measure, which quantifies if one distribution is different from a second.29 Looking at the day of sensor start by day of week did not reveal any trend toward tending to start sensors on a certain day of the week (e.g., always on the weekend), whereby periodic differences in habits could contribute to this trend.
FIG. 1.
Distribution of sensor glucose values on days 1–2 of recommended wear compared with days 11–12 of extended wear as well as days 3–10 of recommended wear. Days 11–12 represent a different distribution from days 1 to 2 due to software incorporated into the sensor that uses different calibration on what is presumed to be a newly inserted sensor than for days 3–10.
FIG. 2.
Ambulatory glucose profiles over times of the day, grouped by days of wear, with percentiles and mean blood glucose values.
Table 1.
Case Comparisons of Days 1–2, 3–10, and 11–12
| Case 1 | Case 2 | |
|---|---|---|
| Mean SG day 1–2 | 142 | 137 |
| Mean SG day 3–10 | 136 | 137 |
| Mean SG day 11–12 | 195 | 141 |
| SD SG day 1–2 | 54 | 36 |
| SD SG day 3–10 | 48 | 38 |
| SD SG day 11–12 | 65 | 38 |
| P-value different distributions between days 1–2 and 11–12 | <0.001 | 0.008 |
| P-value different distributions between days 1–2 and 3–10 | 0.01 | 0.14 |
| Percent time BG <54 mg/dL day 1–2 | 0.41% | 0.00% |
| Percent time BG <70 mg/dL day 1–2 | 4.90% | 1.10% |
| Percent time BG 70–180 mg/dL day 1–2 | 73.72% | 86.13% |
| Percent time BG >180 mg/dL day 1–2 | 21.38% | 12.77% |
| Percent time BG <54 mg/dL day 3–10 | 0.57% | 0.00% |
| Percent time BG <70 mg/dL day 3–10 | 5.09% | 0.65% |
| Percent time BG 70–180 mg/dL day 3–10 | 76.63% | 84.61% |
| Percent time BG >180 mg/dL day 3–10 | 18.28% | 14.74% |
| Percent time BG <54 mg/dL day 11–12 | 0.00% | 0% |
| Percent time BG <70 mg/dL day 11–12 | 1.21% | 0.35% |
| Percent time BG 70–180 mg/dL day 11–12 | 46.88% | 83.91% |
| Percent time BG >180 mg/dL day 11–12 | 51.91% | 15.74% |
SD, standard deviation; SG, Sensor Glucose.
These data indicate that the distributions of SG values appear to be different after a user restart compared with using the device in the initial 10-day period. That both individual's SG averages tended to be higher after the restart is particularly concerning as falsely elevated CGM values may be more likely to falsely reassure a patient that true hypoglycemia is not present or increase the risk for overdosing a correction dose of insulin. While this analysis is small, exploratory, and by no means conclusive, it indicates that extending sensor life beyond its recommended duration may be unsafe. It is notable that changes in stress, illness, and lifestyle between the periods of comparison could have contributed to the differences seen with extended wear in such a small case sample. Larger prospective studies of subjects wearing multiple sensors for a prolonged duration, such as those previously performed by Buckingham,30,31 would certainly better address these questions.
Comment from Dexcom on Restarting the G6 CGM
As due diligence for this article, we reached out to Dexcom for commentary on restarting the G6 CGM. Dexcom asked that we supply the following information and warning to researchers and health care providers:
“The Dexcom G6 CGM algorithm is designed for optimal performance for up to 10 days of sensor wear. Intentionally altering, modifying or hacking the G6 system to extend the sensor usage beyond the labeled 10-day wear period may compromise the system. This can lead to inaccurate CGM readings, resulting in missed hypoglycemia or hyperglycemia.
The Dexcom G6 CGM System was granted De Novo clearance in March 2018 by the US FDA as the first of its kind interoperable CGM (iCGM) System. The FDA also published several special clinical performance metrics for an iCGM. One of these special controls requires manufacturers to demonstrate that “The device must include appropriate measures to ensure that disposable sensors cannot be used beyond its claimed sensor wear period.” Dexcom is obligated to take measures at all times to prevent sensors from restarting and as such must continually evaluate design mitigations to ensure adherence to this special control.”
