Abstract
Background: Inhaled chemotherapeutics may enhance pulmonary drug exposure to malignant lesions in the lung without substantially contributing to systemic toxicities. The pharmacokinetic profile of inhaled submicron particle paclitaxel (NanoPac®) in healthy rodent plasma and lung tissue is evaluated here to determine administration proof-of-principle.
Methods: Healthy male Sprague Dawley rats received paclitaxel in one of three arms: intravenous nab-paclitaxel at 2.9 mg/kg (IVnP), inhaled NanoPac low dose (IHNP-LD) at 0.38 mg/kg, or inhaled NanoPac high dose (IHNP-HD) at 1.18 mg/kg. Plasma and lung tissue paclitaxel concentrations were determined using ultraperformance liquid chromatography tandem mass spectrometry from animals sacrificed at 10 time points ranging up to 2 weeks after administration. Peak concentration (Cmax), apparent residence half-life (T1/2), exposure (AUC(last)), and dose-normalized exposure (AUCD(last)) were determined. Pulmonary histopathology was performed on rats sacrificed at the 336-hour time point.
Results: Paclitaxel was detectable and quantifiable in the rat lung for both inhaled NanoPac arms sampled at the final necropsy, 336 hours postadministration. Substantial paclitaxel deposition and retention resulted in an order of magnitude increase in dose-normalized pulmonary exposure over IVnP. Inhaled NanoPac arms had an order of magnitude lower plasma Cmax than IVnP, but followed a similar plasma T1/2 clearance (quantifiable only to 72 hours postadministration). Pulmonary histopathology found all treated animals indistinguishable from treatment-naive rats.
Conclusion: In the rodent model, inhaled NanoPac demonstrated substantial deposition and retention of paclitaxel in sampled lung tissue. Further research to determine NanoPac's toxicity profile and potential efficacy as lung cancer therapy is underway.
Keywords: : chemotherapy, inhalation, nab-paclitaxel, NanoPac, NSCLC, paclitaxel, pharmacokinetics, rat, rodent
Introduction
The American Cancer Society estimates that in 2018 the United States will diagnose 243,030 new cases of lung cancer, and 154,050 deaths. Lung cancer will be responsible for 25% of all cancer-related deaths, accounting for more than breast, prostate, and colon cancer combined.(1) Surgical treatment for localized disease provides the best prognosis in early stage disease; however, early diagnosis of lung cancer is challenging, with 57% of patients exhibiting distant metastasis upon symptomatic presentation. For patients diagnosed with metastatic disease, it is estimated that only 4.5% will survive an additional 5 years.(1–3)
Surgery for these patients is generally no longer viable, requiring intravenous chemotherapy, immunotherapy, radiotherapy, or a combination thereof to prolong survival, control symptoms, or improve quality of life. Recent advances in targeted- and immunotherapies have substantially increased overall survival for their specific corresponding subpopulations,(4–6) yet the majority of patients with lung cancer will only exhibit a survival benefit of a few months. Therefore, the need remains to provide an additional long-lasting benefit to patients without significantly affecting quality of life.
A review by Nichols et al. evaluated mortality in 100 lung cancer patients, 91 of whom had presented with metastatic disease. The study found that local disease progression as the principle cause of death in 59% of subjects, causative factors included primary tumor burden, pulmonary hemorrhage, pulmonary thromboembolism, and diffuse alveolar damage.(7) Additional therapy administered directly to the primary cancer may, therefore, provide an enhanced survival response even in patients exhibiting extrapulmonary disease. Increasing local drug exposure through conventional intravenous methods widely distributes drug to nontarget organs, leading to severe systemic toxicities and low bioavailability to pulmonary malignancies before clearance by metabolism and excretion.(8)
Inhaled chemotherapy provides an alternate route of administration to overcome limitations associated with intravenous methods; substantial doses can be delivered directly to the lung while avoiding additive toxic exposure to nontarget vital organs.(8–10) Historically, achieving increased pulmonary exposure through inhalation is limited by poor retention of drug within the lung due to clearance mechanisms such as diffusion across the alveolar–capillary barrier, the mucociliary escalator removing material to the gastrointestinal tract, phagocytosis by alveolar macrophages and dendritic cells, and lymphatic drainage.(11–13)
To remain clinically feasible, inhaled chemotherapeutics would preferentially have the ability to evade or minimize pulmonary clearance mechanisms, while continuously releasing drug to maintain constant exposure to malignant lesions.(11) Inhaled cisplatin,(14,15) doxorubicin,(16,17) carboplatin,(18) gemcitabine,(19) and 9-nitro-camptothecin(19) have demonstrated proof-of-principle in clinical safety studies, yet despite promising results, have not progressed to complete efficacy trials.
Paclitaxel, an FDA-approved intravenous cytotoxic taxane that induces mitotic arrest through stabilization of microtubules in the G2/M cell cycle phase,(20,21) has yet to be clinically evaluated for efficacy as an inhaled chemotherapeutic for the treatment of NSCLC. Owing to paclitaxel's poor solubility, commercially available formulations for systemic administration pose unique difficulties for deposition and retention in the lung following inhalation. The first approved paclitaxel formulation Taxol® requires 50% Cremaphor EL as a solvent, and is known to elicit toxic biological effects.(22,23) The subsequently approved albumin-bound paclitaxel (Abraxane®(24)) has been studied preclinically for inhaled administration, which demonstrated remarkably efficient systemic bioavailability.(25)
Varying coated formulations of paclitaxel have exhibited beneficial pharmacokinetic profiles(11,26–29) and antitumor efficacy in both rodent(11,28–32) and canine models.(33,34) Of note, not only did inhaled paclitaxel significantly inhibit tumor growth as measured by lung weight,(30–32) but also significantly inhibited pulmonary metastasis from intravenously administered kidney cancer cells (Renca cell line).31
NanoPac®
NanoPac® is paclitaxel processed utilizing precipitation with compressed antisolvents, into uncoated submicron crystals sized between 600 and 800 nm35,36 that allow for suspension reconstitution in physiological saline containing 0.1% polysorbate 80 (PS80). Designed large enough to avoid clearance by systemic circulation, but with high internal surface area for drug release characteristics of smaller particles, local administration of NanoPac directly at malignant sites provides a depot effect releasing paclitaxel into surrounding fluids and tissues at constant saturation levels.36
Although in vitro dissolution methods have been marginally useful for evaluating the release of paclitaxel from NanoPac, unpublished in vivo trials and a phase 1 intraperitoneal clinical study have demonstrated the extended release profile. Twenty-one patients with peritoneal malignancies received intraperitoneal (IP) doses of 50–275 mg/m2, in up to six cycles (every 28 days); the peritoneal fluid paclitaxel concentrations were 450–2900 times greater than systemic plasma, resulting in fewer toxic-related events in comparison with intravenous trials.36
With its slow depot release properties, aerosolized NanoPac may have the potential to provide a meaningful survival benefit for patients with lung cancer. Following nebulization and deposition within the alveolar sacs, rapid diffusion of the saline would theoretically leave NanoPac particles behind for constant release of bioavailable paclitaxel directly to pulmonary surfactant and malignant cells. In this study we explore the feasibility and pharmacokinetics of nebulized NanoPac for inhalation therapy in a rodent model.
Materials and Methods
All animal procedures were conducted under protocols approved by the Institutional Animal Care and Use Committee (IACUC) at Lovelace Biomedical (Albuquerque, NM), which is accredited by the Association for Assessments and Accreditation of Laboratory Animal Care International.
Animals
Male Sprague Dawley rats (6–8 weeks old) were obtained from Charles River Laboratories (Kingston, NY). The rats were quarantined for 14 days, after which the animals were weighed and randomized for study assignment. Animals were identified by tail marking and cage card. Water, lighting, humidity, and temperature control were maintained and monitored. Rats were fed a standard rodent diet ad libitum during nonexposure hours.
Chemicals and reagents
Paclitaxel USP reference standard (USP Catalog No.: 1491332) and Paclitaxel-13C6 (TRC Catalog No.: P132504) were used as reference and internal standards within the LCMS assay. USP reference standard was used within the HPLC assay as the reference standard.
NanoPac dry powder and reconstitution solution were provided to Lovelace Biomedical by NanOlogy, LLC (Lawrence, KS), and were prepared as follows. In brief, 5.0 mL of 1% Polysorbate 80 (PS80) was added to the dry NanoPac powder vial, which was shaken vigorously and inverted to ensure wetting of all particles. Immediately after shaking, 0.9% sodium chloride solution (Hospira, IL) was added to the NanoPac vial and shaken for at least 1 minute to ensure proper dispersion, 46 mL for the 6.0 mg/mL suspension and 10.3 mL for 20.0 mg/mL suspension. Resultant formulations were left undisturbed for at least 5 minutes to reduce any air/foam in the vial before placing it in a jet nebulizer for aerosolization. The composition of the varying NanoPac concentrations only differs in final concentration of PS80: 0.1% and 0.33% for the 6.0 and 20.0 mg/mL suspensions, respectively.
Lovelace Biomedical obtained nab-paclitaxel, Abraxane (Celgene Corporation, NJ), from a clinical pharmacy and drug was reconstituted to 5.0 mg/mL with saline on the day of dosing, stored and administered per manufacturer's instructions.
Paclitaxel quantification verification from spiked glass fiber filter (GF/A)
Aerosol filter paclitaxel analysis was conducted, non-GLP, with an Agilent 1100 HPLC-ultraviolet (HPLC-UV) (Santa Clara, CA) fitted with a Phenomenex Hypersil ODS (C18) (Torrance, CA). The column temperature was set at 25°C with detection at 227 nm. The flow rate was 1.0 mL/min and the injection volume was 5 μL. Quantification was performed within validated Empower 3 software with a seven-point standard curve between 5 and 300 μg/mL prepared with Paclitaxel USP reference standard. Before utilization, the assay was characterized for linearity, reproducibility, accuracy, and precision.
In addition, a filter (GF/A, Whatman) extraction method was developed and characterized for recovery (target 85–115% recovery) over the range of paclitaxel on filters (90–785 μm). A liquid extraction was performed in 7 mL glass vials with 4 mL of methanol and acetic acid diluent (200:1). The samples were rotated, vortexed, and centrifuged at 13,000 revolutions per minute (rpm), and transferred to autosampler vials for HPLC analysis.
Sample analysis was performed with linearity standards and quality control checks within every run. The filter data were processed within Empower with validated custom fields to determine the amount of paclitaxel on filter and the corresponding paclitaxel aerosol concentration.
Paclitaxel quantification verification from biological samples
Sample (rat plasma and lung tissue) paclitaxel analysis was conducted through a non-GLP ultraperformance liquid chromatography tandem mass spectrometry (UPLC-MS/MS), with an ABSciex API 4000 triple quadrupole mass spectrometer (Framingham, MA) coupled to an Acquity H-Class UPLC (Waters, Milford, MA) with ABSciex Analyst version 1.6 software. Sample extracts were resolved on an Acquity UPLC BEH C(18) column.
Quantification was performed with paclitaxel reference standard (USP) and internal standards (Paclitaxel-13C6) in matrix (Rat plasma K2EDTA and naive lung tissue). Quality control checks were prepared in matrix over the range of each method. Plasma extraction was performed through protein precipitation with acetonitrile (100 μL plasma with 300 μL of acetonitrile). Lung tissue samples included a homogenization in 1 × phosphate-buffered saline (PBS), 1 g lung tissue with 4 mL PBS before the same protein precipitation. Samples were prepared and assayed with quality controls (QCs run in triplicate) at 3, 80, and 800 ng/mL to assess the accuracy and precision of paclitaxel. Curve fitting was performed using linear regression with 1/x2 weighting requiring a produced correlation coefficient ≥0.98.
Within bioanalytical runs, standard criteria were applied for standard inclusion (±15%) and QC criteria (±15%) before run acceptance and release of the sample data. All data below the range of the assay were reported as BQL (below quantification level).
Treatment protocol and sampling
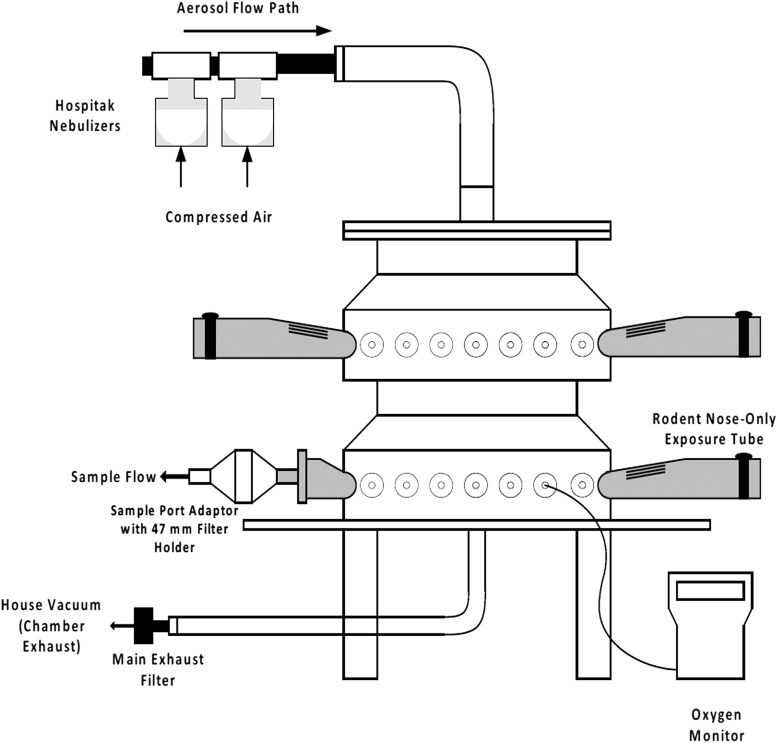
Ninety animals were randomized into three treatment groups (n = 30). The first group of animals were to receive a single intravenous tail vein injection of nab-paclitaxel (IVnP) as a positive treatment control to assess inhaled NanoPac feasibility, and was dosed to a maximum allowable 5.0 mg/kg or corresponding dose associated with the maximum allowable injection volume of 250 μL as set by IACUC. NanoPac suspension formulations of 6.0 mg/mL and 20.0 mg/mL were aerosolized on a single occasion for the inhaled NanoPac low-dose (IHNP-LD) and inhaled NanoPac high-dose (IHNP-HD) arms, respectively. Both formulations were aerosolized separately and were directed through a delivery line into a 32-port nose-only exposure chamber for 65 minutes (Fig. 1).