Conclusions
With this transition from SMBG to factory-calibrated CGM for medical decision making, it is inevitable that there will be resistance to abandoning the perception of “certainty” inherent to SMBG measurements. Garg and Hirsch outlined many key issues with SMBG in the 2018 Advanced Technologies and Treatments for Diabetes yearbook SMBG chapter.32 The key point they raised is that many areas of the world do not have access or affordability for CGM and thus SMBG will not be disappearing any time soon. This certainly argues for retaining SMBG as a mainstay of diabetes therapy in some circumstances, however, should not dissuade factory-calibrated CGM from becoming a new standard for diabetes management when available.
Acknowledgments
Work on this publication by T.K., L.H.M., and S.S. was supported by NSF Grant 1815983 and by G.P.F. was supported by NIH K12 grant DK094712.
Author Disclosure Statement
G.P.F. reports research support from the NIH NIDDK, Medtronic, Tandem, Insulet, Dexcom, Abbott, Novo Nordisk, Type Zero, and Beta Bionics. He has served as an advisory board member for Dexcom, a paid consultant for Medtronic and Abbott, and a speaker for Tandem, Dexcom, and Medtronic. L.H.M. is a Contract Product Trainer for Medtronic Diabetes and consults for Tandem Diabetes Care, Capillary Biomedical, and Clinical Sensors. R.P.W. reports research support from Lexicon, Dexcom, Bigfoot Biomedical, MannKind Corporation, Novo Nordisk, Helmsley Charitable Trust and NIH/NIDDK, advisory board consulting fees from Eli Lilly and Company, and consulting fees from Dexcom. T.K. and S.S. report no conflicts of interest.
References
- 1. Kadish AH, Hall DA: A new method for the continuous monitoring of blood glucose by measurement of dissolved oxygen. Clin Chem 1965;11:869–875 [PubMed] [Google Scholar]
- 2. Ginsbergh BH: The FDA panel advised approval of the first continuous glucose sensor. Diabetes Technol Ther 1999;1:203–204 [DOI] [PubMed] [Google Scholar]
- 3. DirecNet Study Group: The accuracy of the CGMS™ in children with type 1 diabetes: results of the Diabetes Research in Children Network (DirecNet) accuracy study. Diabetes Technol Ther 2003;5:781–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Facchinetti A: Continuous glucose monitoring sensors: past, present and future algorithmic challenges. Sensors (Basel) 2016;16:2093–2104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Slover RH, Tryggestad JB, DiMeglio LA, et al. : Accuracy of a fourth-generation continuous glucose monitoring system in children and adolescents with type 1 diabetes. Diabetes Technol Ther 2018;20:576–584 [DOI] [PubMed] [Google Scholar]
- 6. Choleau C, Klein JC, Reach G, et al. : Calibration of a subcutaneous amperometric glucose sensor. Part 1. Effect of measurement uncertainties on the determination of sensor sensitivity and background current. Biosens Bioelectron 2002;17:641–646 [DOI] [PubMed] [Google Scholar]
- 7. Bailey T, Bode BW, Christiansen MP, et al. : The performance and usability of a factory-calibrated flash glucose monitoring system. Diabetes Technol Ther 2015;17:787–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shah VN, Laffel LM, Wadwa RP, Garg SK: Performance of a factory-calibrated real-time continuous glucose monitoring system utilizing an automated sensor applicator. Diabetes Technol Ther 2018;20:428–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wadwa RP, Laffel LM, Shah VN, Garg SK: Accuracy of a factory-calibrated, real-time continuous glucose monitoring system during 10 days of use in youth and adults with diabetes. Diabetes Technol Ther 2018;20:395–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hoss U, Budiman ES, Liu H, Christiansen MP: Feasibility of factory calibration for subcutaneous glucose sensors in subjects with diabetes. J Diabetes Sci Technol 2014;8:89–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Parkes JL, Slatin SL, Pardo S, Ginsberg BH: A new consensus error grid to evaluate the clinical significance of inaccuracies in the measurement of blood glucose. Diabetes Care 2000;23:1143–1148 [DOI] [PubMed] [Google Scholar]
- 12. Acciaroli G, Vettoretti M, Facchinetti A, et al. : Reduction of blood glucose measurements to calibrate subcutaneous glucose sensors: a Bayesian multi-day framework. IEEE Trans Biomed Eng 2018;65:587–595 [DOI] [PubMed] [Google Scholar]