FIG. 1.
Schematic of the rodent exposure system for nebulized NanoPac aerosol.
NanoPac aerosols were generated with a set of two Up-Mist (Hospitak, ConvaTec, McAllen, TX) compressed air jet nebulizers at a nebulizer pressure of 20 psi (used for up to 40 [±1] minutes), then replaced with a second set of two Up-Mist nebulizers for the remaining exposure duration for a total exposure time of 65 minutes. Oxygen and temperature were monitored and recorded throughout each inhalation exposure.
Three animals (n = 3) from each arm were sacrificed at 0.5 (±10 minutes), 6 (±10 minutes), 12 (±10 minutes), 24 (±30 minutes), 48 (±30 minutes), 72 (±30 minutes), 120 (±30 minutes), 168 (±30 minutes), 240 (±30 minutes), and 336 (±30 minutes) hours postexposure. Blood samples for plasma bioanalytical analysis were collected via cardiac puncture into K2EDTA tubes. Whole lung weight was measured, lung lobes were separated, individually weighed and snap frozen in liquid nitrogen, and stored at −70°C to −90°C. Right lung lobes were designated for bioanalytical analyses and left lung lobes for histopathological review. External surfaces of the body, orifices, and the contents of the cranial, thoracic, and abdominal cavities were examined.
Particle and aerosol characteristic determination
Particle size distribution of aerosols was measured from rodent breathing zone of the nose-only exposure chamber by a Mercer-style, seven-stage cascade impactor (lntox Products, Inc., NM). Cascade impactor samples were collected at a flow rate of 2.0 ± 0.1 L/min. The particle size distributions were determined in terms of mass median aerodynamic diameter (MMAD) and geometric standard deviation (GSD). Aerosolized NanoPac concentration monitoring was conducted by analyzing aerosols on preweighed GF/A 47-mm filters sampled every 10 minutes at a flow rate of 1.0 ± 0.5 L/minute. Filters were weighed to determine the total aerosol concentration in the exposure system, then extracted and analyzed by HPLC methods already mentioned to quantify paclitaxel collected on each filter.
Inhaled dose determination
Deposited dose could not be determined in vivo due to active clearance in the lung and administration occurring for 65 minutes. Deposited pulmonary dose was calculated using Equation 1, using the average paclitaxel aerosol concentration measurements, body weights, and administration windows. Varying MMAD sizes results in different fractions of total aerosol to be deposited within the lung; FDA assumes 10% deposition fraction for rodents inhaling particles with MMAD 1–5 μm.37,38 Previous analysis with Hospitak nebulizers assumed nebulization droplets to fall within this range for the Up-Mist nebulizer.
Equation 1: Inhaled deposited dose calculation.39
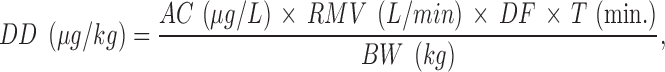 |
where DD is deposited dose, BW is body weight, RMV is respiratory minute volume = 0.608 × BW0.852, AC is aerosol concentration, and DF is deposition fraction.
Pharmacokinetics
Plasma samples were thawed immediately before analysis and were assayed through UPLC-MS methods already mentioned to quantify paclitaxel concentration at the specified necropsy time points. Right lung lobes were thawed, homogenized with PBS at a ratio of 4:1 (water–lung tissue), and underwent a similar protein precipitation with acetonitrile before UPLC-MS analysis. Quantification was conducted with a matrix-based calibration curve, with a lower level of quantification determined from the biological sample verification methods already mentioned. Noncompartmental analysis (Phoenix WinNonlin 6.2 [Certara, Princeton, NJ]) was conducted on average data at each time point from the plasma and lung tissue concentrations. Noncompartmental analysis was performed to calculate Cmax, T1/2, AUC(last), and AUCD(last).
Histopathology
Histopathologic examination was performed on left lung lobes from animals in all treatment arms (n = 3 per arm) necropsied at the final time point (336 hours), alongside three untreated controls. Tissues were processed routinely, paraffin embedded, sectioned at 4 μm, mounted, and stained with hematoxylin and eosin (H&E) for microscopic examination. Findings were graded subjectively and semiquantitatively by a single pathologist experienced in toxicologic pathology. The Provantis™ (Instem LSS Ltd., Staffordshire, England) computer software/database was used for histopathology data acquisition, reporting, and analysis.
Results
Clinical observations, survival, and body weights
All animals survived to their designated necropsy time points and were euthanized within the intended windows. No abnormal clinical observations, signs of distress, or labored breathing were noted throughout the study duration. Every animal had an increased body weight at necropsy in comparison with administration except for a single animal in the IHNP-LD group necropsied 6 hours after administration, which experienced a 9% loss in body weight.
Paclitaxel quantification verification from spiked glass fiber filter (GF/A)
The HPLC assay, before utilization, was shown to be linear between 5 and 300 μg/mL (correlation coefficient of linearity curve [R2] was 0.9989) and reproducible (% RSD of 0.3% between repeated injections). The filter spike recovery showed recovery of no more than 3.6% from target.
Paclitaxel quantification verification from biological samples
The bioanalytical methods (plasma and lung tissue) were characterized before use for accuracy, recovery, precision, and linear range. The assay was linear between 1 and 1000 ng/mL for plasma, and 10–10,000 ng/g for lung tissue, with correlation coefficients of ∼0.99. The accuracy and precision of the assay met standard ±15% range for small molecule criteria. Recovery was assessed in plasma between 3 and 800 ng/mL and in lung tissue between 10 and 7500 ng/g, all of which met standard ±15% range for small molecule criteria.
Particle and aerosol characteristics and dose determination
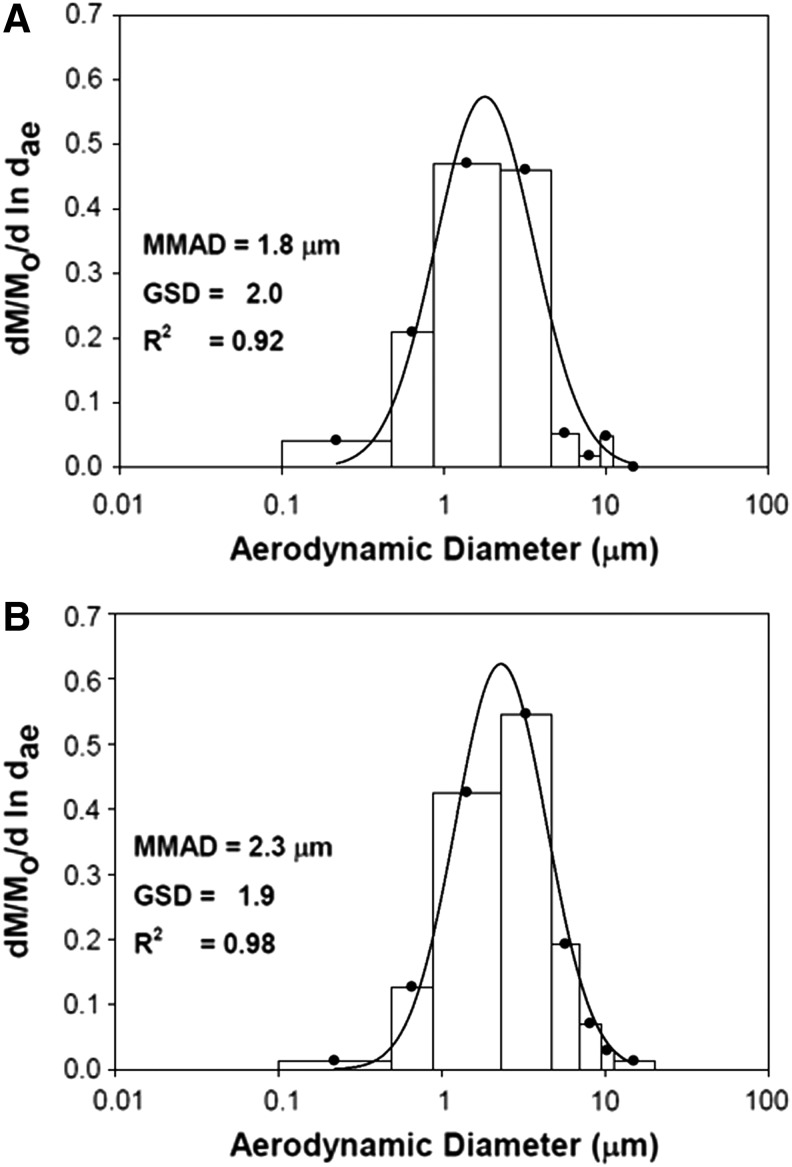
NanoPac aerosols generated by two Up-Mist compressed air jet nebulizers running simultaneously, producing aerosols with MMAD (GSD) of 1.8 (2.0) μm and 2.3 (1.9) μm (Fig. 2) for the aerosolized 6.0 and 20.0 mg/mL suspension formulations, respectively. Filters from both aerosol exposure groups were analyzed for paclitaxel accumulation and evaluated into average paclitaxel aerosol concentration (summarized in Table 1). Average paclitaxel aerosol concentrations (standard deviation) were 85.64 (8.76) μg/L and 262.27 (31.45) μg/L for the 6.0 and 20.0 mg/mL suspension formulations, respectively. Estimated doses of 0.38 and 1.18 mg/kg were calculated for the IHNP-LD and IHNP-HD arms, respectively. Owing to increasing body weights and maximum allowable injection volume of 250 μL, the IVnP arm received an average dose of 2.9 mg/kg.
FIG. 2.
Mass median aerodynamic size distribution of nebulized (A) 6.0 mg/mL NanoPac suspension and (B) 20.0 mg/mL NanoPac suspension. NanoPac aerosols generated by two Up-Mist compressed air jet nebulizers produced aerosols with MMAD (GSD) of 1.8 (2.0) μm and 2.3 (1.9) μm as measured with the Mercer-style cascade impactor for the 6.0 mg/mL and 20.0 mg/mL suspensions, respectively. MMAD, mass median aerodynamic diameter; GSD, geometric standard deviation; R2 = coefficient of correlation.
Table 1.
Inhaled Aerosolized NanoPac Exposure Filter Analysis
| Filter ID No. | Total aerosol concentration (mg/L) | Paclitaxel aerosol concentration (μg/L) | |
|---|---|---|---|
| IHND-LD | FS-1-L | 0.247 | 80.05 |
| FS-2-L | 0.242 | 81.79 | |
| FS-3-L | 0.252 | 87.09 | |
| FS-4-L | 0.296 | 104.38 | |
| FS-5-L | 0.247 | 78.47 | |
| FS-6-L | 0.249 | 82.50 | |
| FS-7-L | 0.244 | 85.19 | |
| Average | 0.25 | 85.64 | |
| SD | 0.02 | 8.76 | |
| % RSD | 7.43 | 10.23 | |
| IHND-HD | FS-1-H | 0.383 | 212.53 |
| FS-2-H | 0.412 | 239.28 | |
| FS-3-H | 0.494 | 291.44 | |
| FS-4-H | 0.516 | 296.56 | |
| FS-5-H | 0.456 | 254.67 | |
| FS-6-H | 0.501 | 289.50 | |
| FS-7-H | 0.431 | 251.88 | |
| Average | 0.46 | 262.27 | |
| SD | 0.05 | 31.45 | |
| % RSD | 10.95 | 11.99 |
IHNP-LD, inhaled NanoPac low-dose arm; IHNP-HD, inhaled NanoPac high-dose arm; SD, standard deviation; % RSD, percentage relative standard deviation.
Pharmacokinetics of inhaled NanoPac
Tables 2 and 3 depict analyzed paclitaxel concentrations from plasma and homogenized right lung tissue, respectively. Table 4 summarizes the calculated pharmacokinetic values for both the plasma and lung tissue. The paclitaxel concentration–time curves throughout the study for the lung tissue and plasma are visualized in Figure 3A and B, respectively.
Table 2.
Paclitaxel Quantification in Rodent Plasma
| Time point (hour) | IVnP | IHNP-LD | IHNP-HD | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Concentration (ng/mL) | Average (ng/mL) | Standard deviation | Concentration (ng/mL) | Average (ng/mL) | Standard deviation | Concentration (ng/mL) | Average (ng/mL) | Standard deviation | |
| 0.5 | 153 | 206 | 44.1 | 15.6 | 11.6 | 3.5 | 10.8 | 15.9 | 4.3 |
| 205 | 12.1 | 21.3 | |||||||
| 261 | 7.09 | 15.6 | |||||||
| 6 | 70.5 | 62.2 | 9.2 | 3.44 | 2.87 | 0.4 | 6.56 | 5.69 | 1.0 |
| 66.7 | 2.37 | 4.35 | |||||||
| 49.3 | 2.81 | 6.15 | |||||||
| 12 | 18.9 | 20.0 | 0.9 | 5.29 | 3.35 | 1.4 | 7.14 | 4.95 | 1.6 |
| 20 | 2.08 | 3.47 | |||||||
| 21.1 | 2.67 | 4.23 | |||||||
| 24 | 9.46 | 15.3 | 4.4 | BQL | 1.26 | 0.1 | 1.47 | 1.96 | 0.8 |
| 16.3 | 1.16 | 3.11 | |||||||
| 20.1 | 1.36 | 1.31 | |||||||
| 48 | 5.08 | 2.98 | 1.5 | BQL | BQL | N/A | 1.21 | 1.21 | N/A |
| 1.56 | BQL | BQL | |||||||
| 2.3 | BQL | BQL | |||||||
| 72 | BQL | 1.05 | N/A | BQL | BQL | N/A | BQL | 1.06 | N/A |
| 1.05 | BQL | 1.06 | |||||||
| BQL | BQL | BQL | |||||||
| 120 | BQL | BQL | N/A | BQL | BQL | N/A | BQL | BQL | N/A |
| BQL | BQL | BQL | |||||||
| BQL | BQL | BQL | |||||||
| 168 | BQL | BQL | N/A | BQL | BQL | N/A | BQL | BQL | N/A |
| BQL | BQL | BQL | |||||||
| BQL | BQL | BQL | |||||||
| 240 | BQL | BQL | N/A | BQL | BQL | N/A | BQL | BQL | N/A |
| BQL | BQL | BQL | |||||||
| BQL | BQL | BQL | |||||||
| 336 | BQL | BQL | N/A | BQL | BQL | N/A | BQL | BQL | N/A |
| BQL | BQL | BQL | |||||||
| BQL | BQL | BQL | |||||||
IVnP, intravenous nab-paclitaxel arm (2.9 mg/kg); IHNP-LD, inhaled NanoPac-low dose (0.38 mg/kg); IHNP-HD, inhaled NanoPac-high dose (1.18 mg/kg); BQL, below quantifiable level; N/A, not assessable.