- 13. Dzyadevych SV, Arkhypova VN, Soldatkin AP, et al. : Amperometric enzyme biosensors: past, present and future. IRBM 2008;29:171–180 [Google Scholar]
- 14. Hoss U, Budiman ES: Factory-calibrated continuous glucose sensors: the science behind the technology. Diabetes Technol Ther 2017;19(S2):S44–S50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Acciaroli G, Vettoretti M, Facchinetti A, Sparacino G: Toward calibration-free continuous glucose monitoring sensors: Bayesian Calibration Approach Applied to Next-Generation Dexcom Technology. Diabetes Technol Ther 2018;20:59–67 [DOI] [PubMed] [Google Scholar]
- 16. Helton KL, Ratner BD, Wisniewski NA: Biomechanics of the sensor-tissue interface-effects of motion, pressure, and design on sensor performance and foreign body response-part II: examples and application. J Diabetes Sci Technol 2011;5:647–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Klueh U, Liu Z, Feldman B, et al. : Metabolic biofouling of glucose sensors in vivo: role of tissue microhemorrhages. J Diabetes Sci Technol 2011;5:583–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Acciaroli G, Vettoretti M, Facchinetti A, et al. : From two to one per day calibration of dexcom G4 platinum by a time-varying day-specific Bayesian Prior. Diabetes Technol Ther 2016;18:472–479 [DOI] [PubMed] [Google Scholar]
- 19. De Nicolao G, Sparacino G, Cobelli C: Nonparametric input estimation in physiological systems: problems, methods, and case studies. Automatica 1997;33:851–870 [Google Scholar]
- 20. Vettoretti M, Facchinetti A, Del Favero S, et al. : Online calibration of glucose sensors from the measured current by a time-varying calibration function and Bayesian Priors. IEEE Trans Biomed Eng 2016;63:1631–1641 [DOI] [PubMed] [Google Scholar]
- 21. Ekhlaspour L, Mondesir D, Lautsch N, et al. : Comparative accuracy of 17 point-of-care glucose meters. J Diabetes Sci Technol 2017;11:558–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Klonoff DC, Parkes JL, Kovatchev BP, et al. : Investigation of the accuracy of 18 marketed blood glucose monitors. Diabetes Care 2018;41:1681–1688 [DOI] [PubMed] [Google Scholar]
- 23. Hirose T, Mita T, Fujitani Y, et al. : Glucose monitoring after fruit peeling: pseudohyperglycemia when neglecting hand washing before fingertip blood sampling: wash your hands with tap water before you check blood glucose level. Diabetes Care 2011;34:596–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Arakawa M, Ebato C: Influence of fruit juice on fingertips and patient behavior on self-monitoring of blood glucose. Diabetes Res Clin Pract 2012;96:e50–e52 [DOI] [PubMed] [Google Scholar]
- 25. Olamoyegun MA, Oloyede T, Adewoye OG, et al. : Pseudohyperglycemia: effects of unwashed hand after fruit peeling or handling on fingertips blood glucose monitoring results. Ann Med Health Sci Res 2016;6:362–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Messer LH, Johnson R, Driscoll KA, Jones J: Best friend or spy: a qualitative meta-synthesis on the impact of continuous glucose monitoring on life with Type 1 diabetes. Diabet Med 2018;35:409–418 [DOI] [PubMed] [Google Scholar]
- 27. Sherr JL, Tauschman M, Battelino T, et al. : ISPAD clinical practice consensus guidelines 2018 diabetes technologies. Pediatr Diabetes 2018;19 Suppl 27:302–325 [DOI] [PubMed] [Google Scholar]
- 28. Koh A, Nichols SP, Schoenfisch MH: Glucose sensor membranes for mitigating the foreign body response. J Diabetes Sci Technol 2011;5:1052–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Seghouane AK, Amari S: The AIC criterion and symmetrizing the Kullback-Leibler divergence. IEEE Trans Neural Netw 2007;18:97–106 [DOI] [PubMed] [Google Scholar]
- 30. DeSalvo DJ, Maahs DM, Messer L, et al. : Effect of lipohypertrophy on accuracy of continuous glucose monitoring in patients with type 1 diabetes. Diabetes Care 2015;38:e166–e167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Forlenza GP, Deshpande S, Ly TT, et al. : Application of zone model predictive control artificial pancreas during extended use of infusion set and sensor: a randomized crossover-controlled home-use trial. Diabetes Care 2017;40:1096–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Garg SK, Hirsch IB: Self-monitoring of blood glucose. Diabetes Technol Ther 2018;20(S1):S-3–S-12 [DOI] [PubMed] [Google Scholar]