Table 3.
Paclitaxel Quantification in the Right Rodent Lung
| Time point (hour) | IVnP | IHNP-LD | IHNP-HD | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Concentration (ng/g) | Average (ng/g) | Standard deviation | Concentration (ng/g) | Average (ng/g) | Standard deviation | Concentration (ng/g) | Average (ng/g) | Standard deviation | |
| 0.5 | 5850 | 5800 | 430.1 | 19,450 | 21,000 | 3503.1 | 40,400 | 41,600 | 1557.8 |
| 5250 | 17,700 | 43,800 | |||||||
| 6300 | 25,850 | 40,600 | |||||||
| 6 | 2665 | 2730 | 106.4 | 6700 | 4990 | 1219.1 | 15,500 | 20,800 | 4499.6 |
| 2880 | 3945 | 20,400 | |||||||
| 2645 | 4325 | 26,500 | |||||||
| 12 | 1045 | 1170 | 113.7 | 6200 | 5368 | 764.1 | 17,050 | 14,700 | 1661.8 |
| 1145 | 5550 | 13,500 | |||||||
| 1320 | 4355 | 13,550 | |||||||
| 24 | 386 | 647 | 188.6 | 2325 | 3008 | 1170.0 | 10,300 | 11,433 | 838.0 |
| 825 | 2045 | 11,700 | |||||||
| 730 | 4655 | 12,300 | |||||||
| 48 | 307 | 244 | 48.1 | 850 | 1247 | 288.9 | 6000 | 6700 | 535.4 |
| 190 | 1530 | 7300 | |||||||
| 237 | 1360 | 6800 | |||||||
| 72 | 101 | 145 | 54.0 | 950 | 950 | 355.2 | 4375 | 3953 | 863.5 |
| 221 | 1385 | 4735 | |||||||
| 113 | 515 | 2750 | |||||||
| 120 | BQL | BQL | N/A | 1500 | 1045 | 327.1 | 1570 | 1923 | 846.1 |
| BQL | 890 | 1110 | |||||||
| BQL | 745 | 3090 | |||||||
| 168 | BQL | BQL | N/A | 309 | 377 | 236.1 | 3395 | 2143 | 889.4 |
| BQL | 695 | 1410 | |||||||
| BQL | 129 | 1625 | |||||||
| 240 | BQL | BQL | N/A | 58 | 109 | 38.4 | 271 | 430 | 122.8 |
| BQL | 151 | 448 | |||||||
| BQL | 117 | 570 | |||||||
| 336 | BQL | BQL | N/A | BQL | 55.5 | N/A | 233 | 272 | 67.5 |
| BQL | 55.5 | 367 | |||||||
| BQL | BQL | 216 | |||||||
IVnP, intravenous nab-paclitaxel arm (2.9 mg/kg); IHNP-LD, inhaled NanoPac-low dose (0.38 mg/kg); IHNP-HD, inhaled NanoPac-high dose (1.18 mg/kg); BQL, below quantifiable level; N/A, not assessable.
Table 4.
Paclitaxel Pharmacokinetic Profiles
| Group | Average paclitaxel aerosol concentration μg/L (std dev) | Dose mg/kg (std dev) | Plasma | Lung tissue | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cmax ng/mL (std dev) | T1/2 hours | AUC(last) hr*ng/mL | AUCD(last) hr*ng*mg/mL*kg | Cmax ng/g (std dev) | T1/2 hours | AUC(last) hr*ng/g | AUCD(last) hr*ng*mg/g*kg | |||
| IVnP | N/A | 2.9 (0.16) | 206 (44.1) | 8.7 | 1,517 | 528 | 5,800 (430.1) | 19.9 | 62,870 | 23,112 |
| IHNP-LD | 85.64 (8.76) | 0.38 (0.003) | 11.6 (3.5) | 7.9 | 101 | 264 | 21,000 (3503.1) | 56.3 | 342,877 | 914,095 |
| IHNP-HD | 262.27 (31.45) | 1.18 (0.01) | 15.9 (4.3) | 8.6 | 228 | 193 | 41,600 (1557.8) | 56.0 | 1,155,662 | 997,985 |
Cmax, maximum concentration; T1/2, apparent residence half-life; AUC(last), exposure (area under the curve); AUCD(last), dose-normalized exposure; std dev, standard deviation.
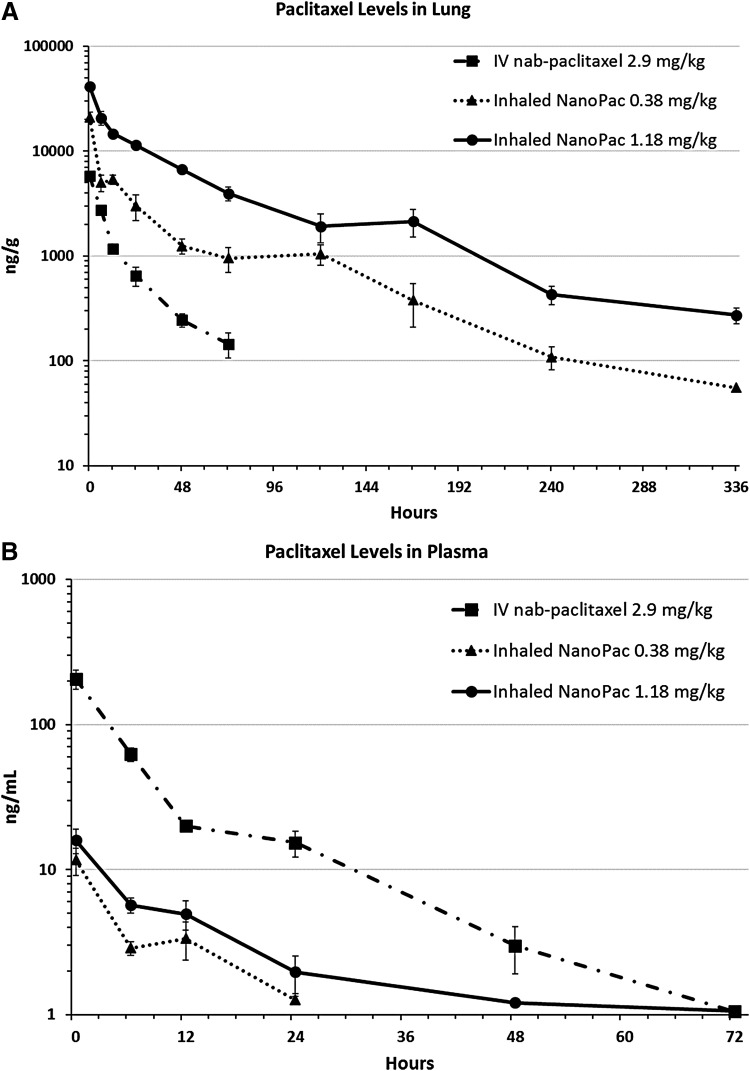
FIG. 3.
Postadministration paclitaxel concentration levels (A) in the lung tissue and (B) in the plasma. Male Sprague Dawley rats (6–8 weeks old) were administered paclitaxel on a single occasion in one of three treatment arms (n = 30 each): inhaled NanoPac in a nose-only exposure at a low dose of 0.38 mg/kg or a high dose of 1.18 mg/kg, or intravenous nab-paclitaxel administered through tail vein injection at 2.9 mg/kg. Three animals from each arm were sacrificed at 0.5, 6, 12, 24, 48, 72, 120, 168, 240, and 336 hours postexposure for lung tissue and plasma collections. Lung tissue (A) and plasma (B) were assayed through ultraperformance liquid chromatography tandem mass spectrometry to quantify paclitaxel concentration as a function of time with a lower level of quantification of 50 ng/g and 1 ng/mL, respectively (mean ±1SEM).
The initial paclitaxel exposure to the right lung lobes of the rats was higher in the IHNP-LD and IHNP-HD arms than in IVnP, with Cmax values 3.5- and 7-fold greater, respectively. Although paclitaxel quantification was not performed on the left lung lobes, they were assumed to have similar concentration profiles as the right lobes.40 After deposition, inhaled NanoPac appeared to clear from the lung at a slower rate than that of IVnP, a roughly threefold increase in T1/2 in both arms. IVnP paclitaxel levels were only quantifiable to the 72-hour time point, whereas both inhaled NanoPac arms were quantifiable to study completion (336-hour time point).
The paclitaxel deposition and retention resulted in a pulmonary AUC(last) roughly 5.5- and 18-times greater in the IHNP-LD and IHNP-HD arms than in the IVnP arm, respectively. When dose normalized, IHNP-LD and IHNP-HD arms exhibited 39- and 43-fold greater paclitaxel exposure per drug unit dose than the IVnP arm.
The plasma paclitaxel concentration was higher in the IVnP arm than in the IHNP-LD and IHNP-HD arms, with Cmax values in the plasma roughly 17.75- and 13-fold greater, respectively. All arms exhibited similar clearance from the plasma, all T1/2 values being within a range of 7.9–8.7 hours. Furthermore, IVnP and IHNP-HD were only quantifiable to the 72-hour time point, and IHNP-LD only to 24-hour time point. These plasma paclitaxel levels resulted in an AUC(last) exposure 15- and 6.5-fold lower in the IHNP-LD and IHNP-HD than in IVnP, respectively. Dose-normalized plasma exposures were also greater in the IVnP arm by roughly 2- and 2.75-times than in the IHNP-LD and IHNP-HD arms, respectively.
Histopathology
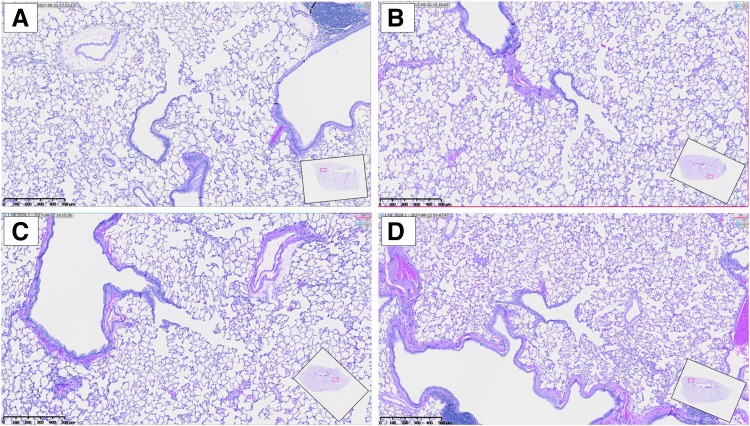
No abnormalities were noted within the trachea or left lung lobes of the animals sacrificed 336 hours postadministration in any arm. When compared with untreated control tissue samples, treated tissues were microscopically indistinguishable (Fig. 4). Although not be construed as equivalent to a thorough histologic evaluation, the results indicate that 2 weeks postsingle administration of NanoPac at 1.18 mg/kg, substantial pathology does not occur within the trachea or lungs.
FIG. 4.
Histology slides from (A) an untreated rat, (B) a rat treated with intravenous nab-paclitaxel at 2.9 mg/kg, (C) a rat treated with inhaled NanoPac at 0.38 mg/kg, and (D) a rat treated with inhaled NanoPac at 1.18 mg/kg (bar = 500 μm). Tissues from fixed left lung lobes of rats sacrificed at the 336-hour time point were processed routinely, paraffin embedded, sectioned at 4 μm, mounted, and stained with hematoxylin and eosin for microscopic examination.
Discussion
Inhalation of aerosolized NanoPac was successful in this study via compressed air jet nebulization. Particle characteristic analysis found both NanoPac suspensions nebulized with MMADs ∼2 μm, falling within the 1–3 μm range required for efficient distribution and deposition throughout the lung when translating into the clinic.41–47 Paclitaxel quantification on the right lung lobes found retention of drug beyond 14 days, whereas systemic plasma concentrations fell BQL 3 days postadministration. This extended retention of paclitaxel at malignant sites may allow for increased efficacy when combined with conventional therapies to treat diseases such as NSCLC, without substantially contributing to systemic toxicities.
Although the total paclitaxel concentration analyzed within the right lung lobes is considerable, the amount of bioavailable paclitaxel is still to be determined as release from NanoPac is gated by saturation levels in the surrounding environment. Although residual NanoPac crystals may remain within the lung up to 2 weeks after inhalation, histologic evaluation of the left lung lobes from rats sacrificed at 336 hours postadministration revealed treatment arms indistinguishable from untreated controls. Multiple administration pharmacology and toxicology studies are underway to further research inhaled NanoPac's potential as lung cancer therapy.
Ethical Adherences
This study complied with all applicable sections of the Final Rules of the Animal Welfare Act regulations (9 CFR Parts 1, 2, and 3), as well as the Guide for the Care and Use of Laboratory Animals (2011). Lovelace Biomedical is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care.
Author Disclosure Statement
All funding for the study detailed in the article is provided by NanOlogy, LLC. The following authors reported receiving remuneration, holding a consultant/advisory role, or receiving funding from NanOlogy, LLC: James Verco, William Johnston, Michael Baltezor, Philip J. Kuehl, Andrew Gigliotti, Steven A. Belinsky, Anita Lopez, Ronald Wolff, Lauren Hylle, and Gere diZerega.
Reviewed by: Remi Rosiere
References
- 1. Cancer Facts & Figures 2018. American Cancer Society, Atlanta, GA; 2018 [Google Scholar]
- 2. SEER Cancer Stat Facts: Lung and Bronchus Cancer; 2017. https://seer.cancer.gov/statfacts/html/lungb.html
- 3. Non-Small Cell Lung Cancer: NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). 3.2018 ed. National Comprehensive Cancer Network; 2018 [Google Scholar]
- 4. Hanna N, Johnson D, Temin S, Baker S, Jr., Brahmer J, Ellis PM, Giaccone G, Hesketh PJ, Jaiyesimi I, Leighl NB, Riely GJ, Schiller JH, Schneider BJ, Smith TJ, Tashbar J, Biermann WA, and Masters G: Systemic therapy for stage IV non-small-cell lung cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2017;35:3484–3515 [DOI] [PubMed] [Google Scholar]
- 5. Ruiz-Ceja KA, and Chirino YI: Current FDA-approved treatments for non-small cell lung cancer and potential biomarkers for its detection. Biomed Pharmacother. 2017;90:24–37 [DOI] [PubMed] [Google Scholar]
- 6. Lazzari C, Bulotta A, Ducceschi M, Vigano MG, Brioschi E, Corti F, Gianni L, and Gregorc V: Historical evolution of second-line therapy in non-small cell lung cancer. Front Med (Lausanne). 2017;4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nichols L, Saunders R, and Knollmann FD: Causes of death of patients with lung cancer. Arch Pathol Lab Med. 2012;136:1552–1557 [DOI] [PubMed] [Google Scholar]
- 8. Mangal S, Gao W, Li T, and Zhou QT: Pulmonary delivery of nanoparticle chemotherapy for the treatment of lung cancers: Challenges and opportunities. Acta Pharmacol Sin. 2017;38:782–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zarogoulidis P, Chatzaki E, Porpodis K, Domvri K, Hohenforst-Schmidt W, Goldberg EP, Karamanos N, and Zarogoulidis K: Inhaled chemotherapy in lung cancer: Future concept of nanomedicine. Int J Nanomedicine. 2012;7:1551–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Abdelaziz HM, Gaber M, Abd-Elwakil MM, Mabrouk MT, Elgohary MM, Kamel NM, Kabary DM, Freag MS, Samaha MW, Mortada SM, Elkhodairy KA, Fang JY, and Elzoghby AO: Inhalable particulate drug delivery systems for lung cancer therapy: Nanoparticles, microparticles, nanocomposites and nanoaggregates. J Control Release. 2018;269:374–392 [DOI] [PubMed] [Google Scholar]
- 11. Rosiere R, Van Woensel M, Gelbcke M, Mathieu V, Hecq J, Mathivet T, Vermeersch M, Van Antwerpen P, Amighi K, and Wauthoz N: New folate-grafted chitosan derivative to improve delivery of paclitaxel-loaded solid lipid nanoparticles for lung tumor therapy by inhalation. Mol Pharm. 2018;15:899–910 [DOI] [PubMed] [Google Scholar]
- 12. Tsuda A, and Gehr P: Nanoparticles in the Lung: Environmental Exposure and Drug Delivery. CRC Press, Taylor & Francis Group, Boca Raton, FL; 2015 [Google Scholar]
- 13. Lehnert BE: Pulmonary and thoracic macrophage subpopulations and clearance of particles from the lung. Environ Health Perspect. 1992;97:17–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wittgen BP, Kunst PW, van der Born K, van Wijk AW, Perkins W, Pilkiewicz FG, Perez-Soler R, Nicholson S, Peters GJ, and Postmus PE: Phase I study of aerosolized SLIT cisplatin in the treatment of patients with carcinoma of the lung. Clin Cancer Res. 2007;13:2414–2421 [DOI] [PubMed] [Google Scholar]
- 15. Zarogoulidis P, Darwiche K, Krauss L, Huang H, Zachariadis GA, Katsavou A, Hohenforst-Schmidt W, Papaiwannou A, Vogl TJ, Freitag L, Stamatis G, and Zarogoulidis K: Inhaled cisplatin deposition and distribution in lymph nodes in stage II lung cancer patients. Future Oncol. 2013;9:1307–1313 [DOI] [PubMed] [Google Scholar]
- 16. Otterson GA, Villalona-Calero MA, Sharma S, Kris MG, Imondi A, Gerber M, White DA, Ratain MJ, Schiller JH, Sandler A, Kraut M, Mani S, and Murren JR: Phase I study of inhaled Doxorubicin for patients with metastatic tumors to the lungs. Clin Cancer Res. 2007;13:1246–1252 [DOI] [PubMed] [Google Scholar]
- 17. Otterson GA, Villalona-Calero MA, Hicks W, Pan X, Ellerton JA, Gettinger SN, and Murren JR: Phase I/II study of inhaled doxorubicin combined with platinum-based therapy for advanced non-small cell lung cancer. Clin Cancer Res. 2010;16:2466–2473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zarogoulidis P, Eleftheriadou E, Sapardanis I, Zarogoulidou V, Lithoxopoulou H, Kontakiotis T, Karamanos N, Zachariadis G, Mabroudi M, Zisimopoulos A, and Zarogoulidis K: Feasibility and effectiveness of inhaled carboplatin in NSCLC patients. Invest New Drugs. 2012;30:1628–1640 [DOI] [PubMed] [Google Scholar]
- 19. Lemarie E, Vecellio L, Hureaux J, Prunier C, Valat C, Grimbert D, Boidron-Celle M, Giraudeau B, le Pape A, Pichon E, Diot P, el Houfia A, and Gagnadoux F: Aerosolized gemcitabine in patients with carcinoma of the lung: Feasibility and safety study. J Aerosol Med Pulm Drug Deliv. 2011;24:261–270 [DOI] [PubMed] [Google Scholar]
- 20. Gautam A, and Koshkina N: Paclitaxel (taxol) and taxoid derivates for lung cancer treatment: Potential for aerosol delivery. Curr Cancer Drug Targets. 2003;3:287–296 [DOI] [PubMed] [Google Scholar]
- 21. Barbuti AM, and Chen ZS: Paclitaxel through the ages of anticancer therapy: Exploring its role in chemoresistance and radiation therapy. Cancers (Basel). 2015;7:2360–2371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gelderblom H, Verweij J, Nooter K, and Sparreboom A: Cremophor EL: The drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer. 2001;37:1590–1598 [DOI] [PubMed] [Google Scholar]
- 23. Taxol® [package insert]: Drugs@FDA: FDA Approved Drug Products. 2011 [Google Scholar]
- 24. Abraxane® [prescribing information]: Abraxane for Injectable Suspension. 2015 [Google Scholar]
- 25. Desai N, De T, Yang A, Beals B, and Soon-Schlong P: #3672 Pulmonary delivery of a novel, cremaphor-free, protein-based nanoparticle preparation of paclitaxel. American Association for Cancer Research; Philadelphia, PA: 2003 [Google Scholar]
- 26. Gill KK, Nazzal S, and Kaddoumi A: Paclitaxel loaded PEG(5000)-DSPE micelles as pulmonary delivery platform: Formulation characterization, tissue distribution, plasma pharmacokinetics, and toxicological evaluation. Eur J Pharm Biopharm. 2011;79:276–284 [DOI] [PubMed] [Google Scholar]
- 27. Koshkina NV, Knight V, Gilbert BE, Golunski E, Roberts L, and Waldrep JC: Improved respiratory delivery of the anticancer drugs, camptothecin and paclitaxel, with 5% CO2-enriched air: Pharmacokinetic studies. Cancer Chemother Pharmacol. 2001;47:451–456 [DOI] [PubMed] [Google Scholar]
- 28. Joshi N, Shirsath N, Singh A, Joshi KS, and Banerjee R: Endogenous lung surfactant inspired pH responsive nanovesicle aerosols: Pulmonary compatible and site-specific drug delivery in lung metastases. Sci Rep. 2014;4:7085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Luo T, Loira-Pastoriza C, Patil HP, Ucakar B, Muccioli GG, Bosquillon C, and Vanbever R: PEGylation of paclitaxel largely improves its safety and anti-tumor efficacy following pulmonary delivery in a mouse model of lung carcinoma. J Control Release. 2016;239:62–71 [DOI] [PubMed] [Google Scholar]
- 30. Koshkina NV, Golunski E, Roberts LE, Gilbert BE, and Knight V: Cyclosporin A aerosol improves the anticancer effect of paclitaxel aerosol in mice. J Aerosol Med. 2004;17:7–14 [DOI] [PubMed] [Google Scholar]
- 31. Koshkina NV, Waldrep JC, Roberts LE, Golunski E, Melton S, and Knight V: Paclitaxel liposome aerosol treatment induces inhibition of pulmonary metastases in murine renal carcinoma model. Clin Cancer Res. 2001;7:3258–3262 [PubMed] [Google Scholar]
- 32. Knight V, Koshkina NV, Golunski E, Roberts LE, and Gilbert BE: Cyclosporin A aerosol improves the anticancer effect of paclitaxel aerosol in mice. Trans Am Clin Climatol Assoc. 2004;115:395–404; discussion 404 [PMC free article] [PubMed] [Google Scholar]
- 33. Hershey AE, Kurzman ID, Forrest LJ, Bohling CA, Stonerook M, Placke ME, Imondi AR, and Vail DM: Inhalation chemotherapy for macroscopic primary or metastatic lung tumors: Proof of principle using dogs with spontaneously occurring tumors as a model. Clin Cancer Res. 1999;5:2653–2659 [PubMed] [Google Scholar]
- 34. Khanna C, and Vail DM: Targeting the lung: Preclinical and comparative evaluation of anticancer aerosols in dogs with naturally occurring cancers. Curr Cancer Drug Targets. 2003;3:265–273 [DOI] [PubMed] [Google Scholar]
- 35. Roby KF, Niu F, Rajewski RA, Decedue C, Subramaniam B, and Terranova PF: Syngeneic mouse model of epithelial ovarian cancer: Effects of nanoparticulate paclitaxel, Nanotax. Adv Exp Med Biol. 2008;622:169–181 [DOI] [PubMed] [Google Scholar]
- 36. Williamson SK, Johnson GA, Maulhardt HA, Moore KM, McMeekin DS, Schulz TK, Reed GA, Roby KF, Mackay CB, Smith HJ, Weir SJ, Wick JA, Markman M, diZerega GS, Baltezor MJ, Espinosa J, and Decedue CJ: A phase I study of intraperitoneal nanoparticulate paclitaxel (Nanotax®) in patients with peritoneal malignancies. Cancer Chemother Pharmacol. 2015;75:1075–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tepper JS, Kuehl PJ, Cracknell S, Nikula KJ, Pei L, and Blanchard JD: Symposium summary: “Breathe in, breathe out, its easy: What you need to know about developing inhaled drugs”. Int J Toxicol. 2016;35:376–392 [DOI] [PubMed] [Google Scholar]
- 38. Wolff RK, and Dorato MA: Toxicologic testing of inhaled pharmaceutical aerosols. Crit Rev Toxicol. 1993;23:343–369 [DOI] [PubMed] [Google Scholar]
- 39. Alexander DJ, Collins CJ, Coombs DW, Gilkison IS, Hardy CJ, Healey G, Karantabias G, Johnson N, Karlsson A, Kilgour JD, and McDonald P: Association of Inhalation Toxicologists (AIT) working party recommendation for standard delivered dose calculation and expression in non-clinical aerosol inhalation toxicology studies with pharmaceuticals. Inhal Toxicol. 2008;20:1179–1189 [DOI] [PubMed] [Google Scholar]
- 40. Brain JD, Knudson DE, Sorokin SP, and Davis MA: Pulmonary distribution of particles given by intratracheal instillation or by aerosol inhalation. Environ Res. 1976;11:13–33 [DOI] [PubMed] [Google Scholar]
- 41. Hussain M, Madl P, and Khan A: Lung deposition predictions of airborne particles and the emergence of contemporary disease Part-I. theHealth. 2011;2:51–59 [Google Scholar]
- 42. Darwiche K, Zarogoulidis P, Karamanos NK, Domvri K, Chatzaki E, Constantinidis TC, Kakolyris S, and Zarogoulidis K: Efficacy versus safety concerns for aerosol chemotherapy in non-small-cell lung cancer: A future dilemma for micro-oncology. Future Oncol. 2013;9:505–525 [DOI] [PubMed] [Google Scholar]
- 43. Labiris NR, and Dolovich MB: Pulmonary drug delivery. Part I: Physiological factors affecting therapeutic effectiveness of aerosolized medications. Br J Clin Pharmacol. 2003;56:588–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fernandez Tena A, and Casan Clara P: Deposition of inhaled particles in the lungs. Arch Bronconeumol. 2012;48:240–246 [DOI] [PubMed] [Google Scholar]
- 45. Yang W, Peters JI, and Williams RO, 3rd: Inhaled nanoparticles—A current review. Int J Pharm. 2008;356:239–247 [DOI] [PubMed] [Google Scholar]
- 46. Sharma S, White D, Imondi AR, Placke ME, Vail DM, and Kris MG: Development of inhalational agents for oncologic use. J Clin Oncol. 2001;19:1839–1847 [DOI] [PubMed] [Google Scholar]
- 47. Carvalho TC, Peters JI, and Williams RO, 3rd: Influence of particle size on regional lung deposition—What evidence is there? Int J Pharm. 2011;406:1–10 [DOI] [PubMed] [Google Scholar]